1. Introduction
Solitary enchondromas are benign bone tumors arising from the persisting cartilaginous cells from the growth plate. They are located in the hand bones in more than 50% of cases [
1]. The incidence of this pathology is frequently underestimated because, in many cases, the enchondroma evolves in the absence of any symptoms. The diagnosis of the enchondroma is established frequently by an accidental X-ray or due to local pain, swelling or a pathological fracture. The radiographs show a well-defined lytic lesion, which usually does not affect the bone cortex or soft tissues, with or without calcification [
2]. Takigawa has developed probably, the most reliable radiological classification with type A—central; type B—eccentric; type C—associated; type D—polycentric and type E—giant form [
3].
Treatment of benign tumors of the hand bones, including enchondroma, consists of tumor curettage or curettage and filling of the remaining cavity with autologous bone or bone substitute [
4]. Although the use of autologous bone is the gold standard for the treatment of bone defects, to avoid complications and morbidity at the donor sites, bone substitutes are increasingly used. In cases of bone defects of the hand, the most common sites used for cancellous bone harvesting are the distal radius or olecranon of the ulna. These sites usually offer enough cancellous bone for the defects in carpal bones, metacarpals and phalanges and are preferred because they are present in the same operation field and under the same regional anesthesia [
5,
6].
Initially, bone substitutes were rarely used in the treatment of hand bone defects, but currently, an increasing number of studies report their results for this pathology [
7]. An effective bone substitute is a synthetic or natural material that promotes bone healing by at least one of the following properties: osteogenesis, osteoinduction or osteoconduction [
8,
9]. The biological mechanisms of the various types of bone substitutes vary due to their different composition and mechanical strength. Hydroxyapatite and tricalcium phosphate are bone substitutes whose use and efficacy have been reported in the literature. Other relatively new techniques of bone bioengineering are those that involve biological components such as mesenchymal stem cells, platelet-rich plasma, platelet-rich fibrin, bone morphogenetic proteins, deantigenated heterologous bone matrix or their association with ceramics [
10,
11]. These substitutes are available in different forms, such as pellets, granules or fluids [
12,
13]. Regarding the use of bone graft or solid bone substitutes, the surgeon must adjust the shape of the implant to that of the bone defect. This process results in bone loss, additional trauma and prolonged operating time. In contrast, chitosan-based injectable bone substitutes are easy to handle and ensure complete filling of the defect [
14]. k-IBS
® is a fully resorbable injectable substituent that comes in prefilled, ease-to-use syringes. It contains rapidly oseointegrable ceramic granules due to its components: Hydroxyapatite (HA) (Ca
10 (PO
4)
6 (OH)
2) and β-Tricalcium Phosphate (β-TCP) (Ca
3 (PO
4)
2). The granules are disseminated in a liquid polymeric matrix consisting of chitosan ((C
6H
11O
4N)
n) and polyethylene glycol ((C
2H
4O)
n H
2O). The HA/β-TCP ratio is 3/1 in the k-IBS
® bone substitute, and the ceramic granule dimensions are between 125 and 355 µm [
15]. The HA/β-TCP ratio in the composition of ceramics seems to be a good indicator of new bone formation, according to recent studies. The ceramics that have 100% HA or those with 75% HA and 25% β-TCP showed the best results in terms of new bone formation and resorbability in vivo [
16].
Our study is based on a series of 15 patients diagnosed with enchondroma of the hand: eight of them with type A, four with type B and three with type D enchondroma according to the Takigawa classification. All the tumors were treated by curettage. The resulting bone defects were left to heal naturally in four cases, and in the other 11 cases after curettage, either autologous bone or liquid bone substitute was used, and in some cases, osteosynthesis material was associated with increased bone strength until bone defect consolidation.
2. Materials and Methods
Our report included 15 patients, 11 men and 4 women, aged between 30 and 68. The patients were admitted and underwent surgery in the Plastic and Reconstructive Surgery Department of the “Sf. Spiridon” Emergency Clinical Hospital Iasi, as well as in the Orthopedics and Traumatology Department of the Vaslui Emergency Clinical Hospital between January 2016 and December 2018. The follow-up period was at least 2 years for each case. All the patients gave their consent for surgery and participation in this study. An agreement from the Ethics Committee of the Hospital was obtained. All cases were diagnosed with solitary enchondroma, and we did not include patients with multiple sites of enchondroma in this study. The suspicion of enchondroma was raised by plain radiographic findings and clinical examination of the hand, and the diagnosis was confirmed by histopathology. Nine of the patients included in this report presented without symptoms. In these cases, the tumor was found accidentally on an X-ray taken for another reason. Three patients presented to the hospital for pain or swelling. In the other three cases, the first sign was a pathologic bone fracture. The fracture occurred during daily activities in the absence of trauma. All cases from this study had surgical indications due to the large volume of the tumor, affecting more than 50% of the cortical circumference. The surgical procedure consisted of curettage of the tumor cavity followed by filling or natural healing. In 9 of the 15 cases, the procedure was performed under regional anesthesia with a bloodless operative field by exsanguination and the use of a pneumatic tourniquet. The other six cases were performed under local anesthesia using the WALANT technique (Wide Awake Local Anesthesia No Tourniquet), using a solution of 1% Lidocaine with Epinephrine in a concentration of 1:100,000 [
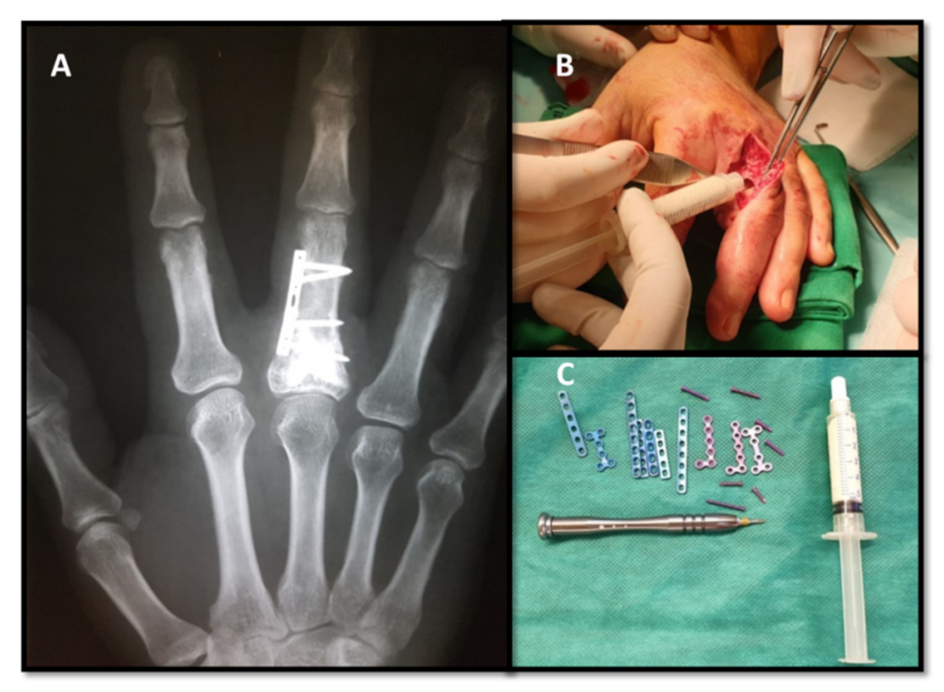
17]. After curettage, all the resected tissue was sent for anatomopathological examination and diagnosis establishment. In cases in which the tumor was located in the distal phalanx, after curettage, the cavity was left to heal naturally, and the finger required immobilization for 3 weeks in a plastic splint for middle phalanx, distal interphalangeal joint and distal phalanx in full extension (
Figure 1).
In cases in which the tumor was located in the middle or proximal phalanx, the bone defect was filled with autologous cancellous bone graft harvested from the distal radius in four cases. The cancellous bone was harvested from the distal radius, radially to the Lister’s tubercle from the floor of the second extensor compartment. A skin incision of 3 cm was performed over the extensor carpi radialis brevis (ECRB) tendon; the ECRB and extensor carpi radialis longus are retracted to the radial side, a hinge is used to perforate the cortex of the distal radius and the cancellous bone is harvested with a curette. The bone cortex and periosteum are closed, and the skin is sutured in anatomical layers.
In the other seven cases, the bone defects were filled with injectable bone substitutes based on chitosan (k-IBS
®). K-IBS
® is presented in prefilled syringes with the ready-to-use bone substitute, so the bone substitute does not need any prior preparation before use. Osteosynthesis was performed with low profile miniplate and screws only in cases that presented signs of bone instability after tumor resection (
Figure 2). Protected active and passive movement in the dynamic splint was performed on the 5th postoperative day in all cases.
We obtained early postoperative X-rays and compared them at 1, 6, 12 and 24 months in order to observe bone defect healing and the eventual tumor recurrence. The functional results were assessed using Disabilities of the Arm, Shoulder and Hand score (DASH) and Range of Motion (ROM) at 1, 3, 6 and 12 months. The severity of pain in the first 5 postoperative days was assessed with the Visual Analogue Scale (VAS). In order to minimize the risk of bias, the measurements were performed by two different assessors, and in the cases with significant discrepancies, a third assessor was involved.
3. Results
During the radiological examination, the well-defined lytic lesion was accidentally discovered, or a symptomatic patient raised the suspicion of enchondroma. The location of the tumors was at the proximal phalanx of the ring finger in six cases, at the distal phalanx of the little finger in four cases, at the proximal phalanx of the little finger in two cases, at the proximal phalanx of the middle finger in two cases and at the middle phalanx of the ring finger in one case.
According to the Takigawa classification, eight cases had a type A tumor, four cases had type B and three cases had type C. We did not include any case with multiple tumors or with a large tumor in this study.
The common surgical step in the three treatment groups was tumor resection by curettage. The average operating time in the group of patients who received an autologous bone graft was 150 min, 72 min in the group with injectable bone substitute and 40 min in the group with natural healing. It is worth mentioning that performing plate osteosynthesis represents approximately 35% of the operating time. No intraoperative or immediate postoperative complications such as wound infection, neurovascular injury, tendon injury, postoperative fractures or fractures during physical therapy were recorded. The mean VAS pain score in the patients who received autologous bone graft was 4.2, and it was 2.5 in the group of patients in which the defect was allowed to heal on its own, and the lowest score was recorded in the group which received injectable bone substitute.
Postoperative X-rays showed a radiolucent cavity in cases where the enchondroma was removed without filling the defect.
In all cases, the histopathological examination revealed fragments of hyaline cartilage encased by bone and covered by perichondrium, decreased cellularity and low vascularization, chondroblasts containing monomorphic chondrocytes and with no mitotic activity. Necrosis is common due to avascularity. These morphological aspects are distinctive for enchondroma.
In the cases in which an injectable bone substituent was used, a thin radiolucent line was observed between the substituent and the recipient’s bone. Subsequent postoperative X-rays revealed a reduced radiolucency of this line due to the development of new bone tissue in the k-IBS
® substituent, a process favored by its osteoconductive properties. The ceramic granules in k-IBS
® composition are rapidly osteointegrated due to their chemical composition similar to human bone. On 1-year follow-up X-rays, a complete replacement of the substituent by spongy bone tissue was observed in all cases (
Figure 3).
In the cases in which an autologous graft was used, small areas of radiolucency were detected in areas where the graft did not have close contact with the recipient’s bone.
All the cases with type B enchondroma, according to Takigawa, were managed with curettage and natural healing with very good results, while in all the cases with type C and D, the surgeon considered it necessary to fill the defect after curettage.
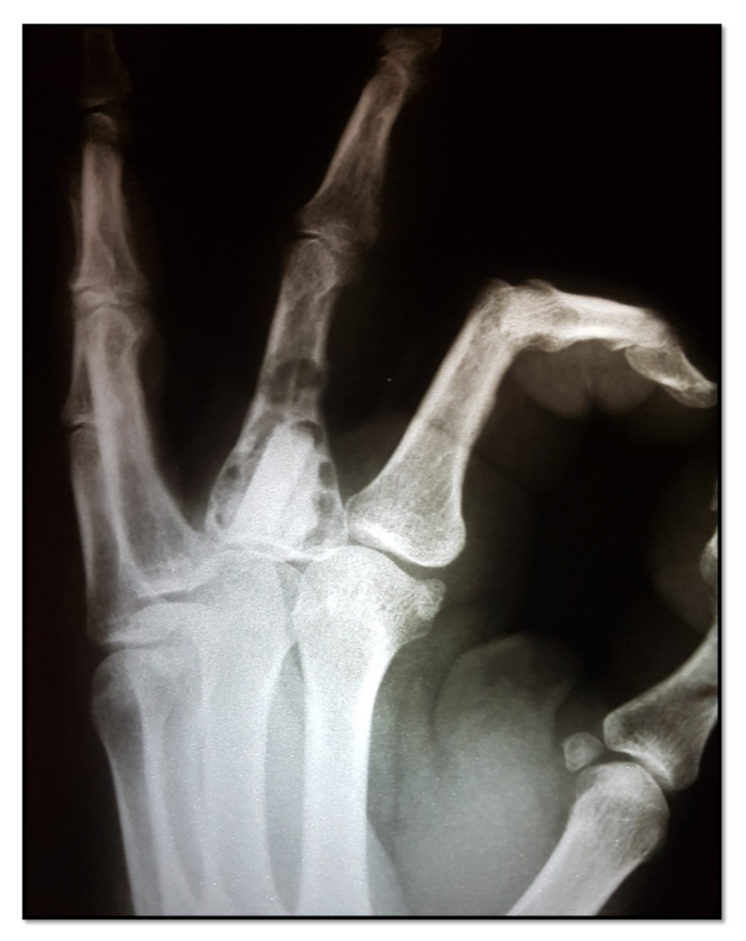
In this study, the recurrence rate was 6.6%, 1 of the 15 cases (
Figure 4).
The mean DASH disability score of the whole study group was 2.0, with no significant differences between patients in which different methods of treating bone defects were used. All patients resumed work and social activities without significant sequelae. All patients reported full satisfaction with the functional and aesthetic outcomes.
Range of motion (ROM) was measured by digital goniometer in the metacarpophalangeal (MCP), proximal interphalangeal (PIP) and distal interphalangeal (DIP) joints and then compared with the other hand. According to the Evaluation System of the American Society for Surgery of the Hand, 93% of the patients included in the present study attained “excellent” TAM (total active motion) at a 6-month follow-up (
Table 1). The exception was the patient with recurrent enchondroma, who had a 20-degree flexion deficit in the MF joint (middle finger joint) and of 10 degrees in the PIP joint, which required tenolysis of the extensor apparatus, refused by the patient due to functional adaptation (
Figure 5).
4. Discussion
The incidence of enchondromas is underestimated in the general population because, in many cases, enchondromas are asymptomatic and do not require treatment. Enchondroma usually arises in the metaphysis of the tubular bones, most often affecting the bones of the hand, followed by the humerus and femur [
18]. The proximal phalanges are most commonly involved, followed by the middle phalanges, the metacarpal bones and the distal phalanges. Carpal bones are affected in only 1–3% of cases of hand enchondroma [
1]. Although all 15 cases included in this study had solitary enchondromas, cases of multiple enchondromas were described in 3.4% of the cases. Enchondromas are thought to be tumors that begin to form before the 54th day of intrauterine life, which then separates into the bones of the same digit ray during the embryological development of the hand [
19]. Multiple enchondromas should not be confused with Ollier’s disease or Maffucci’s syndrome. Ollier’s disease is characterized by multiple enchondromatosis with a typically asymmetrical distribution, most commonly found in the phalanges and metacarpals of the hand [
20]. Maffucci syndrome is a rare genetic abnormality characterized by multiple enchondromatosis and vascular abnormalities, the most common being spindle cell hemangioma (SCH), a painful, aggressive and frequently disabling lesion [
21].
The treatment of choice for enchondroma is tumor curettage. The autologous bone graft used to consolidate bone defects is still considered the gold standard because it is the only biological material that possesses osteoinductive, osteogenetic and osteoconductive properties, fully integrated and transformed into trabecular bone [
7,
22]. However, a large number of complications are reported at the bone graft donor site, especially in the iliac crest. Complications include persistent postoperative pain, damage to the lateral femoral cutaneous nerve or ilioinguinal nerve, development of a hematoma or infection [
23]. Autologous bone graft requires a longer operating time, sometimes a more laborious type of anesthesia and higher costs [
24,
25]. The postoperative recovery protocol depends on the strength of the remaining bone and the stability of the bone fixation mechanism. That is the reason why in some cases, fixation was performed with low-profile miniplates, which allow early motion from 5th day postoperatively [
13]. In seven of our study cases, because of the large bone defect affecting over ½ of the cortical circumference, it was decided to use miniplates for osteosynthesis that allowed the early active and passive motion. Analyzing the results of this study, we found that there are no differences between DASH and TAM score values in patients receiving autologous bone graft or bone substitute, but the bone graft group recorded higher postoperative pain according to the VAS score, longer operative time and significantly higher costs. An additional advantage of using injectable bone substitutes could be the immediate mechanical stability they provide [
12,
26]. Gaasbek et al. reported good results following the use of calcium phosphate bone substitutes in 19 patients with enchondroma of the hand, as the material resorbed, on average, in 10 weeks and was progressively replaced by trabecular bone [
27]. Yusuda reported good functional results after using calcium phosphate in the treatment of a series of patients with enchondroma of the hand or foot [
28]. A possible disadvantage of synthetic bone substitutes could be its delayed integration, insufficient defect consolidation or immune rejection, complications not encountered in this study [
29]. In the cases studied by us, we observed that in Takigawa type B enchondromas, we did not need bone grafting or bone substitutes to obtain good results. This could be due to smaller dimensions of the tumor with unicortical affecting. The type A and D enchondromas from our study benefitted from curettage and filling of bone defect with autologous bone graft or injectable bone substitute with similar results.
Recurrences are rare but can occur even 16 years after surgery [
30]. The recurrence rate reported by some studies ranges from 7% to 14.3%. Because enchondromas are slow-growing benign tumors, recurrences can remain asymptomatic for a long time. Nevertheless, the use of bone cement can help in the early radiological observation of a lytic bone tumor. Because cement forms a highly radiopaque mass, the recurrence can be easily identified at the cement–bone interface [
31]. Long-term follow-up is recommended to determine accurate recurrence rates [
30,
32]. Despite our small sample size (15 patients) and the potential for performance bias, a recurrence (6.6%) was detected on the 1-year follow-up X-ray (
Figure 3).
5. Conclusions
In the case of solitary enchondromas of the hand, the results do not show major differences in their excision, but there is controversy over the method of filling the remaining defect. Although the autologous bone graft still meets the gold standard criteria, the injectable bone substitute gains ground against the autologous graft, demonstrating greater efficiency and accessibility through shorter operating time and lower postoperative pain. The Takigawa classification could predict the optimal treatment option. According to the results of this study, the Takigawa type B enchondromas can be treated with curettage and natural healing. The small sample of each group lowers the power of the study; hence, further studies with a larger number of participants would be useful.
Author Contributions
Conceptualization, P.C. and B.P.; methodology, A.P., I.R. and N.F.; software, S.A. and R.T.; validation, A.C., V.N. and V.P.; formal analysis, V.P.; investigation, P.C.; resources, R.T.; data curation, I.R.; writing—original draft preparation, P.C., B.P. and V.P.; writing—review and editing, A.C. and V.N.; visualization, R.T.; supervision, I.R.; project administration, P.C.; funding acquisition, R.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Emergency Clinical Hospital “Sf. Spiridon” (Nr.199/14.09.21).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data supporting reported results can be found in patient medical files located in the archive of “Sf. Spiridon” Emergency Clinical Hospital.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gaulke, R. The distribution of solitary enchondromata at the hand. J. Hand Surg. Br. 2002, 27, 444–445. [Google Scholar] [CrossRef] [PubMed]
- Lubahn, J.D.; Bachoura, A. Enchondroma of the Hand: Evaluation and Management. J. Am. Acad. Orthop. Surg. 2016, 24, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, T. Chondromas of the bones of the hand. A review of 110 cases. J. Bone Jt. Surg. 1971, 53, 1591–1600. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, J.; Dai, X.; Schilling, A.F. Modified technique for one-stage treatment of proximal phalangeal enchondromas with pathologic fractures. J. Hand Surg. Am. 2014, 39, 1757–1760. [Google Scholar] [CrossRef]
- Bruno, R.J.; Cohen, M.S.; Berzins, A.; Sumner, D.R. Bone graft harvesting from the distal radius, olecranon, and iliac crest: A quantitative analysis. J. Hand Surg. Am. 2001, 26, 135–141. [Google Scholar] [CrossRef]
- Vyrva, O.; Thirkannad, S.M. Distal Radius Bone Graft From the Second Extensor Compartment: A Safe and Effective Technique. Tech. Hand Up. Extrem. Surg. 2016, 20, 147–150. [Google Scholar] [CrossRef]
- Liodaki, E.; Kraemer, R.; Mailaender, P.; Stang, F. The Use of Bone Graft Substitute in Hand Surgery. A Prospective Observational Study. Medicine 2016, 95, e3631. [Google Scholar] [CrossRef]
- Hidaka, C.; Cunningham, M.E.; Rodeo, S.A.; Maher, S.A.; Zhu, W. Modern biologics used in orthopaedic surgery. Curr. Opin. Rheumatol. 2006, 18, 74–79. [Google Scholar] [CrossRef]
- Alexa, O.; Pertea, M.; Malancea, R.I.; Puha, B.; Veliceasa, B. Our experience in the surgical treatment of acetabular fractures using ”spring plate” technique. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2019, 123, 275–281. [Google Scholar]
- Lammens, J.; Maréchal, M.; Delport, H.; Geris, L.; Oppermann, H.; Vukicevic, S.; Luyten, F.P. A cell-based combination product for the repair of large bone defects. Bone 2020, 138, 115511. [Google Scholar] [CrossRef]
- Stokovic, N.; Ivanjko, N.; Maticic, D.; Luyten, F.P.; Vukicevic, S. Bone Morphogenetic Proteins, Carriers, and Animal Models in the Development of Novel Bone Regenerative Therapies. Materials 2021, 14, 3513. [Google Scholar] [CrossRef] [PubMed]
- Botez, P.; Sîrbu, P.D.; Simion, L.; Munteanu, F.; Antoniac, I. Application of a biphasic macroporous synthetic bone substitutes CERAFORM (R): Clinical and histological results. Eur. J. Orthop. Surg. Traumatol. 2009, 19, 387–395. [Google Scholar] [CrossRef]
- Bickels, J.; Wittig, J.C.; Kollender, Y.; Kellar-Graney, K.; Mansour, K.L.; Meller, I.; Malawer, M.M. Enchondromas of the hand: Treatment with curettage and cemented internal fixation. J. Hand Surg. Am. 2002, 27, 870–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.H.K.; Weir, M.D.; Simon, C.G. Injectable and strong nano-apatite scaffolds for cell/growth factor delivery and bone regeneration. Dent. Mater. 2008, 24, 1212–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Li, H.; Cheng, W.; Yang, Y.; Zhu, M.; Zhou, C. Novel injectable calcium phosphate/chitosan composites for bone substitute materials. Acta Biomater. 2006, 2, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.I.; Asahina, I.; Ohmamiuda, K.; Takahashi, K.; Yokota, S.; Enomoto, S. Evaluation of ceramics composed of different hydroxyapatite to tricalcium phosphate ratios as carriers for rhBMP-2. Biomaterials 2001, 22, 1643–1651. [Google Scholar] [CrossRef]
- Pertea, M.; Grosu, O.M.; Veliceasa, B.; Velenciuc, N.; Ciobanu, P.; Tudor, R.; Poroch, V.; Lunca, S. Effectiveness and Safety of Wide Awake Local Anesthesia no Tourniquet (WALANT) Technique in Hand Surgery. Rev. Chim. 2019, 70, 3587–3591. [Google Scholar]
- Herget, G.W.; Strohm, P.; Rottenburger, C.; Kontny, U.; Krauß, T.; Bohm, J.; Sudkamp, N.; Uhl, M. Insights into enchondroma, enchondromatosis and the risk of secondary chondrosarcoma. Review of the literature with an emphasis on the clinical behaviour, radiology, malignant transformation and the follow up. Neoplasma 2014, 61, 365–378. [Google Scholar] [CrossRef] [Green Version]
- Miwa, S.; Okamoto, H.; Yamada, S.; Kawaguchi, Y.; Endo, K.; Aiba, H.; Hayashi, K.; Kimura, H.; Sekiya, I.; Otsuka, T.; et al. Distribution of Solitary and Multiple Enchondromas of the Hand. In Vivo 2019, 33, 2235–2240. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Jain, V.K.; Bharadwaj, M.; Arya, R.K. Ollier disease: Pathogenesis, diagnosis, and management. Orthopedics 2015, 38, e497–e506. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.C.; Swee, R.G.; Shives, T.C.; Unni, K.K. Chondrosarcoma in Maffucci’s syndrome. J. Bone Jt. Surg. Am. 1985, 67, 1214–1219. [Google Scholar] [CrossRef]
- Kuur, E.; Hansen, S.L.; Lindequist, S. Treatment of solitary enchondromas in fingers. J. Hand Surg. 1989, 14, 109–112. [Google Scholar] [CrossRef]
- Grob, D. Problems at the donor site in autologous bone transplantation. Unfallchirurg 1986, 89, 339–345. (In German) [Google Scholar] [PubMed]
- Schaller, P.; Baer, W. Operative treatment of enchondromas of the hand: Is cancellous bone grafting necessary? Scand. J. Plast. Reconstr. Surg. Hand Surg. 2009, 43, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Poroch, V.; Grosu, O.M.; Lunca, S. Study on Epinephrine Used in Local Anesthesia Controversy and certainty. Rev. Chim. 2018, 69, 169–171. [Google Scholar] [CrossRef]
- Sîrbu, P.D.; Petreus, T.; Munteanu, F.; Pertea, M.; Lunca, S.; Poroch, V.; Botez, P. Clinical Experience with a Macroporous Synthetic Bone Substituite (Eurocer) in the Treatment of the Patitents with Bone Defects. In Proceedings of the International Conference on Advancements of Medicine and Health Care through Technology IFMBE Proceedings 2011, Cluj-Napoca, Romania, 29 August–2 September 2011; Volume 36, pp. 358–368. [Google Scholar]
- Gaasbeek, R.D.; Rijnberg, W.J.; van Loon, C.J.; Meyers, H.; Feith, R. No local recurrence of enchondroma after curettage and plaster filling. Arch. Orthop. Trauma Surg. 2005, 125, 42–45. [Google Scholar] [CrossRef]
- Yusuda, M.; Masada, K.; Takeuchi, E. Treatment of enchondroma of the hand with injectable calcium phosphate bone cement. J. Hand Surg. 2006, 31, 98–102. [Google Scholar] [CrossRef]
- Shibuya, N.; Jupiter, D.C. Bone graft substitute: Allograft and xenograft. Clin. Podiatr. Med. Surg. 2015, 32, 21–34. [Google Scholar] [CrossRef]
- Gaulke, R.; Suppelna, G. Solitary enchondroma at the hand. Long-term follow-up study after operative treatment. J. Hand Surg. Br. 2004, 29, 64–66. [Google Scholar] [CrossRef]
- Fraquet, N.; Faizon, G.; Rosset, P.; Phillipeau, J.; Waast, D.; Gouin, F. Long bones giant cells tumors: Treatment by curretage and cavity filling cementation. Orthop. Traumatol. Surg. Res. 2009, 95, 402–406. [Google Scholar] [CrossRef] [Green Version]
- Sassoon, A.A.; Fitz-Gibbon, P.D.; Harmsen, W.S.; Moran, S.L. Enchondromas of the hand: Factors affecting recurrence, healing, motion, and malignant transformation. J. Hand Surg. Am. 2012, 37, 1229.e34. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).