Abstract
This article provides the results of the elemental composition study of Carassius auratus organs living in the Komsomolsk mine tailings pond, in which the water contains elevated concentrations of metals and metalloids. It was determined with an electrotomography survey that the pond is bordered vertically and laterally with highly conductive zones, in which pore waters are high-mineralized solutions. Due to the constant infiltration of solutions into the pond water, a stable composition is formed with elevated concentrations of a number of elements (sulfate-ion, As, Sb, Zn, Cd, Se, and others) exceeding background values. In the organs of Carassius auratus living in the pond, the accumulation of many elements occurs, the largest Sb, As, Cd, Hg, and Pb and to a lesser extent Al, Cu, Zn, and Se. Moreover, the concentration of elements is selective. In total, the greatest excess of element concentrations over background values was determined in the heart (15,000 times) and gills (4100 times) of fish, the smallest in muscles (1900 times) and liver (2000 times). The impact of the accumulation of metals and metalloids in fish organs influenced the metabolic rate, a decrease in the growth rate of Carassius auratus, and the appearance of dwarf forms.
1. Introduction
Mining and ore industry tailings containing high amounts of metals and metalloids have a serious impact on the environment, biota, and humans [1,2,3,4]. Special attention was directed to the study of the mine water, drainage flows, and surface water due to high concentrations of toxic elements [5,6,7] and the influence of mining tailings on water, soil, plants, and humans [8].
However, not all metals and not all amounts of these metals are toxic for certain. Some heavy metals are important as trace elements in intracellular biochemical reactions. A chemical element is considered necessary when its lack causes functional disorders (hypomicroelementoses) to occur in the human body, such as Menkes disease (copper deficiency) [9], congenital malformations due to zinc deficiency [10,11], and diabetes due to manganese deficiency [12]. Some metal ions serve as therapeutic agents. Zinc carboxylates, for example, are used against bacteria that cause the disease “athlete’s legs”, and lithium is used in the treatment of manic depression [13]. However, with the accumulation of heavy metals in the body, the toxic effects of many of them are of serious concern.
Fish are ideal targets of toxigenoma studies as biomarkers of exposure. They respond to toxicants in the same way as higher vertebrates; hence, they can be used to screen for chemicals potentially teratogenic and carcinogenic to humans [14]. Cadmium genotoxicity has been demonstrated in cyprinid fish [15,16] and revealed genome disorders under the influence of hexavalent chromium [17]. In catfish, researchers found the development of leukemia under the influence of arsenic [18]. The negative effect of heavy metals on the organogenesis and viability of fish embryos has been proven [19,20,21,22,23,24], found pathologies in the functioning of the endocrine and reproductive systems [25], showed negative effects on the nervous system and sensory organs [26], as well as disorders of physiological processes and structure of tissues of different organs [27,28,29,30]. Experimental studies have established the effect of even low concentrations of lead on morphological abnormality of zebrafish Danio rerrio [31].
As one of the important foods of humans and animals, fish draw the attention of researchers to the field of studying the distribution and dynamics of heavy metal accumulation in organs and tissues [32,33,34,35,36,37] when evaluating the migration of toxic elements along the food chain [38].
The silver crucian Carassius auratus is an important fishery site in western Siberia. In many shallow lakes, ichthyofauna is represented exclusively by this species [39]. Therefore, the issue of human safety of the quality of this food is extremely relevant, and the results of studies on the distribution and accumulation of heavy metals in the body of crucian carp [29,30] attract the attention of specialists in various fields—in ecology, medicine, and biochemistry.
The aim of this paper was to investigate the migration and distribution of metals and metalloids into the silver crucian Carassius auratus, which inhabits Komsomolsk pond, to study the patterns of their accumulation in visceral organs and the influence on the morphological characteristics of the fish.
2. Study Object
The Komsomolsk gold deposit is a system of gold-arsenopyrite-quartz veins with a large reserve of gabbroids. The deposit is located in the Eastern Kemerovo region of Russia [40]. The deposit was developed from 1937 to 1999. Primary sulfide minerals are represented by pyrite, pyrrhotite, and arsenopyrite with a lower content of galena, sphalerite, and chalcopyrite (2–2.5% of the total deposit). The most common gangue minerals are mica, quartz, and feldspar, and some carbonate grains (calcite and dolomite) are also present (~6%). The ores of the Komsomolsk deposit were processed with NaCN cyanidation at the Komsomolsk Gold Extraction Plant (KGEP).
In addition, KGEP produced gold extracted from antimony sludge (residual product after leaching of antimonite concentrates using Na2S and NaOH) from the Kadamjai plant. This sludge was a small part of the mine waste (0.5 tons of sludge was added for every 100 tons of ore) but was rich in metals.
The KGEP tailings are located in the territory of the Komsomolsk settlement (Figure 1). These tailings appeared in 1964 and represent a natural ravine filled with slurry runoff. The tailings surface area is 146,000 m2, and the amount of solid accumulated material is ≈1.1 million m3, or 3.5 million tons. The storage is topographically enclosed on three sides and by a bulk diamond on the fourth side. A tailings pond formed at the surface due to particle accumulation and consolidation. The pond area is ≈60,000 m2, and the average depth is 2 m. Over time, after the plant stopped, the pond took on the appearance of a natural lake. Shallow areas have been growing over with aquatic vegetation, zoo- and phytoplankton appeared, and fish (crucian carp) originated, presumably by birds transferring caviar from neighboring reservoirs. Currently, the main source of replenishment of the pond is seasonal precipitation. The population of the Komsomolsk settlement has perceived the water body as a “natural” pond, and it is actively used for domestic purposes, including for fishing (Figure 1).
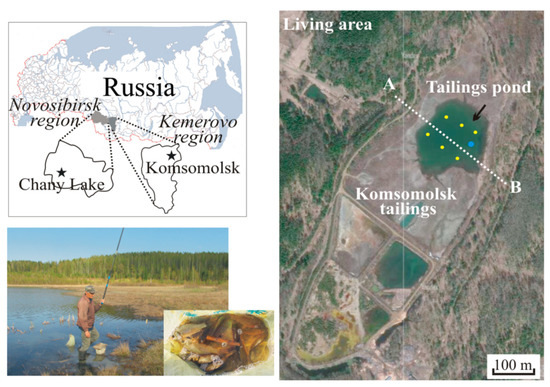
Figure 1.
Geographical position of study sites (Komsomolsk settlement and Chany Lake marked with stars on the map), sampling scheme at Komsomolsk pond, and photograph “Fisherman and his catch”. Yellow points are water samples, blue circle is site of catching fish, and white line is the elecrotomography profile.
Chany Lake, set as a background reservoir for comparison with the Komsomolsk pond, is the largest reservoir of the West Siberian Plain. It is located on the territory of the Barabinsk lowland of the Ob-Irtysh interfluve. At present time, the surface area of the lake reaches 1800 km2. Livestock grazing limited anthropogenic impacts on the aquatic ecosystem. Therefore, in terms of water quality, Chany Lake can be considered as a background habitat for hydrobionts.
3. Materials and Methods
3.1. Field Sampling
3.1.1. Water
The collection of water samples was processed throughout the whole Komsomolsk pond area. The samples were taken into two 0.5 L plastic bottles from a depth of ~0.3–0.5 m, where a trap for carp was set. The pH parameters and redox potential (Eh) were measured in situ using a portable waterproof pH Meter (HI 9025 C, Hanna Instruments, Padova, Italy), a combined glass electrode for pH (HI 1230 B, Hanna Instruments), and a platinum electrode for Eh measurements (Oxidation Reduction Potential Electrode, Hanna Instruments). The electrical conductivity was measured with a Cond 315 i conductivity meter (WTW, Ann Arbor, MI, USA). A part of the water samples (1 bottle) was filtered at the sampling site through a <0.25 µm hydrophobic membrane and acidified with distilled HNO3 (purity of 99%) for subsequent analysis for cations and trace elements. The second bottle was stored as is for an anionic composition analysis.
In Chany Lake, 10 water samples were taken at the fish catch site. The measurements of the parameters and sample preparation in situ were carried out according to the same scheme as in the Komsomolsk pond.
3.1.2. Fish
The fish capture in Komsomolsk pond and Chany Lake was carried out with a single method using a passive trap with a capacity of 15 L with one entrance. The traps were installed in the coastal zone of the objects for 5–6 h. After the fish accumulated in the traps, they were removed, and the fish were placed into the refrigerator until they were delivered to the laboratory.
3.2. Laboratory Analyses
3.2.1. Laboratory Preparation of Fish
The laboratory investigation of the fish was carried out according to standard methods [41]. The length and weight of the body of each individual was measured. The gender and stage of maturity of the gonads were determined. The filling of the intestinal tract and fat deposits in the body cavity were estimated on a six-point scale. The age of the fish was determined by the number of annual rings on the scales. A visual assessment of the external integument of the body and internal organs was also carried out.
The size, body weight of the tested fish, and the growth rate of the population from Komsomolsk pond were compared to C. auratus samples from the hypereutrophic Chany Lake. In total, biological indicators of 177 fish are included in the analysis of the dimensional-age structure of populations.
The prepared internal organs (weighed muscles, gills, gonads, heart, liver, and kidneys) in Petri dishes were placed in a drying cabinet (model SNOL 67/350) where they were dehydrated at a temperature of +60 °C for further analysis of the microelement composition.
3.2.2. Method of Analyses
The analysis of the main cations (Al, Fe, Ca, Mg, K, Na, Si) and trace elements in water samples were carried out using an ICP-AES (IRIS Advantage) at the Analytical Center of the Institute of Geology and Mineralogy SB RAS. When analyzing the water, every third sample was tested twice. The ionic balance error was less than 15%. Another aliquot was analyzed for major anions (SO42−, Cl−, HCO3−) using capillary electrophoresis on a “Rapel-105M” instrument.
Samples of fish organs (dry weight) were analyzed for chemical elements with an ICP-MS using a NexION 300D mass spectrometer (PerkinElmer, USA, Shelton, CT) (PNIL “Water”, Tomsk). A pre-prepared sample of 0.2–0.3 g was placed in a 50 cm3 test tube and 2 cm3 of especially pure concentrated HNO3 and 1 cm3 of H2O2 were added to it. The next step was to decompose the sample in a microwave at a temperature of 190 °C for 15 min. The resulting mixture was brought to a volume of 50 cm3 with a 3% nitric acid solution. A solution of Li, Mg, Co, Y, and Tl in 2% nitric acid with a concentration of 1 μg/L for each determined element (“ICP-MS Tuning Solution”, Agilent Technologies, Mulgrave, Australia) was used for the adjustment. The three sigma method detection limits (MDLs) and 10 sigma Limit of Quantitation (MLOQs) were calculated from 10 measurements of the preparation blank. All measurements were conducted in three replicates for each element. All validations and sample analyses were performed in conjunction with appropriate QA/QC, including blank samples prepared using distilled de-ionized water (>15 MΩ) and high purity nitric acid for ICP-MS spectrometry. The ICP-MS control was prepared using a 1.00 ± 0.05 mg/L standard multielement solution for ICP-MS (SKAT, Novosibirsk, Russia).
Element concentrations were determined with an external calibration. Reference materials of muscle tissue of Baikal perch (Perca fluviatilis, L.) CRM BOk-2 (GSO 9055-2008, Institute of Geochemistry SB RAS) CO KOOMET 0068-2009-RU were used for the standard curve including at least five points with a correlation coefficient of 0.998. In each sample of crucian organs, the concentration of 72 chemical elements (Li–U) was analyzed. The relative error of the determinations is 15–30% depending on the content of elements. The element bioaccumulation in fish organs was expressed mg/kg dry wt.
3.2.3. Coefficients
The following concentration factors were used to obtain quantitative indicators of the pond water composition and the level of element accumulation in different crucian organs:
where IconcW is the index of water pollution, Cel pond is the concentration of the element in the water of Komsomolsk pond, and Cel backgr is the concentration of element in water of the background reservoir.
where IconcF is the index of excess of the element concentration in the fish organ, Cel Fpond is the element concentration in the organ of the fish from the tailings pond, and Cel Fbackgr is element concentration in organ of fish from the background reservoir.
The analysis of the obtained results was carried out using mathematical statistics; patterns of distribution and localization of trace elements in the fish body were estimated on the basis of cluster analysis results using the Past Program for PC.
4. Results and Discussion
4.1. Composition of the Pond Water
The pond water composition is stable in all analyzed samples, and it is characterized by a slightly alkaline or alkaline pH values, oxidizing conditions, and a relatively low total dissolved solids (TDS) concentration level of 0.5 g L−1. The waters are of sulfate-Ca type, which is typical for mine tailings. The concentrations of the observed elements in most cases do not exceed the MPCfp levels [42] (Table 1). The main toxic elements are As and Sb, and the concentrations are relatively stable. In particular, the contents of As and Sb exceed MPCfp by an average of 22 and 180 times, respectively. Concentrations of Al, Cu, Zn, and Mo are at or slightly higher than MPCfp.

Table 1.
Composition of water in Komsomolsk pond and Chany Lake, Eh in mV, electroconductivity S in µS/cm, SO42−–Sb in mg/L, Al–Mo in µg/L.
The most excess over the concentrations of a background reservoir (IconcW > 5) was determined for Sb, As, Cd, Se, Cr, Ca, and SO42−, which indicates contrasting conditions in terms of water composition in Komsomolsk pond and Chany Lake.
Komsomolsk pond currently exists due to the replenishment of water with seasonal precipitation—rain and snow waters. The plant was stopped in 1999, and pulp effluents from ore processing have not been received into it since that time. A substantial reduction in metal and metalloid concentrations in the pond water would be expected. However, the water composition with high concentrations of many elements, the main ones being arsenic and antimony, has remained stable for over 20 years [40,43,44].
In 2017, geophysical surveys of internal zonality of the Komsomolsk tailings using electrical resistivity tomography (ERT) were carried out [40]. The infiltration of highly mineralized acidic waters at a depth of 20 m and contamination of drinking water in the Komsomolsk settlement were detected. One of the ERT profiles crossed the tailings pond, capturing surface water area of the pond (Figure 2). The geoelectric structure of the section indicates the transition of highly conductive zones (the pond: blue and cyan layers) to underlying diorites with low conductivity (yellow and red layers). Low-resistance zones (<60 Ohm∙m) beneath the water/solid boundary extend to a depth of ~10 m, spreading along the coastal zone of the pond at a ~ of 25 m from the water. We interpret such zones as some flooded areas with a constant interaction of the material of the tailings and pore waters within. Moreira et al. [45] showed that existence of such saturated sections within waste rock pile as permanent features provides sulfide minerals oxidation during the entire hydrological cycle.
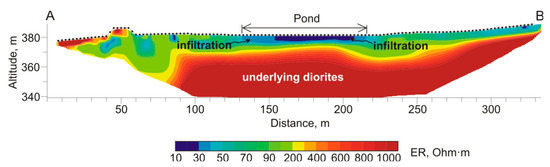
Figure 2.
The geoelectric section through Komsomolsk tailings pond along line A–B (Figure 1).
As a result, dissolved forms of many elements accumulate in pore solutions, in particular arsenic—up to 29 g/L, antimony—up to 10 g/L [46]. The main mechanism for the metal release from solid tailings into solutions is the oxidative dissolution of sulfides and oxides, such as following.
- (1)
- Oxidation of arsenopyrite FeAsS with dissolved oxygen [47,48]:4FeAsS + 11O2 + 6H2O→4Fe2+ + 4H3AsO3 + 4SO42−
4Fe2+ + O2 + 10H2O→4Fe(OH)3 + 8H+
2H3AsO3 + O2→2HAsO42− + 4H+
2H3AsO3 + O2→2H2AsO42− + 1H+ - (2)
- Bacterial oxidation of pyrite and arsenopyrite [49]:2FeS2 + 7O2 + 2H2O → 2Fe2+ + 4SO42−+ 4H+
4Fe2+ + O2 + H+ → 4Fe3+ + 2H2O
4FeAsS + 13O2 + 6H2O →4Fe2+ + 4SO42− + 4H3AsO4 - (3)
- Bacterial oxidation of antimony oxide or in the presence of the Fe2+/Fe3+ (Mn2+/Mn3+) system with the transition to the mobile Sb(VI) form [50,51]:Sb2O3 + 3H2O→2Sb(OH)3
Sb(OH)3 + 2H2O + 0.5O2→Sb(OH)6− + H+
The exchange of the pond water and the pore solutions from underlying horizons supports stable concentrations of elements in the pond.
4.2. Mutational Changes of Crucian Carp from the Tailings Pond
4.2.1. Biological Parameters of Crucian Carp
The population of crucian carp from Komsomolsk pond is represented by dwarf-shaped individuals. The body length of five-year-old mature adult individuals did not exceed 100 mm (Figure 3). According to the results of a visual examination of fish, no pathologies were detected on the scaly and skin of the body, fins, gills, as well as on internal organs.

Figure 3.
Linear dimensions of Carassius auratus at the age of 5+ from Komsomolsk pond.
The ratio of females to males was 1:1. Attention is drawn to the low gastrointestinal filling rate. Perhaps this is due to the consumption of energy reserves in the winter-spring period, which is typical for many cyprinid fish. However, these signs may indicate a limited food supply.
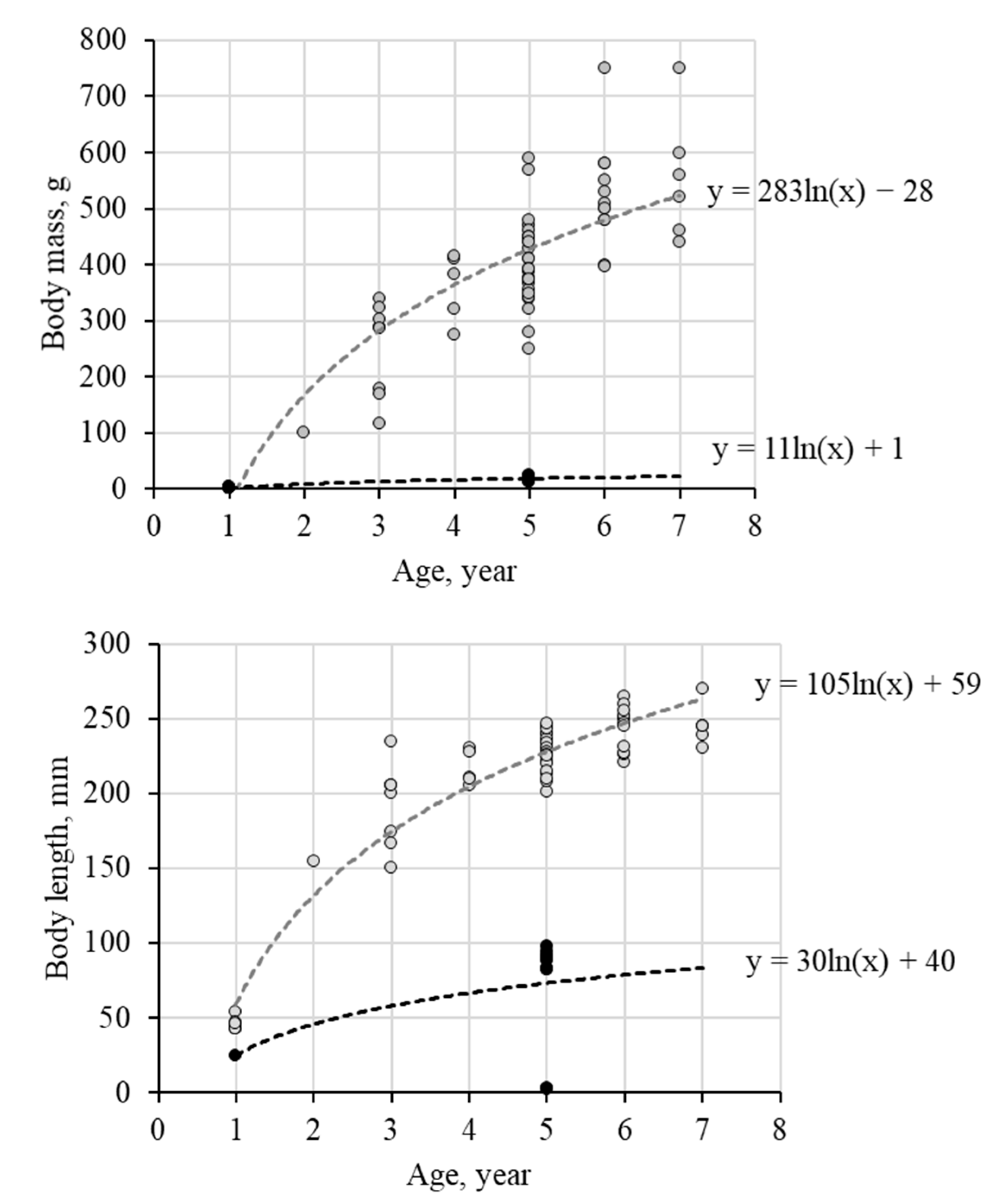
The appearance of dwarfism reflects differences in the growth rates of the populations of crucian carp from Komsomolsk pond and Chany Lake (Figure 4). It is significant that usually crucian carp grow faster in lake systems rather than riverside, which is associated not as much with the level of development of the feed base as with a higher water temperature in stagnation zones. With respect to the population from Komsomolsk pond, the possibility of aggressive effects of the high concentration of metals and metalloids on fish growth should not be ruled out. A comparison of logarithmic functions (Figure 4) shows that the linear growth rate of crucians in Chany Lake is three times higher than in the fish from Komsomolsk pond, and by mass—almost thirty times.
Figure 4.
The growth rate of crucian carp in the studied reservoirs in terms of weight and body length (light circles—fish from Chany Lake, black—from Komsomolsk pond).
4.2.2. Elemental Composition of Crucian Carp Organs
In crucian carp organs, some elements are distributed selectively (Figure 5). The concentration of Ca, Sr, and Al in the gills of fish from the background reservoir (Chany Lake) is much higher than their concentration in other organs. Aluminum serves no known biological function and is both toxic to aquatic life and bioaccumulative [52]. The accumulation of these elements in the gills can be associated with the entry of a thin suspension of carbonate and clay minerals from the water. Iron is contrasted; the main organ of its concentration is the heart. In a smaller amount, it is contained in the kidneys and gills, and the muscles and gonads contain iron an order of magnitude lower. Additionally, elevated concentrations of Fe and Mn can cause neurological, genotoxic, pulmonary, and brain disorders in fish [53,54]. Zinc and copper are distributed more evenly across organs. It has been noticed that prolonged exposure to Cu can evoke an acclimation response, allowing fish not only to survive but also withstand further exposure to higher concentrations. However, there are reproductive impairments, reduced growth, and behavioral changes [55].
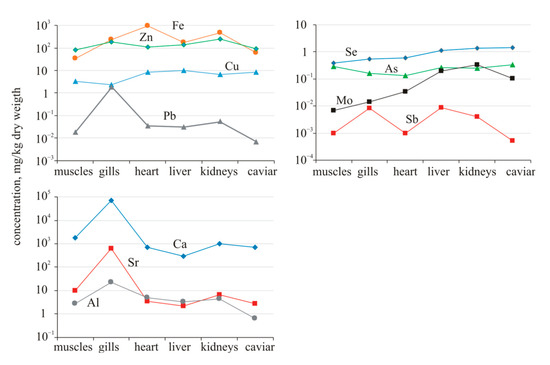
Figure 5.
Distribution of elements in organs of Carassius gibelio from Chany Lake (background reservoir).
Lead, just like calcium, strontium, and aluminum, predominantly accumulates in the gills. The concentrations of metalloids (As, Sb, and Se) are very close in all organs, varying within (μg/g dry weight) 0.13–0.34 (As), 0.00052–0.0089 (Sb), 0.38–1.4 (Se). It should be noted that the content of Pb, Cd, Hg, and Sb in gonads is significantly lower than in other organs.
In the organs of crucian carp from Komsomolsk pond, an increase in many elements is determined compared to the background (Figure 6). Antimony accumulated the most: in the heart IconcF was more than 10,000, in all other organs—more than 1000. Recall that in the water of Komsomolsk pond, the greatest value of IconcW is determined precisely for antimony—350. Other metalloids and metals also accumulated in significant amounts. The IconcF (As) was more than 100 in the gills, heart, liver, and kidneys (the concentration of arsenic in the pond water is 40 times higher than the background reservoir).
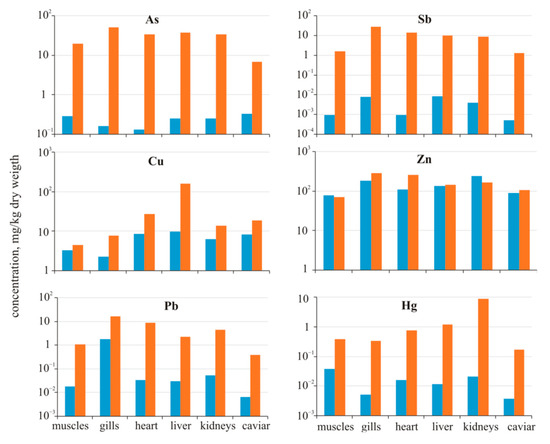
Figure 6.
Comparison of element concentrations in organs of Carassius gibelio from Chany Lake (blue columns) and Komsomolsk pond (orange columns).
According to [56], the bioaccumulation of As in the muscular tissue of P. lineatus seems to be linked to its detritivorous feeding behavior, where this element is accumulated via ingestion of sediments with As high concentrations.
A high positive correlation (r = 0.98) between the composition of the pond water and the level of element accumulation in organs was found. At the same time, the assimilation of some elements occur in different ways. For example, IconcW(Cu) in the pond water was 1.6 and IconcF(Cu) in organs was 1.4 (muscle) to 16 times (liver). Meanwhile, higher concentrations of zinc in water (IconcW(Zn) = 3.8) were much lower in organs; the muscles and kidneys contained zinc lower than the background values, the liver was at the same level as in the background crucian carp, and the other organs had insignificant concentrations (1.2 (caviar)—2.3 (heart)). The IconcW(Pb) was 2.5. However, lead accumulated rather more in organs; the IconcF(Pb) varies from 9.1 (gills) up to 250 (heart). It should be especially focused on the mercury accumulation. Despite the fact that its content in the water of Komsomolsk pond and the background lake was low (<0.01 μg/L), there was an intensive accumulation of mercury in the crucian carp organs from the pond; the largest value of IconcF(Hg) was determined in the kidneys (420) and liver (100). In other organs, it varies from 10 to 64. These findings suggest different biogeochemical mechanisms of entry and accumulation of elements in fish.
Heavy metals can enter fish in several ways: direct adsorption through the skin surface, ion exchange in gills, ingestion of food or suspended particles [57,58]. Bioaccumulation and biomagnification of certain metals in fish tissues has been observed in many studies [59,60]. For most situations, metals can be divided into biologically essential and nonessential. The nonessential metals (Al, Cd, Hg, Sn, and Pb) have no proven biological function (also called xenobiotics or foreign elements), and their toxicity rises with increasing concentration. Essential metals (Cu, Zn, Cr, Ni, Co, Mo, and Fe) on the other hand, have a known biological role, and toxicity occurs either at metabolic deficiencies or at high concentrations. The deficiency of an essential metal can therefore cause an adverse health effect, whereas it’s high concentration can also result in negative impacts which are equivalent to or worse than those caused by nonessential metals [61]. The most commonly found heavy metals in fish organisms are cadmium, lead, mercury, zinc, copper, nickel, cobalt, molybdenum, chromium, and tin. Amongst them, the most frequently studied, with respect to fish deformities, include cadmium, copper, lead, zinc, mercury, and chromium. The feature of nutrition is an important factor affecting the concentration of metals in fish. Heavy metal concentrations tend to be higher in carnivorous fish species than in omnivores and herbivores [62]. However, the habitat of some fish species was found to affect heavy metal accumulation to a greater extent than eating habits [63].
The results of a cluster analysis indicate the uneven accumulation of toxicants in different organs of the tested fish (Figure 7). A relatively high correlation of Cu content in all organs except the liver indicates its uniform distribution throughout the fish and the existence of Cu accumulation mechanisms in the liver. The predominant concentration of Zn and Pb in the gills was expressed in the large difference in the correlations of their contents in other crucian carp organs while zinc, as can be seen from the dendrogram, is more unevenly distributed.
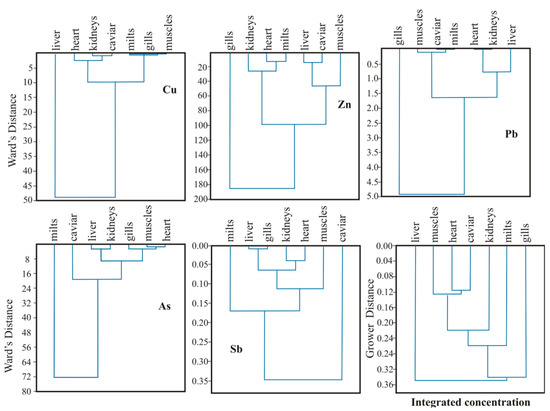
Figure 7.
Results of cluster analysis on the distribution of some elements in the organs of carassius.
Note that with respect to metalloids (As and Sb), the greatest difference in their concentrations is found in gonads—caviar and milt. It is likely that the accumulation of these elements and their impact on the future generation is the main cause of morphological changes in fish and the appearance of dwarf individuals. In this regard, it can be argued that the development of pathologies in the vital activity of fish is a direct consequence of the vulnerability of different organs to the penetration and accumulation of different toxicant substances.
5. Conclusions
Geophysical studies with electrical resistivity tomography revealed the presence of highly conductive zones bordering Komsomolsk pond vertically and laterally, pore waters in which there are highly mineralized solutions. The composition of the water of Komsomolsk pond is formed due to the interaction of seasonal precipitation with tailings material and the infiltration of pore water into the pond from coastal and underlying areas. As a result of the continuous dissolution processes of the solid tailings (mainly, sulfides and oxides), the pond water has a stable composition with an excess of >5 relative to the background according to As, Sb, Cd, Se, Cr, and Mn.
In the organisms of crucian carp, Carassius auratus, in Komsomolsk pond, there is a process of intensive accumulation of many chemical elements, the main of which are As, Sb, Cd, Pb, and Hg. The highest concentrations of As and Sb are recorded in the gills and heart of crucians, Cd in the liver and gonads, Pb in the heart, and Hg in the kidneys and liver.
The impact of the accumulation of chemical elements (including hazard classes 1–2) in fish organs leads to a decrease in the rate of the linear growth of crucian carp in Komsomolsk pond compared to the background reservoir (Chany Lake) by 3 times and by weight—almost thirty times and the appearance of dwarf forms. We consider the inhibitory effect of the complex of metals and metalloids on the metabolic rate of fish as one of the hypotheses for the formation of dwarf forms of crucian carp,. Corresponding to [61], the effect of heavy metal intoxicating could also be indirect; vertebral deformities can be the result of a nutritional deficiency as fish exposed to heavy metals stop feeding (which promotes vitamin C deficiency, etc.).
The elemental composition of the studied crucian organs indicates their high toxicity and the inadmissibility of using fish from Komsomolsk pond for food since the accumulated metals and metalloids are in bioavailable, easily digestible forms.
Author Contributions
Conceptualization, S.B.B. and E.N.Y.; methodology, A.Y.S., V.V.O. and A.A.K.; software, E.N.Y. and N.V.Y.; validation, S.B.B., T.V.K. and V.V.O.; formal analysis, T.V.K. and A.A.K.; investigation, E.N.Y. and S.B.B.; resources, A.Y.S.; data curation, A.A.K.; writing—original draft preparation, S.B.B. and E.N.Y.; writing—review and editing, S.B.B.; visualization, N.V.Y.; supervision, S.B.B.; project administration, S.B.B.; funding acquisition, S.B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This researches was funding by Ministry of Education and Science of Russian Federation, project numbers FWZZ-2022-0028 of IPGG SB RAS and FWGS-2021-0002 of ISEA SB RAS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon request to the corresponding author.
Acknowledgments
Many thanks are due to A. Yadrenkin for his help during the sampling sessions, S. Volynkin for help in describing the chemistry of processes, and K. Tulisova for technical support. The authors are in debt with the Editor, two anonymous reviewers, and Bangjun Liu whose comments and suggestions improved an early version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Robles-Arenas, V.M.; Rodríguez, R.; García, C.; Manteca, J.I.; Candela, L. Sulphide-mining impacts in the physical environment: Sierra de Cartagena-La Unión (SE Spain) case study. Environ. Geol. 2006, 51, 57–64. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M.; Thakur, L.S. Heavy metal Cu, Ni and Zn: Toxicity, health hazards and their removal techniques. Int. J. Plant Anim. Environ. Sci. 2013, 3, 143–157. [Google Scholar]
- Park, J.H.; Hodge, V.; Gerstenberger, S.; Stave, K. Mobilization of Toxic Elements from an Abandoned Manganese Mine in the Arid Metropolitan Las Vegas (NV, USA) Area. Appl. Sci. 2014, 4, 240–254. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, H.K.; Lee, J.C. Hydrogeochemistry of mine, surface and groundwaters from the Sanggok mine creek in the upper Chungju Lake, Republic of Korea. Environ. Geol. 2001, 40, 482–494. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Blowes, D.W.; Ptacek, C.J. Hydrogeochemistry and microbiology of mine drainage: An update. Appl. Geochem. 2015, 57, 3–16. [Google Scholar] [CrossRef]
- Yurkevich, N.; Osipova, P.; Tsibizov, L.; Tsibizova, E.; Fadeeva, I.; Volynkin, S.; Tulisova, K.; Kuleshova, T. Current State of the Gold Mining Waste from the Ores of the Ursk Deposit (Western Siberia, Russia). Appl. Sci. 2022, 12, 10610. [Google Scholar] [CrossRef]
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. 2016, 23, 8244–8259. [Google Scholar] [CrossRef]
- Vest, K.E.; Hashemi, H.F.; Cobine, P.A. The Copper Metallome in Eukaryotic Cells. Met. Ions Life Sci. 2013, 12, 451–478. [Google Scholar]
- Corsello, G.; Giuffrè, M. Congenital malformations. J. Matern. Fetal. Neonatal. Med. 2012, 25, 25–29. [Google Scholar] [CrossRef]
- Salnikova, E.V. Human needs for zinc and its sources (review). Trace Elem. Med. 2016, 17, 11–15. [Google Scholar] [CrossRef][Green Version]
- Hajra, B.; Orakzai, B.A.; Faryal, U.; Hassan, M.; Rasheed, S.; Wazir, S. Insulin Sensitivity to Trace Metals (Chromium, Manganese) In Type 2 Diabetic Patients And Non Diabetic Individuals. J. Ayub. Med. Coll. Abbottabad. 2016, 28, 534–536. [Google Scholar] [PubMed]
- Suldina, T. Heavy metal content in food and its effect on the organism. Ration. Nutr. Diet. Suppl. Biostimulant. 2016, 1, 136–140. (In Russian) [Google Scholar]
- Das, S.; Unni, B.; Bhattacharjee, M.; Wann, S.B.; Rao, P.G. Toxicological effects of arsenic exposure in a freshwater teleost fish, Channa punctatus. Afr. J. Biotech. 2012, 11, 4447–4454. [Google Scholar]
- Aramphongphan, A.; Laovitthayanggoon, S.; Himakoun, L. Snakehead-fish cell line, SSN-1 (Ophicephalus striatus) as a model for cadmium genotoxicity testing. Toxicol. Vitr. 2009, 23, 963–968. [Google Scholar] [CrossRef]
- Gasulla, J.; Picco, S.J.; Carriquiriborde, P.; Dulout, F.N.; Ronco, A.E.; de Luca, J.C. Genotoxic effects induced by Cd+2, Cr+6, Cu+2 in the gill and liver of Odontesthes bonariensis (piscies, Atherinopsidae). Bull. Environ. Contam. Toxicol. 2016, 96, 591–595. [Google Scholar] [CrossRef]
- Arunachalam, K.; Annamalai, S.; Kuruva, J. In-vivo evaluation of hexavalent chromium induced DNA damage by alkaline cometassay and oxidative stress in Catla catla. Am. J. Environ. Sci. 2013, 9, 470–482. [Google Scholar] [CrossRef]
- Pichhode, M.; Gaherwal, S. Toxicological effects of arsenic trioxide exposure on haematolical profile in catfish, Clarias Batrachus. Int. J. Curr. Res. Rev. 2019, 11, 9–12. [Google Scholar] [CrossRef]
- Jezierska, B.; Ługowska, K.; Witeska, M. The effects of heavy metals on embryonic development of fish (a review). Fish Physiol. Biochem. 2008, 35, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wu, Z.-F.; Li, J.; Wang, G.-X. Single and joint action toxicity of heavy metals on early developmental stages of Chinese rare minnow (Gobiocypris Rarus). Ecotoxicol. Environ. Saf. 2011, 74, 2193–2202. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Liu, L.; Li, D.-L.; Ling, F.; Wang, G.-X. Developmental toxicity in rare minnow (Gobiocypris Rarus) embryos exposed to Cu, Zn and CD. Ecotoxicol. Environ. Saf. 2014, 104, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Witeska, M.; Sarnowski, P.; Ługowska, K.; Kowal, E. The effects of cadmium and copper on embryonic and larval development of IDE Leuciscus Idus L. Fish Physiol. Biochem. 2013, 40, 151–163. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, Y.-Y.; Ji, Y.-H.; Liu, M.-Z.; Chen, Y.; Zeng, Y.-L.; Zhang, Y.-G.; Jin, L. Cuins 2/ZNS QD exposure induces developmental toxicity, oxidative stress and DNA damage in rare minnow (Gobiocypris rarus) embryos and larvae. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 2017, 198, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Lian, D.; Su, T.; Wang, Y.Y.; Zhang, D.; Wang, Z.; Gimeno, S.; You, J. Species and life-stage sensitivity of Chinese rare minnow (gobiocypris rarus) to Chemical Exposure: A critical review. Environ. Toxicol. Chem. 2021, 40, 2680–2692. [Google Scholar] [CrossRef]
- Gárriz, Á.; Miranda, L.A. Effects of metals on sperm quality, fertilization and hatching rates, and embryo and larval survival of Pejerrey Fish (odontesthes bonariensis). Ecotoxicology 2020, 29, 1072–1082. [Google Scholar] [CrossRef]
- Baatrup, E. Structural and functional effects of heavy metals on the nervous system, including sense organs, of fish. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1991, 100, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Golovanova, I.L. Effects of heavy metals on the physiological and biochemical status of fishes and aquatic invertebrates. Inland Water Biol. 2008, 1, 93–101. [Google Scholar] [CrossRef]
- Mahboob, S. Environmental pollution of heavy metals as a cause of oxidative stress in fish: A review. Life Sci. J. 2013, 10, 336–347. [Google Scholar]
- Khan, S.A.; Liu, X.; Shah, B.R. Impact of acute toxicity of lead acetate on the level of essential trace metals and histopathological changes in Crucian carp. (Carassius Auratus Gibelio). J. Anim. Plant Sci. 2014, 24, 1405–1414. [Google Scholar]
- Şenol, N.; Özan, S. The Histomorphological Changes in Carassius carassius (Linnaeus, 1758), Liver and Kidney Tissues of Some Heavy Metals. Indian J. Geomar. Sci. 2016, 45, 1123–1127. [Google Scholar]
- Curcio, V.; Macirella, R.; Sesti, S.; Pellegrino, D.; Ahmed, A.I.; Brunelli, E. Morphological and molecular alterations induced by lead in embryos and larvae of danio rerio. Appl. Sci. 2021, 11, 7464. [Google Scholar] [CrossRef]
- Gül, A.; Yılmaz, M.; Benzer, S.; Taşdemir, L. Investigation of zinc, copper, lead and cadmium accumulation in the tissues of Sander Lucioperca (L., 1758) living in Hirfanlı Dam Lake, Turkey. Bull. Environ. Contam. Toxicol. 2011, 87, 264–266. [Google Scholar] [CrossRef]
- Zubcov, E.; Zubcov, N.; Ene, A.; Biletchi, L. Assessment of copper and zinc levels in fish from freshwater ecosystems of Moldova. Environ. Sci. Pollut. R. 2012, 19, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Subotić, S.; Višnjić Jeftić, Ž.; Spasić, S.; Hegediš, A.; Krpo-Ćetković, J.; Lenhardt, M. Distribution and accumulation of elements (as, Cu, Fe, hg, Mn, and Zn) in tissues of fish species from different trophic levels in the Danube River at the confluence with the Sava River (Serbia). Environ. Sci. Pollut. Res. 2013, 20, 5309–5317. [Google Scholar] [CrossRef] [PubMed]
- Vaseem, H.; Banerjee, T.K. Contamination of metals in different tissues of rohu (labeo rohita, Cyprinidae) collected from the Indian River Ganga. Bull. Environ. Contam. Toxicol. 2013, 91, 36–41. [Google Scholar] [CrossRef]
- Boalt, E.; Miller, A.; Dahlgren, H. Distribution of cadmium, Mercury, and lead in different body parts of Baltic Herring (Clupea Harengus) and perch (Perca fluviatilis): Implications for environmental status assessments. Mar. Pollut. Bull. 2014, 78, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Zolotaryov, K.; Mikhailova, M.; Nakhod, K.; Mikhailov, A. Algorithm to analyse distribution of heavy metals in fish tissues on the example of northern pike. Princ. Ecol. 2018, 28, 34–47. [Google Scholar] [CrossRef]
- Monikh, F.A.; Maryamabadi, A.; Savari, A.; Ghanemi, K. Heavy metals concentration in sediment, shrimp and two fish species from the northwest Persian Gulf. Toxicol. Ind. Health 2013, 31, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Yadrenkina, E.N. Distribution of alien fish species in lakes within the temperate climatic zone of Western Siberia. Russ. J. Biol. Invasions 2012, 3, 145–157. [Google Scholar] [CrossRef]
- Bortnikova, S.; Olenchenko, V.; Gaskova, O.; Yurkevich, N.; Abrosimova, N.; Shevko, E.; Edelev, A.; Korneeva, T.; Provornaya, I.; Eder, L. Characterization of a gold extraction plant environment in assessing the hazardous nature of accumulated wastes (Kemerovo Region, Russia). Appl. Geochem. 2018, 93, 145–157. [Google Scholar] [CrossRef]
- Romanov, V.; Petlina, A.; Babkina, I. Methods of Research of Freshwater Fish of Siberia; Tomsk State University: Tomsk, Russia, 2012; 252 p. (In Russian) [Google Scholar]
- Order of the Ministry of Agriculture of the Russian Federation of 13.12.2016 No. 552 (Revised from 10 March 2020) «On Approval of Water Quality Standards for Water Bodies of Fishery Significance, Including Standards for Maximum Permissible Concentrations of Harmful Substances in the Waters of Water Bodies of Fishery Significance». Available online: http://publication.pravo.gov.ru/Document/View/0001202006160052/ (accessed on 14 November 2020).
- Gaskova, O.; Bortnikova, S.; Airiyants, A.; Kolmogorov, Y.; Pashkov, M. Geochemical Features of an Anthropogenic Impoundment with Cyanidation Wastes of Gold-Arsenopyrite-Quartz Ores. Geochem. Int. 2000, 38, 281–291. [Google Scholar]
- Shuvaeva, O.; Bortnikova, S.; Korda, T.; Lazareva, E. Arsenic Speciation in a Contaminated Gold Processing Tailings Dam. Geostand. Geoanal. Res. 2000, 24, 247–252. [Google Scholar] [CrossRef]
- Moreira, C.A.; Casagrande, M.F.; de Siqueira Büchi, F.M.; Targa, D.A. Hydrogelogical characterization of a waste rock pile and bedrock affected by acid mine drainage from geophysical survey. SN Appl. Sci. 2020, 2, 1236. [Google Scholar] [CrossRef]
- Kirillov, M.V.; Bortnikova, S.B.; Gaskova, O.L. Authigenic gold formation in the cyanidation tailings of gold–arsenopyrite–quartz ore of Komsomolsk Deposit (Kuznetski Alatau, Russia). Environ. Earth Sci. 2016, 75, 1050. [Google Scholar] [CrossRef]
- Walker, F.P.; Schreiber, M.E.; Rimstidt, J.D. Kinetics of arsenopyrite oxidative dissolution by oxygen. Geoch. Cosmoch. Acta 2006, 70, 1668–1676. [Google Scholar] [CrossRef]
- Craw, D.; Falconer, D.; Youngson, J.H. Environmental arsenopyrite stability and dissolution: Theory, experiment, and field observations. Chem. Geol. 2003, 199, 71–82. [Google Scholar] [CrossRef]
- Komnitsas, K.; Xenidis, A.; Adam, K. Oxidation of pyrite and arsenopyrite in sulphidic spoils in Lavrion. Min. Eng. 1995, 8, 1443–1454. [Google Scholar] [CrossRef]
- Oorts, K.; Smolders, E.; Degryse, F.; Buekers, J.; Gascó, G.; Cornelis, G.; Mertens, J. Solubility and Toxicity of Antimony Trioxide (Sb2O3) in Soil. Environ. Sci. Technol. 2008, 42, 4378–4383. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Cui, P.; Huang, M.; Liu, C.; Dang, F.; Wang, Z.; Alves, M.E.; Zhou, D.; Wang, Y. Oxidative dissolution of Sb2O3 mediated by surface Mn redox cycling in oxic aquatic systems. Water Res. 2022, 217, 118403. [Google Scholar] [CrossRef] [PubMed]
- Cantanhêde, S.M.; de Carvalho, I.S.; Hamoy, M.; Corrêa, J.A.; de Carvalho, L.M.; Barbas, L.A.; Montag, L.F.; Amado, L.L. Evaluation of cardiotoxicity in Amazonian Fish Bryconops caudomaculatus by acute exposure to aluminium in an acidic environment. Aquat. Toxicol. 2022, 242, 106044. [Google Scholar] [CrossRef]
- Marins, K.; Lazzarotto, L.M.; Boschetti, G.; Bertoncello, K.T.; Sachett, A.; Schindler, M.S.; Chitolina, R.; Regginato, A.; Zanatta, A.P.; Siebel, A.M.; et al. Iron and manganese present in underground water promote biochemical, genotoxic, and behavioral alterations in zebrafish (danio rerio). Environ. Sci. Pollut. Res. 2019, 26, 23555–23570. [Google Scholar] [CrossRef]
- Gimenes, L.L.; Freschi, G.P.; Bianchini Júnior, I.; Cunha Santino, M.B. Growth of the aquatic macrophyte Ricciocarpos natans (L.) corda in different temperatures and in distinct concentrations of aluminum and Manganese. Aquat. Toxicol. 2020, 224, 105484. [Google Scholar] [CrossRef] [PubMed]
- Grosell, M. Fish Physiology. In Homeostasis and Toxicology of Essential Metals; Wood, C.M., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 31, Part A, pp. 53–133. [Google Scholar] [CrossRef]
- Viana, L.F.; Crispim, B.D.A.; Kummrow, F.; Nascimento, V.A.; Melo, E.S.; de Lima, N.A.; Barufatti, A. Bioaccumulation, genotoxicity, and risks to native fish species from inorganic contaminants in the Pantanal Sul-Mato-Grossense, Brazil. Environ. Pollut. 2022, 314, 120204. [Google Scholar] [CrossRef] [PubMed]
- Squadrone, S.; Prearo, M.; Brizio, P.; Gavinelli, S.; Pellegrino, M.; Scanzio, T.; Guarise, S.; Benedetto, A.; Abete, M.C. Heavy metals distribution in muscle, liver, kidney and Gill of European catfish (Silurus glanis) from Italian rivers. Chemosphere 2013, 90, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Voigt, C.L.; da Silva, C.P.; Doria, H.B.; Randi, M.A.; de Oliveira Ribeiro, C.A.; de Campos, S.X. Bioconcentration and bioaccumulation of metal in freshwater neotropical fish geophagus brasiliensis. Environ. Sci. Pollut. Res. 2014, 22, 8242–8252. [Google Scholar] [CrossRef]
- Borrell, A.; Tornero, V.; Bhattacharjee, D.; Aguilar, A. Trace element accumulation and trophic relationships in aquatic organisms of the Sundarbans mangrove ecosystem (Bangladesh). Sci. Total. Environ. 2016, 545–546, 414–423. [Google Scholar] [CrossRef]
- Chouvelon, T.; Brach-Papa, C.; Auger, D.; Bodin, N.; Bruzac, S.; Crochet, S.; Degroote, M.; Hollanda, S.J.; Hubert, C.; Knoery, J.; et al. Chemical contaminants (trace metals, persistent organic pollutants) in albacore tuna from western Indian and south-eastern Atlantic Oceans: Trophic influence and potential as tracers of populations. Sci.Total. Environ. 2017, 596, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.J. Toxicology | The Toxicology of Metals in Fishes. In Encyclopedia of Fish Physiology; PH: Simon Fraser University: Burnaby BC, Canada, 2011; Volume 3, pp. 2061–2068. [Google Scholar] [CrossRef]
- Hosseini, M.; Nabavi, S.M.; Nabavi, S.N.; Pour, N.A. Heavy metals (CD, Co, Cu, ni, pb, Fe, and hg) content in four fish commonly consumed in Iran: Risk assessment for the consumers. Environ. Monit. Assess. 2015, 187, 237. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Hu, X.; Tao, X.; Yu, H.; Zhang, X. Risk and toxicity assessments of heavy metals in sediments and fishes from the Yangtze River and Taihu Lake, China. Chemosphere 2013, 93, 1887–1895. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).