First Report on the Bioremediation of Textile Industrial Effluents by Piptoporus Betulinus IEBL-3 by Using Response Surface Methodology
Abstract
:1. Introduction
2. Methodology
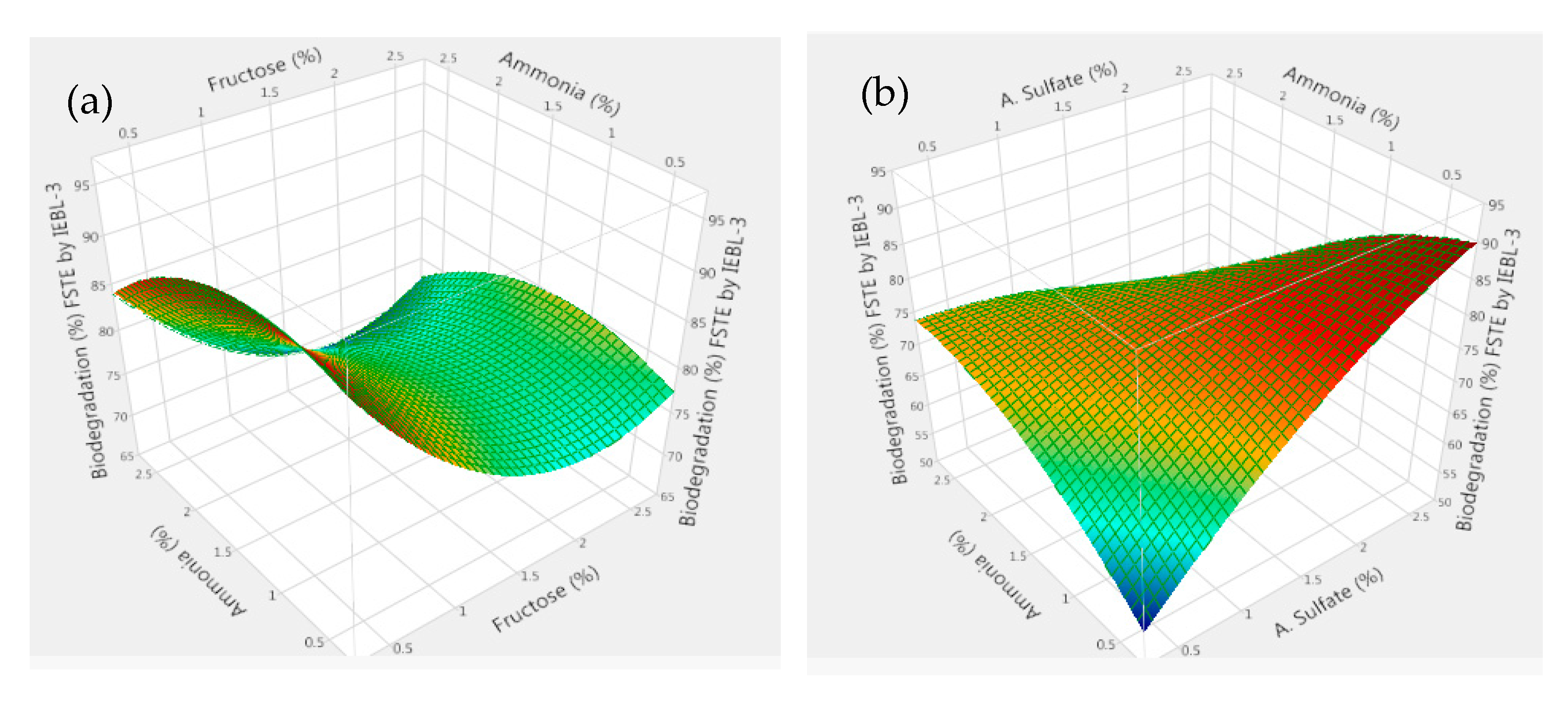
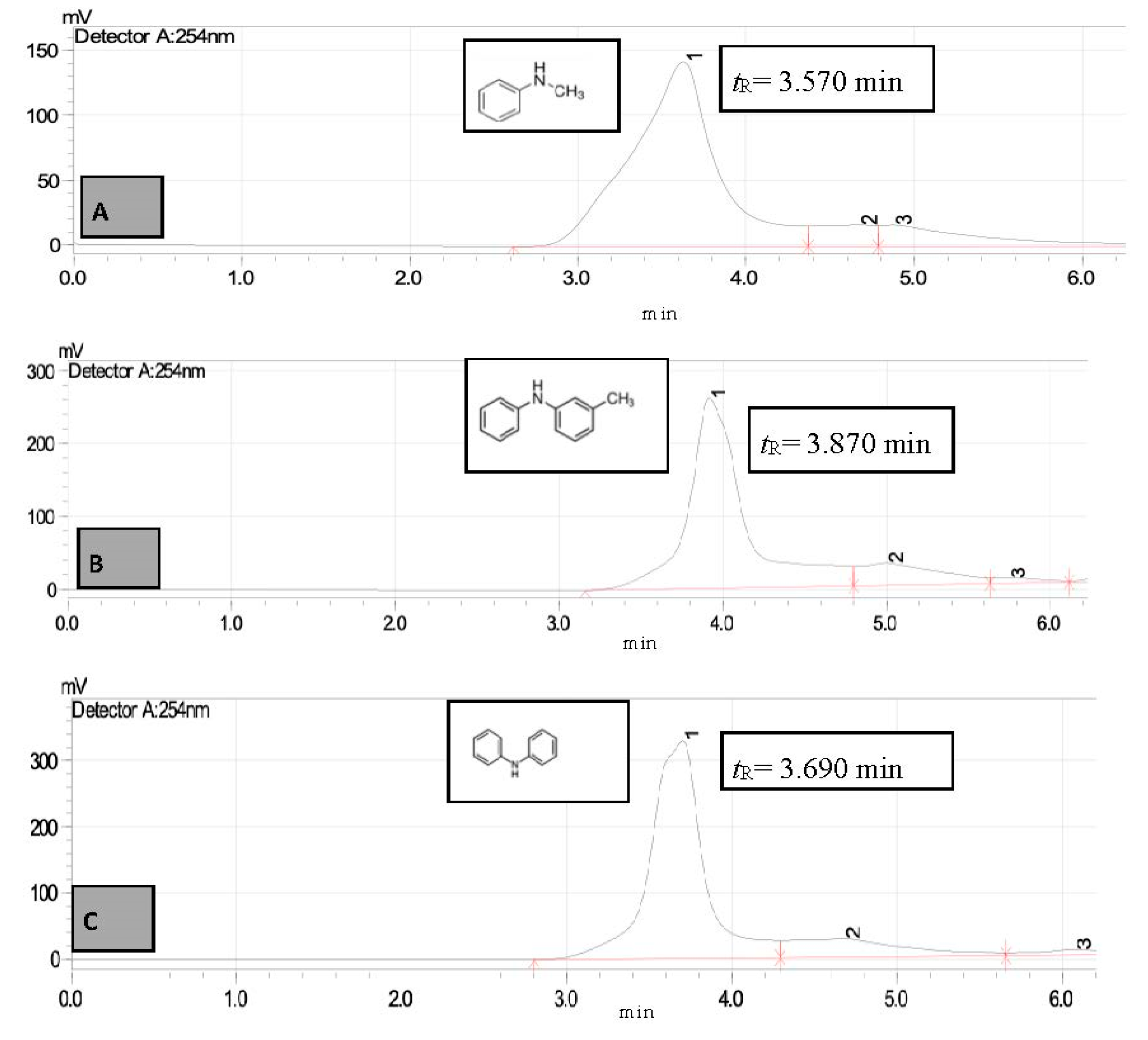
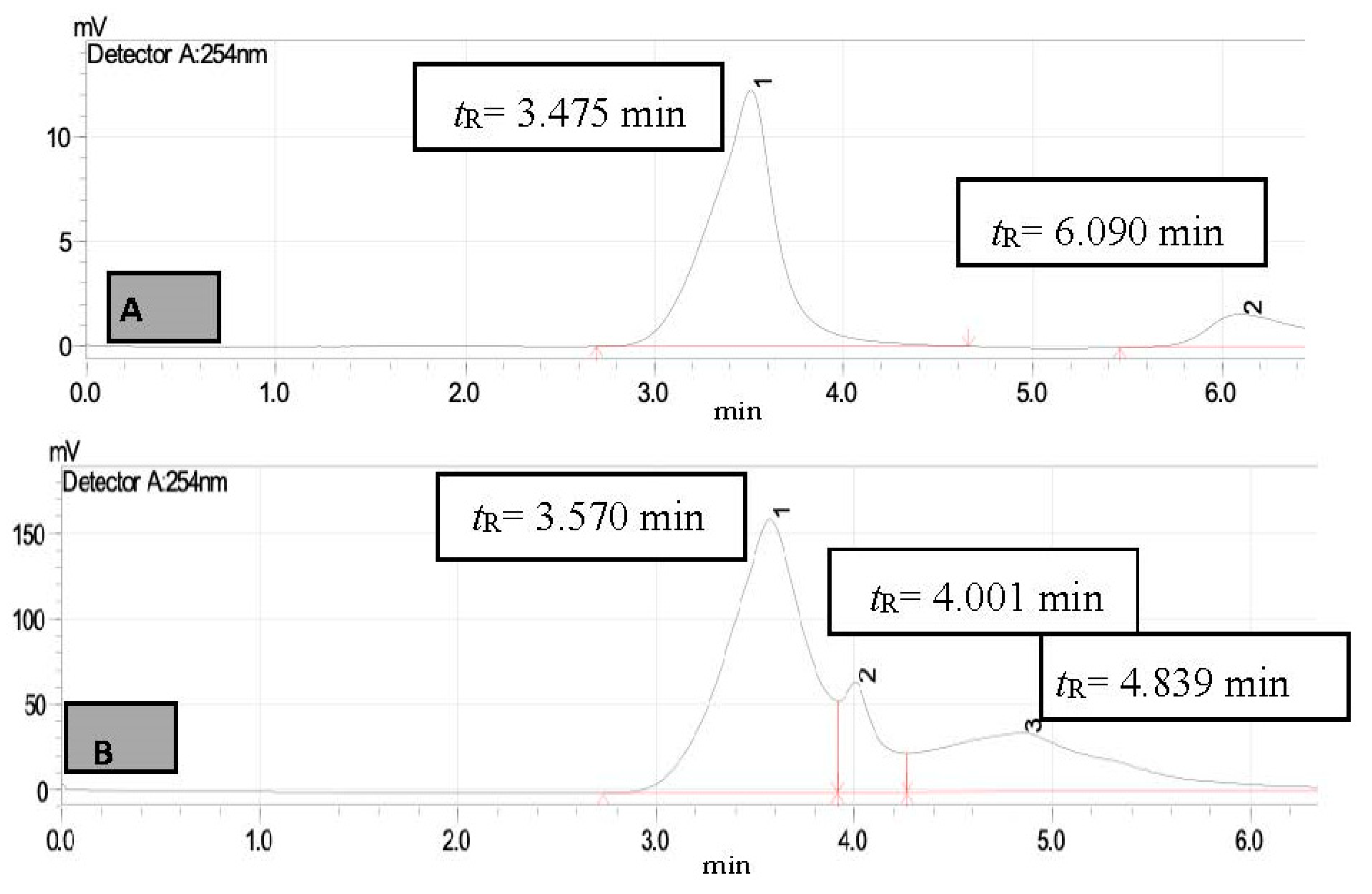
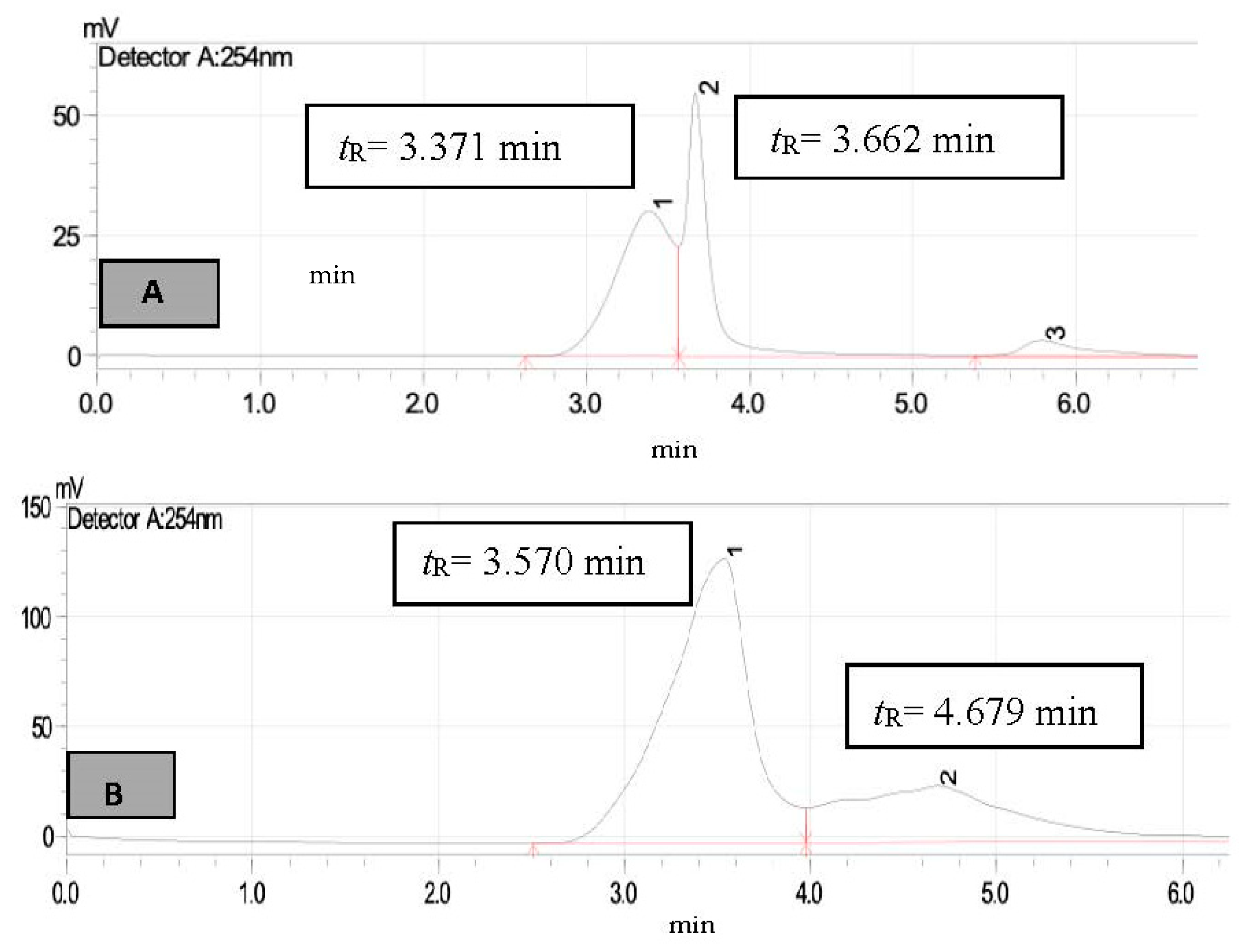
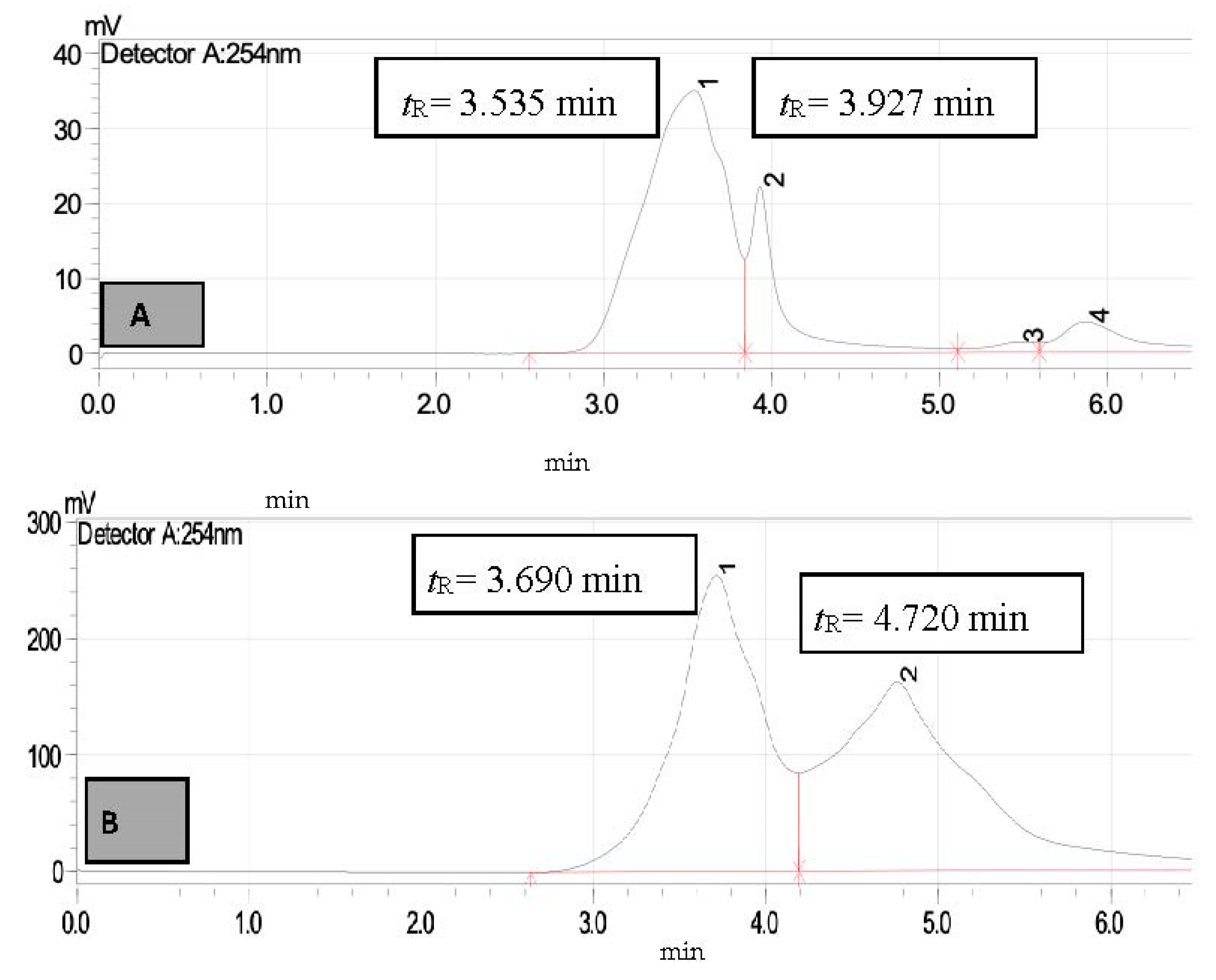
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asgher, M.; Yasmeen, Q.; Iqbal, H.M.N. Enhanced decolorization of solar brilliant red 80 textile dye by an indigenous white rot fungus Schizophyllum commune IBL-06. Saud. J. Biol. Sci. 2013, 20, 347–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rani, B.; Kumar, V.; Singh, J.; Bisht, S.; Teotia, P.; Sharmaand, S.; Kela, R. Bioremediation of dyes by fungi isolated from contaminated dye effluent sites for bio-usability. Braz. J. Microbiol. 2014, 45, 1055–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, L.; Singh, V.P. Decolourization of Azo (Acid red) and Anthraquinonic (Basic blue) Dyes by the Fungus Aspergillus flavus. Int. J. Biomed. Engine Clin. Sci. 2017, 3, 1–5. [Google Scholar]
- Vijayalakshmidevi, S.; Muthukumar, K. Improved biodegradation of textile dye effluent by coculture. Ecotoxicol. Environ. Saf. 2015, 114, 23–30. [Google Scholar] [CrossRef]
- Asgher, M.; Jamil, F.; Iqbal, H.M.N. Bioremediation Potential of Mixed White Rot Culture of Pleurotus Ostreatus IBL-02 and Coriolus Versicolor IBL-04 for Textile Industry Wastewater. J. Bioremediation Biodegrad. 2012, 6, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Roselin, L.S.; Selvin, R. Photocatalytic treatment and reusability of textile dyeing effluents from cotton dyeing industries. Sci. Adv. Mat. 2011, 3, 113–119. [Google Scholar] [CrossRef]
- Ajaz, M.; Rehman, A.; Khan, Z.; Nisar, M.A.; Hussain, S. Degradation of azo dyes by Alcaligenes aquatilis 3c and its potential use in the wastewater treatment. AMB Express 2019, 9, 64. [Google Scholar] [CrossRef]
- Singh, L.; Singh, V.P. Microbial degradation and decolourization of dyes in semi-solid medium by the fungus-Trichoderma harzianum. Environ. We Int. J. Sci. Technol. 2010, 5, 147–153. [Google Scholar]
- Abd El-Rahim, W.M.; Moawad, H.; Azeiz, A.Z.A.; Sadowsky, M.J. Biodegradation of azo dyes by bacterial or fungal consortium and identification of the biodegradation products. Egypt. J. Aquat. Res. 2021, 47, 269–276. [Google Scholar] [CrossRef]
- Iqbal HM, N.; Asgher, M. Characterization and decolorization applicability of xerogel matrix immobilized manganese peroxidase produced from Trametes versicolor IBL-04. Protein Pep. Lett. 2013, 20, 591–600. [Google Scholar] [CrossRef]
- Mahmood, R.T.; Asad, M.J.; Asgher, M.; Gulfraz, M.; Mukhtar, T.; Akram, M. Study of disperse dyes biodegradation and lignolytic enzymes production potential of indigenous Coniophora puteana IBL-01, a brown rot fungi. Adv. Environ. Bio. 2015, 9, 139–150. [Google Scholar]
- Mahmood, R.T.; Asad, M.J.; Asgher, M.; Gulfraz, M.; Mukhtar, T. Analysis of lingolytic enzymes and decolorization of disperse violet S3RL, yellow brown S2RFL, red W4BS, yellow SRLP and red S3B by brown rot fungi. Pak. J. Agric. Sci. 2017, 54, 407–413. [Google Scholar]
- Kaushik, P.; Malik, A. Process optimization for efficient dye removal by Aspergillus lentulus FJ172995. J. Hazard. Mater. 2011, 185, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Pavko, A. Fungal decolourization and degradation of synthetic dyes some chemical engineering aspects. Waste Water Treat. Reutili. 2011, 2, 65–88. [Google Scholar]
- Kaushik, P.; Malik, A. Effect of nutritional conditions on dye removal from textile effluent by Aspergillus lentulus. World J. Microbiol. Biotechnol. 2010, 26, 1957–1964. [Google Scholar] [CrossRef]
- Mahmood, R.T.; Asad, M.J.; Asgher, M.; Zainab, T.; Zafar, M.; Hadri, S.H.; Ali, I.; Zaman, N.; Wattoo, F.H. First report on implementation of response surface methodology for the biodegradation of textile industrial effluents by Coniophora puteana IEBL-1. Arch. Environ. Proc. 2019, 45, 48–59. [Google Scholar]
- Tien, M.; Kirk, T.K. Lignin peroxidases of Phanerochaete chrysosporium. Methods Enzymol. 1988, 161, 238–249. [Google Scholar]
- Wariishi, H.; Vallim, K.; Gold, M.H. Manganese(II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium kinetic mechanism and role of chelators. J. Biol. Chem. 1992, 267, 23688–23695. [Google Scholar] [CrossRef]
- Wolfenden, B.S.; Willson, R.L. Radical-cations as reference chromogens in kinetic studies of ono-electron transfer reactions: Pulse radiolysis studies of 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate). J. Chem. Soc. Perkin Trans. 1982, 2, 805–812. [Google Scholar] [CrossRef]
- Greenberg, A.E.; Trussell, R.R.; Clesceri, L.S.; Franson, M.A.H. Standard Methods for the Examination of Water and Wastewater, 16th ed.; American Public Health Association: Washington, DC, USA, 1985. [Google Scholar] [CrossRef]
- Zhao, X.; Hardin, I.R. HPLC and spectrophotometric analysis of biodegradation of azo dyes by Pleurotus ostreatus. Dye. Pigment. 2007, 73, 322–325. [Google Scholar] [CrossRef]
- Lade, H.; Govindwar, S.; Paul, D. Low-Cost Biodegradation and Detoxification of Textile Azo Dye C.I. Reactive Blue 172 by Providencia rettgeriStrain HSL1. J. Chem. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Luther, K.L. Ammonia Toxicity in Bacteria and Its Implications for Treatment of and Resource Recovery from Highly Nitrogenous Organic Wastes. P.D. Dissertation, The State University of New Jersey, New Brunswick, NJ, USA, 2015. [Google Scholar]
- Jafaria, N.; Soudib, M.R.; Kermanshahic, R.K. Biodegradation Perspectives of Azo Dyes by Yeasts. Microbiology 2014, 83, 484–497. [Google Scholar] [CrossRef]
- Kumar, V.V.; Kirupha, S.D.; Periyaraman, P.; Sivanesan, S. Screening and induction of laccase activity in fungal species and its application in dye decolorization. Afr. J. Microbiol. Res. 2011, 5, 1261–1267. [Google Scholar] [CrossRef]
- Shahid, A.; Singh, J.; Bisht, S.; Teotia, P.; Kumar, V. Biodegradation of Textile Dyes by Fungi Isolated from North Indian Field Soil. EnvironmentAsia 2013, 6, 51–57. [Google Scholar]
- Kunjadia, P.D.; Sanghvi, G.V.; Kunjadia, A.P.; Mukhopadhyay, P.N.; Dave, G.S. Role of ligninolytic enzymes of white rot fungi (Pleurotus spp.) grown with azo dyes. SpringerPlus 2016, 5, 1487. [Google Scholar] [CrossRef] [Green Version]
- Wesenberg, D.; Kyriakides, I.; Agathos, S.N. White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol. Adv. 2003, 22, 161–187. [Google Scholar] [CrossRef] [PubMed]
- Porwal, H.; Mane, A.; Velhal, S. Biodegradation of dairy effluent by using microbial isolates obtained from activated sludge. Water Resour. Ind. 2015, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Park, C.; Lee, M.; Lee, B.; Kim, S.W.; Chase, H.A.; Lee, J.; Kim, S. Biodegradation and biosorption for decolorization of synthetic dyes by Funalia trogii. Biochem. Eng. J. 2007, 36, 59–65. [Google Scholar] [CrossRef]
- El-Kassas, H.Y.; Mohamed, L.A. Bioremediation of the textile waste effluent by Chlorella vulgaris. Egypt J. Aquat. Res. 2014, 40, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Prasad, A.S.A.; Rao, K. Aerobic biodegradation of azo dye Acid Black-24 by Bacillus halodurans. J. Environ. Biol. 2014, 35, 549–554. [Google Scholar] [PubMed]
- Prosser, R.S.; Parrott, J.L.; Galicia, M.; Shires, K.; Sullivan, C.; Toito, J.; Bartlett, A.J.; Milani, D.; Gillis, P.L.; Balakrishnan, V.K. Toxicity of sediment-associated substituted phenylamine antioxidants on the early life stages ofPimephales promelasand a characterization of effects on freshwater organisms. Environ. Toxicol. Chem. 2017, 36, 2730–2738. [Google Scholar] [CrossRef] [PubMed]
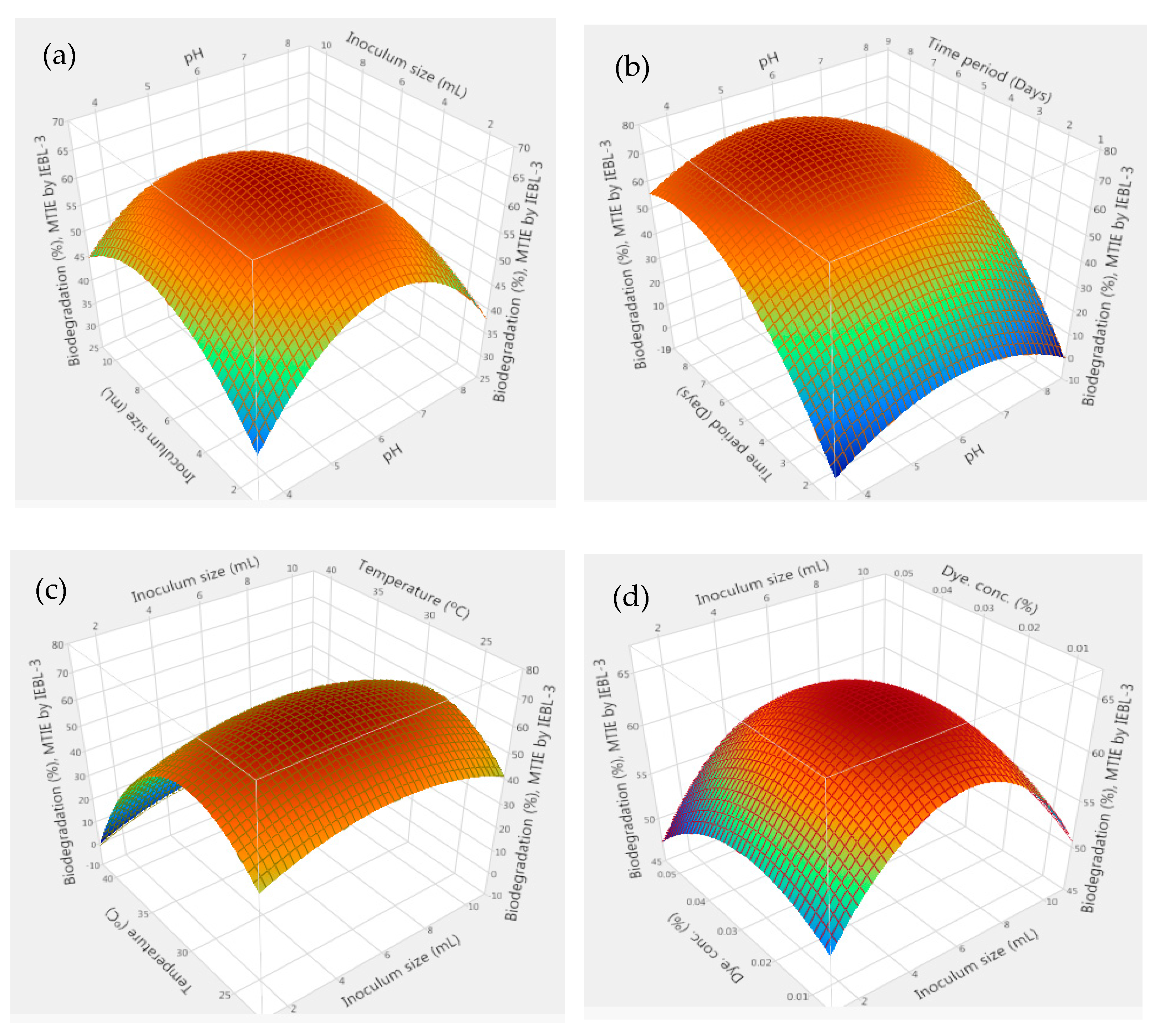


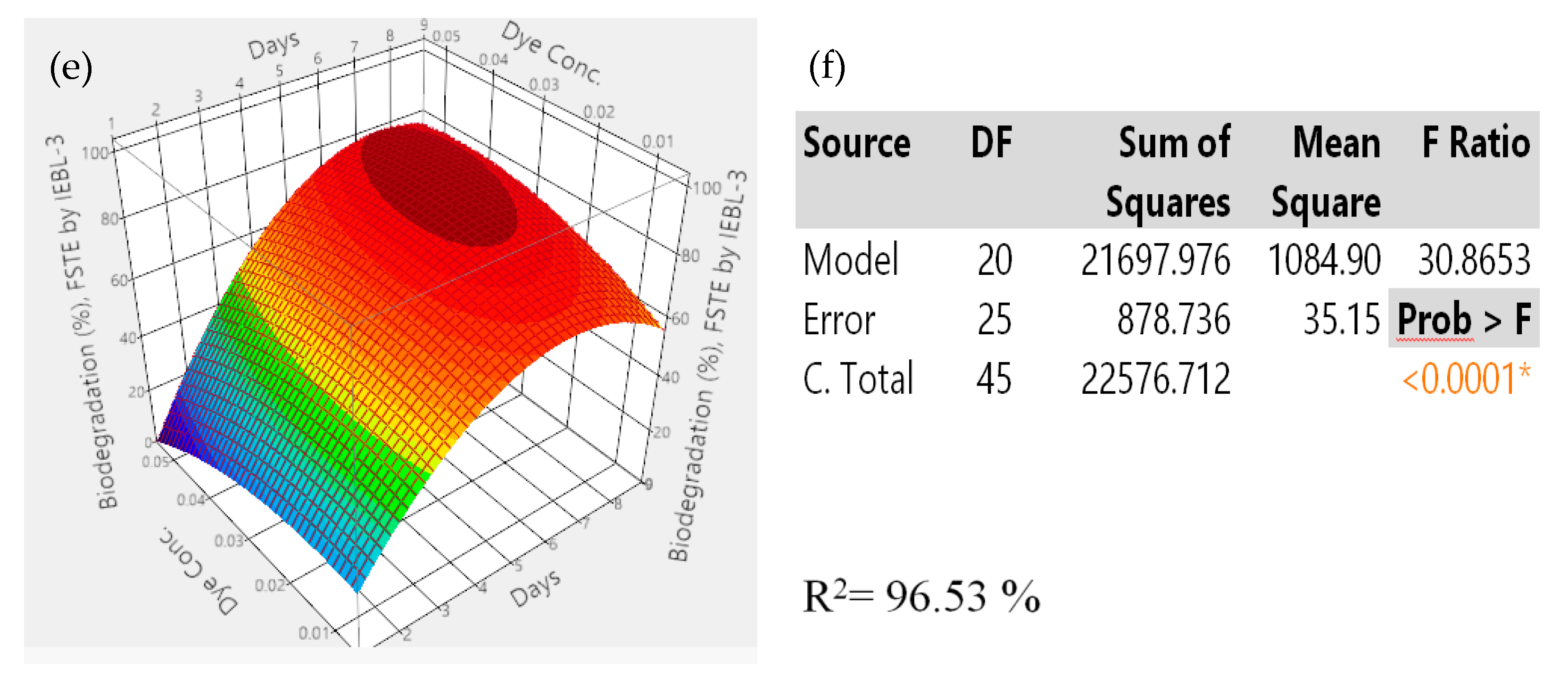
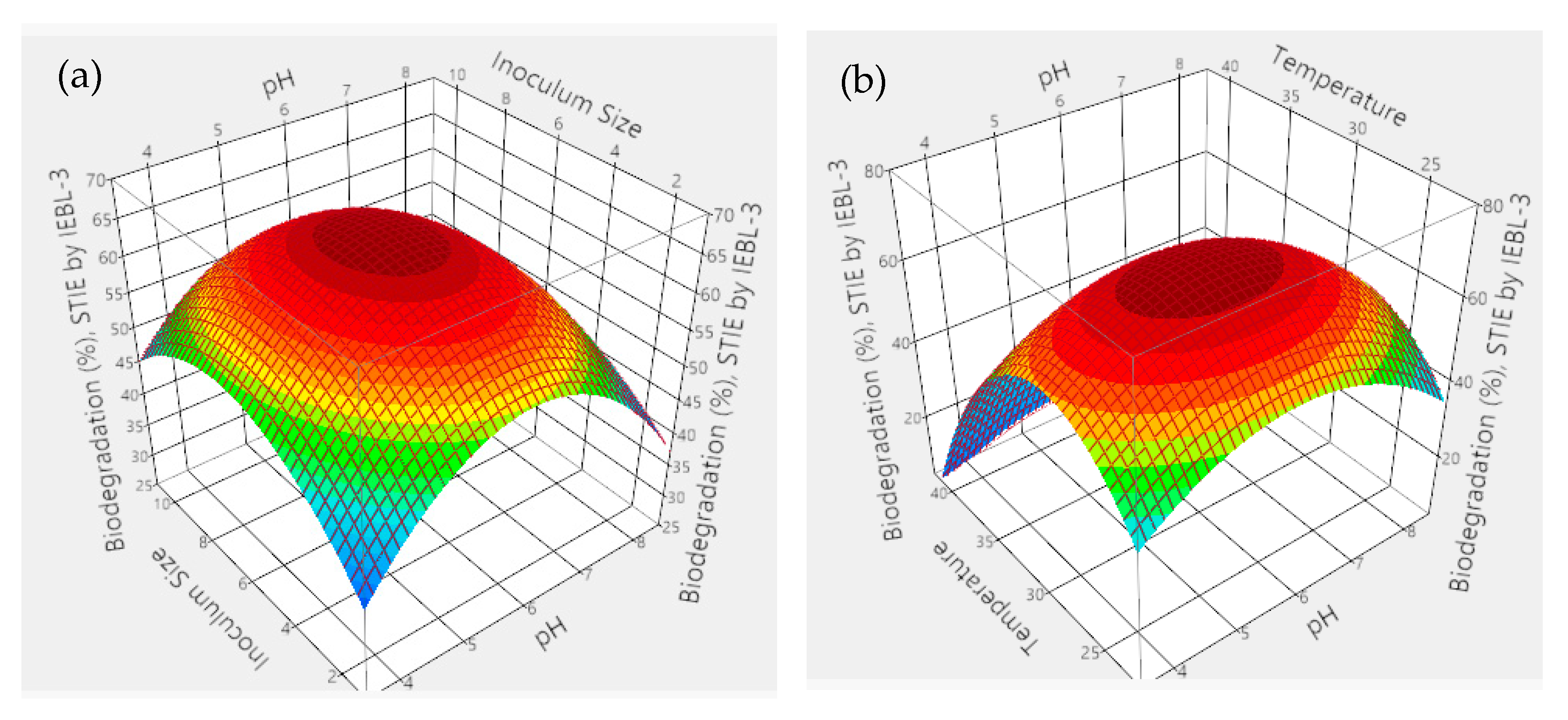


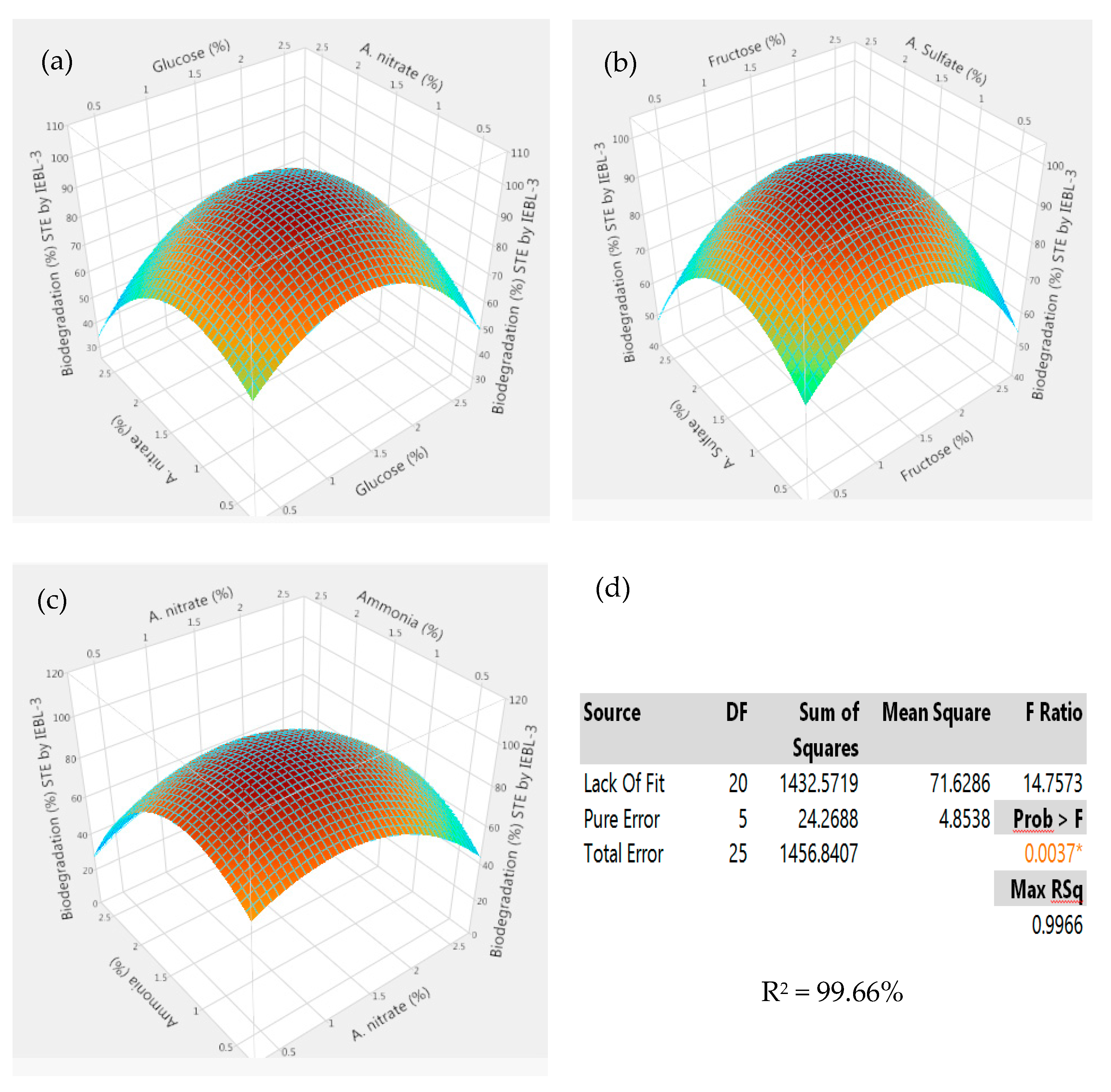

| No. | pH | Fungal Inoculum Size (mL) | Days | Temp. (°C) | Effluent Conc. (%) | No. | pH | Fungal Inoculum Size (mL) | Days | Temp. (°C) | Effluent Conc. (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 8.0 | 6.0 | 02 | 32.5 | 0.030 | 6.0 | 6.0 | 05 | 32.5 | 0.030 | ||
| 4.0 | 6.0 | 02 | 32.5 | 0.030 | 6.0 | 6.0 | 05 | 32.5 | 0.030 | ||
| 8.0 | 6.0 | 05 | 32.5 | 0.010 | 6.0 | 6.0 | 02 | 32.5 | 0.010 | ||
| 4.0 | 6.0 | 05 | 40.0 | 0.030 | 8.0 | 6.0 | 05 | 32.5 | 0.050 | ||
| 4.0 | 6.0 | 05 | 32.5 | 0.010 | 6.0 | 6.0 | 02 | 40.0 | 0.030 | ||
| 6.0 | 2.0 | 08 | 32.5 | 0.030 | 4.0 | 6.0 | 05 | 32.5 | 0.050 | ||
| 4.0 | 10.0 | 05 | 32.5 | 0.030 | 6.0 | 6.0 | 08 | 32.5 | 0.010 | ||
| 6.0 | 6.0 | 05 | 40.0 | 0.050 | 6.0 | 10.0 | 05 | 32.5 | 0.010 | ||
| 6.0 | 6.0 | 05 | 32.5 | 0.030 | 6.0 | 6.0 | 08 | 25 | 0.030 | ||
| 4.0 | 6.0 | 05 | 25.0 | 0.030 | 8.0 | 6.0 | 05 | 40.0 | 0.030 | ||
| 6.0 | 6.0 | 02 | 25.0 | 0.030 | 6.0 | 10.0 | 05 | 40.0 | 0.030 | ||
| 6.0 | 2.0 | 05 | 32.5 | 0.010 | 6.0 | 2.0 | 05 | 25.0 | 0.030 | ||
| 8.0 | 6.0 | 08 | 32.5 | 0.030 | 6.0 | 10.0 | 05 | 25.0 | 0.030 | ||
| 6.0 | 6.0 | 02 | 32.5 | 0.050 | 6.0 | 6.0 | 08 | 40.0 | 0.030 | ||
| 4.0 | 2.0 | 05 | 32.5 | 0.030 | 6.0 | 10.0 | 02 | 32.5 | 0.030 | ||
| 6.0 | 10.0 | 08 | 32.5 | 0.030 | 6.0 | 2.0 | 05 | 32.5 | 0.050 | ||
| 6.0 | 10.0 | 05 | 32.5 | 0.050 | 6.0 | 6.0 | 05 | 32.5 | 0.030 | ||
| 8.0 | 2.0 | 05 | 32.5 | 0.030 | 6.0 | 6.0 | 05 | 25.0 | 0.050 | ||
| 6.0 | 2.0 | 05 | 40.0 | 0.030 | 6.0 | 2.0 | 02 | 32.5 | 0.030 | ||
| 6.0 | 6.0 | 05 | 25.0 | 0.010 | 6.0 | 6.0 | 05 | 32.5 | 0.030 | ||
| 6.0 | 6.0 | 05 | 32.5 | 0.030 | 6.0 | 6.0 | 05 | 40.0 | 0.010 | ||
| 8.0 | 6.0 | 05 | 25.0 | 0.030 | 6.0 | 6.0 | 08 | 32.5 | 0.050 | ||
| 4.0 | 6.0 | 08 | 32.5 | 0.030 | 8.0 | 10.0 | 05 | 32.5 | 0.030 |
| No. | Glucose (%) | Fructose (%) | Amm. Nitrate (%) | Amm. Sulfate (%) | Ammonia (%) | No. | Glucose (%) | Fructose (%) | Amm. Nitrate (%) | Amm. Sulfate (%) | Ammonia (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.00 | 2.00 | 1.00 | 1.00 | 2.00 | ||
| 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 1.00 | 1.00 | 2.00 | 1.00 | ||
| 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 2.00 | 1.00 | 2.00 | 1.00 | 2.00 | ||
| 1.00 | 1.00 | 1.00 | 1.00 | 2.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| 1.00 | 1.00 | 1.00 | 2.00 | 2.00 | 2.00 | 1.00 | 1.00 | 1.00 | 2.00 | ||
| 1.00 | 1.00 | 2.00 | 2.00 | 2.00 | 1.00 | 2.00 | 1.00 | 2.00 | 1.00 | ||
| 1.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 1.00 | 2.00 | 2.00 | 1.00 | ||
| 2.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 2.00 | 2.00 | 2.00 | 1.00 | ||
| 2.00 | 2.00 | 1.00 | 1.00 | 1.00 | 2.00 | 2.00 | 1.00 | 2.00 | 2.00 | ||
| 2.00 | 2.00 | 2.00 | 1.00 | 1.00 | 1.00 | 2.00 | 2.00 | 1.00 | 2.00 | ||
| 2.00 | 2.00 | 2.00 | 2.00 | 1.00 | 2.50 | 1.50 | 1.50 | 1.50 | 1.50 | ||
| 2.00 | 1.00 | 2.00 | 1.00 | 1.00 | 1.50 | 1.50 | 1.50 | 1.50 | 0.50 | ||
| 2.00 | 2.00 | 1.00 | 1.00 | 2.00 | 1.50 | 1.50 | 1.50 | 2.50 | 1.50 | ||
| 1.00 | 2.00 | 1.00 | 2.00 | 2.00 | 1.50 | 1.50 | 1.50 | 0.50 | 1.50 | ||
| 1.00 | 1.00 | 2.00 | 1.00 | 2.00 | 0.50 | 1.50 | 1.50 | 1.50 | 1.50 | ||
| 2.00 | 1.00 | 1.00 | 2.00 | 2.00 | 1.50 | 1.50 | 0.50 | 1.50 | 1.50 | ||
| 2.00 | 2.00 | 2.00 | 1.00 | 2.00 | 1.50 | 2.50 | 1.50 | 1.50 | 1.50 | ||
| 1.00 | 1.00 | 1.00 | 2.00 | 1.00 | 1.50 | 1.50 | 2.50 | 1.50 | 1.50 | ||
| 1.00 | 2.00 | 2.00 | 1.00 | 1.00 | 1.50 | 1.50 | 1.50 | 1.50 | 2.50 | ||
| 2.00 | 1.00 | 2.00 | 2.00 | 2.00 | 1.50 | 0.50 | 1.50 | 1.50 | 1.50 | ||
| 1.00 | 1.00 | 2.00 | 2.00 | 2.00 | 1.00 | 1.50 | 1.50 | 0.50 | 1.00 | ||
| 2.00 | 2.00 | 1.00 | 1.00 | 1.00 | 1.50 | 1.00 | 1.00 | 1.50 | 0.50 | ||
| 2.00 | 2.00 | 1.00 | 2.00 | 1.00 | 0.50 | 0.50 | 1.00 | 1.00 | 1.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmood, R.T.; Asad, M.J.; Asgher, M.; Khan, F.S.; Muzammil, K.; Nasir, N.; Anwar, P.; Awais, M. First Report on the Bioremediation of Textile Industrial Effluents by Piptoporus Betulinus IEBL-3 by Using Response Surface Methodology. Appl. Sci. 2022, 12, 1090. https://doi.org/10.3390/app12031090
Mahmood RT, Asad MJ, Asgher M, Khan FS, Muzammil K, Nasir N, Anwar P, Awais M. First Report on the Bioremediation of Textile Industrial Effluents by Piptoporus Betulinus IEBL-3 by Using Response Surface Methodology. Applied Sciences. 2022; 12(3):1090. https://doi.org/10.3390/app12031090
Chicago/Turabian StyleMahmood, Raja Tahir, Muhammad Javaid Asad, Muhammad Asgher, Falak Sher Khan, Khursheed Muzammil, Nazim Nasir, Pervez Anwar, and Muhammad Awais. 2022. "First Report on the Bioremediation of Textile Industrial Effluents by Piptoporus Betulinus IEBL-3 by Using Response Surface Methodology" Applied Sciences 12, no. 3: 1090. https://doi.org/10.3390/app12031090
APA StyleMahmood, R. T., Asad, M. J., Asgher, M., Khan, F. S., Muzammil, K., Nasir, N., Anwar, P., & Awais, M. (2022). First Report on the Bioremediation of Textile Industrial Effluents by Piptoporus Betulinus IEBL-3 by Using Response Surface Methodology. Applied Sciences, 12(3), 1090. https://doi.org/10.3390/app12031090






