Abstract
Orthodontic tooth movement (OTM) is based on intermitted or continuous forces applied to teeth, changing the mechanical loading of the system and arousing a cellular response that leads to bone adaptation. The traditional orthodontic movement causes a remodeling of the alveolar bone and changes in the periodontal structures that lead to tooth movement. The use of a piezoelectric instrument in orthodontic surgery has already shown great advantages. The purpose of this study is to rank the behavior of inflammatory mediators in accelerating orthodontic tooth movement. Ten patients with malocclusion underwent orthodontic surgical treatment, which included a first stage of surgically guided orthodontic movement (monocortical tooth dislocation and ligament distraction, MTDLD) to accelerate orthodontic movements. In all cases, corticotomy was performed by Piezosurgery. Bone and dental biopsy was executed to evaluate changes in the cytokines IL-1beta, TNF-alpha and IL-2 in different time intervals (1, 2, 7, 14 and 28 days). The molecular mediators are IL-1 beta, TNF-alpha and IL-2. Immediately after the surgical procedure there was a mild expression of the three molecular markers, while the assertion of IL-1 beta and TNF-alpha reached the maximum value after 24 h and 48 h, indicating a strong activation of the treated tissues. The Piezosurgery® surgical technique induces an evident stress in short times, within 24–48 h from the treatment, but it decreases significantly during the follow-up.
1. Introduction
OTM is a biological event that involves a series of signal transduction processes due to the remodeling of the periodontal ligament and alveolar bone. Chemical changes will in turn directly, or indirectly through other biologically active substances, stimulate a particular cell differentiation [1,2].
In the compression side of periodontal membrane, osteoclasts resorb bone and consent the tooth to move; in the tension side, osteoblasts induce formation bone.
Henneman et al. explained the main processes occurring after an orthodontic force application [3]. Four different moments are involved: the tension of the matrix and the flow of fluid, the cellular tension, the cellular activation and the differentiation, and finally the remodeling.
OTM begins with the expression of cytokines in the periodontal ligament determining strong modification in the periodontal ligament. Interleukin (IL)-1beta’s concentration is the highest at the first level of orthodontic motion due to its effect on the resorption of the surrounding alveolar bone [4].
The level of IL-1 beta in human fibroblasts build up during all the orthodontic movements. In vitro, the periodontal ligament and gingival fibroblasts exposed to IL-1 beta let out prostaglandin, PGE2 and secrete metal proteinase of matrix (MMPs), which are among the major proteolytic enzymes involved in a process where collagens and other extracellular matrix (ECM) molecules become weaker. During inflammatory tissue responses, various subtypes of MMPs, MMP-2 and MMP-9 manifest. It stimulates the production of PG, other cytokines such as IL-2 and the activation and recruitment of other cells of the immune system [5,6,7]. IL-1beta plays a crucial role in the survival, blend and setting in motion of osteoclasts and assumes an important role since the amount of tooth movement is correlated with the correct amount of bone remodeling in the alveolar process [8,9]. IL-2 is a pro-inflammatory cytokine secreted by activated T cells and acts on both T and B lymphocytes and on cells of innate immunity such as mononuclear phagocytes and NK (natural killer) cells also. It is involved in the stimulation of osteoclast activity for bone remodeling and has an active role in the pathogenesis of periodontal condition [10].
Tumor necrosis factor-alpha (TNF-alpha) is a growth factor belonging to the cytokine group. It is a 17-kD protein, which is secreted by monocytes and macrophages at the site of inflammation. TNF-alpha precedes interleukin-1 beta (IL-1 beta) during the response to classic inflammatory inducers, such as bacterial endotoxin.
Orthodontic force induces expression of TNF-alpha in the compression side of the periodontal ligament, and has demonstrated to play an important role in OTM inducing osteoclast formation and regulating the distance of tooth movement. Moreover, TNF-alpha has an important role in increasing the expression level of RANK expressed on the surface of osteoclast precursor cells during OTM [11,12].
To date, there are numerous procedures in dentistry that require remodeling of the alveolar bone and orthodontics plays a crucial role among these [13,14,15,16].
In orthodontic treatment, with any type of appliance and material, including traditional brackets, ceramic brackets and EverStick, the number of adult patients has been increasing [17,18]. During the diagnostic phase it is essential to check the periodontal biotype, oral hygiene, dental structure (enamel and dentin) and the oral microbiology of the patient [19,20,21,22,23]. This kind of treatment must be performed looking at the cosmetic aspects and the minimum timing to reach the final goal. Indeed, the most frequent complaint is the long duration of the orthodontic therapy and hence the reason why most patients avoid the treatment [24].
For this reason, a series of surgical techniques have been described in the literature, so as to be able to effectively reduce orthodontic treatment time and significantly reduce the number of complications [25,26,27,28,29,30,31]. These include osteogenic distraction, corticotomies, cortical incisions, piezoelectric osteotomy, decortication, ligament dislocation and distraction technique (MTDLD). The MTDLD has optimized the orthodontic movement technique by shortening treatment time and preventing damage to periodontal tissue [32,33].
The application of a piezoelectric tool in orthodontic surgery has already shown great advantages, both from the psychological point of view of the patient, and by reducing the treatment time itself.
Piezosurgery® is the first ultrasonic instrument dedicated to bone cutting. The two principle concepts of bone microsurgery are:
- Reduced surgical invasiveness: compared to traditional techniques, post-operative pain and swelling are reduced, also leading to favorable healing and reduced morbidity for the patient.
- High predictability: this technique increases the therapeutic efficacy, the easy intraoperative control of the instrument and reduces bleeding. The precise and selective cut together with tissue healing optimizes surgical results even in cases of complex anatomy [34,35,36].
The aspiration of this study is to consider the performance of inflammatory mediators during tooth displacement through orthodontic microsurgery with Piezosurgery®.
2. Materials and Methods
2.1. Patients
At the Faculty of Medicine in Cluj Napoca (Romania) and in private practice, 10 Caucasian patients (aged range between 18 and 30 years old) were treated. The exclusion criteria were age below 18 years, acute or chronic local infections, bisphosphonate intake and therapy with corticosteroids, autoimmune disorders, oncological diseases, metabolic disorders and psychiatric disease that could interfere with treatment. The inclusion criteria were Class II or III malocclusion and no radiographic evidence of periodontal disease performed by full-mouth periapical radiograph examination. Before the treatment, all the patients received a standardized oral hygiene instruction by the same dental specialist and had a good oral hygiene profile.
The research was conducted in accordance with the Declaration of Helsinki.
Patients were told about both the advantages and risk involved in the therapy and gave their written informed consent for treatment and participation in the study.
2.2. Treatment
These patients underwent orthodontic surgical treatment, which included a first stage of surgically guided orthodontic movement (MTDLD) to speed up orthodontic movements. None of the patients on arrival declared to be suffering from relevant pathologies. Facial and intraoral orthodontic photography, cephalometric analysis were performed.
In all cases, corticotomy was performed by Piezosurgery® (Piezosurgery III—Mectron, Carasco, Italy), with the “cortical bone” setting and irrigation level 4. The surgery was performed by the same surgeon under local anesthesia with Mepivacaine 2% and 0.5 mL of Adrenaline 1:100,000.
A circumvestibular incision was made with a 15 blade scalpel on the gingiva exposing the periosteum with a periosteal elevator on the buccal bone surface of the maxilla or the mandible. Osteotomies were performed using the OT7 and OT7s insert. A Vycril 4-0 resorbable thread was used (Ethicon, Inc., Somerville, NJ, USA). Antibiotic therapy included administering oral amoxicillin associated with clavulanic acid (1 gr three times a day for five days), 8 mg dexamethasone one hour before and one hour after surgery to avoid post-operative edema, 20 mg omeprazole once a day for 12 days, while the pain was monitored with 100 mg Ketoprofen as needed.
The biomechanical orthodontic forces were applied the day before the surgery by the orthodontist. Self-ligating brackets with 0.22 of slots (SDS Ormco, Ultradent Product, 3 m and Unitech) and NiTi wires (0.32 inches, 0.17 at 0.25 and 0.32) were used. Orthodontic refinements were performed, whenever needed, using conventional techniques.
Follow-up examinations were performed at 1, 2, 7, 14 and 28 days, and subsequently every two weeks for the following two months.
2.3. Biomolecular Analysis
Bone and dental biopsy was performed to evaluate changes in the cytokines IL-1beta, TNF-alpha and IL-2 in various time intervals and it consisted of bone harvesting with the dental, trabecular and cortical component on the buccal side. The mucosa was infiltrated with 2% Mepivacaine associated with Adrenaline 1:100,000 and was collected with a micro incision (2 mm) using a Piezosurgery® scalpel, with an EX1 insert at the level of the dental apex. The biopsy site was filled with a swine absorbable gelatin sponge (Spongostan, Ethicon). Polysorb 4-0 suture threads were used in order to fix the sponge. The sample was stored in a 2% Gluteraldehyde solution at 4°. The samples for the biomolecular evaluation were collected at 8, 24, 48 h and at 7, 14 and 28 days and analyzed by real-time RT—PCR technique.
The TRIzol reagent (Molecular Research Center Inc., Cincinnati, OH, USA) was applied to extract the total RNA from each sample, according to the methods used in the literature [37]. A microgram of total-RNA was subjected to be reported and transformed into cDNA using the Promega reverse transcription system (Promega Corp., Madison, WI, USA). Reverse transcriptase was summed up to the mixture that contained RT POX buffer, 2.5 mM of MgCl2, Oligo (dT) primer, dNTP Mix and Rnasin. The reagents were kept at 42 °C for 15 min and then heated at 95 °C for 5 min. Real-time RT-PCR was conducted with ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). PCR was used in a total volume of 25 μL, which contained 50 ng of cDNA samples, 1X TaqMan Universal PCR Master Mix, 300 nM of each primer and 250 nM of TaqMan probe. The phase conditions for IL-1 Beta, TNF and IL-2 were 30 s at 95 °C, 30 s at 60 °C, then 30 s at 72 °C. cDNA for IL-1 beta, TNF-alpha and IL-2 were then amplified for 40 cycles. In PCR, probes were used for hybridization of oligonucleotides, branded with 6-carboxyfluorescein to highlight fluorescence and with 6-carboxy-tetramethyl-rhodamine to terminate fluorescence. For each patient treated, the analysis of the expression values of the single mRNAs was carried out using the ANOVA test.
3. Results
The molecular mediators are IL-1 beta, TNF-alpha and IL-2. Expression values were analyzed at different time points (8, 24, 48 h and 7, 14, 28 days after surgery). The results of all mediators are statistically significant, so the change in values is due to time in which the variable was measured (and consequently to the treatment with respect to time 0). (Figure 1, Figure 2 and Figure 3) The change is not due to factors related to the subjects or to the withdrawal or random analytical errors. All tests were performed with level = 0.05.
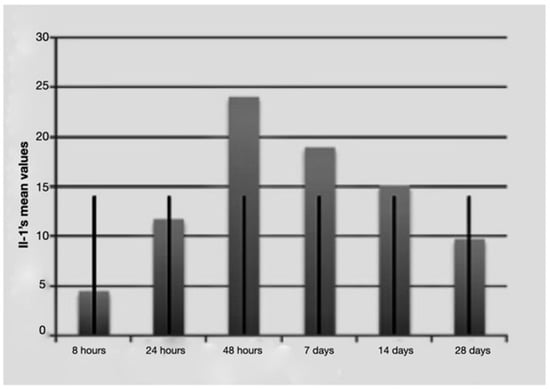
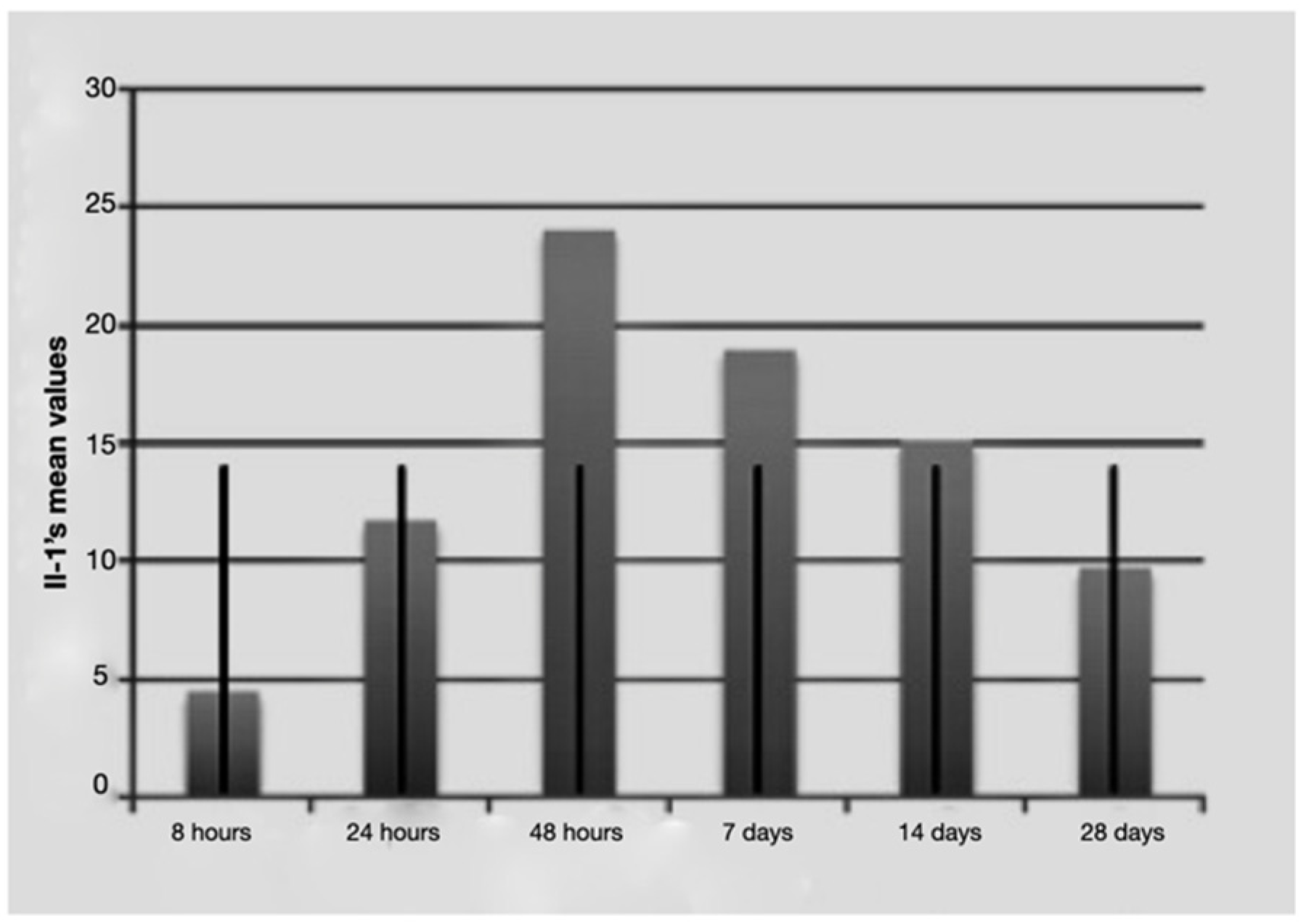
Figure 1.
Average IL-1 beta values over time with standard deviation.
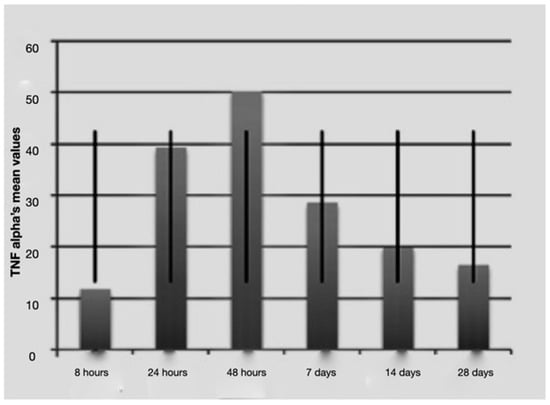
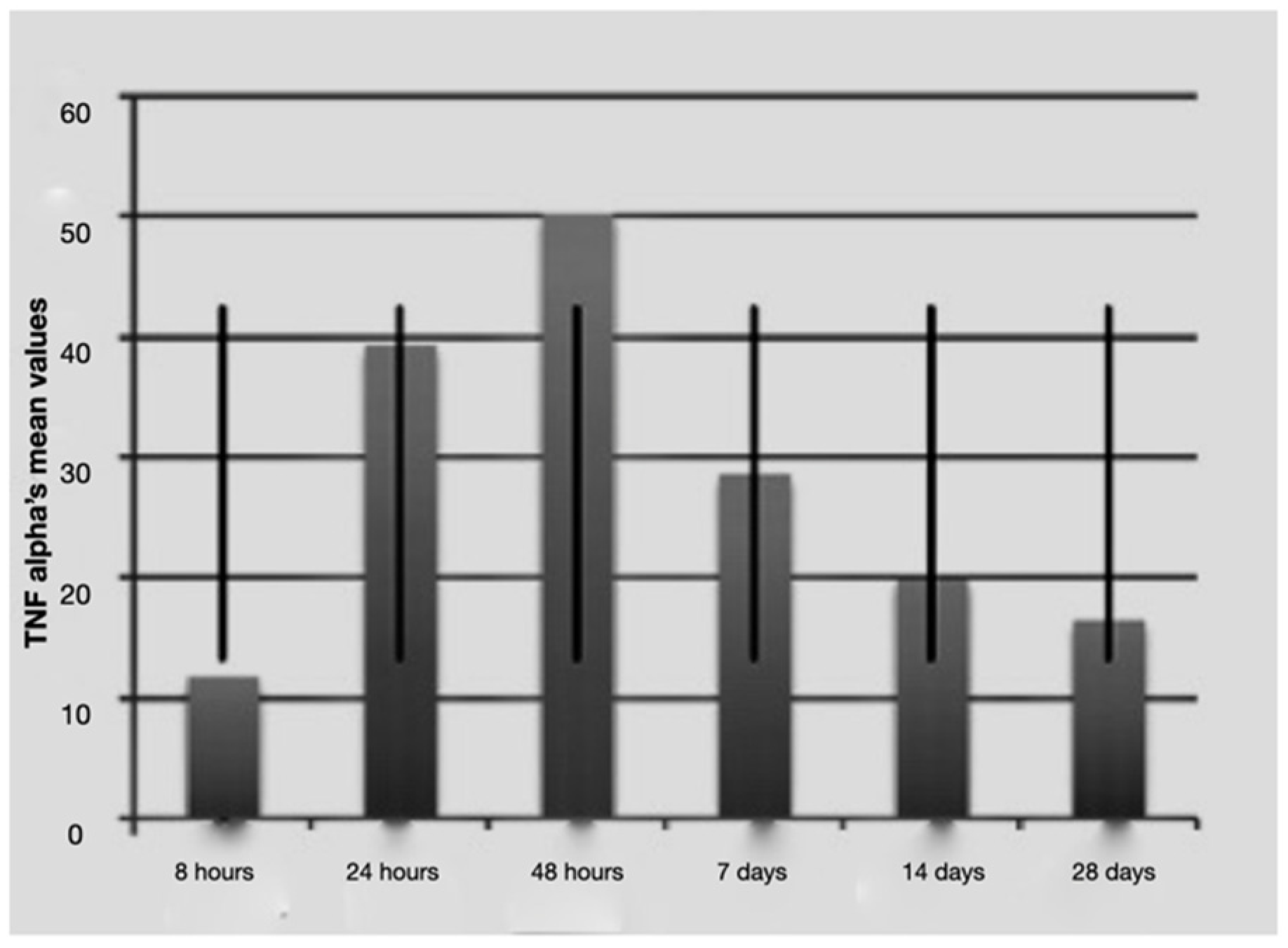
Figure 2.
Average TNF-alfa values over time with standard deviation.
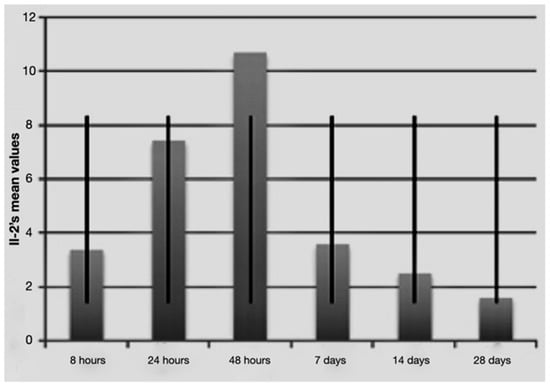
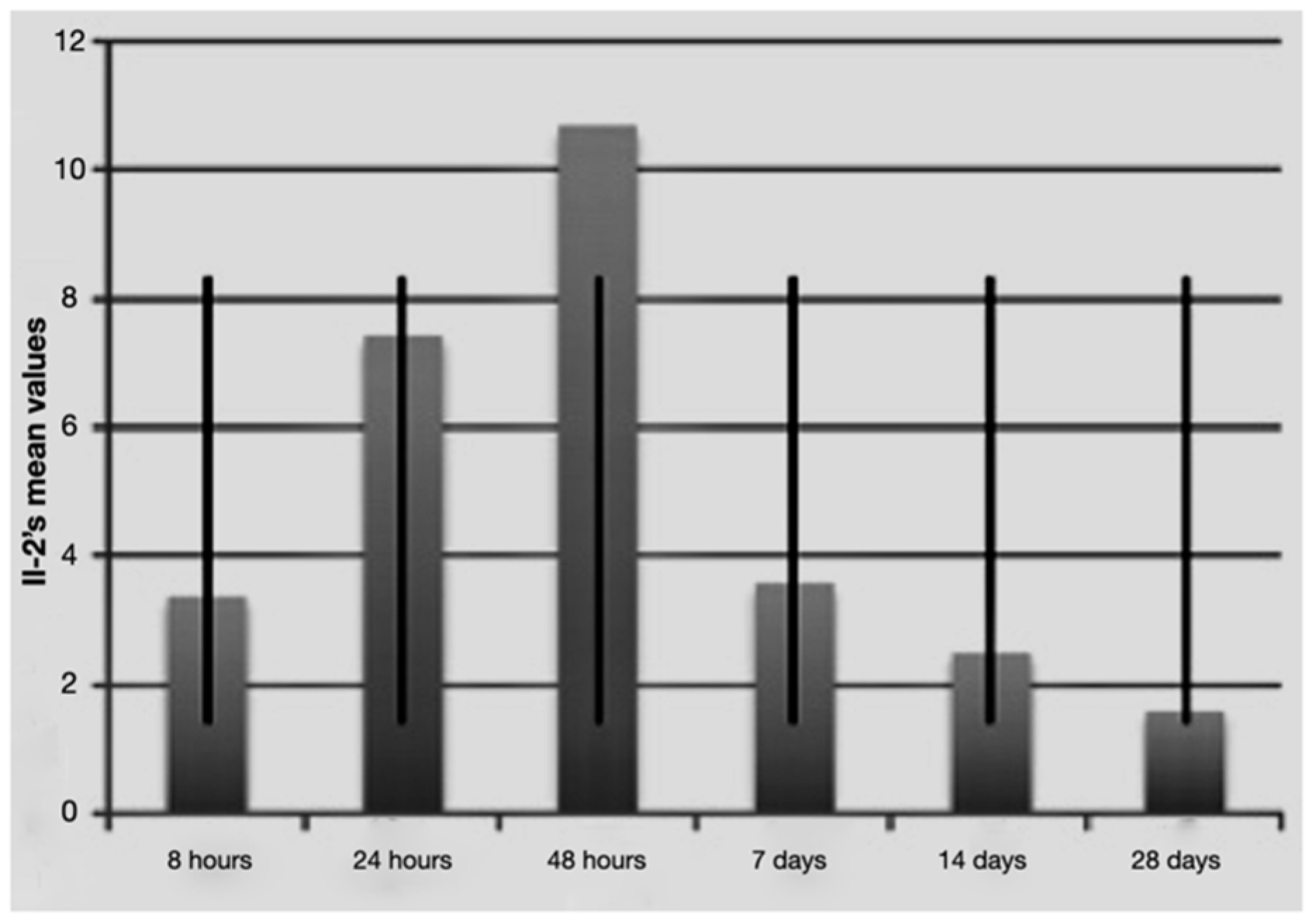
Figure 3.
Average IL-2 values over time with standard deviation.
Subsequently, in order to verify whether and at which times the expression values of the mediators’ mRNA are significantly changed, the Friedman test was used, and it was highlighted at what time the highest response was found.
It is notable that immediately after the surgical procedure, there was a mild expression of the three molecular markers, while after 24 h and 48 h, IL-1 beta and TNF-alpha reached the maximum value, indicating a strong activation of the treated tissues. The expression of IL-2 increased at longer times (48 h and 7 days post-surgery and load activation). In the later observation times (7, 14 and 28 days), the expression of the mRNA of the single markers seemed to diminish, indicating a significantly lower degree of inflammation and tissue stress. Above all the levels, IL-1 beta underwent a sharp decrease at 14 and 28 days.

Table 1.
IL-1 beta’s values.

Table 2.
TNF-alpha’s values.

Table 3.
IL-2′s values.
4. Discussion
As demonstrated in the literature, traditional orthodontics treatment acts as a trigger on the periodontal tissues [26,38,39]. Traditional orthodontic techniques have been described as causing damage to dental and periodontal tissues, such as recessions and root resorption [33,40,41,42]. Based on the protocols used in conventional orthodontics, profound changes in the periodontium were observed, such as thickening in the traction’s zone and thinning in the compression’s zone, with the appearance of areas of necrosis and bone remodeling.
The current gold standard is represented by Piezosurgery®, which aims to improve tooth alignment without adverse effects on periodontal structures through a standardized surgical practice protocol [43,44,45,46,47,48].
Gingival inflammation is related to the increase in inflammatory cytokines and/or MMPs in the periodontal ligament, and the turning on of inflammatory tissue destruction by gingival fibroblasts and periodontal ligament has been proposed.
Despite these functional and regional corrections, the role of gum tissue has been neglected with regard to orthodontic treatment [49,50]. To focus on biological events in gingival tissue, a prerequisite for this study was the exclusion of gingival tissue subjected to orthodontic improvement and the observation of primary subcellular mutations. In this study, a real-time RT-PCR was necessary since this allows a relatively reliable quantification of RNA, unlike conventional RT-PCR; without taking into account the number of PCR cycles, it quickly shows results in time real.
The IL-1 beta and MMP-9 mRNA levels were significantly high at all pressure sites for a relatively long period of time (2 weeks), and no substantial change was noted in the group of false activation, indicating that the changes were mainly due to pressure stimulation on the gum tissue.
Although many studies have pointed out growing pro-inflammatory cytokines in the periodontal ligament, the direct efficiency of the mechanical load on beta IL-1 from gingival fibroblasts is not yet clear.
Bode et al. demonstrated a voltage-induced increase in IL-6 secretion in tendon fibroblasts [51]. Itoh et al. found that the inflammatory response of tendon fibroblasts depended on the intensity of the traction, with an anti-inflammatory response at low levels and a proinflammatory response at high levels of strength [52]. Changes in IL-1 beta mRNA levels in this research mostly agree with those described in a previous report on IL-1beta secretion from gingival crevicular fluid. However, a relatively constant increase can be noted.
The decline in IL-1 beta on day 14 can be attributable to a decrease in the level of forces caused by the absence of reactivation, but the cytokines may be refractory to the application of a reactivation [53]. Iwasaki et al. reported that the concentration of IL-1 beta in gingival crevicular fluid rose and returned to baseline levels in 14–28 days [54]. However, a sustained increase of IL-1beta in the pressure side of the gingival tissue set against with the traction site was a new discovery of our study, implying a possible role of gingival soft tissues in tissue remodeling [50,54].
In our research, the expression of MMP-9 was increased by performing orthodontic microsurgery followed by orthodontic loading. MMP-2 and MMP-9 were previously found, both in the pressure and PDL side, during orthodontic movements to assist collagen abasement.
Distler et al. reported, through in situ hybridization technique, that MMP-9 mRNA in the periodontal ligament was increased within 7 days of orthodontic loading and decreased after 14 days [55].
The primary increase in those key mRNA points in the gingiva suggest the following possibilities:
- (i)
- mRNAs could be directly implicated in gingival inflammation and/or destruction of the soft tissues surrounding the moving tooth;
- (ii)
- Inflammation mediators afforded in the gingiva could play a regulatory role in inducing bone remodeling in PDL.
However, a growth in inflammatory mediators under orthodontic loading in the gingiva put forward that the active biological effects of pressure on the gingival soft tissues are evident.
Kubota et al. showed the differences in cytokines and other metabolites between pressure and tension sides using gingival crevicular fluid and periodontal ligament [56,57]. A comparative examination on the pressure and tension side of the gingiva as well as on periodontal ligament cells will therefore be helpful for a broad understanding of the results expressed here. A study of cell cultures in vitro may also help to clarify the interplay between mediators.
Although the number of patients for this pilot clinical study is rather small (n = 10), in the subjects examining the analysis of the expression of mRNA levels for IL-1beta, TNF-alpha and IL-2 showed interesting differences during the observation period.
The Piezosurgery® surgical technique has been shown to induce an evident stress in short times, within 24–48 h from the treatment, but that decreases significantly at longer observation times [43,44,46].
This phenomenon indicates a re-adaptation of the treated tissue and a return within 14–28 days to the physiological conditions of the tissue.
5. Conclusions
In this study, the markedly lower level of molecular markers of inflammation and tissue stress one or two weeks from Piezosurgery® indicates that the surgical technique is minimally invasive and that after 28 days there is a restitutio ad integrum of the treated tissues, confirmed also by histological analyses [4].
Furthermore, it can be stated that Piezosurgery® should be considered as a helpful surgical technique in orthodontics due to the reduction of stress time to periodontal tissues.
Author Contributions
Conceptualization, D.B. and C.C.; Data curation, C.C., A.L. and G.R.; Formal analysis, G.C., A.S. and G.S.; Investigation, D.D., A.L.M.M. and G.R.; Methodology, D.D. and L.C.C.; Project administration, A.S.; Resources, G.C., L.C.C. and A.L.M.M.; Validation, P.F.N.; Visualization, P.F.N.; Writing—review & editing, D.B. and A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data of each patient are not publicly available due to the privacy of the patients.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schwarz, A.M. Tissue changes incident to orthodontic tooth movement. Int. J. Orthod. 1932, 18, 331–352. [Google Scholar]
- Tsuge, A.; Noda, K.; Nakamura, Y. Early tissue reaction in the tension zone of PDL during orthodontic tooth movement. Arch. Oral. Biol. 2016, 65, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Henneman, S.; Von den Hoff, J.W.; Maltha, J.C. Mechanobiology of tooth movement. Eur. J. Orthod. 2008, 30, 299–306. [Google Scholar] [CrossRef]
- Bertossi, D.; Galzignato, P.F.; Conti, G.; Luciano, U.; Gualdi, A.; Corega, C.; Faccioni, P.; Donadello, D.; Lanaro, L.; Grendene, E.; et al. Histological evaluation of periodontal ligament in human after orthodontic treatment with piezosurgery and monolateral tooth dislocation and ligament distraction technique: A first morphologic and histologic evaluation. J. Biol. Regul. Homeost. Agents 2018, 32 (Suppl. 2), 9–13. [Google Scholar]
- Rodriguez y Baena, R.R.; Pastorino, R.; Gherlone, E.F.; Perillo, L.; Lupi, S.M.; Lucchese, A. Histomorphometric evaluation of two different bone substitutes in sinus augmentation procedures: A randomized controlled trial in humans. Int. J. Oral. Maxillofac. Implant. 2017, 32, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Conti, G.; Bertossi, D.; Dai Prè, E.; Cavallini, C.; Scupoli, M.T.; Ricciardi, G.; Parnigotto, P.; Saban, Y.; Sbarbati, A.; Nocini, P.F. Regenerative potential of the Bichat fat pad determined by the quantification of multilineage differentiating stress enduring cells. Eur. J. Histochem. 2018, 62, 2900. [Google Scholar] [CrossRef]
- Gameiro, G.H.; Schultz, C.; Trein, M.P.; Mundstock, K.S.; Weidlich, P.; Goularte, J.F. Association among pain, masticatory performance, and proinflammatory cytokines in crevicular fluid during orthodontic treatment. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 967–973. [Google Scholar] [CrossRef]
- Monga, N.; Chaurasia, S.; Kharbanda, O.P.; Duggal, R.; Rajeswari, M.R. A study of interleukin 1β levels in peri-miniscrew crevicular fluid (PMCF). Prog. Orthod. 2014, 15, 30. [Google Scholar] [CrossRef]
- Iwasaki, L.R.; Haack, J.E.; Nickel, J.C.; Reinhardt, R.A.; Petro, T.M. Human interleukin-1 beta and interleukin-1 receptor antagonist secretion and velocity of tooth movement. Arch. Oral. Biol. 2001, 46, 185–189. [Google Scholar] [CrossRef]
- Basaran, G.; Özer, T.; Kaya, F.A.; Hamamci, O. Interleukins 2, 6, and 8 levels in human gingival sulcus during orthodontic treatment. Am. J. Orthod. Dentofac. Orthop. 2006, 130, 7.e1–7.e6. [Google Scholar] [CrossRef]
- Ogawa, S.; Kitaura, H.; Kishikawa, A.; Qi, J.; Shen, W.-R.; Ohori, F.; Noguchi, T.; Marahleh, A.; Nara, Y.; Ochi, Y.; et al. TNF-α is responsible for the contribution of stromal cells to osteoclast and odontoclast formation during orthodontic tooth movement. PLoS ONE 2019, 14, e0223989. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Kitaura, H.; Ogawa, S.; Qi, J.; Shen, W.-R.; Ohori, F.; Marahleh, A.; Nara, Y.; Pramusita, A.; Mizoguchi, I. TNF-α stimulates the expression of RANK during orthodontic tooth T movement. Arch. Oral. Biol. 2020, 117, 104796. [Google Scholar] [CrossRef]
- Rigo, L.; Viscioni, A.; Franco, M.; Lucchese, A.; Zollino, I.; Brunelli, G.; Carinci, F. Overdentures on implants placed in bone augmented with fresh frozen bone. Minerva Stomatol. 2011, 60, 5–14. [Google Scholar] [CrossRef]
- Traini, T.; Danza, M.; Zollino, I.; Altavilla, R.; Lucchese, A.; Sollazzo, V.; Trapella, G.; Brunelli, G.; Carinci, F. Histomorphometric evaluation of an immediately loaded implant retrieved from human mandible after 2 years. Int. J. Immunopathol. Pharmacol. 2011, 24 (Suppl. 2), 31–36. [Google Scholar] [CrossRef] [PubMed]
- Danza, M.; Zollino, I.; Avantaggiato, A.; Lucchese, A.; Carinci, F. Distance between Implants Hasa Potential Impact of CrestalBone Resorption. Saudi Dent. J. 2011, 23, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Lo Giudice, A.; Ortensi, L.; Farronato, M.; Lucchese, A.; Lo Castro, E.; Isola, G. The step further smile virtual planning: Milled versus prototyped mock-ups for the evaluation of the designed smile characteristics. BMC Oral Health 2020, 20, 165. [Google Scholar] [CrossRef] [PubMed]
- Lucchese, A.; Carinci, F.; Brunelli, G.; Monguzzi, R. Everstick® and Ribbond® fiber reinforced composites: Scanning Electron Microscope (SEM) comparative analysis. Eur. J. Inflamm. 2011, 9, 73–79. [Google Scholar]
- Manuelli, M. A peaceful man. Prog. Orthod. 2012, 13, 1. [Google Scholar] [CrossRef]
- Gandini, P.; Schiavi, A.; Manuelli, M.; Camassa, D. Epidemiological survey of caries occurrence in school age children. Mondo Ortod. 1989, 14, 63–72. [Google Scholar]
- Roncati, M.; Polizzi, E.; Cingano, L.; Gherlone, E.F.; Lucchese, A. An oral health aid for disabled patients. Dent. Cadmos. 2013, 81, 447–452. [Google Scholar] [CrossRef]
- Prati, C.; Chersoni, S.; Lucchese, A.; Pashley, D.H.; Mongiorgi, R. Dentin permeability after toothbrushing with different toothpastes. Am. J. Dent. 1999, 12, 190–193. [Google Scholar]
- Matarese, G.; Isola, G.; Ramaglia, L.; Dalessandri, D.; Lucchese, A.; Alibrandi, A.; Fabiano, F.; Cordasco, G. Periodontal biotype: Characteristic, prevalence and dimensions related to dental malocclusion. Minerva Stomatol. 2016, 65, 231–238. [Google Scholar]
- Lucchese, A.; Bondemark, L.; Marcolina, M.; Manuelli, M. Changes in oral microbiota due to orthodontic appliances: A systematic review. J. Oral. Microbiol. 2018, 3, 1476645. [Google Scholar] [CrossRef] [PubMed]
- Bertossi, D.; Giampaoli, G.; Lucchese, A.; Manuelli, M.; Albanese, M.; Nocini, R.; Nocini, P.F. The skin rejuvenation associated treatment-Fraxel laser, Microbotox, and low G prime hyaluronic acid: Preliminary results. Lasers Med. Sci. 2019, 34, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xu, L.; Liang, C.; Jiang, J. Class III orthognathic surgical cases facilitated by accelerated osteogenic orthodontics: A preliminary report. Aust. Orthod. J. 2015, 31, 226–235. [Google Scholar] [CrossRef]
- Ericsson, I.; Thilander, B.; Lindhe, J.; Okamoto, H. The effect of orthodontic tilting movements on the periodontal tissues of infected and non-infected dentitions in dogs. J. Clin. Periodontol. 1977, 4, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Zotti, F.; Capocasale, G.; Lonardi, F.; Zambotti, T.; Nocini, R.; Albanese, M. Trigeminal trophic syndrome: Strange evolution of maxillofacial surgery. EXCLI J. 2019, 18, 931–935. [Google Scholar]
- Wilcko, M.T.; Wilcko, W.M.; Pulver, J.J.; Bissada, N.F.; Bouquot, J.E. Accelerated osteogenic orthodontics technique: A 1-stage surgically facilitated rapid orthodontic technique with alveolar augmentation. J. Oral. Maxillofac. Surg. 2009, 67, 2149–2159. [Google Scholar] [CrossRef]
- Olate, S.; Pozzer, L.; Unibazo, A.; Huentequeo-Molina, C.; Martinez, F.; De Moraes, M. LeFort I segmented osteotomy experience with piezosurgery in orthognathic surgery. Int. J. Clin. Exp. Med. 2014, 7, 2092–2095. [Google Scholar]
- Murphy, N.C. Accelerated osteogenic orthodontics. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 2. [Google Scholar] [CrossRef]
- De Santis, D.; Sinigaglia, S.; Pancera, P.; Faccioni, P.; Luciano, U.; Setti, A.P.; Bursi, P.; Nocini, R.; Nocini, P.F.; Bertossi, D. An overview of guided bone regeneration. J. Biol. Regul. Homeost. Agents 2019, 33, 49–53. [Google Scholar] [PubMed]
- Sadowsky, C.; BeGole, E.A. Long-term effects of orthodontic treatment on periodontal health. Am. J. Orthod. 1981, 80, 156–172. [Google Scholar] [CrossRef]
- Ericsson, I. The combined effects of plaque and physical stress on periodontal tissues. J. Clin. Periodontol. 1986, 13, 918–922. [Google Scholar] [CrossRef]
- Sivolella, S.; Berengo, M.; Scarin, M.; Mella, F.; Martinelli, F. Autogenous particulate bone collected with a piezo-electric surgical device and bone trap: A microbiological and histomorphometric study. Arch. Oral. Biol. 2006, 51, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Vercellotti, T. Technological characteristics and clinical indications of piezoelectric bone surgery. Minerva Stomatol. 2004, 53, 207–214. [Google Scholar]
- Gleizal, A.; Bera, J.C.; Lavandier, B.; Beziat, J.L. Piezoelectric osteotomy: A new technique for bone surgery-advantages in craniofacial surgery. Childs Nerv. Syst. 2007, 23, 509–513. [Google Scholar] [CrossRef]
- Ribaudo, R.; Gilman, M.; Kingston, R.E.; Chomczynski, P.; Sacchi, N. Preparation of RNA from tissues and cells. Curr. Protoc. Neurosci. 2001, 10, 10–11. [Google Scholar]
- Zachrisson, B.U.; Alnaes, L. Periodontal condition in orthodontically treated and untreated individuals. I. Loss of attachment, gingival pocket depth and clinical crown height. Angle Orthod. 1973, 43, 402–411. [Google Scholar]
- Kessler, M. Interrelationships between orthodontics and periodontics. Am. J. Orthod. 1976, 70, 154–172. [Google Scholar] [CrossRef]
- Wennstrom, J.L.; Stokland, B.L.; Nyman, S.; Thilander, B. Periodontal tissue response to orthodontic movement of teeth with infrabony pockets. Am. J. Orthod. Dentofac. Orthop. 1993, 103, 313–319. [Google Scholar] [CrossRef]
- Killiany, D.M. Root resorption caused by orthodontic treatment: An evidence-based review of literature. Semin. Orthod. 1999, 5, 128–133. [Google Scholar] [CrossRef]
- Eriksoon, L. Different dental plaque indices and their applications. Tandlakartidningen 1978, 70, 912–920. [Google Scholar]
- Kotrikova, B.; Wirtz, R.; Krempien, R.; Blank, J.; Eggers, G.; Samiotis, A.; Mühling, J. Piezosurgery—A new safe technique in cranial osteoplasty? Int. J. Oral. Maxillofac. Surg. 2006, 35, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Kramer, F.J.; Ludwig, H.C.; Materna, T.; Gruber, R.; Merten, H.A.; Schliephake, H. Piezoelectric osteotomies in craniofacial procedures: A series of 15 pediatric patients. Technical note. J. Neurosurg. 2006, 104, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Bertossi, D.; Albanese, M.; Mortellaro, C.; Malchiodi, L.; Kumar, N.; Nocini, R.; Nocini, P.F. Osteotomy in Genioplasty by Piezosurgery. J. Craniofac. Surg. 2018, 29, 2156–2159. [Google Scholar] [CrossRef] [PubMed]
- Bertossi, D.; Nocini, R.; Luciano, U.; Galzignato, P.F.; Ricciardi, G.; Lucchese, A.; Tacchino, U.; Donadello, D.; Lanaro, L.; Gualdi, A.; et al. Piezoelectric surgery inserts vs conventional burst: A clinical investigation. J. Biol. Regul. Homeost. Agents 2018, 32 (Suppl. 2), 15–19. [Google Scholar] [PubMed]
- Lazzarotto, A.; Franz, L.; Stella, E.; Tel, A.; Sembronio, S.; Costa, F.; Bertossi, D.; Nocini, R.; Robiony, M. Volumetric Analysis of Fat Injection by Computerized Tomography in Orthognathic Surgery: Preliminary Report on a Novel Volumetric Analysis Process for the Quantification of Aesthetic Results. J. Craniofac. Surg. 2019, 30, 771–776. [Google Scholar] [CrossRef]
- Spinelli, G.; Lazzeri, D.; Conti, M.; Agostini, T.; Mannelli, G. Comparison of piezosurgery and traditional saw in bimaxillary orthognathic surgery. J. Craniomaxillofac. Surg. 2014, 42, 1211–1220. [Google Scholar] [CrossRef]
- Ngan, P.W.; Crock, B.; Varghese, J.; Lanese, R.; Shanfeld, J.; Davidovitch, Z. Immunohistochemical assessment of the effect of chemical and mechanical stimuli on cAMP and prostaglandin E levels in human gingival fibroblasts in vitro. Arch. Oral. Biol. 1988, 33, 163–174. [Google Scholar] [CrossRef]
- Richards, D.; Rutherford, R.B. The effects of interleukin 1 on collagenolytic activity and prostaglandin-E secretion by human periodontal-ligament and gingival fibroblast. Arch. Oral. Biol. 1988, 33, 237–243. [Google Scholar] [CrossRef]
- Bode, W.; Maskos, K. Structural basis of the matrix metalloproteinases and their physiological inhibitors, the tissue inhibitors of metalloproteinases. Biol. Chem. 2003, 384, 863–872. [Google Scholar] [CrossRef]
- Itoh, T.; Matsuda, H.; Tanioka, M.; Kuwabara, K.; Itohara, S.; Suzuki, R. The role of matrix metalloproteinase-2 and matrix metalloproteinase-9 in antibody-induced arthritis. J. Immunol. 2002, 169, 2643–2647. [Google Scholar] [CrossRef]
- Tanaka, A.; Kumagai, S.; Kawashiri, S.; Takatsuka, S.; Nakagawa, K.; Yamamoto, E.; Matsumoto, N. Expression of matrix metalloproteinase-2 and -9 in synovial fluid of the temporomandibular joint accompanied by anterior disc displacement. J. Oral Pathol. Med. 2001, 30, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, L.R.; Chandler, J.R.; Marx, D.B.; Pandey, J.P.; Nickel, J.C. IL-1 gene polymorphisms, secretion in GCF, and speed of human tooth orthodontic movement. Orthod. Craniofac. Res. 2009, 12, 129–140. [Google Scholar] [CrossRef]
- Distler, J.H.; Jungel, A.; Huber, L.C.; Seemayer, C.A.; Reich, C.F., 3rd; Gay, R.E.; Michel, B.A.; Fontana, A.; Gay, S.; Pisetsky, D.S.; et al. The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc. Natl. Acad. Sci. USA 2005, 102, 2892–2897. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Matsuki, Y.; Nomura, T.; Hara, K. In situ hybridization study on tissue inhibitors of metalloproteinases (TIMPs) of mRNA-expressing cells in human inflamed gingival tissue. J. Periodontal Res. 1997, 32, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Lerario, F.; Roncati, M.; Gariffo, A.; Atorresi, E.; Lucchese, A.; Galanakis, A.; Palaia, G.; Romeo, U. Non-surgical periodontal treatment of peri-implant diseases with the adjunctive use of diode laser: Preliminary clinical study. Lasers Med. Sci. 2016, 31, 1–6. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).