Effect of Photoperiod and Glycerol Supplementation on the Biomass Productivity and Protein Production of Spirulina sp. LEB 18 Cultures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture Conditions
2.2. Evaluation of Growth Parameters
2.3. Biomass Harvest
2.4. Biomass Composition
2.5. Glycerol Consumption
2.6. Determination of Chlorophylls
2.7. Statistical Analysis
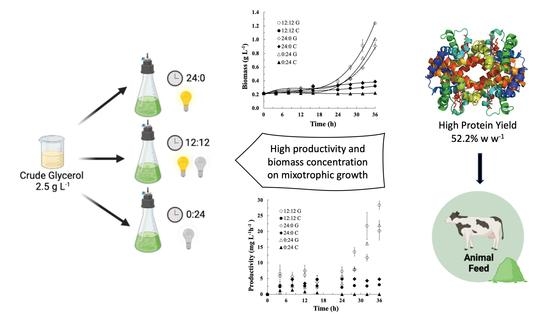
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morais, M.G.; da Silva Vaz, B.; Morais, E.G.; Costa, J.A.V. Biologically Active Metabolites Synthesized by Microalgae. BioMed Res. Int. 2015, 2015, 835761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitaya, Y.; Xiao, L.; Masuda, A.; Ozawa, T.; Tsuda, M.; Omasa, K. Effects of temperature, photosynthetic photon flux density, photoperiod and O2 and CO2 concentrations on growth rates of the symbiotic dinoflagellate, Amphidinium sp. J. Appl. Phycol. 2009, 20, 287–292. [Google Scholar] [CrossRef]
- Matos, Â.P.; Cavanholi, M.G.; Moecke, E.H.S.; Sant’Anna, E.S. Effects of different photoperiod and trophic conditions on biomass, protein and lipid production by the marine alga Nannochloropsis gaditana at optimal concentration of desalination concentrate. Bioresour. Technol. 2017, 224, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Wahidin, S.; Idris, A.; Shaleh, S.R.M. The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresour. Technol. 2013, 129, 7–11. [Google Scholar] [CrossRef]
- Daneshvar, E.; Ok, Y.S.; Tavakoli, S.; Sarkar, B.; Shaheen, S.M.; Hong, H.; Luo, Y.; Rinklebe, J.; Song, H.; Bhatnagar, A. Insights into upstream processing of microalgae: A review. Bioresour. Technol. 2021, 329, 124870. [Google Scholar] [CrossRef]
- Deng, X.; Chen, B.; Xue, C.; Li, D.; Hu, X.; Gao, K. Biomass production and biochemical profiles of a freshwater microalga Chlorella kessleri in mixotrophic culture: Effects of light intensity and photoperiodicity. Bioresour. Technol. 2018, 273, 358–367. [Google Scholar] [CrossRef]
- Abad, S.; Turon, X. Valorization of biodiesel derived glycerol as a carbon source to obtain added-value metabolites: Focus on polyunsaturated fatty acids. Biotechnol. Adv. 2012, 30, 733–741. [Google Scholar] [CrossRef]
- Luo, X.; Ge, X.; Cui, S.; Li, Y. Value-added processing of crude glycerol into chemicals and polymers. Bioresour. Technol. 2016, 215, 144–154. [Google Scholar] [CrossRef] [Green Version]
- Morais, E.G.; Druzian, J.I.; Nunes, I.L.; Morais, M.G.; Costa, J.A.V. Glycerol increases growth, protein production and alters the fatty acids profile of Spirulina (Arthrospira) sp. LEB 18. Process Biochem. 2018, 76, 40–45. [Google Scholar] [CrossRef]
- Morais, E.G.; Nunes, I.L.; Druzian, J.I.; de Morais, M.G.; da Rosa, A.P.C.; Costa, J.A.V. Increasing the cell productivity of mixotrophic growth of Spirulina sp. LEB 18 with crude glycerol. Biomass Convers. Biorefinery 2022, 1, 3. [Google Scholar] [CrossRef]
- Morais, E.G.; Nunes, I.L.; Druzian, J.I.; Morais, M.G.; da Rosa, A.P.C.; Costa, J.A.V. Increase in Biomass Productivi-ty and Protein Content of Spirulina sp. LEB 18 (Arthrospira) Cultivated with Crude Glycerol. Biomass Convers. Biorefinery 2020, 12, 597–605. [Google Scholar] [CrossRef]
- Paternina, L.P.R.; Moraes, L.; Santos, T.D.; Morais, M.G.; Costa, J.A.V. Spirulina and Açai as Innovative Ingredients in the Development of Gummy Candies. J. Food Process. Preserv. 2022, 1, e17261. [Google Scholar] [CrossRef]
- Veiga, M.C.; Fontoura, M.M.; de Oliveira, M.G.; Costa, J.A.V.; Santos, L.O. Magnetic Fields: Biomass Potential of Spirulina Sp. for Food Supplement. Bioprocess Biosyst. Eng. 2020, 43, 1231–1240. [Google Scholar] [CrossRef]
- Benemann, J. Microalgae for Biofuels and Animal Feeds. Energies 2013, 6, 5869–5886. [Google Scholar] [CrossRef] [Green Version]
- Paranjape, K.; Leite, G.B.; Hallenbeck, P.C. Effect of Nitrogen Regime on Microalgal Lipid Production during Mixo-trophic Growth with Glycerol. Bioresour. Technol. 2016, 214, 778–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paranjape, K.; Leite, G.B.; Hallenbeck, P.C. Strain variation in microalgal lipid production during mixotrophic growth with glycerol. Bioresour. Technol. 2016, 204, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Baldisserotto, C.; Sabia, A.; Guerrini, A.; Demaria, S.; Maglie, M.; Ferroni, L.; Pancaldi, S. Mixotrophic cultivation of Thalassiosira pseudonana with pure and crude glycerol: Impact on lipid profile. Algal Res. 2021, 54, 102194. [Google Scholar] [CrossRef]
- Morais, M.G.; Reichert, C.D.C.; Dalcanton, F.; Durante, A.J.; Marins, L.F.; Costa, J.V. Isolation and Characterization of a New Arthrospira Strain. Z. Fur Nat. Sect. C J. Biosci. 2008, 63, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, P.L.L.; da Silva, A.C.M.S.; Filho, J.A.M.; Druzian, J.I. Impact of different by-products from the biodiesel industry and bacterial strains on the production, composition, and properties of novel polyhydroxyalkanoates containing achiral building blocks. Ind. Crops Prod. 2015, 69, 212–223. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Colla, L.M.; Filho, P.D.; Kabke, K.; Weber, A.; Vieira Costa, J.A.; Colla, L.M.; Filho, P.D.; Kabke, K.; We-ber, A.; et al. Modelling of Spirulina platensis growth in fresh water using response surface methodology. World J. Microbiol. Biotechnol. 2002, 18, 603–607. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Marsh, J.B.; Weinstein, D.B. Simple charring method for determination of lipids. J. Lipid Res. 1966, 7, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Bondioli, P.; Della Bella, L. An alternative spectrophotometric method for the determination of free glycerol in biodiesel. Eur. J. Lipid Sci. Technol. 2005, 107, 153–157. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Mitra, D.; van Leeuwen, J.; Lamsal, B. Heterotrophic/mixotrophic cultivation of oleaginous Chlorella vulgaris on industrial co-products. Algal Res. 2012, 1, 40–48. [Google Scholar] [CrossRef]
- Chojnacka, K.; Noworyta, A. Evaluation of Spirulina sp. growth in photoautotrophic, heterotrophic and mixotrophic cultures. Enzym. Microb. Technol. 2004, 34, 461–465. [Google Scholar] [CrossRef]
- Li, T.; Kirchhoff, H.; Gargouri, M.; Feng, J.; Cousins, A.B.; Pienkos, P.T.; Gang, D.R.; Chen, S. Assessment of photosynthesis regulation in mixotrophically cultured microalga Chlorella sorokiniana. Algal Res. 2016, 19, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Cheirsilp, B.; Suwannarat, W.; Niyomdecha, R. Mixed culture of oleaginous yeast Rhodotorula glutinis and microalga Chlorella vulgaris for lipid production from industrial wastes and its use as biodiesel feedstock. New Biotechnol. 2011, 28, 362–368. [Google Scholar] [CrossRef]
- Holdmann, C.; Schmid-Staiger, U.; Hornstein, H.; Hirth, T. Keeping the light energy constant—Cultivation of Chlorella sorokiniana at different specific light availabilities and different photoperiods. Algal Res. 2018, 29, 61–70. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Han, F.; Mu, J.; Chen, D.; Feng, B.; Zeng, H. Regulation of lipid metabolism in the green microalga Chlorella protothecoides by heterotrophy–photoinduction cultivation regime. Bioresour. Technol. 2014, 192, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Narayan, M.S.; Manoj, G.P.; Vatchravelu, K.; Bhagyalakshmi, N.; Mahadevaswamy, M. Utilization of Glycerol as Carbon Source on the Growth, Pigment and Lipid Production in Spirulina platensis. Int. J. Food Sci. Nutr. 2005, 56, 521–528. [Google Scholar] [CrossRef] [PubMed]


| Xmax (g L−1) | Pmax (mg L−1 h−1) | µmax (h−1) | tg (h) | Proteins (% w w−1) | Carbohydrates (% w w−1) | Lipids (% w w−1) | |
|---|---|---|---|---|---|---|---|
| 24:0 | |||||||
| Glycerol | 0.91 b ± 0.05 | 20.16 b ± 2.91 | 0.08 a ± 0.01 | 8.88 a ± 0.45 | 45.3 b ± 2.73 | 23.8 a ± 1.17 | 7.3 a ± 0.54 |
| Control | 0.39 c ± 0.01 | 4.76 c ± 0.42 | - | - | 47.6 b ± 0.36 | 14.5 b ± 2.86 | 8.4 a ± 0.58 |
| 12:12 | |||||||
| Glycerol | 1.24 a ± 0.01 | 28.36 a ± 1.38 | 0.10 a ± 0.01 | 7.01 b ± 0.44 | 52.2 b ± 3.92 | 15.9 b ± 0.35 | 8.0 a ± 0.94 |
| Control | 0.32 d ± 0.02 | 3.01 d ± 0.47 | - | - | 50.5 b ± 2.13 | 14.7 b ± 0.47 | 8.9 a ± 0.48 |
| 0:24 | |||||||
| Glycerol | 1.01 b ± 0.01 | 22.02 b ± 1.60 | 0.10 a ± 0.01 | 7.10 b ± 0.35 | 58.1 a ± 1.69 | 11.4 c ± 3.69 | 7.3 a ± 0.98 |
| Control | 0.22 e ± 0.01 | 1.35 e ± 0.89 | - | - | 43.1 b ± 1.76 | 11.4 c ± 1.00 | 8.9 a ± 0.54 |
| Fatty Acid | Control | Glycerol | ||||
|---|---|---|---|---|---|---|
| 24:0 | 12:12 | 0:24 | 24:0 | 12:12 | 0:24 | |
| C11:0 | 15.98 b ± 2.58 | 20.48 a ± 1.15 | 6.67 c ± 2.54 | n.d. | 3.41 c ± 0.82 | 4.88 c ± 1.57 |
| C16:0 | 185.39 a ± 7.58 | 175.26 a ± 36.51 | 111.48 b ± 13.54 | 84.07 b ± 12.34 | 95.11 b ± 18.89 | 92.29 b ± 4.71 |
| C16:1w7 | 8.01 b ± 5.33 | 11.80 b ± 2.25 | 44.59 a ± 10.03 | 30.00 a ± 2.37 | 37.72 a ± 7.87 | 32.29 a ± 12.21 |
| C18:1w9c | 4.48 b ± 0.28 | 4.33 b ± 0.86 | 347.63 a ± 45.87 | 250.34 a ± 50.45 | 300.53 a ± 54.94 | 268.19 a ± 46.51 |
| C18:2w6c | 60.14 a ± 5.56 | 54.31 a ± 9.77 | 21.43 b ± 4.54 | 16.42 b ± 1.45 | 17.53 b ± 4.38 | 16.14 b ± 3.71 |
| C18:3w6 | 87.94 a ± 4.25 | 76.85 a ± 8.00 | 31.30 b ± 12.32 | 24.97 b ± 8.54 | 27.32 b ± 4.69 | 31.56 b ± 12.47 |
| C20:1w9 | n.d. | 15.83 b ± 13.54 | 32.57 a ± 9.62 | n.d. | 0.66 c ± 0.66 | 12.03 b ± 3.65 |
| Outros * | 22.55 a ± 0.07 | 19.45 a ± 4.92 | 7.45 b ± 2.34 | 7.79 b ± 1.23 | 6.63 b ± 0.99 | 10.96 b ± 0.76 |
| Total | 384.50 b ± 14.99 | 378.31 b ± 77.01 | 603.12 a ± 89.45 | 413.60 b ± 45.76 | 488.91 a,b ± 94.51 | 468.33 a,b ± 86.5 |
| Saturated | 220.31 a ± 6.62 | 213.93 a ± 43.00 | 125.61 b ± 23.45 | 87.22 b ± 12.87 | 105.15 b ± 23.29 | 106.58 b ± 7.07 |
| Unsaturated | 163.43 c ± 7.61 | 164.38 c ± 34.00 | 477.51 a ± 12.34 | 326.37 b ± 21.12 | 383.77 a,b ± 71.22 | 361.76 a,b ± 93.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Morais, E.G.; Conceição, J.d.A.; Nunes, I.L.; Druzian, J.I.; de Morais, M.G.; da Rosa, A.P.C.; Costa, J.A.V. Effect of Photoperiod and Glycerol Supplementation on the Biomass Productivity and Protein Production of Spirulina sp. LEB 18 Cultures. Appl. Sci. 2022, 12, 12329. https://doi.org/10.3390/app122312329
de Morais EG, Conceição JdA, Nunes IL, Druzian JI, de Morais MG, da Rosa APC, Costa JAV. Effect of Photoperiod and Glycerol Supplementation on the Biomass Productivity and Protein Production of Spirulina sp. LEB 18 Cultures. Applied Sciences. 2022; 12(23):12329. https://doi.org/10.3390/app122312329
Chicago/Turabian Stylede Morais, Etiele Greque, Jenyfer de Almeida Conceição, Itaciara Larroza Nunes, Janice Izabel Druzian, Michele Greque de Morais, Ana Priscila Centeno da Rosa, and Jorge Alberto Vieira Costa. 2022. "Effect of Photoperiod and Glycerol Supplementation on the Biomass Productivity and Protein Production of Spirulina sp. LEB 18 Cultures" Applied Sciences 12, no. 23: 12329. https://doi.org/10.3390/app122312329