Combustion, Pyrolysis, and Gasification of Waste-Derived Fuel Slurries, Low-Grade Liquids, and High-Moisture Waste: Review
Abstract
:1. Introduction
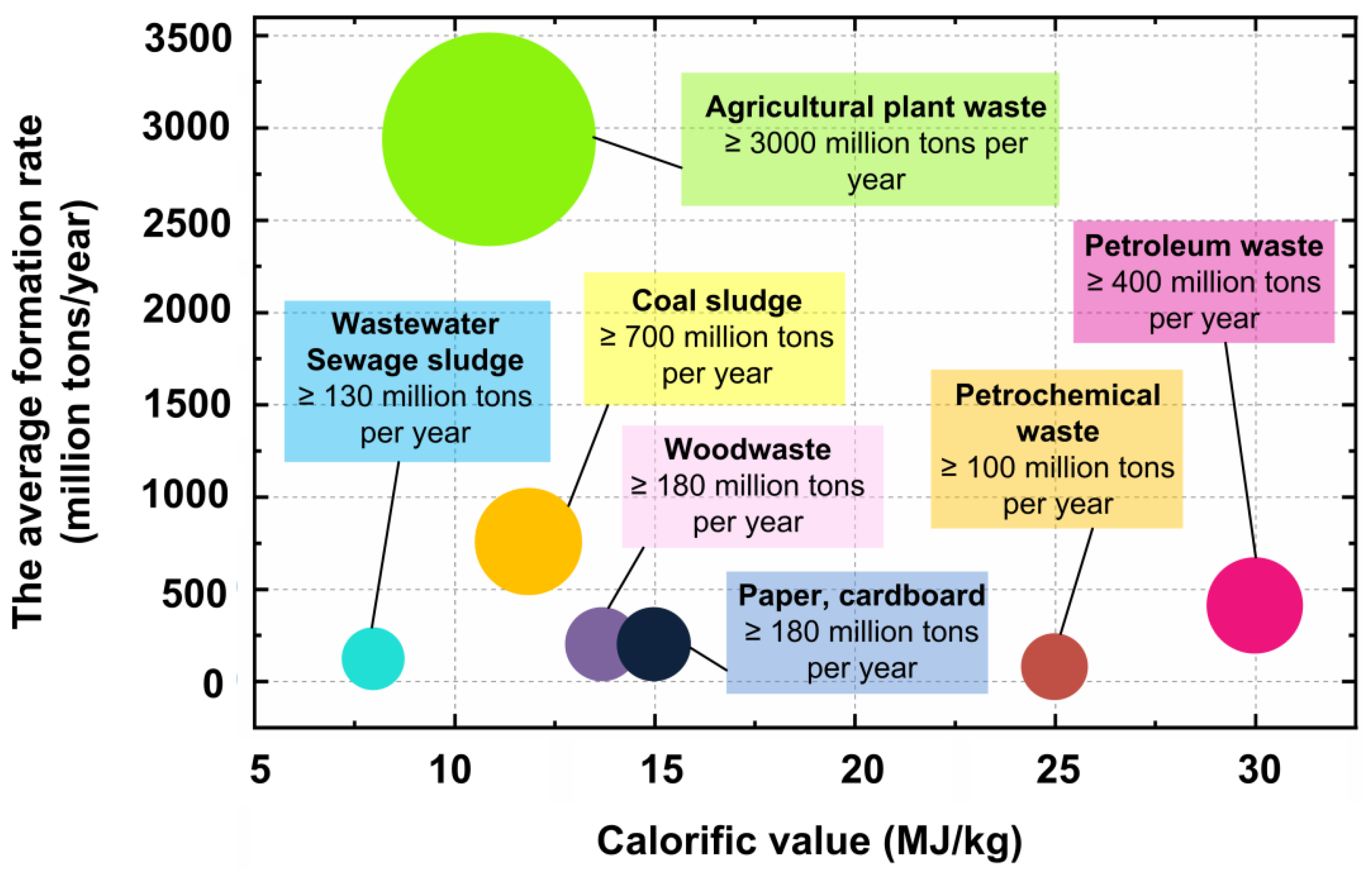
2. Main Types of Combustible Waste
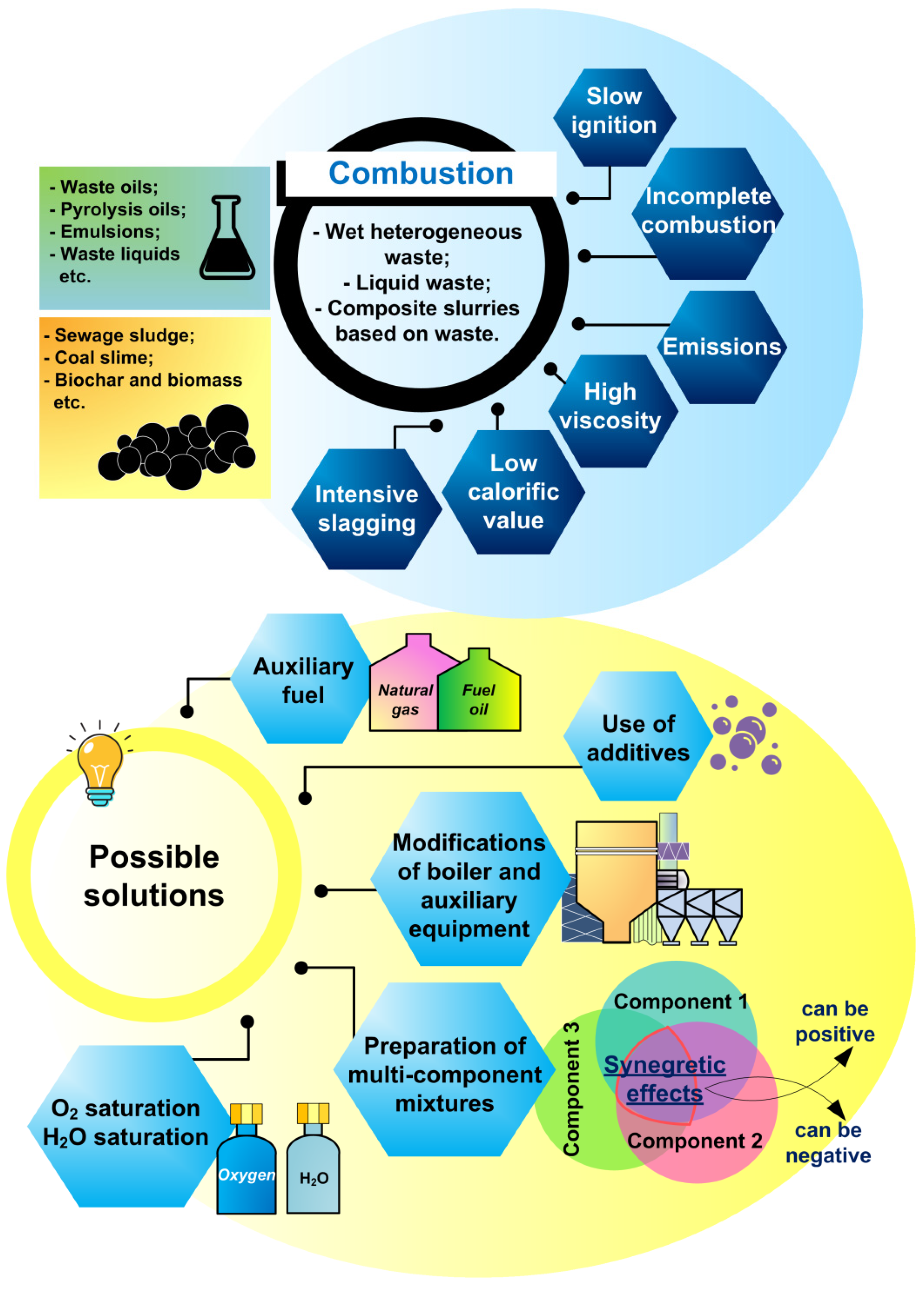
3. Combustion of Non-Conventional Liquid, High-Moisture, and Slurry Fuels
3.1. Influence of the Composition of the Oxidizing Atmosphere on the Ignition and Combustion
3.2. Synergism of Components at Ignition and Burnout of Composite Fuels
3.3. Combustion of Wet Sludge, Process Fluids, and Waste Cooking Oils as Part of Fuel Mixtures
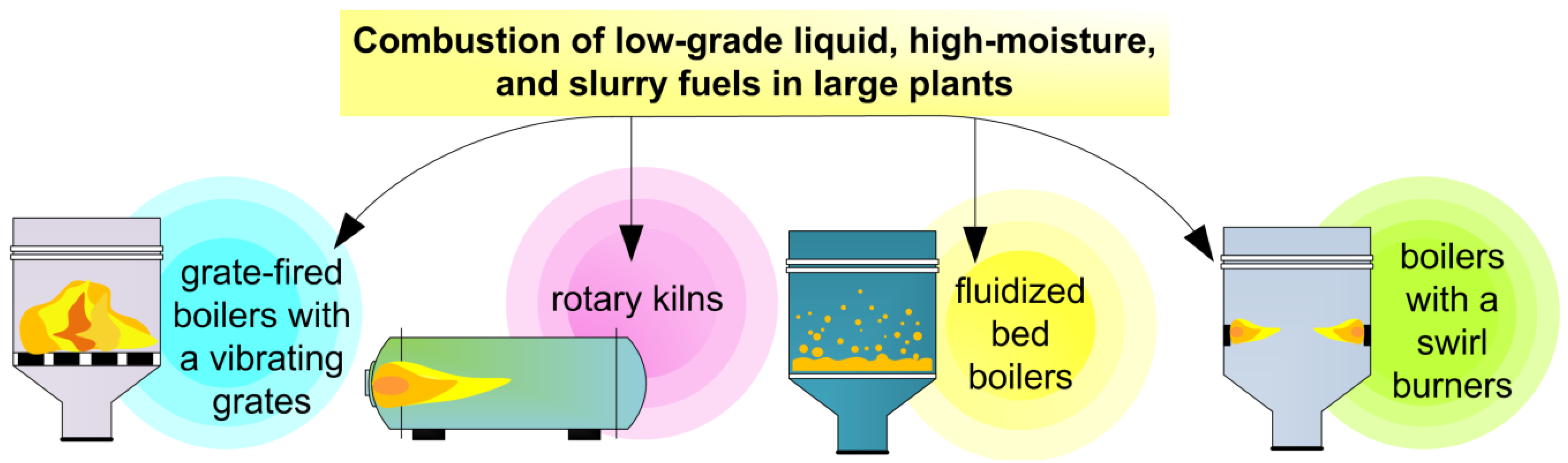
3.4. Combustion of Low-Grade Liquid, Wet, and Slurry Fuels in Large Experimental and Pilot-Scale Plants
3.5. Summarizing the Results on Combustion of Low-Grade Liquid, Wet, and Slurry Fuels
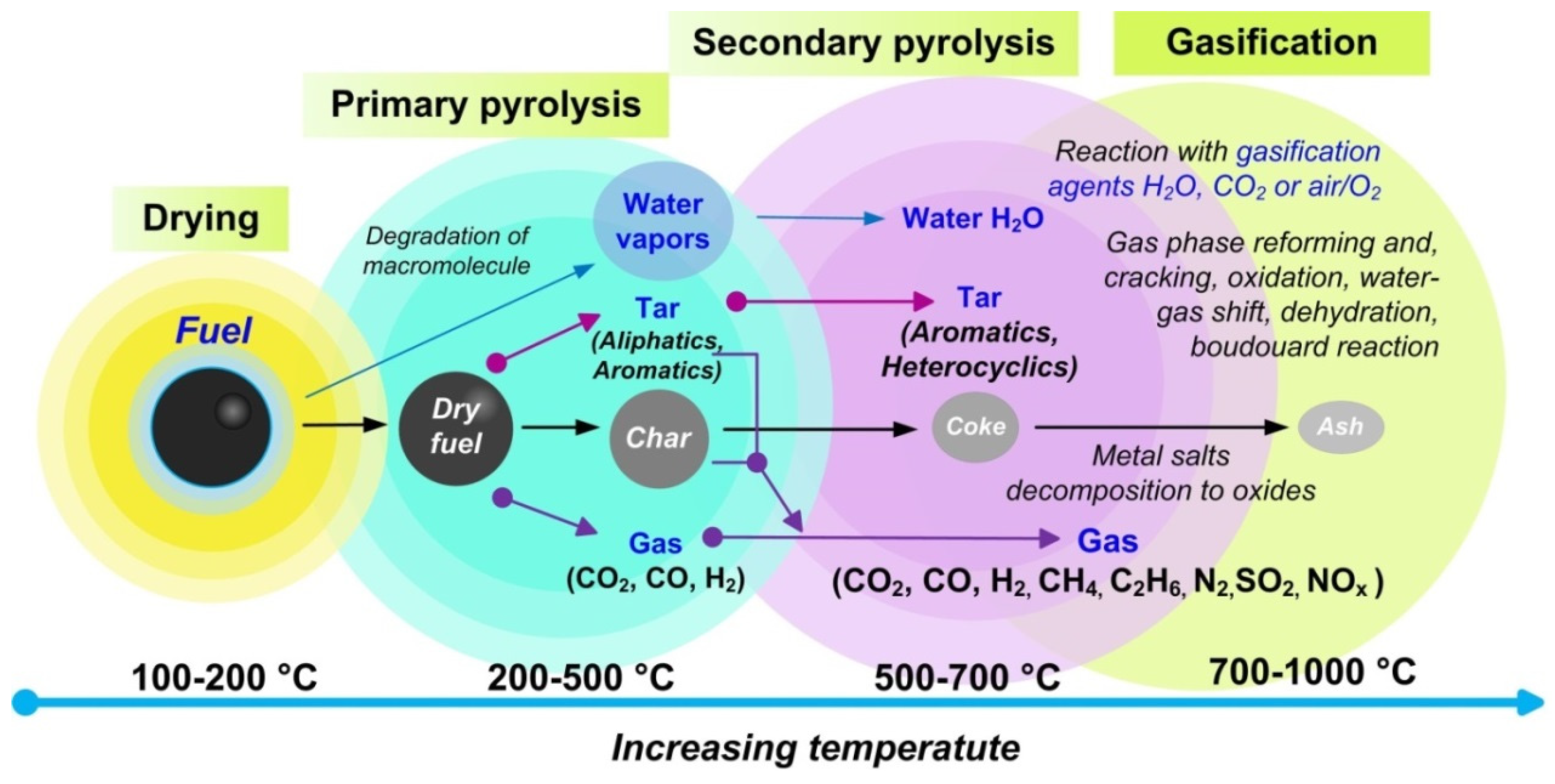
4. Waste Conversion for Fuel Gas Production
4.1. Pyrolysis and Gasification of Coal–Water Slurries with Industrial Waste Additives
4.2. Influence of External Conditions on the Characteristics of the Pyrolysis and Gasification Products
4.3. Specialized Additives and Catalysts to Increase the Efficiency of Pyrolysis and Gasification of Waste
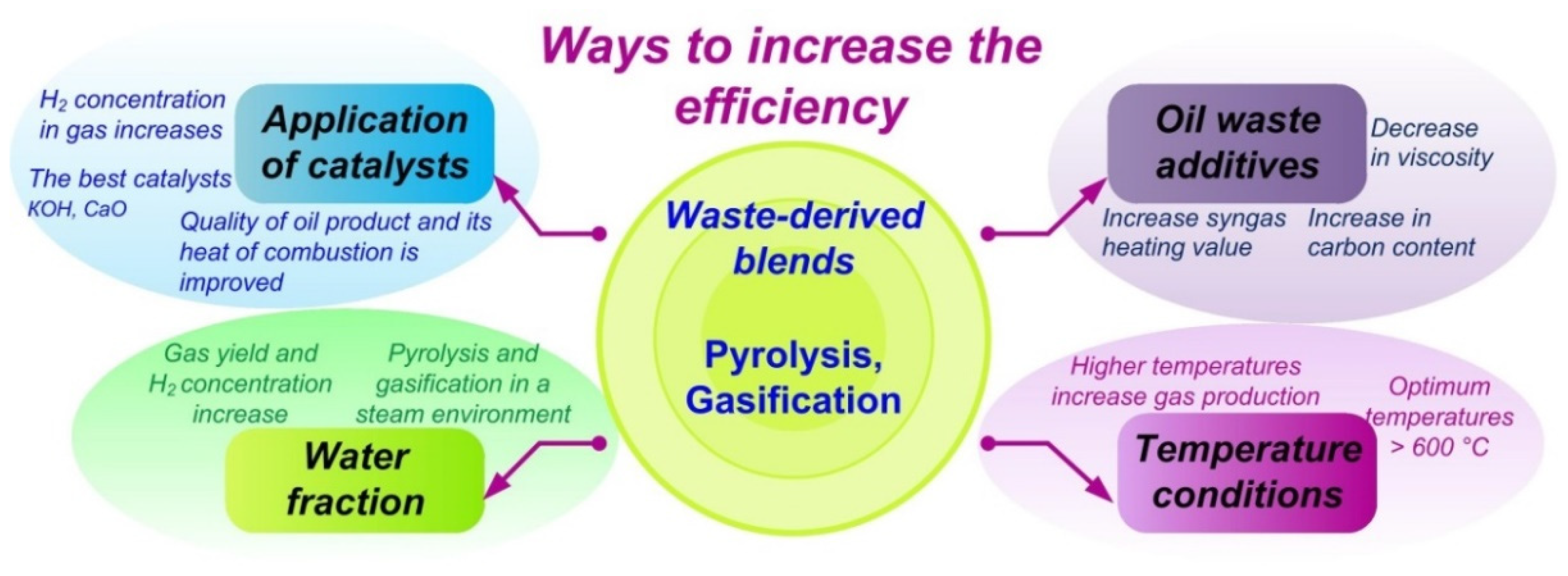
4.4. Summarizing the Results on Pyrolysis and Gasification of Low-Grade Liquid, Wet, and Slurry Fuels
- (i)
- Petroleum waste can significantly increase the efficiency (up to 70%) of pyrolysis and gasification of slurries due to high volatiles and carbon content, low ash content, and high calorific value. The use of even a small amount of petroleum-based additive (from 5 wt% to 20 wt%) in the conventional CWS increases the carbon content without changing the fuel viscosity;
- (ii)
- Water in slurries can positively affect the composition of the gas since gasification in a vapor medium is more effective in terms of maximizing the yield of hydrogen compared to air;
- (iii)
- In terms of the gas yield, temperature above 600 °C is optimal. Under these conditions, the reactions of dehydrogenation, steam reforming, and water–gas conversion are intensified. However, the choice of thermal conditions should be determined based on the purpose of the pyrolysis and gasification products.
- (iv)
- Alkaline catalysts contribute to H2 production during pyrolysis and gasification of waste.
- (v)
- Future research in the field of pyrolysis and gasification of slurry and mixed fuels has the following promising directions: torrefaction of components for the preparation of wet slurries; a detailed study of the influence of heating rate, residence time, and pressure of the pyrolysis and gasification.
5. Conclusions
- (i)
- Almost all typical industrial and solid domestic waste has a calorific value comparable to low-grade fuels. The average calorific value of such waste is 7–12 MJ/kg. As a consequence, this waste can be considered promising for energy production. This approach helps to reduce the rate of depletion of fossil fuels, expand the fuel base of many countries, and reduce their economic and energy dependence.
- (ii)
- The combustion of waste-derived liquid, high-moisture, and slurry fuels can be efficiently implemented using boilers of various modifications such as vortex combustion chambers, boilers with burners, and nozzles for fuel injection, grate, and fluidized bed boilers.
- (iii)
- The combustion of waste-derived fuel slurries and high-moisture fuels can be complicated by a long ignition delay time, low combustion temperature, and low specific calorific value. However, this approach provides wide possibilities for varying the composition of the mixture, atomization, increasing the completeness of fuel burnout, and reducing some hazardous emissions.
- (iv)
- The combustion of fuel mixtures, in contrast to the combustion of individual components, provides more opportunities for stabilizing important process parameters (combustion temperature, calorific value, etc.) and obtaining synergistic benefits (for example, a significant reduction in sulfur oxide emissions).
- (v)
- The present review of pyrolysis and gasification of waste-derived fuel slurries summarizes the main parameters influencing the optimization of the yield of gaseous products. Temperature is one of the most important factors affecting the yield and properties of the end products of pyrolysis and gasification. Optimal process temperatures in terms of maximizing gas yield are above 600 °C. High temperatures and long residence times favor the formation of non-condensable gaseous products due to secondary decomposition reactions.
- (vi)
- Pyrolysis and gasification in a steam medium are more efficient in terms of maximizing the yield of hydrogen compared to air. From this point of view, the use of water-containing slurry fuels is a promising direction. A substantial part of the gasifier steam requirement can be covered by the generation of steam from the slurry.
- (vii)
- The processes of pyrolysis and gasification of oil wastes in the slurry fuels is an effective option for maximizing gas and liquid products and a promising way to dispose of accumulated waste as fuel with low energy losses. This could conserve fossil fuels and solve the problem of increasing energy demand.
Author Contributions
Funding
Conflicts of Interest
References
- Li, D.; Wu, D.; Xu, F.; Lai, J.; Shao, L. Literature overview of Chinese research in the field of better coal utilization. J. Clean. Prod. 2018, 185, 959–980. [Google Scholar] [CrossRef]
- Mallakpour, S.; Sirous, F.; Hussain, C.M. Sawdust, a versatile, inexpensive, readily available bio-waste: From mother earth to valuable materials for sustainable remediation technologies. Adv. Colloid Interface Sci. 2021, 295, 102492. [Google Scholar] [CrossRef] [PubMed]
- Kazamias, G.; Zorpas, A.A. Drill cuttings waste management from oil & gas exploitation industries through end-of-waste criteria in the framework of circular economy strategy. J. Clean. Prod. 2021, 322, 129098. [Google Scholar] [CrossRef]
- Mishra, A.; Siddiqi, H.; Kumari, U.; Behera, I.D.; Mukherjee, S.; Meikap, B.C. Pyrolysis of waste lubricating oil/waste motor oil to generate high-grade fuel oil: A comprehensive review. Renew. Sustain. Energy Rev. 2021, 150, 111446. [Google Scholar] [CrossRef]
- Zhao, R.; Dai, R.; Chen, T.; Qin, J.; Zhang, J.; Wu, J. Investigation on combustion, gaseous pollutants emission and ash characteristics during co-combustion of semicoke and coal slime. J. Environ. Chem. Eng. 2021, 9, 106249. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, F.; Zhou, L.; Guo, Z.; Miao, Z.; Liu, H.; Zhang, X.; Wu, J.; Zhang, Y. Investigation on co-combustion of coal gasification fine slag residual carbon and sawdust char blends: Physiochemical properties, combustion characteristic and kinetic behavior. Fuel 2021, 292, 120387. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahman, S.M.; Rahman, M.S.; Hasan, M.A.; Shoaib, S.A.; Rushd, S. Greenhouse Gas Emissions from Solid Waste Management in Saudi Arabia—Analysis of Growth Dynamics and Mitigation Opportunities. Appl. Sci. 2021, 11, 1737. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, J.; Wang, L.; Zhu, G.; Li, M.; Liu, J.; Li, Z.; Gong, H.; Wu, C.; Yin, G. Multiple classes of chemical contaminants in soil from an e-waste disposal site in China: Occurrence and spatial distribution. Sci. Total Environ. 2021, 752, 141924. [Google Scholar] [CrossRef]
- Khan, H.M.; Iqbal, T.; Yasin, S.; Irfan, M.; Kazmi, M.; Fayaz, H.; Mujtaba, M.A.; Ali, C.H.; Kalam, M.A.; Soudagar, M.E.M.; et al. Production and utilization aspects of waste cooking oil based biodiesel in Pakistan. Alexandria Eng. J. 2021, 60, 5831–5849. [Google Scholar] [CrossRef]
- Furubayashi, T.; Nakata, T. Analysis of woody biomass utilization for heat, electricity, and CHP in a regional city of Japan. J. Clean. Prod. 2021, 290, 125665. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, L.; Guo, J.; Wang, B.; Zhang, L.; Xiang, J.; Jin, Y. Thermogravimetric analysis of co-combustion between municipal sewage sludge and coal slime: Combustion characteristics, interaction and kinetics. Thermochim. Acta 2021, 706, 179056. [Google Scholar] [CrossRef]
- United Nations. The Paris Agreement, FCCC/CP/2015/L.9/Rev.1; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank: Washington, DC, USA, 2018; ISBN 978-1-4648-1329-0. [Google Scholar]
- Habib, M.A.; Ahmed, M.M.; Aziz, M.; Beg, M.R.A.; Hoque, M.E. Municipal Solid Waste Management and Waste-to-Energy Potential from Rajshahi City Corporation in Bangladesh. Appl. Sci. 2021, 11, 3744. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, J.; Liu, J.W.; Zhou, J.; Cheng, L.; Zhao, J.; Shao, Z.; Iris, Ç.; Pan, B.; Li, X.; et al. A review of China’s municipal solid waste (MSW) and comparison with international regions: Management and technologies in treatment and resource utilization. J. Clean. Prod. 2021, 293, 126144. [Google Scholar] [CrossRef]
- Sun, Y.; Qin, Z.; Tang, Y.; Huang, T.; Ding, S.; Ma, X. Techno-environmental-economic evaluation on municipal solid waste (MSW) to power/fuel by gasification-based and incineration-based routes. J. Environ. Chem. Eng. 2021, 9, 106108. [Google Scholar] [CrossRef]
- Wei, C.; Yu, Z.; Zhang, X.; Ma, X. Co-combustion behavior of municipal solid waste and food waste anaerobic digestates: Combustion performance, kinetics, optimization, and gaseous products. J. Environ. Chem. Eng. 2021, 9, 106028. [Google Scholar] [CrossRef]
- Yang, W.; Pudasainee, D.; Gupta, R.; Li, W.; Wang, B.; Sun, L. An overview of inorganic particulate matter emission from coal/biomass/MSW combustion: Sampling and measurement, formation, distribution, inorganic composition and influencing factors. Fuel Process. Technol. 2021, 213, 106657. [Google Scholar] [CrossRef]
- Holubčík, M.; Klačková, I.; Ďurčanský, P. Pyrolysis Conversion of Polymer Wastes to Noble Fuels in Conditions of the Slovak Republic. Energies 2020, 13, 4849. [Google Scholar] [CrossRef]
- Bala-Litwiniak, A.; Radomiak, H. Possibility of the Utilization of Waste Glycerol as an Addition to Wood Pellets. Waste Biomass Valoriz. 2019, 10, 2193–2199. [Google Scholar] [CrossRef] [Green Version]
- Dudyński, M.; Dudyński, K.; Kluska, J.; Ochnio, M.; Kazimierski, P.; Kardaś, D. Gasification of leather waste for energy production: Laboratory scale and industrial tests. Int. J. Energy Res. 2021, 45, 18540–18553. [Google Scholar] [CrossRef]
- Rueda-Avellaneda, J.F.; Rivas-García, P.; Gomez-Gonzalez, R.; Benitez-Bravo, R.; Botello-Álvarez, J.E.; Tututi-Avila, S. Current and prospective situation of municipal solid waste final disposal in Mexico: A spatio-temporal evaluation. Renew. Sustain. Energy Transit. 2021, 1, 100007. [Google Scholar] [CrossRef]
- Shahnazari, A.; Rafiee, M.; Rohani, A.; Bhushan Nagar, B.; Ebrahiminik, M.A.; Aghkhani, M.H. Identification of effective factors to select energy recovery technologies from municipal solid waste using multi-criteria decision making (MCDM): A review of thermochemical technologies. Sustain. Energy Technol. Assess. 2020, 40, 100737. [Google Scholar] [CrossRef]
- Kumar, A.; Sah, B.; Singh, A.R.; Deng, Y.; He, X.; Kumar, P.; Bansal, R.C. A review of multi criteria decision making (MCDM) towards sustainable renewable energy development. Renew. Sustain. Energy Rev. 2017, 69, 596–609. [Google Scholar] [CrossRef]
- Tong, C.; Yang, X.; Chen, G.; Zhang, Y.; Chen, L.; Zhou, Y.; He, T.; Jin, B. Experimental investigation for the combustion characteristics of blends of three kinds of coal. Fuel 2021, 300, 120937. [Google Scholar] [CrossRef]
- Yang, X.; Luo, Z.; Liu, X.; Yu, C.; Li, Y.; Ma, Y. Experimental and numerical investigation of the combustion characteristics and NO emission behaviour during the co-combustion of biomass and coal. Fuel 2021, 287, 119383. [Google Scholar] [CrossRef]
- Paraschiv, L.S.; Serban, A.; Paraschiv, S. Calculation of combustion air required for burning solid fuels (coal/biomass/solid waste) and analysis of flue gas composition. Energy Rep. 2020, 6, 36–45. [Google Scholar] [CrossRef]
- Quesada, L.; Pérez, A.; Godoy, V.; Peula, F.J.; Calero, M.; Blázquez, G. Optimization of the pyrolysis process of a plastic waste to obtain a liquid fuel using different mathematical models. Energy Convers. Manag. 2019, 188, 19–26. [Google Scholar] [CrossRef]
- Su, G.; Ong, H.C.; Mofijur, M.; Mahlia, T.M.I.; Ok, Y.S. Pyrolysis of waste oils for the production of biofuels: A critical review. J. Hazard. Mater. 2022, 424, 127396. [Google Scholar] [CrossRef] [PubMed]
- Ferraz de Campos, V.A.; Silva, V.B.; Cardoso, J.S.; Brito, P.S.; Tuna, C.E.; Silveira, J.L. A review of waste management in Brazil and Portugal: Waste-to-energy as pathway for sustainable development. Renew. Energy 2021, 178, 802–820. [Google Scholar] [CrossRef]
- Shahbaz, M.; AlNouss, A.; Ghiat, I.; Mckay, G.; Mackey, H.; Elkhalifa, S.; Al-Ansari, T. A comprehensive review of biomass based thermochemical conversion technologies integrated with CO2 capture and utilisation within BECCS networks. Resour. Conserv. Recycl. 2021, 173, 105734. [Google Scholar] [CrossRef]
- Dufour, A. Thermochemical Conversion of Biomass for Energy and Chemicals Production; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 1–160. [Google Scholar] [CrossRef]
- Mammino, L. Biomass Burning in Sub-Saharan Africa: Chemical Issues and Action Outreach; Springer: Dordrecht, The Netherlands, 2020. [Google Scholar]
- International Energy Agency. Key World Energy Statistics (Statistics Report); International Energy Agency: Paris, France, 2020. [Google Scholar]
- Junginger, M.; Goh, C.S.; Faaij, A. International Bioenergy Trade: History, Status & Outlook on Securing Sustainable Bioenergy Supply, Demand and Markets; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- David, C. Wilson Global Waste Management Outlook 2015; International Solid Waste Association General Secretariat: Vienna, Austria, 2015. [Google Scholar]
- World Energy Council. World Energy Resources; World Energy Council: London, UK, 2016. [Google Scholar]
- Li, D.; Liu, J.; Wang, S.; Cheng, J. Study on coal water slurries prepared from coal chemical wastewater and their industrial application. Appl. Energy 2020, 268, 114976. [Google Scholar] [CrossRef]
- Lázaro, M.J.; Moliner, R.; Suelves, I.; Domeo, C.; Nerín, C. Co-pyrolysis of a mineral waste oil/coal slurry in a continuous-mode fluidized bed reactor. J. Anal. Appl. Pyrolysis 2002, 65, 239–252. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Liu, L.; Wang, X.; Zhang, Z. Environmental investigation on co-combustion of sewage sludge and coal gangue: SO2, NOx and trace elements emissions. Waste Manag. 2016, 50, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Glushkov, D.O.; Paushkina, K.K.; Shabardin, D.P. Co-combustion of coal processing waste, oil refining waste and municipal solid waste: Mechanism, characteristics, emissions. Chemosphere 2020, 240, 124892. [Google Scholar] [CrossRef] [PubMed]
- Vershinina, K.Y.; Strizhak, P.A. Ignition of coal suspensions based on water of different quality. Coke Chem. 2016, 59, 437–440. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, R.; Ge, L.; Wu, J.; Yin, Q.; Wang, C. Energy utilization of coal-coking wastes via coal slurry preparation: The characteristics of slurrying, combustion, and pollutant emission. Energy 2019, 168, 609–618. [Google Scholar] [CrossRef]
- Lin, B.; Wang, J.; Huang, Q.; Chi, Y. Effects of potassium hydroxide on the catalytic pyrolysis of oily sludge for high-quality oil product. Fuel 2017, 200, 124–133. [Google Scholar] [CrossRef]
- Zou, H.; Liu, C.; Evrendilek, F.; He, Y.; Liu, J. Evaluation of reaction mechanisms and emissions of oily sludge and coal co-combustions in O2/CO2 and O2/N2 atmospheres. Renew. Energy 2021, 171, 1327–1343. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Zhong, Z.; Ding, K.; Deng, A.; Min, M.; Chen, P.; Ruan, R. Catalytic fast co-pyrolysis of bamboo residual and waste lubricating oil over an ex-situ dual catalytic beds of MgO and HZSM-5: Analytical PY-GC/MS study. Energy Convers. Manag. 2017, 139, 222–231. [Google Scholar] [CrossRef]
- Staroń, A.; Kowalski, Z.; Staroń, P.; Banach, M. Studies on CWL with glycerol for combustion process. Environ. Sci. Pollut. Res. 2019, 26, 2835–2844. [Google Scholar] [CrossRef] [Green Version]
- Bae, J.S.; Lee, D.W.; Park, S.J.; Lee, Y.J.; Hong, J.C.; Ra, H.W.; Han, C.; Choi, Y.C. High-pressure gasification of coal water ethanol slurry in an entrained flow gasifier for bioethanol application. Energy Fuels 2012, 26, 6033–6039. [Google Scholar] [CrossRef]
- Boumanchar, I.; Chhiti, Y.; M’hamdi Alaoui, F.E.; Elkhouakhi, M.; Sahibed-dine, A.; Bentiss, F.; Jama, C.; Bensitel, M. Investigation of (co)-combustion kinetics of biomass, coal and municipal solid wastes. Waste Manag. 2019, 97, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.S.; Tsang, Y.F.; Yek, P.N.Y.; Liew, R.K.; Osman, M.S.; Peng, W.; Lee, W.H.; Park, Y.K. Co-processing of oil palm waste and waste oil via microwave co-torrefaction: A waste reduction approach for producing solid fuel product with improved properties. Process Saf. Environ. Prot. 2019, 128, 30–35. [Google Scholar] [CrossRef]
- Sukiran, M.A.; Abnisa, F.; Wan Daud, W.M.A.; Abu Bakar, N.; Loh, S.K. A review of torrefaction of oil palm solid wastes for biofuel production. Energy Convers. Manag. 2017, 149, 101–120. [Google Scholar] [CrossRef]
- Bach, Q.V.; Trinh, T.N.; Tran, K.Q.; Thi, N.B.D. Pyrolysis characteristics and kinetics of biomass torrefied in various atmospheres. Energy Convers. Manag. 2017, 141, 72–78. [Google Scholar] [CrossRef]
- Medic, D.; Darr, M.; Shah, A.; Potter, B.; Zimmerman, J. Effects of torrefaction process parameters on biomass feedstock upgrading. Fuel 2012, 91, 147–154. [Google Scholar] [CrossRef]
- Cheng, Z.; Jin, H.; Liu, S.; Guo, L.; Xu, J.; Su, D. Hydrogen production by semicoke gasification with a supercritical water fluidized bed reactor. Int. J. Hydrogen Energy 2016, 41, 16055–16063. [Google Scholar] [CrossRef]
- Gaber, C.; Wachter, P.; Demuth, M.; Hochenauer, C. Experimental investigation and demonstration of pilot-scale combustion of oil-water emulsions and coal-water slurry with pronounced water contents at elevated temperatures with the use of pure oxygen. Fuel 2020, 282, 118692. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Z.; Ao, W.; Li, J.; Liu, G.; Fu, J.; Ran, C.; Mao, X.; Kang, Q.; Liu, Y.; et al. Microwave-assisted pyrolysis of textile dyeing sludge using different additives. J. Anal. Appl. Pyrolysis 2017, 127, 140–149. [Google Scholar] [CrossRef]
- Staroń, A.; Banach, M.; Kowalski, Z.; Staroń, P. Impact of waste soot on properties of coal-water suspensions. J. Clean. Prod. 2016, 135, 457–467. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, W.; Huang, J.; Li, M.; Gong, M. Emission characteristics of PCDD/Fs, PAHs and PCBs during the combustion of sludge-coal water slurry. J. Energy Inst. 2015, 88, 105–111. [Google Scholar] [CrossRef]
- Fu, B.; Liu, G.; Mian, M.M.; Zhou, C.; Sun, M.; Wu, D.; Liu, Y. Co-combustion of industrial coal slurry and sewage sludge: Thermochemical and emission behavior of heavy metals. Chemosphere 2019, 233, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Kuan, Y.H.; Wu, F.H.; Chen, G.B.; Lin, H.T.; Lin, T.H. Study of the combustion characteristics of sewage sludge pyrolysis oil, heavy fuel oil, and their blends. Energy 2020, 201, 117559. [Google Scholar] [CrossRef]
- Luo, Z.; Xu, D.; Ma, Y.; Cheng, Q. Experimental Study on Co-Firing of Coal and Brewery Wastewater Sludge. Appl. Sci. 2020, 10, 7589. [Google Scholar] [CrossRef]
- Wan, G.; Yu, J.; Wang, X.; Sun, L. Study on the pyrolysis behavior of coal-water slurry and coal-oil-water slurry. J. Energy Inst. 2022, 100, 10–21. [Google Scholar] [CrossRef]
- Moliner, R.; Lázaro, M.; Suelves, I. Valorization of Lube Oil Waste by Pyrolysis. Energy Fuels 1997, 11, 1165–1170. [Google Scholar] [CrossRef]
- Tamošiūnas, A.; Gimžauskaitė, D.; Aikas, M.; Uscila, R.; Praspaliauskas, M.; Eimontas, J. Gasification of Waste Cooking Oil to Syngas by Thermal Arc Plasma. Energies 2019, 12, 2612. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.; Wu, H. Synergy on particulate matter emission during the combustion of bio-oil/biochar slurry (bioslurry). Fuel 2018, 214, 546–553. [Google Scholar] [CrossRef]
- Zhou, H.; Li, Y.; Li, N.; Cen, K. Experimental investigation of ignition and combustion characteristics of single coal and biomass particles in O2/N2 and O2/H2O. J. Energy Inst. 2019, 92, 502–511. [Google Scholar] [CrossRef]
- D’Jesús, P.; Boukis, N.; Kraushaar-Czarnetzki, B.; Dinjus, E. Gasification of corn and clover grass in supercritical water. Fuel 2006, 85, 1032–1038. [Google Scholar] [CrossRef] [Green Version]
- Malika, A.; Mohammed, A.; Guhel, Y. Energetic Combustion Characteristics and Environmental Impact of Moroccan Biomass Wastes and Their Solid Biofuel. Waste Biomass Valoriz. 2019, 10, 1311–1322. [Google Scholar] [CrossRef]
- Variny, M.; Varga, A.; Rimár, M.; Janošovský, J.; Kizek, J.; Lukáč, L.; Jablonský, G.; Mierka, O. Advances in Biomass Co-Combustion with Fossil Fuels in the European Context: A Review. Processes 2021, 9, 100. [Google Scholar] [CrossRef]
- Khan, A.A.; de Jong, W.; Jansens, P.J.; Spliethoff, H. Biomass combustion in fluidized bed boilers: Potential problems and remedies. Fuel Process. Technol. 2009, 90, 21–50. [Google Scholar] [CrossRef]
- Xinjie, L.; Shihong, Z.; Xincheng, W.; Jinai, S.; Xiong, Z.; Xianhua, W.; Haiping, Y.; Hanping, C. Co-combustion of wheat straw and camphor wood with coal slime: Thermal behaviour, kinetics, and gaseous pollutant emission characteristics. Energy 2021, 234, 121292. [Google Scholar] [CrossRef]
- Seepana, S.; Arumugam, S.; Sivaramakrishnan, K.; Muthukrishnan, M. Evaluation of feasibility of pelletized wood co-firing with high ash Indian coals. J. Energy Inst. 2018, 91, 1126–1135. [Google Scholar] [CrossRef]
- Johansson, A.-C.; Molinder, R.; Vikström, T.; Wiinikka, H. Particle formation during suspension combustion of different biomass powders and their fast pyrolysis bio-oils and biochars. Fuel Process. Technol. 2021, 218, 106868. [Google Scholar] [CrossRef]
- Skoglund, N.; Bäfver, L.; Fahlström, J.; Holmén, E.; Renström, C. Fuel design in co-combustion of demolition wood chips and municipal sewage sludge. Fuel Process. Technol. 2016, 141, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Jianzhong, L.; Ruikun, W.; Jianfei, X.; Junhu, Z.; Kefa, C. Pilot-scale investigation on slurrying, combustion, and slagging characteristics of coal slurry fuel prepared using industrial wasteliquid. Appl. Energy 2014, 115, 309–319. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, W.; Yu, A.; Wang, C.-H. Co-firing of coal and biomass under pressurized oxy-fuel combustion mode: Experimental test in a 10 kWth fluidized bed. Chem. Eng. J. 2021, 431 Pt 4, 133457. [Google Scholar] [CrossRef]
- Hamadeh, H.; Toor, S.Y.; Douglas, P.L.; Sarathy, S.M.; Dibble, R.W.; Croiset, E. Techno-Economic Analysis of Pressurized Oxy-Fuel Combustion of Petroleum Coke. Energies 2020, 13, 3463. [Google Scholar] [CrossRef]
- Chansa, O.; Luo, Z.; Yu, C. Study of the kinetic behaviour of biomass and coal during oxyfuel co-combustion. Chin. J. Chem. Eng. 2020, 28, 1796–1804. [Google Scholar] [CrossRef]
- Chen, C.; Yang, S. The energy demand and environmental impacts of oxy-fuel combustion vs. post-combustion capture in China. Energy Strategy Rev. 2021, 38, 100701. [Google Scholar] [CrossRef]
- Huang, X.; Guo, J.; Liu, Z.; Zheng, C. Opportunities and Challenges of Oxy-Fuel Combustion. In Oxy-Fuel Combustion; Academic Press: Cambridge, MA, USA, 2018; pp. 1–12. [Google Scholar] [CrossRef]
- Feng, P.; Li, X.; Wang, J.; Li, J.; Wang, H.; He, L. The mixtures of bio-oil derived from different biomass and coal/char as biofuels: Combustion characteristics. Energy 2021, 224, 120132. [Google Scholar] [CrossRef]
- Lei, K.; Zhang, R.; Ye, B.Q.; Cao, J.; Liu, D. Study of Sewage Sludge/Coal Co-Combustion by Thermogravimetric Analysis and Single Particle Co-Combustion Method. Energy Fuels 2018, 32, 6300–6308. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, J.; Jia, W.; Guo, F.; Qiu, G.; Wang, R.; Zhang, Y.; Dai, B. Evaluation of the thermal behavior, synergistic catalysis, and pollutant emissions during the co-combustion of sewage sludge and coal gasification fine slag residual carbon. Catalysts 2021, 11, 1142. [Google Scholar] [CrossRef]
- Van Grinsven, A.; van den Toorn, E.; van der Veen, R.; Kampman, B. Used Cooking Oil (UCO) as Biofuel Feedstock in the EU; CE Delft: Delft, Netherlands, 2020. [Google Scholar]
- Gad, M.S.; Mahfouz, A.; Emara, A. Spray and combustion characteristics for light diesel/waste cooking oils blended with fuel additives inside an industrial boiler. Fuel 2021, 286, 119247. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lee, W.J.; Mwangi, J.K.; Wang, L.C.; Wu, J.L.; Lin, S.L. Reduction of persistent organic pollutant emissions during incinerator start-up by using crude waste cooking oil as an alternative fuel. Aerosol Air Qual. Res. 2017, 17, 899–912. [Google Scholar] [CrossRef] [Green Version]
- Ortner, M.E.; Müller, W.; Schneider, I.; Bockreis, A. Environmental assessment of three different utilization paths of waste cooking oil from households. Resour. Conserv. Recycl. 2016, 106, 59–67. [Google Scholar] [CrossRef]
- Hong, F.; Yan, G.; Gao, M. The operation control and application of CFB boiler unit with high blending ratio of coal slurry. Control Eng. Pract. 2019, 85, 80–89. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, M.; Wang, Z.; Wei, L.; Zhao, S. A novel suspension-floating-circulating fluidized combustion technology for coal slurry. Int. J. Coal Sci. Technol. 2016, 3, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Burra, K.G.; Gupta, A.K. Thermochemical Reforming of Wastes to Renewable Fuels. In Energy for Propulsion; Springer: Singapore, 2018; pp. 395–428. [Google Scholar] [CrossRef]
- Li, W.; He, S.; Li, S. Experimental Study and Thermodynamic Analysis of Hydrogen Production through a Two-Step Chemical Regenerative Coal Gasification. Appl. Sci. 2019, 9, 3035. [Google Scholar] [CrossRef] [Green Version]
- Heinze, C.; Langner, E.; May, J.; Epple, B. Determination of a Complete Conversion Model for Gasification of Lignite Char. Appl. Sci. 2020, 10, 1916. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Dai, Z.; Guo, Q.; Yu, G. Effects of in-situ interactions between steam and coal on pyrolysis and gasification characteristics of pulverized coals and coal water slurry. Appl. Energy 2017, 187, 627–639. [Google Scholar] [CrossRef]
- Efika, C.E.; Onwudili, J.A.; Williams, P.T. Influence of heating rates on the products of high-temperature pyrolysis of waste wood pellets and biomass model compounds. Waste Manag. 2018, 76, 497–506. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An overview of advances in biomass gasification. Energy Environ. Sci. 2016, 9, 2939–2977. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.M.; Alobaid, F.; Dieringer, P.; Epple, B. Biomass-Based Chemical Looping Gasification: Overview and Recent Developments. Appl. Sci. 2021, 11, 7069. [Google Scholar] [CrossRef]
- Neves, D.; Thunman, H.; Matos, A.; Tarelho, L.; Gómez-Barea, A. Characterization and prediction of biomass pyrolysis products. Prog. Energy Combust. Sci. 2011, 37, 611–630. [Google Scholar] [CrossRef]
- Sato, T.; Kurosawa, S.; Smith, R.L.; Adschiri, T.; Arai, K. Water gas shift reaction kinetics under noncatalytic conditions in supercritical water. J. Supercrit. Fluids 2004, 29, 113–119. [Google Scholar] [CrossRef]
- Svoboda, K.; Pohořelý, M.; Jeremiáš, M.; Kameníková, P.; Hartman, M.; Skoblja, S.; Šyc, M. Fluidized bed gasification of coal–oil and coal–water–oil slurries by oxygen–steam and oxygen–CO2 mixtures. Fuel Process. Technol. 2012, 95, 16–26. [Google Scholar] [CrossRef]
- Feng, P.; Lin, W.; Jensen, P.A.; Song, W.; Hao, L.; Raffelt, K.; Dam-Johansen, K. Entrained flow gasification of coal/bio-oil slurries. Energy 2016, 111, 793–802. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Yuan, H. Simultaneous production of hydrogen and carbon nanotubes from cracking of a waste cooking oil model compound over Ni-Co/SBA-15 catalysts. Int. J. Energy Res. 2020, 44, 11564–11582. [Google Scholar] [CrossRef]
- Kruse, A.; Meier, D.; Rimbrecht, P.; Schacht, M. Gasification of Pyrocatechol in Supercritical Water in the Presence of Potassium Hydroxide. Ind. Eng. Chem. Res. 2000, 39, 4842–4848. [Google Scholar] [CrossRef]
- Song, G.; Huang, D.; Li, H.; Wang, X.; Ren, Q.; Jiang, L.; Wang, Y.; Su, S.; Hu, S.; Xiang, J. Pyrolysis reaction mechanism of typical Chinese agriculture and forest waste pellets at high heating rates based on the photo-thermal TGA. Energy 2022, 244, 123164. [Google Scholar] [CrossRef]
- Morf, P.; Hasler, P.; Nussbaumer, T. Mechanisms and kinetics of homogeneous secondary reactions of tar from continuous pyrolysis of wood chips. Fuel 2002, 81, 843–853. [Google Scholar] [CrossRef]
- Xie, Q.; Peng, P.; Liu, S.; Min, M.; Cheng, Y.; Wan, Y.; Li, Y.; Lin, X.; Liu, Y.; Chen, P.; et al. Fast microwave-assisted catalytic pyrolysis of sewage sludge for bio-oil production. Bioresour. Technol. 2014, 172, 162–168. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, D.K. An experimental study of oil recovery from sewage sludge by low-temperature pyrolysis in a fluidised-bed. Fuel 2003, 82, 465–472. [Google Scholar] [CrossRef]
- Nie, F.; He, D.; Guan, J.; Zhang, K.; Meng, T.; Zhang, Q. The influence of abundant calcium oxide addition on oil sand pyrolysis. Fuel Process. Technol. 2017, 155, 216–224. [Google Scholar] [CrossRef]
- Sinag, A.; Kruse, A.; Schwarzkopf, V. Key Compounds of the Hydropyrolysis of Glucose in Supercritical Water in the Presence of K2CO3. Ind. Eng. Chem. Res. 2003, 42, 3516–3521. [Google Scholar] [CrossRef]
| Component | Ultimate Analysis (wt%) | Proximate Analysis (wt%) | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | S | Moisture | Volatile Matter | Fixed Carbon | Ash | Calorific Value (MJ/kg) | ||
| Shenhua coal | 69.55 | 3.74 | 10.14 | 0.83 | 0.25 | 8.28 | 29.55 | 54.96 | 7.21 | 27.07 | [38] |
| Samca coal | 75.9 | 5.3 | 12.27 | 0.7 | 5.8 | - | 36.9 | - | 22.8 | - | [39] |
| Coal gangue | 17.5 | 1.26 | - | 0.56 | 1.28 | 0.75 | 15.07 | 16.31 | 68.62 | 4.82 | [40] |
| Coal slime | 87.2 | 5.1 | 4.5 | 2.1 | 1.1 | - | 23.1 | - | 26.5 | 24.83 | [41] |
| Semicoke powders | 69.12 | 1.35 | 10.33 | 0.89 | 0.71 | 0.7 | 15.74 | 67.36 | 16.9 | - | [54] |
| Pyrolytic carbon black | 93.5 | 2.84 | <0.01 | 0.46 | 3.2 | - | - | - | 25 | 26 | [55] |
| Textile dyeing sludge | 15.53 | 3.44 | 16.47 | 2.43 | 1.38 | 1.37 | 36.53 | 1.35 | 60.75 | 5.99 | [56] |
| Waste soot | 74.6 | 1.6 | - | 0.2 | 1.35 | 68.6 | - | - | - | 28.1 | [57] |
| Sewage sludge | 24.83 | 3.31 | 14.39 | 4.47 | 1.13 | 97.95 | 42.74 | 5.39 | 44.58 | 0.77 | [58] |
| Sewage sludge | 13.22 | 2.91 | 19.7 | 2.12 | 0.57 | 5.29 | 31.31 | 2.06 | 61.34 | 5.215 | [59] |
| Coking sludge | 24.48 | 3.15 | 23.68 | 2.36 | 0.94 | 78.97 | 45.48 | 9.14 | 45.38 | 8.49 | [60] |
| Brewery wastewater sludge | 17.6 | 2.93 | - | 2.41 | - | 2.33 | 37.72 | 0.09 | 59.86 | 6.56 | [61] |
| Waste lubricating oil | 83.53 | 13.32 | 2.83 | 0.15 | 0.17 | - | - | - | - | - | [62] |
| Mineral waste oil | 83.2 | 13.0 | 1.2 | - | 1.2 | - | - | - | - | [39] | |
| Lubricating Oil Wastes | 83.2 | 13 | 1.2 | - | 1.2 | - | - | - | - | 44.33 | [63] |
| Waste lubricating oil | 84.02 | 13.31 | 1.92 | - | 0.75 | - | - | - | - | - | [46] |
| Waste cooking oil | 71.84 | 10.14 | 17.71 | 0.06 | 0.01 | 0.08 | 99.15 | 0.56 | 0.24 | 39.24 | [64] |
| Oily sludge | 63.9 | 7.3 | 25.3 | 1.2 | 2.3 | 33.4 | 69.3 | - | 21.2 | 23 | [44] |
| Bio-oil (from pyrolysis of pine) | 41.47 | 6.37 | 52.05 | 0.11 | - | 24.7 | 73.1 | 2.1 | 0.1 | 16.9 | [65] |
| Corn stalk | 32.01 | 3.44 | 24.0 | 1.02 | 0.22 | 6.77 | 52.1 | 8.61 | 32.52 | 11.87 | [66] |
| Coal slime | 53.29 | 3.89 | 9.41 | 0.83 | 0.65 | 0.95 | 27.51 | 36.62 | 34.92 | 22.07 | [59] |
| Bamboo residual | 55.51 | 6.12 | 42.05 | 0.21 | 0.11 | - | - | - | - | - | [46] |
| Corn silage | 43.40 | 6.17 | 46.70 | 1.02 | 0.93 | - | - | - | - | - | [67] |
| Clover grass | 44.90 | 6.8 | 43.30 | 2.2 | 0.3 | - | - | - | - | - | [67] |
| Biochar (from pyrolysis of pine) | 86.83 | 3.34 | 9.7 | 0.13 | - | 2.4 | 16.4 | 80.6 | 3.0 | 28.3 | [65] |
| Fuel | Installation | Temperature Conditions | Ref. |
|---|---|---|---|
| Stem wood, bark, forest residue, willow, and reed canary grass and pyrolysis oil and solid residue from them | Tube furnace blown by gas mixtures (air, N2, O2) | <1400 °C | [73] |
| Emulsion based on water and heating oil; slurry based on water and pyrolytic soot | Chamber with industrial burners with a total power of 1.2 MW | Temperature of flue gases > 1100 °C Maximum operating temperature 1430 °C | [55] |
| Spherical particles of corn stalk and bituminous coal | Reactor (electrical quartz tube), blown by mixtures of O2/N2 and O2/H2O | 800 °C | [66] |
| Sewage sludge with coal–water slurry (CWS) | Large scale fluidized bed incinerator | >1000 °C | [58] |
| Wet sewage sludge with wood chips | Grate-fired boiler with a vibrating grate | >1000 °C | [74] |
| Pyrolysis oil from sewage sludge, heavy fuel oil | Laboratory setup with heat sources in the form of two plates | Temperature of the plates is 500, 550, 600 °C | [60] |
| Slurry based on coal, water and waste soot | Rotary kiln | 800 °C | [57] |
| Slurries based on coal and liquid waste from petrochemical industry | Pilot-scale combustion system | 1100–1300 °C at steady combustion | [75] |
| Fuel | Process | Characteristics of the Plant | Temperature | Key Result | Ref. |
|---|---|---|---|---|---|
| Coal–oil–water slurry (COWS) (coal 45–55 wt%, oil 10–20 wt%; water 35 wt%) | Pyrolysis | Laboratory tube furnace. The carrier gas: N2, flow rate 0.8 L/min. Experiment time: 30 min. Particle size: 75–100 μm. | 800, 900 and 1000 °C | An increase in the temperature and the proportion of water in the fuel contributed to an increase in the gas yield up to 2.8 times, while the char yield decreased to 1.4 times. The addition of waste oil resulted in a decrease in CO and CO2, and an increase in CH4 and H2. Pyrolysis gas composition: H2: 80–270 mL/g; CO: 35–110 mL/g; CO2: 22–120 mL/g; CH4: 60–150 mL/g. | [62] |
| Coal wastewater slurry (CWWS) (coal 57.2–62 wt%, water 42.8–38 wt%). | Gasification | Industrial CWS gasifier to produce syngas and synthesize ammonia. Syngas output 515,116.8 m3/day. Particle size: 40 μm. | 1350–1400 °C | The syngas produced by the CWWS gasification has a higher effective gas component (CO + H2) than the CWS. In addition, the use of a waste-based slurry increased cold gas efficiency by 1.57% and carbon conversion by 0.45% in industrial processes. Syngas composition: H2: 30.5%; CO: 48.1%; CO2: 16.3%; CH4: 0.9%; N2: 4.2%. | [38] |
| Waste oil/coal slurry (coal 50 wt%, mineral waste oil 50 wt%). | Pyrolysis | Laboratory fluidized bed reactor. Feeding rate 550 g/h. Fuel mass 3 kg. | 625 °C | The quality of waste oil/coal slurry pyrolysis products was higher compared to coal pyrolysis products. During the slurry pyrolysis, the gas yield increased from 14.2% to 31.6%, and the liquid yield increased from 17.4 to 29.1% in comparison with coal. At the same time, the concentrations of CH4, H2, C2H4, and C2H6 increased by 3.3, 2.5, 32, and 10 times, respectively. Pyrolysis gas composition: H2: 0.5 wt%; CO: 1.6 wt%; CO2: 3.4 wt%; CH4: 4.9 wt%, C2H4, 6.4 wt%; C2H6 3 wt%. | [39] |
| Lubricating Oil Wastes (LOW) | Pyrolysis | Laboratory pyrolysis unit. Reactor is heated by an electrical oven. Feeding rate 0.5 g/min. Experiment time 20 min. | 600–700 °C | Pyrolysis gas composition: H2: 0.01–0.02 g/kg; CO: 0.03–0.04 g/kg; CO2: 0.04–0.08 g/kg; CH4: 0.35–0.93 g/kg; C2H4: 0.5–1 g/kg; C2H6: 0.25–0.47 g/kg. Product Yield by Pyrolysis: char: 0.45–0.6 g/kg; liquids: 3.57–6.04 g/kg; gases: 3.46–5.97 g/kg; | [63] |
| Bamboo residual (BR) and waste lubricating oil (WLO) | Pyrolysis | Pyrolyzer with dual catalytic beds HZSM-5 and MgO. Fast pyrolysis: heating rate 2000 °C/s. Particle size: 0.15 μm. | 500–700 °C | The temperature of 600 °C was optimal due to the relatively high yields of furans and phenols. | [46] |
| Coal water ethanol slurry (CWES) (coal 57 wt%, water 36 wt%, ethanol 7 wt%). | Gasification | Pilot-scale entrained flow gasifier. Feeding rate at 20 bar: 96.15 kg/h. | 1100 °C | When ethanol was used in the slurry, an increase was recorded in syngas heating value (by 9%), syngas flow rate (by 38%), syngas production per 1 kg of slurry (by 25%), cold gas efficiency (by 39%) and carbon conversion efficiency (by 15%). Syngas composition: H2: 34.50 vol%; CO: 29.69 vol%; CO2: 35.33 vol%; CH4: 0.47 vol%. | [48] |
| Textile dyeing sludge (DS) with 20–30 wt% additives (CaO, Ca-bentonite, Kaolin and Fe) | Pyrolysis | Two-mode microwave device with 2.45 GHz frequency and the maximum power of 3 kW. Particle size: <1 mm. | 450–750 °C | Addition of CaO and Fe increased the char yield (in 1.2 times) and H2 contents (in 2.5 times), and decreased CO2 content in the non-condensable gas. Pyrolysis gas composition: Without additives: H2: 20–33 vol%; CO: 12–15 vol%; CO2: 0–65 vol%; CH4: 0–5 vol%. With additives: H2: 12–62 vol%; CO: 15–20 vol%; CO2: 45–65 vol%; CH4: 4–15 vol%. Product Yield by Pyrolysis: char: 60–80 wt%; liquids: 10–14 wt%; gases: 4–15 wt% | [56] |
| Corn starch, clover grass, and corn silage in supercritical water | Gasification in supercritical water | Continuous flow reactor | 500–700 °C | Gasification of biomass in supercritical water is highly temperature-dependent. Almost complete conversion of the feed can be achieved at 700 °C. As the temperature rises, the H2 yield increases, but the CO concentration decreases. Syngas composition: H2: 29.7–34.4 vol%; CO: 0.62–2.8 vol%; CO2: 39.7–43.9 vol%; CH4: 15–20.5 vol%; C2H2: 2.6–4.8 vol%. | |
| Water–semicoke slurry (semicoke 10–30 wt%). | Gasification in supercritical water | Supercritical water fluidized bed reactor system. Pressure 23 MPa. Water flow rate 40 g/min, slurry flow rate 20 g/min/ Particle size: <100 μm | 540–660 °C | The temperature of 600 °C is the most preferred to provide full gasification of the fixed carbon is realized. The use of K2CO3 as a catalyst made it possible to increase the hydrogen yield by 92%. Syngas composition: H2: 50–55 vol%; CO: 2–3 vol%; CO2: 35–38 vol%; CH4: 10–12 vol%. | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vershinina, K.; Nyashina, G.; Strizhak, P. Combustion, Pyrolysis, and Gasification of Waste-Derived Fuel Slurries, Low-Grade Liquids, and High-Moisture Waste: Review. Appl. Sci. 2022, 12, 1039. https://doi.org/10.3390/app12031039
Vershinina K, Nyashina G, Strizhak P. Combustion, Pyrolysis, and Gasification of Waste-Derived Fuel Slurries, Low-Grade Liquids, and High-Moisture Waste: Review. Applied Sciences. 2022; 12(3):1039. https://doi.org/10.3390/app12031039
Chicago/Turabian StyleVershinina, Ksenia, Galina Nyashina, and Pavel Strizhak. 2022. "Combustion, Pyrolysis, and Gasification of Waste-Derived Fuel Slurries, Low-Grade Liquids, and High-Moisture Waste: Review" Applied Sciences 12, no. 3: 1039. https://doi.org/10.3390/app12031039







