Investigation of the Possibility of Listeria monocytogenes Growth in Alternatively Cured Cooked Sausages—A Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sausages
2.2. Growth Potential
2.3. Chemical Determinations
2.4. Water Activity Measurement
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halter, E.L.; Neuhaus, K.; Scherer, S. Listeria weihenstephanensis sp.nov., isolated from the water plant Lemna trisulca of German fresh water pond. Int. J. Syst. Evol. Microbiol. 2013, 63, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Walczycka, M. Methods to inhibit and prevent the growth of listeria monocytogenes in meat products. Żywn. Nauk. Technol. Jakość 2005, 43, 61–72. Available online: https://wydawnictwo.pttz.org/wp-content/uploads/2015/02/05_Walczycka.pdf (accessed on 20 May 2021).
- Muskalska, K.B.; Szymczak, B. Progress in research on the genus Listeria. Adv. Microbiol. 2015, 54, 123–132. Available online: http://pm.microbiology.pl/postepy-badan-nad-bakteriami-rodzaju-listeria/ (accessed on 20 May 2021).
- Mazaheri, T.; Cervantes-Huamán, B.R.H.; Bermúdez-Capdevila, M.; Ripolles-Avila, C.; Rodríguez-Jerez, J.J. Listeria monocytogenes biofilms in the food industry: Is the current hygiene program sufficient to combat the persistence of the pathogen? Microorganisms 2021, 9, 181. [Google Scholar] [CrossRef]
- Dewey-Mattia, D.; Manikonda, K.; Wise, M.E.; Crowe, S.J. Surveillance for foodborne disease outbreaks United States, 2009–2015. MMWR Surveill. Summ. 2018, 67, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.A.L.; Botelho, C.V.; Martins, B.T.F.; Tavares, R.M.; Camargo, A.C.; Yamatogi, R.S.; Bersot, L.S.; Nero, L.A. Listeria monocytogenes from farm to fork in a Brazilian pork production chain. J. Food Prot. 2020, 83, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02005R2073-20200308&from=EN (accessed on 20 May 2021).
- Zadernowska, A.; Chajęcka-Wierzchowska, W.; Zadernowski, M.; Zakrzewski, A.; Zarzecka, U. Challenge tests: Predicting and eliminating potential microbiological risks. Listeria monocytogenes in food. Przem. Spoż. 2018, 72, 1–3. [Google Scholar]
- Aureli, P.; Fiorucci, G.C.; Caroli, D.; Marchiaro, G.; Novara, O.; Leone, L.; Salmaso, S. An outbreak of febrile gastroenteritis associated with corn contaminated by Listeria monocytogenes. N. Engl. J. Med. 2000, 342, 1236–1241. [Google Scholar] [CrossRef]
- Zadernowska, A.; Chajęcka-Wierzchowska, W.; Kłębukowska, L.; Zarzecka, U.; Łaniewska-Trokenheim, Ł. Protective bacterial cultures and their use for inhibition of growth of Listeria monocytogenes in meat and meat products. Kosmos 2017, 66, 59–65. [Google Scholar]
- Commission Regulation (EU) No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives. Official J. European Union 2011, L295, 1–177. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32011R1129&from=PL (accessed on 20 May 2021).
- Ma, G.; Wang, Z.; Yu, Q.; Han, L.; Chen, C.; Guo, Z. Effects of low-dose sodium nitrite on the structure of yak meat myoglobin during wet curing. Food Chem. X 2022, 15, 100434. [Google Scholar] [CrossRef] [PubMed]
- Nader, M.; Hosseininezhad, B.; Berizi, E.; Mazloomi, S.M.; Hosseinzadeh, S.; Zare, M.; Derakhshan, Z.; Oliveri Conti, G.; Ferrante, M. The residual nitrate and nitrite levels in meat products in Iran: A systematic review, meta-analysis and health risk assessment. Environ. Res. 2022, 207, 112180. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Jeddi, S.; Azizi, F.; Ghasemi, A.; Hadaegh, F. Nitrate and nitrite content of vegetables, fruits, grains, legumes, dairy products, meats and processed meats. J. Food Comp. Anal. 2016, 51, 93–105. [Google Scholar] [CrossRef]
- Corpet, D.E. Red meat and colon cancer: Should we become vegetarians, or can we make meat safer? Meat Sci. 2011, 89, 310–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraqueza, M.J.; Laranjo, M.; Elias, M.; Patarata, L. Microbiological hazards associated with salt and nitrite reduction in cured meat products: Control strategies based on antimicrobial effect of natural ingredients and protective microbiota. Cur. Opin. Food Sci. 2021, 38, 32–39. [Google Scholar] [CrossRef]
- Gonzalez-Fandos, E.; Vazquez de Castro, M.; Martinez-Laorden, A.; Perez-Arnedo, I. Behavior of Listeria monocytogenes and other microorganisms in sliced Riojano chorizo (Spanish dry-cured sausage) during storage under modified atmospheres. Microorganisms 2021, 9, 1384. [Google Scholar] [CrossRef]
- European Commission (EC). EURL Lm Technical Guidance Document for Conducting Shelf-Life Studies on Listeria monocytogenes in Ready-to-Eat Foods. 2021. Available online: https://eurl-listeria.anses.fr/en/minisite/listeria-monocytogenes/mandate (accessed on 10 August 2022).
- PN-74/A-82114 Polish Standard; Meat and Meat Products. Determination of nitrites and nitrates content. Polish Committee for Standardization: Warsaw, Poland, 1974. (In Polish)
- Zajac, M.; Duda, I.; Skoczylas, L.; Tabaszewska, M. Potential use of hyssopus officinalis and borago officinalis as curing ingredients in pork meat formulations. Animals 2020, 10, 2327. [Google Scholar] [CrossRef]
- PN-ISO 1841-2:2002; Meat and Meat Products Determination of Chloride Content Part 2: Potentiometric Method. Polish Committee for Standardization: Warsaw, Poland, 2002. (In Polish)
- Sucu, C.; Yildiz Turp, G. The investigation of the use of beetroot powder in Turkish fermented beef sausage (sucuk) as nitrite alternative. Meat Sci. 2018, 140, 158–166. [Google Scholar] [CrossRef]
- Cadavez, V.; Gonzales-Barron, U.; Pires, P.; Fernandes, E.; Pereira, A.P.; Gomes, A.; Araújo, J.P.; Lopes-da-Silva, F.; Rodrigues, P.; Fernandes, C.; et al. An assessment of the processing and physicochemical factors contributing to the microbial contamination of salpicão, a naturally-fermented Portuguese sausage. LWT-Food Sci. Technol. 2016, 72, 107–116. [Google Scholar] [CrossRef]
- Bianco Junior, A.; Daguer, H.; Kindlein, L. Baseline sodium nitrate and nitrite concentrations in fresh and processed meats. J. Food Comp. Anal. 2022, 105, 104227. [Google Scholar] [CrossRef]
- Nerbrink, E.; Borch, E.; Blom, H.; Nesbakken, T. A model based on absorbance data on the growth rate of Listeria monocytogenes and including the effects of pH, NaCl, Na-lactate and Na-acetate. Int. J. Food Microbiol. 1999, 47, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Horsch, A.M.; Sebranek, J.G.; Dickson, J.S.; Niebuhr, S.E.; Larson, E.M.; Lavieri, N.A.; Ruther, B.L.; Wilson, L.A. The effect of pH and nitrite concentration on the antimicrobial impact of celery juice concentrate compared with conventional sodium nitrite on Listeria monocytogenes. Meat Sci. 2014, 96, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.A.; Jackson-Davis, A.L.; Schrader, K.D.; Xi, Y.; Kulchaiyawat, C.; Sebranek, J.G.; Dickson, J.S. Survey of naturally and conventionally cured commercial sausages, ham, and bacon for physio-chemical characteristics that affect bacterial growth. Meat Sci. 2012, 92, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.; Popovic, M.; Amatertti, A.; Di Gioia, D.; Rosii, M. Anti–Listeria starters: In vitro selection and production plant evaluation. J. Food Prot. 2014, 77, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Jongberg, S.; Ros, G.; Skibsted, L.H.; Nieto, G. Plant derived ingredients rich in nitrates or phenolics for protection of pork against protein oxidation. Food Res. Int. 2019, 129, 108789. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; Domínguez, R.; Santos, E.M.; Lorenzo, J.M. Cruciferous vegetables as sources of nitrate in meat products. Curr. Opin. Food Sci. 2021, 38, 1–7. [Google Scholar] [CrossRef]
- Jin, S.-K.; Choi, J.S.; Yang, H.-S.; Park, T.-S.; Yim, D.-G. Natural curing agents as nitrite alternatives and their effects on the physicochemical, microbiological properties and sensory evaluation of sausages during storage. Meat Sci. 2018, 146, 34–40. [Google Scholar] [CrossRef]
- Ekici, L.; Ozturk, I.; Karaman, S.; Caliskan, O.; Tornuk, F.; Sagdic, O.; Yetim, H. Effects of black carrot concentrate on some physicochemical, textural, bioactive, aroma and sensory properties of sucuk, a traditional Turkish dry-fermented sausage. LWT-Food Sci. Technol. 2015, 62, 718–726. [Google Scholar] [CrossRef]
- Kurćubić, V.S.; Mašković, P.Z.; Vujić, J.M.; Vranić, D.V.; Vesković-Moračanin, S.M.; Okanović, D.G.; Lilić, S.V. Antioxidant and antimicrobial activity of Kitaibelia vitifolia extract as alternative to the added nitrite in fermented dry sausage. Meat Sci. 2014, 97, 459–467. [Google Scholar] [CrossRef]
| Attribute | Control | Alternatively Cured |
|---|---|---|
| Composition | meat from pork ham (94%), salt, glucose, stabilizer: sodium citrate, aromas, acidity regulator: sodium ascorbate, spices extracts, preservative: sodium nitrite | pork, salt, spices, spices extracts, aromas, dried acerola |
| Additional information | without the addition of monosodium glutamate | 100 g of the product was obtained from 100 g of pork; gluten-free; preservative-free |
| Nutritional value in 100 g | ||
| Energy (kJ/kcal) | 1175/284 | 1304/315 |
| Fat (g) | 25 | 28 |
| Saturated fatty acids | 10 | 10 |
| Carbohydrates | 0.7 | 0 |
| Sugars | 0.7 | 0 |
| Protein | 14 | 15 |
| Salt | 2.1 | 2.3 |
| Attribute | Control | AC |
|---|---|---|
| NaCl (%) | 1.90 b (0.03) | 2.32 a (0.02) |
| Residual nitrite (mg/kg) | 44.89 a (0.50) | 12.10 b (0.38) |
| Water activity | 0.9638 a (0.0023) | 0.9653 a (0.0032) |
| Attribute | Control | AC |
|---|---|---|
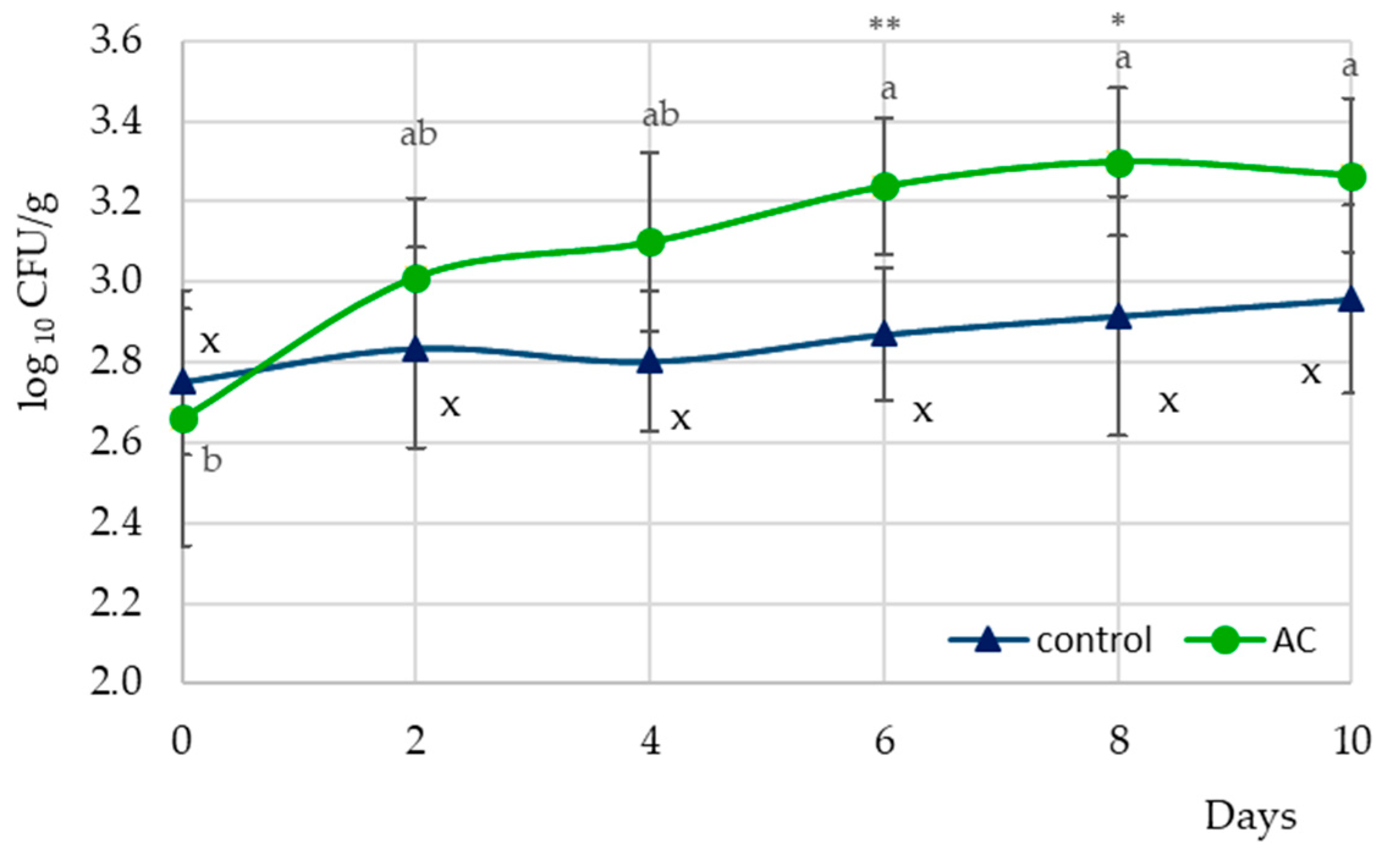
| Initial counts (log10 CFU/g) | 2.75 (0.18) | 2.66 (0.32) |
| The highest counts (log10 CFU/g) | 2.96 (0.24) | 3.30 (0.18) |
| Growth potential | 0.21 | 0.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modzelewska-Kapituła, M.; Lemański, A.; Zduńczyk, W.; Zadernowska, A. Investigation of the Possibility of Listeria monocytogenes Growth in Alternatively Cured Cooked Sausages—A Case Study. Appl. Sci. 2022, 12, 12429. https://doi.org/10.3390/app122312429
Modzelewska-Kapituła M, Lemański A, Zduńczyk W, Zadernowska A. Investigation of the Possibility of Listeria monocytogenes Growth in Alternatively Cured Cooked Sausages—A Case Study. Applied Sciences. 2022; 12(23):12429. https://doi.org/10.3390/app122312429
Chicago/Turabian StyleModzelewska-Kapituła, Monika, Andrzej Lemański, Weronika Zduńczyk, and Anna Zadernowska. 2022. "Investigation of the Possibility of Listeria monocytogenes Growth in Alternatively Cured Cooked Sausages—A Case Study" Applied Sciences 12, no. 23: 12429. https://doi.org/10.3390/app122312429





