Two Red Sea Sponge Extracts (Negombata magnifica and Callyspongia siphonella) Induced Anticancer and Antimicrobial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Identification of Sponge Specimens
2.2. Preparation of NmE and CsE
2.3. GC–MS Analysis of NmE and CsE
2.4. Anticancer Assay
2.4.1. Cell Culture
2.4.2. Measurement of Cytotoxicity by MTT Assay
2.4.3. Protein Extraction and Western Blotting
2.4.4. Cell Cycle Assay with PI
2.4.5. Annexin V-FITC/PI Assay
2.4.6. ROS Assay
2.5. Antimicrobial Assay
2.5.1. Microbial Indicator Strains
2.5.2. Media and Inocula
2.5.3. Antimicrobial Activity Assessment for CsE and NmE
2.6. Statistical Analysis
3. Results
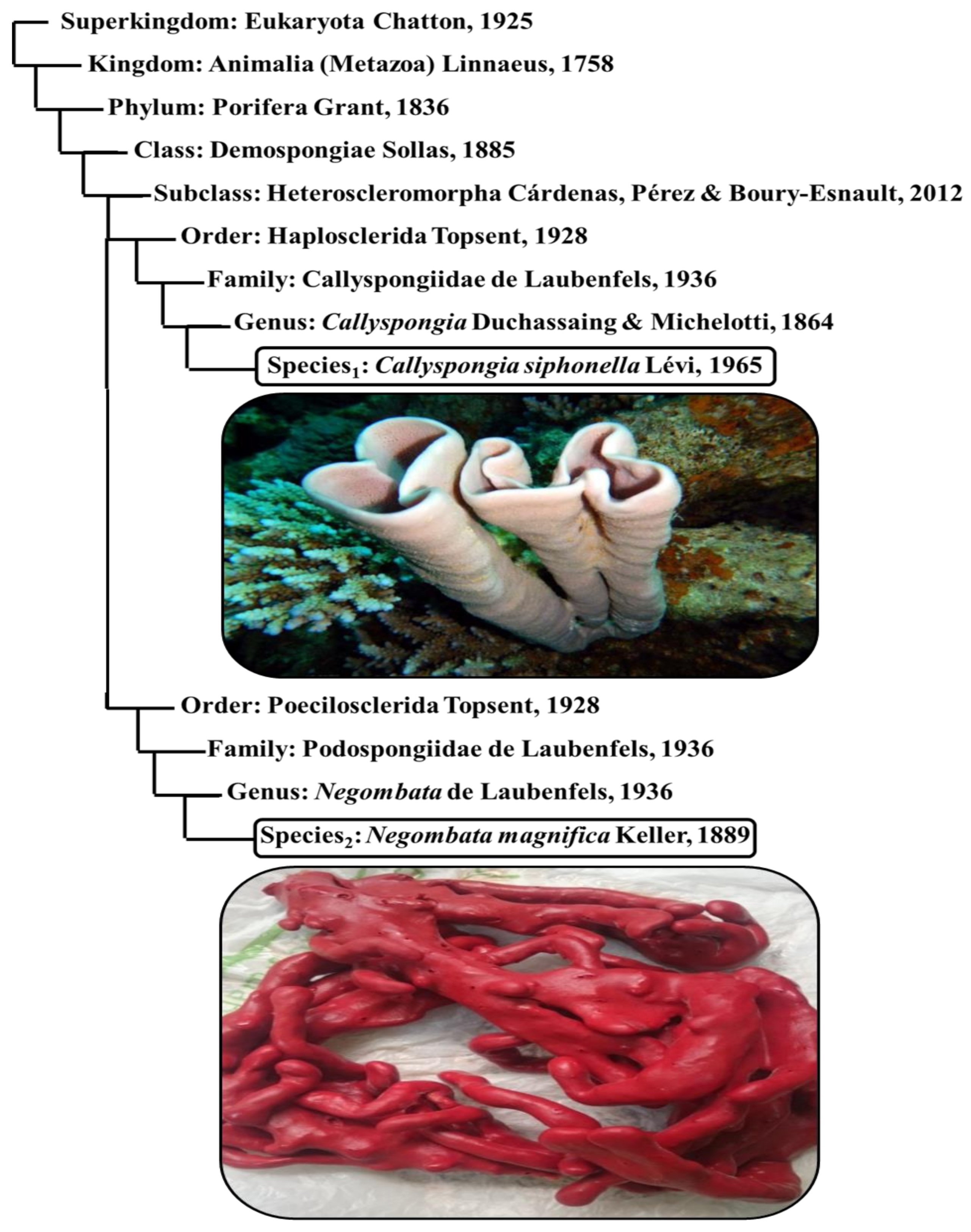
3.1. Sponge Identification
- 1-
- Finger-sponge (Negombata magnifica): It is a reddish-brown, narrow, crooked branched sponge, and lives between coral reefs and rocks.
- 2-
- Tube-sponge (Callyspongia siphonella): It is a cluster of vertical tubes with a common base that lives on sheltered, hard substrate. It is smooth and has a pale purple or reddish-brown color.
3.2. GC–MS Analysis of CsE and NmE
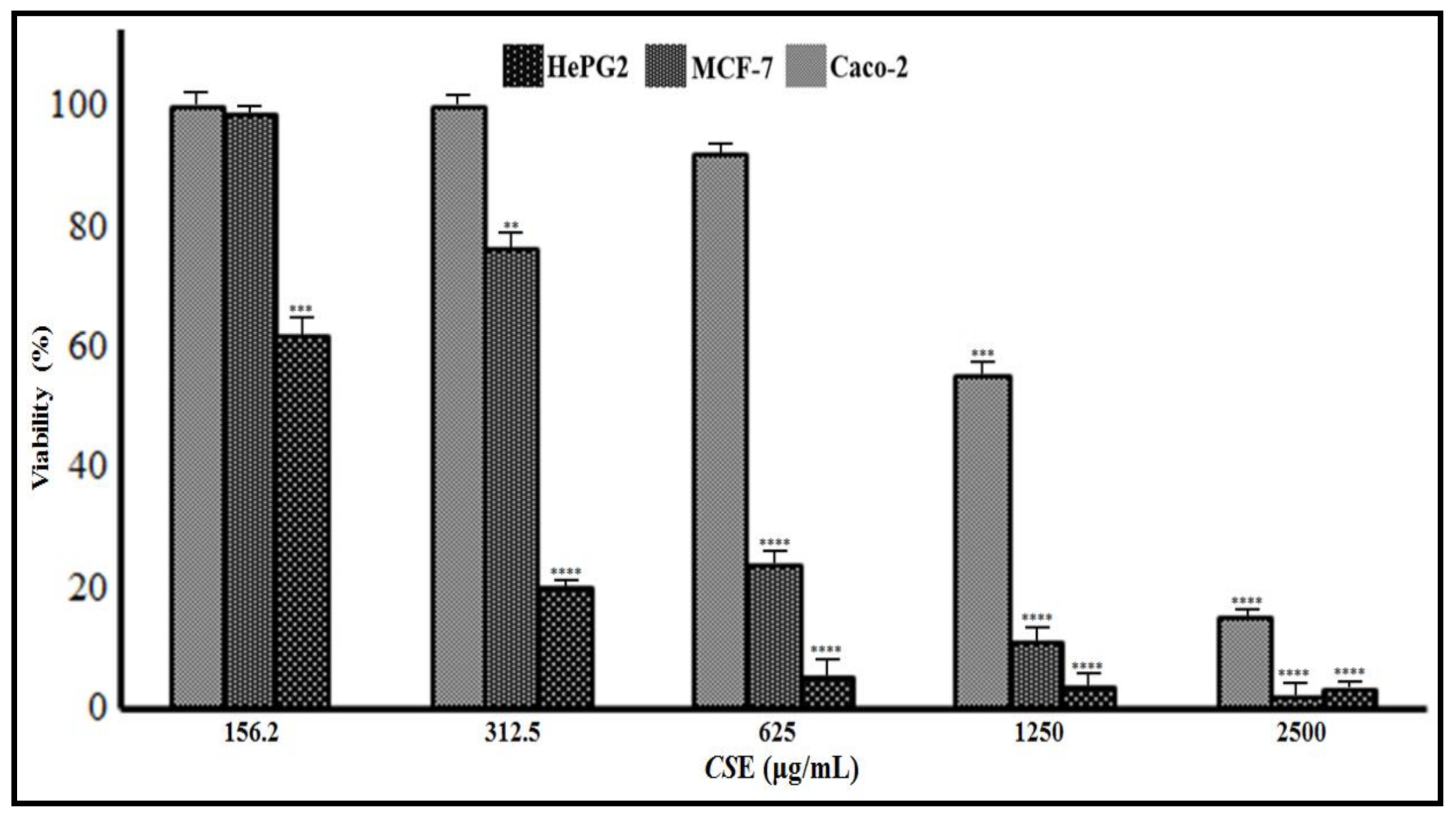
3.3. Anticancer Effects of CsE and NmE
3.3.1. CsE and NmE Effects on HepG2, MCF-7, and Caco-2 Cells Proliferation and Viability
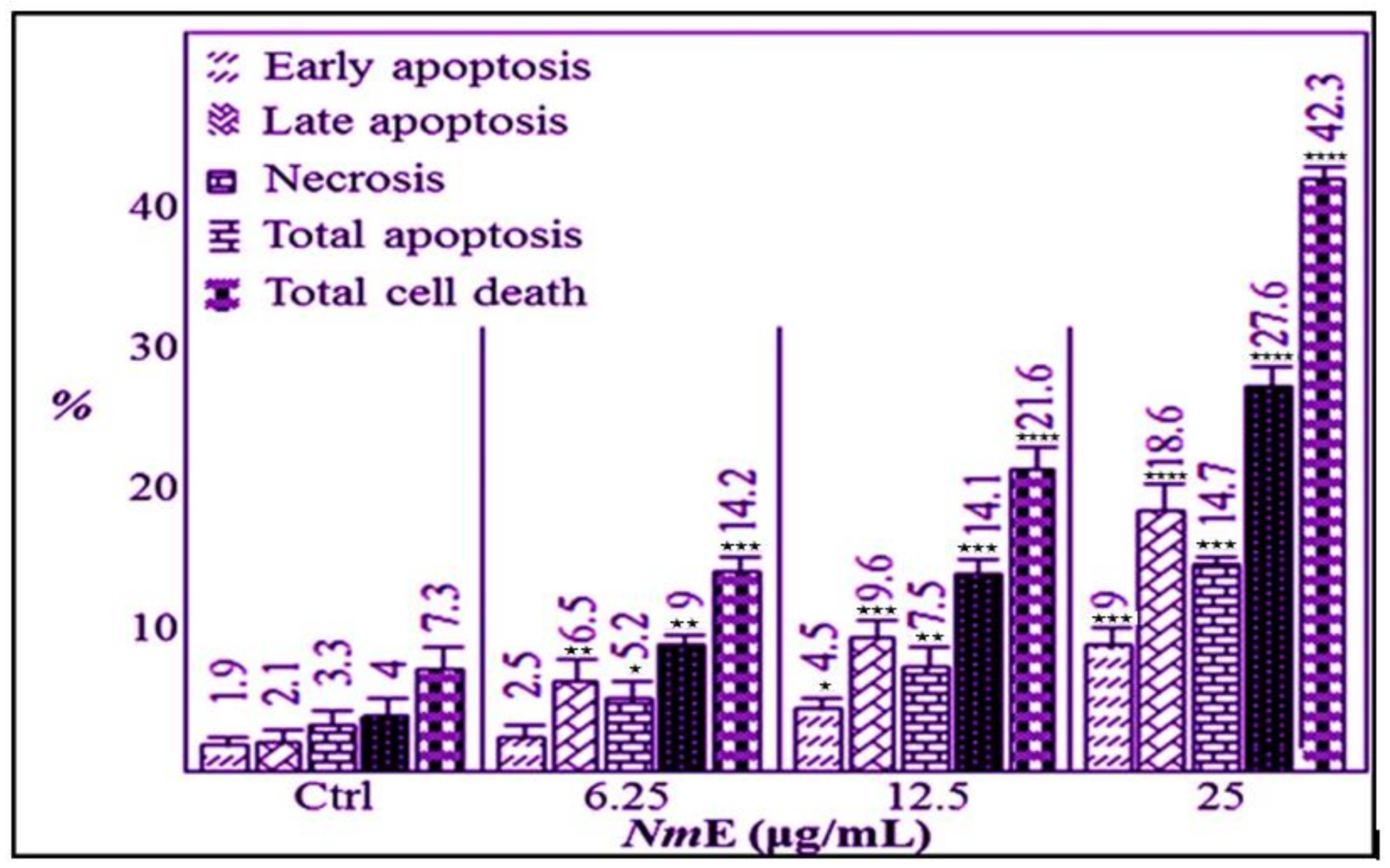
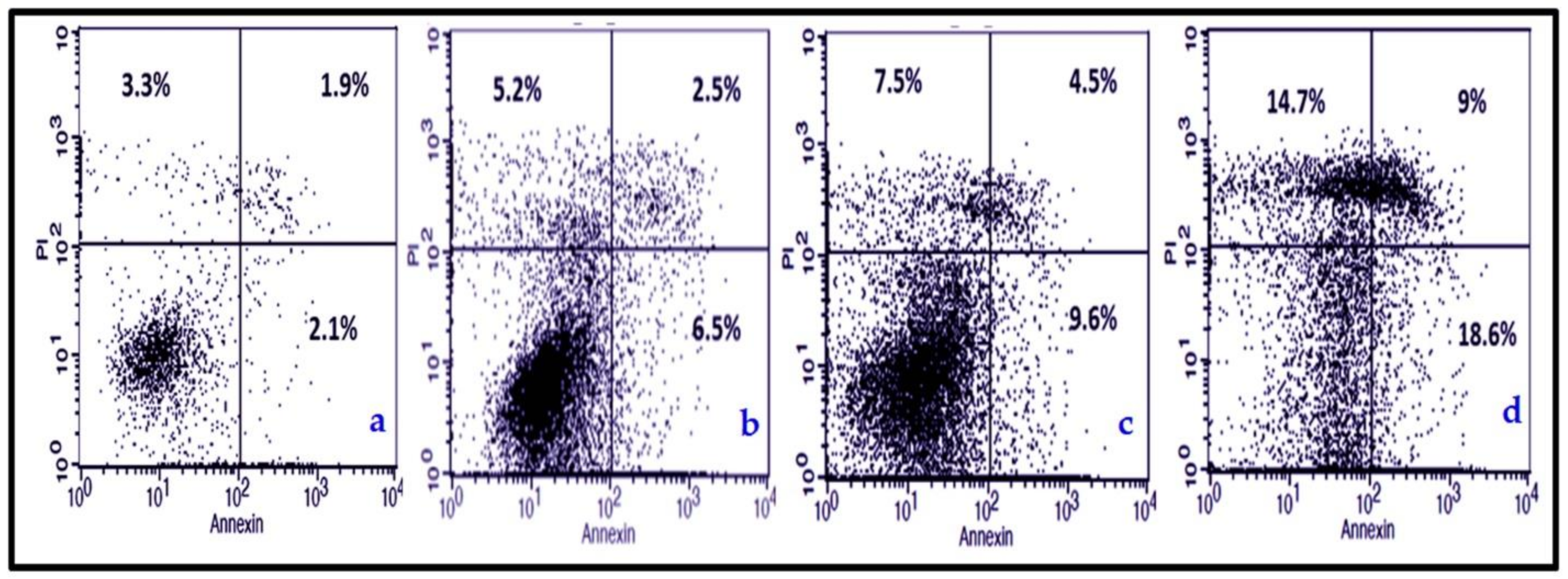
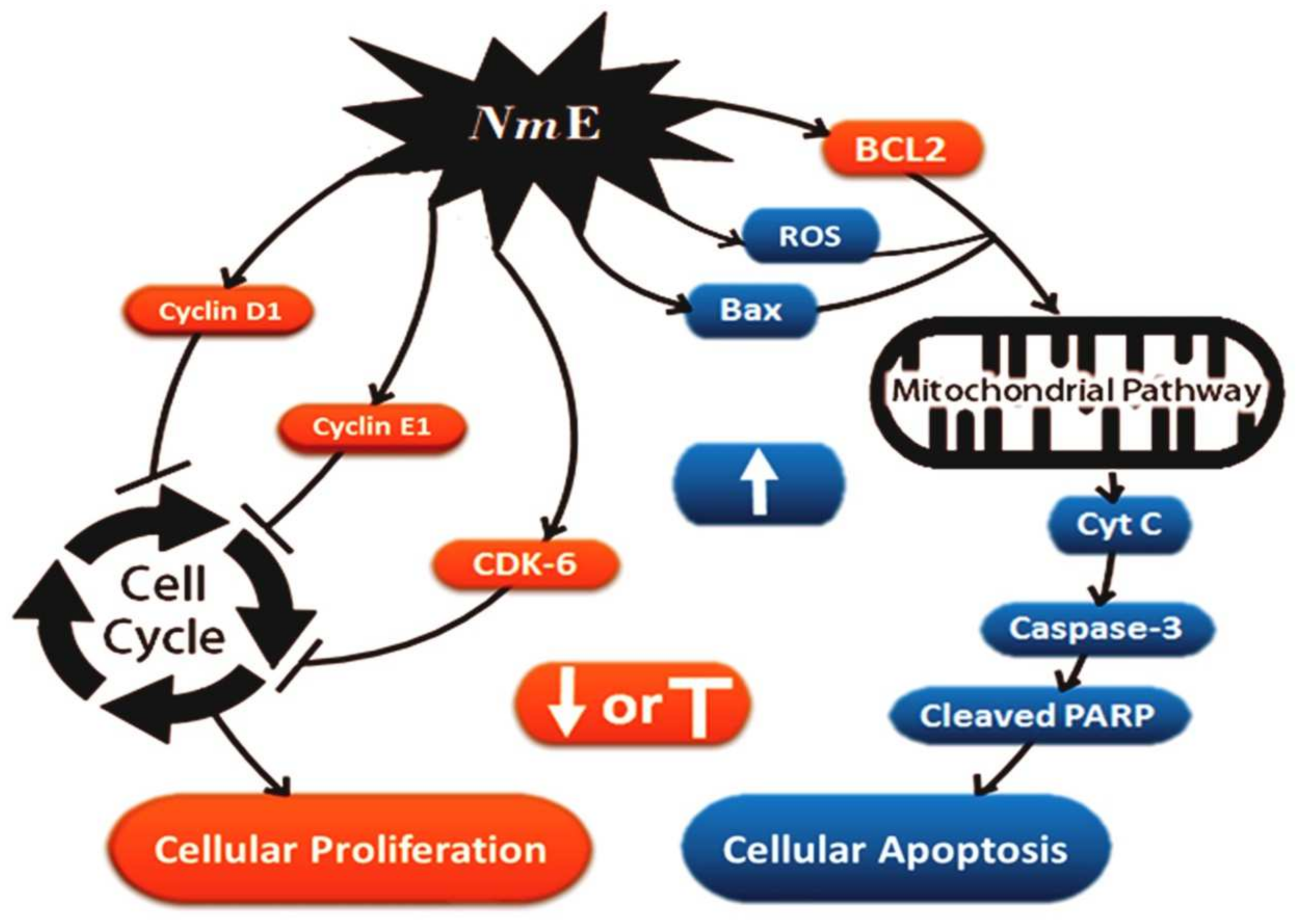
3.3.2. NmE Induced Apoptosis and Increased Bax, Cleavage PARP, and Caspase-3 Expressions in Cancer Cells
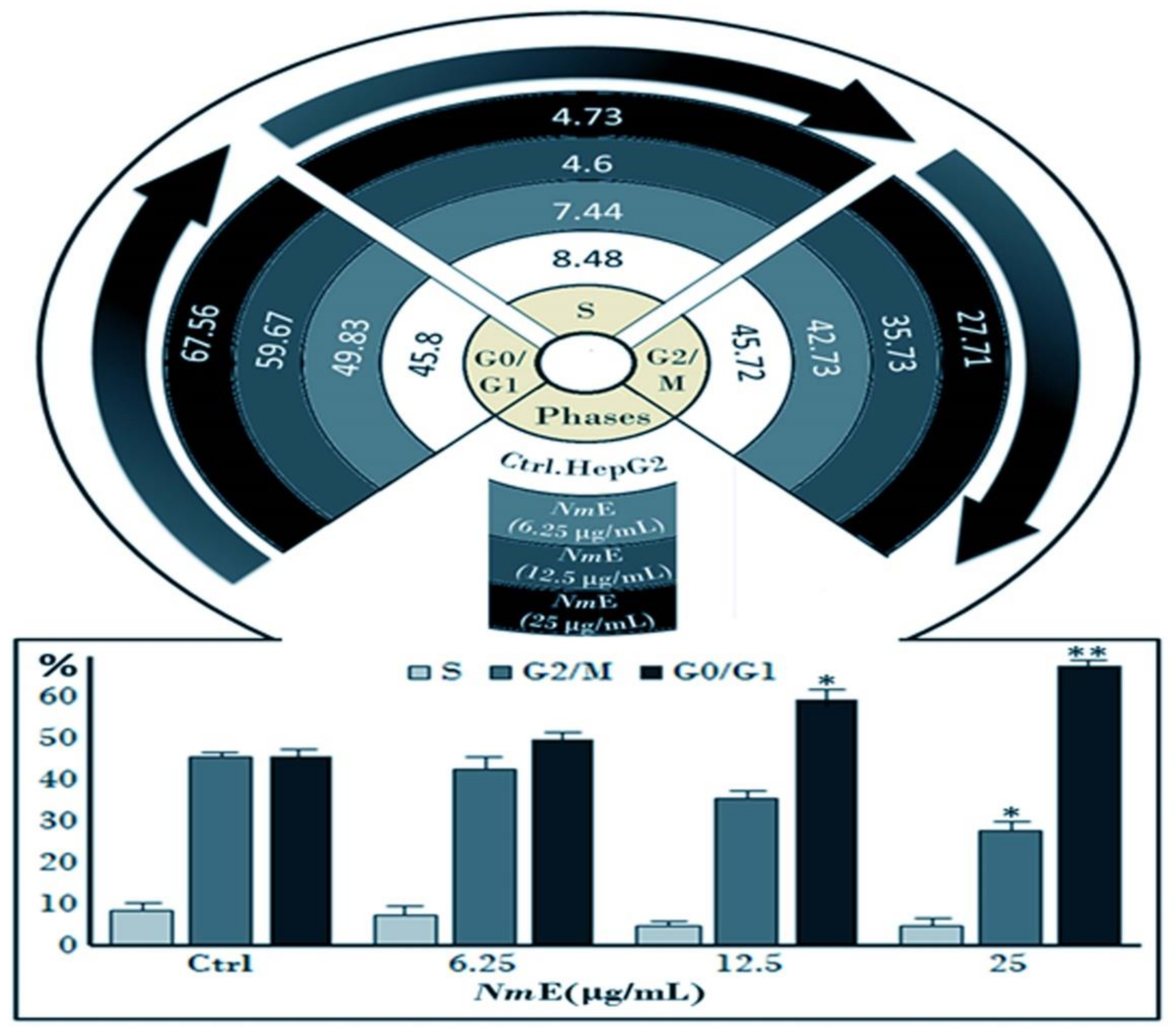
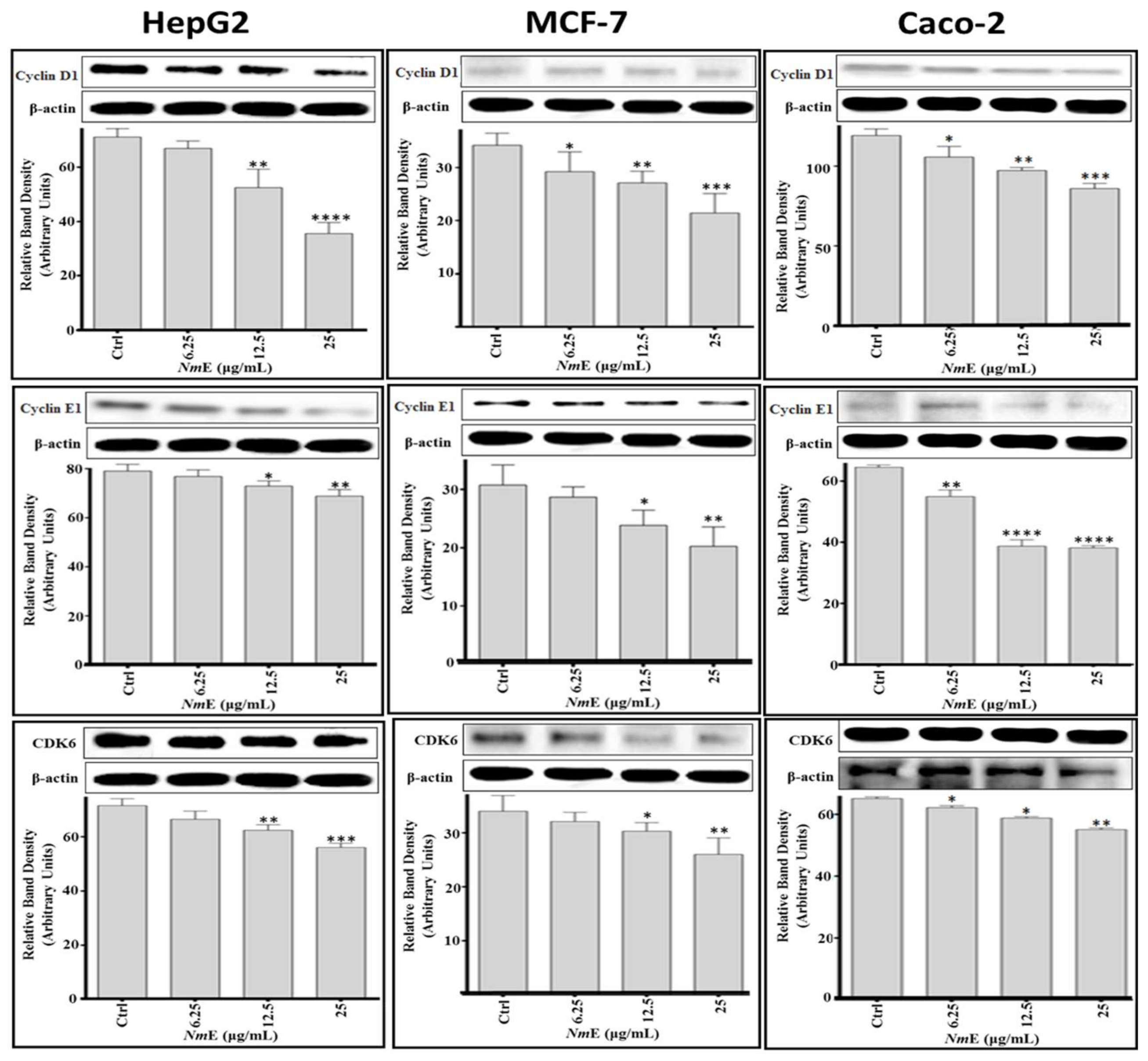
3.3.3. NmE Induced G0/G1-Phase Cell Cycle Arrest and Decreased Cyclins D1 and E1 and CDK6 Expressions in Cancer Cells
3.3.4. NmE Triggered ROS Production in HepG2 Cell Line
3.4. Antimicrobial Activity of CsE and NmE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rady, I.; Bashar, M.A.E. Novel extracts from Callyspongia siphonella and Negombata magnifica sponges from the Red Sea, induced antiproliferative and proapoptotic activity in HepG-2, MCF-7, and Caco-2 cancer cell lines. Egypt. J. Aqua. Biol. Fish. 2020, 24, 319–347. [Google Scholar] [CrossRef]
- Metwally, A.S.; El-Naggar, H.A.; El-Damhougy, K.A.; Bashar, M.A.; Ashour, M.; Abo-Taleb, H.A. GC-MS analysis of bioactive components in six different crude extracts from the Soft Coral (Sinularia maxim) collected from Ras Mohamed, Aqaba Gulf, Red Sea, Egypt. Egypt. J. Aquat. Biol. Fish. 2020, 24, 425–434. [Google Scholar] [CrossRef]
- Cui, H.; Bashar, M.A.E.; Rady, I.; Abd El-Naggar, H.; El-Maoula, L.M.A.; Mehany, A.B.M. Antiproliferative Activity, Proapoptotic Effect, and Cell Cycle Arrest in Human Cancer Cells of Some Marine Natural Product Extract. Oxidative Med. Cell. Longev. 2020, 2020, 7948705. [Google Scholar] [CrossRef] [PubMed]
- Hasaballah, A.I.; El Naggar, H.A.; Abdelbary, S.; Bashar, M.A.E.; Selim, T.A. Eco friendly Synthesis of Zinc Oxide Nanoparticles by Marine Sponge, Spongia ofcinalis: Antimicrobial and Insecticidal Activities against the Mosquito Vectors, Culex pipiens and Anopheles pharoensis. BioNanoScience 2021. [Google Scholar] [CrossRef]
- Mona, M.H.; El-Naggar, H.; El-Gayar, E.E.; Masood, M.F.; Mohamed, E.-S.N. Effect of human activities on biodiversity in Nabq Protected Area, South Sinai, Egypt. Egypt. J. Aquat. Res. 2019, 45, 33–43. [Google Scholar] [CrossRef]
- Shaban, W.; Abdel-Gaid, S.E.; El-Naggar, H.A.; Bashar, M.A.; Masood, M.F.; Salem, E.-S.S.; Alabssawy, A.N. Effects of recreational diving and snorkeling on the distribution and abundance of surgeonfishes in the Egyptian Red Sea northern islands. Egypt. J. Aquat. Res. 2020, 46, 251–257. [Google Scholar] [CrossRef]
- Darweesh, K.F.; Hellal, A.M.; Saber, S.A.; EL-Naggar, H.A.; EL-Kafrawy, S.B. Impact of tourism and fishing on the coral reef health along the west coast of the Gulf of Aqaba, Red Sea, Egypt. Egypt. J. Aquat. Biol. Fish. 2021, 25, 785–805. [Google Scholar] [CrossRef]
- Dibattista, J.D.; Roberts, M.B.; Bouwmeester, J.; Bowen, B.W.; Coker, D.J.; Lozano-Cortés, D.F.; Choat, J.H.; Gaither, M.R.; Hobbs, J.-P.; Khalil, M.; et al. A review of contemporary patterns of endemism for shallow water reef fauna in the Red Sea. J. Biogeogr. 2016, 43, 423–439. [Google Scholar] [CrossRef] [Green Version]
- Rady, I.; Siddiqui, I.A.; Rady, M.; Mukhtar, H. Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy. Cancer Lett. 2017, 402, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Rady, I.; Bloch, M.B.; Chamcheu, R.-C.N.; Mbeumi, S.B.; Anwar, R.; Mohamed, H.; Babatunde, A.S.; Kuiate, J.-R.; Noubissi, F.K.; El Sayed, K.A.; et al. Anticancer Properties of Graviola (Annona muricata): A Comprehensive Mechanistic Review. Oxidative Med. Cell. Longev. 2018, 2018, 1826170. [Google Scholar] [CrossRef] [Green Version]
- Orienti, I.; Gentilomi, G.A.; Farruggia, G. Pulmonary Delivery of Fenretinide: A Possible Adjuvant Treatment in COVID-19. Int. J. Mol. Sci. 2020, 21, 3812. [Google Scholar] [CrossRef]
- Poochi, S.P.; Easwaran, M.; Balasubramanian, B.; Anbuselvam, M.; Meyyazhagan, A.; Park, S.; Bhotla, H.K.; Anbuselvam, J.; Arumugam, V.A.; Keshavarao, S.; et al. Employing bioactive compounds derived from Ipomoea obscura (L.) to evaluate potential inhibitor for SARS-CoV-2 main protease and ACE2 protein. Food Front. 2020, 1, 168–179. [Google Scholar] [CrossRef]
- Gad, A.; Suleiman, W.B.; Beltagy, E.A.; El-Sheikh, H.H.; Ibrahim, H.A.H. Characterization and screening of marine-derived fungi along the coastline of Alexandria, Mediterranean Sea, Egypt. Egypt. J. Aquat. Biol. Fish. 2021, 25, 215–239. [Google Scholar] [CrossRef]
- Farrag, M.M.S.; El-Naggar, H.; Abou-Mahmoud, M.M.A.; Alabssawy, A.N.; Ahmed, H.O.; Abo-Taleb, H.A.; Kostas, K.; Abo-Taleb, H. Marine biodiversity patterns off Alexandria area, southeastern Mediterranean Sea, Egypt. Environ. Monit. Assess. 2019, 191, 367. [Google Scholar] [CrossRef]
- Hashem, A.H.; Suleiman, W.B.; Abu-Elrish, G.M.; El-Sheikh, H.H. Consolidated Bioprocessing of Sugarcane Bagasse to Microbial Oil by Newly Isolated Oleaginous Fungus: Mortierella wolfii. Arab. J. Sci. Eng. 2021, 46, 199–211. [Google Scholar] [CrossRef]
- El-Mehdawy, A.A.; Koriem, M.; Amin, R.M.; El-Naggar, H.A.; Shehata, A.Z.I. The photosensitizing activity of different photosensitizers irradiated with sunlight against aquatic larvae of Culex pipiens L. (Diptera: Culicidae). Egypt. J. Aquat. Biol. Fish. 2021, 25, 661–670. [Google Scholar] [CrossRef]
- Hasaballah, A.I.; Gobaara, I.M.M.; El Naggar, H.A. Larvicidal activity and ultrastructural abnormalities in the ovaries of the housefly “Musca domestica” induced by the soft coral “Ovabunda macrospiculata” synthesized ZnO nanoparticles. Egypt. J. Aquat. Biol. Fish. 2021, 25, 721–738. [Google Scholar] [CrossRef]
- Moitinho-Silva, L.; Bayer, K.; Cannistraci, C.V.; Giles, E.C.; Ryu, T.; Seridi, L.; Ravasi, T.; Hentschel, U. Specificity and transcriptional activity of microbiota associated with low and high microbial abundance sponges from the Red Sea. Mol. Ecol. 2014, 23, 1348–1363. [Google Scholar] [CrossRef]
- El-Damhougy, K.A.; El-Naggar, H.A.; Ibrahim, H.A.H.; Bashar, M.A.E.; Abou-Senna, F.M. Biological activities of some marine sponge extracts from Aqaba Gulf, Red Sea, Egypt. Int. J. Fish. Aquat. Stud. 2017, 5, 652–659. [Google Scholar]
- El-Naggar, H.A.; Hasaballah, A.I. Acute larvicidal toxicity and repellency effect of Octopus cyanea crude extracts against the filariasis vector, Culex pipiens. J. Egypt. Soc. Parasitol. 2018, 48, 721–728. [Google Scholar] [CrossRef]
- Ibrahim, H.A.H.; El-Naggar, H.A.; El-Damhougy, K.A.; Bashar, M.A.E.; Abou-Senna, F.M. Callyspongia crassa and C. siphonella (Porifera, Callyspongiidae) as a potential source for medical bioactive substances, Aqaba Gulf, Red Sea, Egypt. J. Basic Appl. Zool. 2017, 78, 7. [Google Scholar] [CrossRef]
- El-Damhougy, K.A.; Bashar, M.A.E.; El-Naggar, H.A.; Ibrahim, H.A.H.; Abou Senna, F.M. GC-MS analysis of bioactive components of Callyspongia crassa (Porifera) from Gulf of Aqaba, Red Sea (Egypt). Al-Azhar Bull. Sci. 2017, 9, 111–118. [Google Scholar]
- Hasaballah, A.I.; El-Naggar, H.A. Antimicrobial Activities of Some Marine Sponges, and Its Biological, Repellent Effects against Culex pipiens (Diptera: Culicidae). Annu. Res. Rev. Biol. 2017, 12, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Lefranc, F.; Nuzzo, G.; Hamdy, N.A.; Fakhr, I.; Banuls, L.M.Y.; Van Goietsenoven, G.; Villani, G.; Mathieu, V.; van Soest, R.; Kiss, R.; et al. In Vitro Pharmacological and Toxicological Effects of Norterpene Peroxides Isolated from the Red Sea Sponge Diacarnus erythraeanus on Normal and Cancer Cells. J. Nat. Prod. 2013, 76, 1541–1547. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Mooberry, S.L. Hurghadolide A and Swinholide I, Potent Actin-Microfilament Disrupters from the Red Sea Sponge Theonella swinhoei. J. Nat. Prod. 2006, 69, 154–157. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Shaala, L.A.; Mohamed, G.A.; Badr, J.M.; Bamanie, F.H.; Ibrahim, S.R.M. Theonellamide G, a Potent Antifungal and Cytotoxic Bicyclic Glycopeptide from the Red Sea Marine Sponge Theonella swinhoei. Mar. Drugs 2014, 12, 1911–1923. [Google Scholar] [CrossRef] [Green Version]
- Isaacs, S.; Hizi, A.; Kashman, Y. Toxicols A-C and toxiusol—New bioactive hexaprenoid hydroquinones from Toxiclona toxius. Tetrahedron 1993, 49, 4275–4282. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T.A.; Badr, J.M.; Sulaiman, M.; Khedr, A. Bioactive Secondary Metabolites from the Red Sea Marine Verongid Sponge Suberea Species. Mar. Drugs 2015, 13, 1621–1631. [Google Scholar] [CrossRef] [Green Version]
- Shaala, L.A.; Youssef, D.T.A. Cytotoxic Psammaplysin Analogues from the Verongid Red Sea Sponge Aplysinella Species. Biomolecules 2019, 9, 841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youssef, D.T.A. Hyrtioerectines A−C, Cytotoxic Alkaloids from the Red Sea Sponge Hyrtios erectus. J. Nat. Prod. 2005, 68, 1416–1419. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Yamaki, R.K.; Kelly, M.; Scheuer, P.J. Salmahyrtisol A, a Novel Cytotoxic Sesterterpene from the Red Sea Sponge Hyrtios erecta. J. Nat. Prod. 2002, 65, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Alahdal, A.M.; Asfour, H.Z.; Ahmed, S.A.; Noor, A.O.; Al-Abd, A.M.; Elfaky, M.A.; Elhady, S.S. Anti-Helicobacter, Antitubercular and Cytotoxic Activities of Scalaranes from the Red Sea Sponge Hyrtios erectus. Molecules 2018, 23, 978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yosief, T.; Rudi, A.; Kashman, Y. Asmarines A−F, Novel Cytotoxic Compounds from the Marine Sponge Raspailia Species. J. Nat. Prod. 2000, 63, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Grkovic, T.; Abdelmohsen, U.R.; Othman, E.M.; Stopper, H.; Edrada-Ebel, R.; Hentschel, U.; Quinn, R.J. Two new antioxidant actinosporin analogues from the calcium alginate beads culture of sponge-associated Actinokineospora sp. strain EG49. Bioorg. Med. Chem. Lett. 2014, 24, 5089–5092. [Google Scholar] [CrossRef]
- Jomori, T.; Shiroyama, S.; Ise, Y.; Kohtsuka, H.; Matsuda, K.; Kuranaga, T.; Wakimoto, T. Scrobiculosides A and B from the deep-sea sponge Pachastrella scrobiculosa. J. Nat. Med. 2019, 73, 814–819. [Google Scholar] [CrossRef]
- Li, F.; Peifer, C.; Janussen, D.; Tasdemir, D. New Discorhabdin Alkaloids from the Antarctic Deep-Sea Sponge Latrunculia biformis. Mar. Drugs 2019, 17, 439. [Google Scholar] [CrossRef] [Green Version]
- Rubin-Blum, M.; Antony, C.P.; Sayavedra, L.; Martínez-Pérez, C.; Birgel, D.; Peckmann, J.; Wu, Y.-C.; Cardenas, P.; Macdonald, I.; Marcon, Y.; et al. Fueled by methane: Deep-sea sponges from asphalt seeps gain their nutrition from methane-oxidizing symbionts. ISME J. 2019, 13, 1209–1225. [Google Scholar] [CrossRef] [Green Version]
- Ayyad, S.-E.N.; Angawi, R.F.; Saqer, E.; Abdel-Lateff, A.; Badria, F.A. Cytotoxic neviotane triterpene-type from the red sea sponge Siphonochalina siphonella. Pharmacogn. Mag. 2014, 10, 334–341. [Google Scholar] [CrossRef] [Green Version]
- El-Hawary, S.S.; Sayed, A.M.; Mohammed, R.; Hassan, H.M.; Rateb, M.E.; Amin, E.; Mohammed, T.A.; El-Mesery, M.; Bin Muhsinah, A.; Alsayari, A.; et al. Bioactive Brominated Oxindole Alkaloids from the Red Sea Sponge Callyspongia siphonella. Mar. Drugs 2019, 17, 465. [Google Scholar] [CrossRef] [Green Version]
- Youssef, D.T.A.; van Soest, R.W.M.; Fusetani, N. Callyspongenols A−C, New Cytotoxic C22-Polyacetylenic Alcohols from a Red Sea Sponge, Callyspongia Species. J. Nat. Prod. 2003, 66, 679–681. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; van Soest, R.W.M.; Fusetani, N. Callyspongamide A, a New Cytotoxic Polyacetylenic Amide from the Red Sea Sponge Callyspongia fistularis. J. Nat. Prod. 2003, 66, 861–862. [Google Scholar] [CrossRef]
- Abdelmohsen, U.R.; Cheng, C.; Reimer, A.; Kozjak-Pavlovic, V.; Ibrahim, A.K.; Rudel, T.; Hentschel, U.; Edrada-Ebel, R.; Ahmed, S.A. Antichlamydial Sterol from the Red Sea Sponge Callyspongia aff. implexa. Planta Med. 2015, 81, 382–387. [Google Scholar] [CrossRef] [Green Version]
- Hooper, J.N.A. Sponguide: Guide to Sponge Collection and Identification Version; Queensland Museum: South Brisbane, QLD, Australia, 2000; p. 210. [Google Scholar]
- Van Soest, R.W.M.; Boury-Esnault, N.; Hooper, J.N.A.; Rützler, K.; de Voogd, N.J.; Alvarez, B.; Hajdu, E.; Pisera, A.B.; Manconi, R.; Schönberg, C.; et al. World Porifera Database. 2021. Available online: http://www.marinespecies.org/porifera/ (accessed on 19 January 2022).
- Attia, M.S.; El-Naggar, H.A.; Abdel-Daim, M.M.; El-Sayyad, G.S. The potential impact of Octopus cyanea extracts to improve eggplant resistance against Fusarium-wilt disease: In vivo and in vitro studies. Environ. Sci. Pollut. Res. 2021, 28, 35854–35869. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Rady, I.; Chamcheu, R.-C.N.; Siddique, A.B.; Bloch, M.B.; Banang Mbeumi, S.; Babatunde, A.S.; Uddin, M.B.; Noubissi, F.K.; Jurutka, P.W.; et al. Graviola (Annona muricata) Exerts Anti-Proliferative, Anti-Clonogenic and Pro-Apoptotic Effects in Human Non-Melanoma Skin Cancer UW-BCC1 and A431 Cells In Vitro: Involvement of Hedgehog Signaling. Int. J. Mol. Sci. 2018, 19, 1791. [Google Scholar] [CrossRef] [Green Version]
- Suleiman, W.B.; Helal, E.E.-H. Chemical constituents and potential pleiotropic activities of Foeniculum vulgare (Fennel) ethanolic extract; in vitro approach. Egypt. J. Chem. 2022, 65, 5. [Google Scholar] [CrossRef]
- Luparello, C.; Ragona, D.; Asaro, D.M.L.; Lazzara, V.; Affranchi, F.; Celi, M.; Arizza, V.; Vazzana, M. Cytotoxic Potential of the Coelomic Fluid Extracted from the Sea Cucumber Holothuria tubulosa against Triple-Negative MDA-MB231 Breast Cancer Cells. Biology 2019, 8, 76. [Google Scholar] [CrossRef] [Green Version]
- Sanna, V.; Singh, C.K.; Jashari, R.; Adhami, V.M.; Chamcheu, J.C.; Rady, I.; Sechi, M.; Mukhtar, H.; Siddiqui, I.A. Targeted nanoparticles encapsulating (−)-epigallocatechin-3-gallate for prostate cancer prevention and therapy. Sci. Rep. 2017, 7, 41573. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Siddiqui, I.A.; Nihal, M.; Pilla, S.; Rosenthal, K.; Mukhtar, H.; Gong, S. Aptamer-conjugated and doxorubicin-loaded unimolecular micelles for targeted therapy of prostate cancer. Biomaterials 2013, 34, 5244–5253. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Sederstrom, J.M. Assaying Cell Cycle Status Using Flow Cytometry. Curr. Protoc. Mol. Biol. 2015, 111, 28.6.1–28.6.11. [Google Scholar] [CrossRef]
- Crowley, L.; Marfell, B.J.; Scott, A.P.; Waterhouse, N.J. Quantitation of Apoptosis and Necrosis by Annexin V Binding, Propidium Iodide Uptake, and Flow Cytometry. Cold Spring Harb. Protoc. 2016, 2016. [Google Scholar] [CrossRef]
- Degl’Innocenti, D.; Ramazzotti, M.; Sarchielli, E.; Monti, D.; Chevanne, M.; Vannelli, G.B.; Barletta, E. Oxadiazon affects the expression and activity of aldehyde dehydrogenase and acylphosphatase in human striatal precursor cells: A possible role in neurotoxicity. Toxicology 2019, 411, 110–121. [Google Scholar] [CrossRef]
- Kamel, A.; Suleiman, W.B.; Elfeky, A.; El-Sherbiny, G.M.; Elhaw, M. Characterization of bee venom and its synergistic effect combating antibiotic resistance of Pseudomonas aeruginosa. Egypt. J. Chem. 2021, 65. [Google Scholar] [CrossRef]
- Suleiman, W.B. In vitro estimation of superfluid critical extracts of some plants for their antimicrobial potential, phytochemistry, and GC–MS analyses. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 29. [Google Scholar] [CrossRef]
- Yang, R.; Johnson, M.C.; Ray, B. Novel method to extract large amounts of bacteriocins from lactic acid bacteria. Appl. Environ. Microbiol. 1992, 58, 3355–3359. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Gao, T.; Wang, J.; Tian, S.; Yuan, X.; Zhu, H. Structural identification and antitumor activity of the extracellular polysaccharide from Aspergillus terreus. Process. Biochem. 2016, 51, 1714–1720. [Google Scholar] [CrossRef]
- Domenici, L.; Monti, M.; Bracchi, C.; Giorgini, M.; Colagiovanni, V.; Muzii, L.; Panici, P.B. D-mannose: A promising support for acute urinary tract infections in women. A pilot study. Eur. Rev. Med Pharmacol. Sci. 2016, 20, 2920–2925. [Google Scholar]
- Wang, B.; Peng, X.-X.; Sun, R.; Li, J.; Zhan, X.-R.; Wu, L.-J.; Wang, S.-L.; Xie, T. Systematic review of β-Elemene injection as adjunctive treatment for lung cancer. Chin. J. Integr. Med. 2012, 18, 813–823. [Google Scholar] [CrossRef]
- Santana de Oliveira, M.; da Cruz, J.N.; Almeida da Costa, W.; Silva, S.G.; Brito, M.d.P.; de Menezes, S.A.F.; de Jesus Chaves Neto, A.M.; de Aguiar Andrade, E.H.; de Carvalho Junior, R.N. Chemical Composition, Antimicrobial Properties of Siparuna guianensis Essential Oil and a Molecular Docking and Dynamics Molecular Study of its Major Chemical Constituent. Molecules 2020, 25, 3852. [Google Scholar] [CrossRef]
- Do Nascimento, K.F.; Moreira, F.M.F.; Santos, J.A.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; Vieira, M.D.C.; Ruiz, A.L.T.G.; Foglio, M.A.; de Carvalho, J.E.; et al. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef]
- Manoj, G.; Manohar, S.H.; Murthy, H.N. Chemical constituents, antioxidant and antimocrobial activity of essential oil of Pogostemon paniculatus (Willd.). Nat. Prod. Res. 2012, 26, 2152–2154. [Google Scholar] [CrossRef]
- Elshamy, A.I.; El Kashak, W.; Abdallah, H.; Farrag, A.H.; Nassar, M.I. Soft coral Cespitularia stolonifera: New cytotoxic ceramides and gastroprotective activity. Chin. J. Nat. Med. 2017, 15, 105–114. [Google Scholar] [CrossRef]
- Yasa, S.R.; Kaki, S.S.; Poornachandra, Y.; Kumar, C.G.; Penumarthy, V. Synthesis, characterization, antimicrobial and biofilm inhibitory studies of new esterquats. Bioorg. Med. Chem. Lett. 2016, 26, 1978–1982. [Google Scholar] [CrossRef] [PubMed]
- Krishnaveni, M. Docking, simulation studies of desulphosinigrin—Cyclin dependent kinase 2, an anticancer drug target. Int. J. Pharm. Sci. Rev. Res. 2015, 30, 115–118. [Google Scholar]
- Kumar, P.; Sati, S.C.; Khulbe, K.; Pant, P.; Tripathi, A.N.; Sarvendra, K. Phytochemical constituents, antimicrobial and antioxidant activities of Kumaun Himalayan Hoop Pine bark extract. Nat. Prod. Res. 2020, 29, 1–5. [Google Scholar] [CrossRef]
- Li, K.; Ma, T.; Cai, J.; Huang, M.; Guo, H.; Zhou, D.; Luan, S.; Yang, J.; Liu, D.; Jing, Y.; et al. Conjugates of 18β-glycyrrhetinic acid derivatives with 3-(1H-benzo[d]imidazol-2-yl)propanoic acid as Pin1 inhibitors displaying anti-prostate cancer ability. Bioorg. Med. Chem. 2017, 25, 5441–5451. [Google Scholar] [CrossRef]
- Salomón, D.G.; Grioli, S.M.; Buschiazzo, M.; Mascaró, E.; Vitale, C.; Radivoy, G.; Pérez, M.; Fall, Y.; Mesri, E.A.; Curino, A.C.; et al. Novel Alkynylphosphonate Analogue of Calcitriol with Potent Antiproliferative Effects in Cancer Cells and Lack of Calcemic Activity. ACS Med. Chem. Lett. 2011, 2, 503–508. [Google Scholar] [CrossRef] [Green Version]
- Santini, V.; Scappini, B.; Gozzini, A.; Grossi, A.; Villa, P.; Ronco, G.; Douillet, O.; Pouillart, P.; Bernabei, P.A.; Ferrini, P.R. Butyrate-stable monosaccharide derivatives induce maturation and apoptosis in human acute myeloid leukaemia cells. Br. J. Haematol. 1998, 101, 529–538. [Google Scholar] [CrossRef]
- Karrys, A.; Rady, I.; Chamcheu, R.-C.N.; Sabir, M.S.; Mallick, S.; Chamcheu, J.C.; Jurutka, P.W.; Haussler, M.R.; Whitfield, G.K. Bioactive Dietary VDR Ligands Regulate Genes Encoding Biomarkers of Skin Repair That Are Associated with Risk for Psoriasis. Nutrients 2018, 10, 174. [Google Scholar] [CrossRef] [Green Version]
- Su, C.-T.; Hsu, J.T.-A.; Hsieh, H.-P.; Lin, P.-H.; Chen, T.-C.; Kao, C.-L.; Lee, C.-N.; Chang, S.-Y. Anti-HSV activity of digitoxin and its possible mechanisms. Antivir. Res. 2008, 79, 62–70. [Google Scholar] [CrossRef]
- Trenti, A.; Boscaro, C.; Tedesco, S.; Cignarella, A.; Trevisi, L.; Bolego, C. Effects of digitoxin on cell migration in ovarian cancer inflammatory microenvironment. Biochem. Pharmacol. 2018, 154, 414–423. [Google Scholar] [CrossRef]
- López-Lara, I.; Nogales, J.; Pech-Canul, A.D.L.C.; Calatrava-Morales, N.; Bernabéu-Roda, L.M.; Durán, P.; Cuéllar, V.; Olivares, J.; Alvarez, L.; Palenzuela-Bretones, D.; et al. 2-Tridecanone impacts surface-associated bacterial behaviours and hinders plant–bacteria interactions. Environ. Microbiol. 2018, 20, 2049–2065. [Google Scholar] [CrossRef]
- Swantara, M.D.; Rita, W.S.; Suartha, N.; Agustina, K.K. Anticancer activities of toxic isolate of Xestospongia testudinaria sponge. Vet. World 2019, 12, 1434–1440. [Google Scholar] [CrossRef]
- Abou-El-Wafa, G.S.E.; Shaaban, M.; Shaaban, K.A.; El-Naggar, M.E.E.; Maier, A.; Fiebig, H.H.; Laatsch, H. Pachydictyols B and C: New Diterpenes from Dictyota dichotoma Hudson. Mar. Drugs 2013, 11, 3109–3123. [Google Scholar] [CrossRef]
- Figueiredo, C.R.; Matsuo, A.L.; Massaoka, M.H.; Girola, N.; Azevedo, R.A.; Rabaça, A.N.; Farias, C.F.; Pereira, F.V.; Matias, N.S.; Silva, L.P.; et al. Antitumor Activity of Kielmeyera coriacea Leaf Constituents in Experimental Melanoma, Tested in Vitro and in Vivo in Syngeneic Mice. Adv. Pharm. Bull. 2014, 4 (Suppl. 1), 429–436. [Google Scholar] [CrossRef]
- Gelmann, E.P.; Cronan, J.E., Jr. Mutant of Escherichia coli deficient in the synthesis of cis-vaccenic acid. J. Bacteriol. 1972, 112, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Kasanah, N.; Amelia, W.; Mukminin, A.; Triyanto; Isnansetyo, A. Antibacterial activity of Indonesian red algae Gracilaria edulis against bacterial fish pathogens and characterization of active fractions. Nat. Prod. Res. 2019, 33, 3303–3307. [Google Scholar] [CrossRef]
- Khosravani, M.; Dallal, M.M.S.; Norouzi, M. Phytochemical Composition and Anti-efflux Pump Activity of Hydroalcoholic, Aqueous, and Hexane Extracts of Artemisia tournefortiana in Ciprofloxacin-Resistant Strains of Salmonella enterica Serotype Enteritidis. Iran. J. Public Health 2020, 49, 134–144. [Google Scholar] [CrossRef]
- Joel, J.T.; Suluvoy, J.K.; Raja, R.H. Morinda citrifolia L. (Noni) as a free radical scavenger and an anticancer agent. Drug Invent. Today 2018, 10, 3134–3140. [Google Scholar]
- Zhang, Y.; Miller, R.M. Enhanced octadecane dispersion and biodegradation by a Pseudomonas rhamnolipid surfactant (biosurfactant). Appl. Environ. Microbiol. 1992, 58, 3276–3282. [Google Scholar] [CrossRef] [Green Version]
- Mody, N.; Mcilroy, G.D. The mechanisms of Fenretinide-mediated anti-cancer activity and prevention of obesity and type-2 diabetes. Biochem. Pharmacol. 2014, 91, 277–286. [Google Scholar] [CrossRef] [Green Version]
- Goossens, J.-F.; Bailly, C. Ursodeoxycholic acid and cancer: From chemoprevention to chemotherapy. Pharmacol. Ther. 2019, 203, 107396. [Google Scholar] [CrossRef]
- Choi, J.-M.; Lee, E.-O.; Lee, H.-J.; Kim, K.-H.; Ahn, K.-S.; Shim, B.-S.; Kim, N.-I.; Song, M.-C.; Baek, N.-I.; Kim, S.-H. Identification of campesterol from Chrysanthemum coronarium L. and its antiangiogenic activities. Phytother. Res. 2007, 21, 954–959. [Google Scholar] [CrossRef]
- Mekhtiev, A.R.; Timofeev, V.P.; Misharin, A.I. (22R,23R)-3beta-Hydroxy-22,23-epoxy-5alpha-ergost-8(14)-en-15-one: Improved synthesis and toxicity to MCF-7 cells. Bioorg. Khim. 2008, 34, 840–846. [Google Scholar]
- Shawli, G.T.; Adeyemi, O.O.; Stonehouse, N.J.; Herod, M.R. The Oxysterol 25-Hydroxycholesterol Inhibits Replication of Murine Norovirus. Viruses 2019, 11, 97. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; He, H.; Fang, W. Oncogenic roles of the cholesterol metabolite 25-hydroxycholesterol in bladder cancer. Oncol. Lett. 2020, 19, 3671–3676. [Google Scholar] [CrossRef] [Green Version]
- Pandey, P.; Bajpai, P.; Siddiqui, M.H.; Sayyed, U.; Tiwari, R.; Shekh, R.; Mishra, K.; Kapoor, V. Elucidation of the Chemopreventive Role of Stigmasterol against Jab1 in Gall Bladder Carcinoma. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 826–837. [Google Scholar] [CrossRef]
- Yenn, T.W.; Khan, M.A.; Syuhada, N.A.; Ring, L.C.; Ibrahim, D.; Tan, W.-N. Stigmasterol: An adjuvant for beta lactam antibiotics against beta-lactamase positive clinical isolates. Steroids 2017, 128, 68–71. [Google Scholar] [CrossRef]
- Bin Sayeed, M.S.; Ameen, S.S. Beta-Sitosterol: A Promising but Orphan Nutraceutical to Fight against Cancer. Nutr. Cancer 2015, 67, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Sundarraj, S.; Thangam, R.; Sreevani, V.; Kaveri, K.; Gunasekaran, P.; Achiraman, S.; Kannan, S. γ-Sitosterol from Acacia nilotica L. induces G2/M cell cycle arrest and apoptosis through c-Myc suppression in MCF-7 and A549 cells. J. Ethnopharmacol. 2012, 141, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.S.; Ahirwar, B. A steroidal derivative from Trigonella foenum graecum L. that induces apoptosis in vitro and in vivo. J. Food Drug Anal. 2019, 27, 231–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallup, J.M.; Grubor, B.; Barua, A.B.; Mohammadi, G.; Brogden, K.A.; Olson, J.A.; Ackermann, M.R. Repeated intravenous doses of all-trans-retinoyl beta-D-glucuronide is not effective in the treatment of bacterial bronchopneumonia in lambs but is devoid of gross and acute toxicity. Med. Sci. Monit. 2002, 8, 345–353. [Google Scholar]
- Jainab, N.H.; Raja, M. In vitro cytotoxic, antioxidant and GC-MS study of leaf extracts of Clerodendrum phlomidis. Int. J. Pharm. Sci. Res. 2017, 8, 4433–4440. [Google Scholar] [CrossRef]
- Suleiman, W.B.; El Bous, M.M.; El Said, M.; El Baz, H. In vitro evaluation of Syzygium aromaticum L. ethanol extract as biocontrol agent against postharvest tomato and potato diseases. Egypt. J. Bot. 2019, 59, 81–94. [Google Scholar] [CrossRef]
- Abubacker, M.N.; Devi, P.K. In vitro antifungal potentials of bioactive compound oleic acid, 3-(octadecyloxy) propyl ester isolated from Lepidagathis cristata Willd. (Acanthaceae) inflorescence. Asian Pac. J. Trop. Med. 2014, 7 (Suppl. 1), S190–S193. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.Y.; Choi, W.Y.; Heo, S.J.; Kang, D.H.; Lee, H.Y. Anti-Skin Cancer Activities of Apostichopus japonicus Extracts from Low-Temperature Ultrasonification Process. J. Health Eng. 2017, 2017, 6504890. [Google Scholar] [CrossRef] [Green Version]
- Du, L.; Li, Z.-J.; Xu, J.; Wang, J.-F.; Xue, Y.; Xue, C.-H.; Takahashi, K.; Wang, Y.-M. The anti-tumor activities of cerebrosides derived from sea cucumber Acaudina molpadioides and starfish Asterias amurensis in vitro and in vivo. J. Oleo Sci. 2012, 61, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Zakaria, H.Y.; El-Naggar, H. Long-term variations of zooplankton community in Lake Edku, Egypt. Egypt. J. Aquat. Biol. Fish. 2019, 23, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Chari, A.; Mazumder, A.; Lau, K.; Catamero, D.; Galitzeck, Z.; Jagannath, S. A phase II trial of TBL-12 sea cucumber extract in patients with untreated asymptomatic myeloma. Br. J. Haematol. 2018, 180, 296–298. [Google Scholar] [CrossRef] [Green Version]
- Dhinakaran, D.I.; Lipton, A.P. Antimicrobial potential of the marine sponge Sigmadocia pumila from the south eastern region of India. World J. Fish Mar. Sci. 2012, 4, 344–348. [Google Scholar] [CrossRef]
- Gab-Alla, A.A.F.A.; Kilada, R.W.; Shalaby, I.M.; Helmy, T. Antimicrobial activity of some sponges from the Gulf of Aqaba. Egypt. J. Biol. 2000, 2, 28–33. Available online: https://www.ajol.info/index.php/ejb/article/view/29901 (accessed on 22 January 2022).
- Shawky, M.; Suleiman, W.B.; Farrag, A.A. Antibacterial Resistance Pattern in Clinical and Non-clinical Bacteria by Phenotypic and Genotypic Assessment. J. Pure Appl. Microbiol. 2021, 15, 2270–2279. [Google Scholar] [CrossRef]
- Soliman, M.O.; Suleiman, W.B.; Roushdy, M.M.; Elbatrawy, E.N.; Gad, A.M. Characterization of some bacterial strains isolated from the Egyptian Eastern and Northern coastlines with antimicrobial activity of Bacillus zhangzhouensis OMER4. Acta Oceanol. Sin. 2021, 41, 1–8. [Google Scholar] [CrossRef]
- Gad, A.M.; Suleiman, W.B.; Beltagy, E.A.; El-Sheikh, H.; Ibrahim, H.A. Antimicrobial and antifouling activities of the cellulase produced by marine fungal strain; Geotrichum candidum MN638741.1. Egypt. J. Aquat. Biol. Fish. 2021, 25, 49–60. [Google Scholar] [CrossRef]
- Saleem, M.D.; Feldman, S.R. Desoximetasone 0.25% spray, adrenal suppression and efficacy in extensive plaque psoriasis. J. Dermatol. Treat. 2018, 29, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.D.; Negus, D.; Feldman, S.R. Topical 0.25% desoximetasone spray efficacy for moderate to severe plaque psoriasis: A randomized clinical trial. J. Dermatol. Treat. 2018, 29, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Al-Rabia, M.W.; Mohamed, G.A.; Ibrahim, S.R.M.; Asfour, H.Z. Anti-inflammatory ergosterol derivatives from the endophytic fungus Fusarium chlamydosporum. Nat. Prod. Res. 2020, 35, 5011–5020. [Google Scholar] [CrossRef]
- El-Naggar, H.; Allah, H.M.K.; Masood, M.F.; Shaban, W.M.; Bashar, M.A. Food and feeding habits of some Nile River fish and their relationship to the availability of natural food resources. Egypt. J. Aquat. Res. 2019, 45, 273–280. [Google Scholar] [CrossRef]
- Zakaria, H.Y.; Hassan, A.-K.M.; Abo-Senna, F.M.; El-Naggar, H. Abundance, distribution, diversity and zoogeography of epipelagic copepods off the Egyptian Coast (Mediterranean Sea). Egypt. J. Aquat. Res. 2016, 42, 459–473. [Google Scholar] [CrossRef]
- Zakaria, H.Y.; Hassan, A.-K.M.; El-Naggar, H.A.; Abo-Senna, F.M. Biomass determination based on the individual volume of the dominant copepod species in the Western Egyptian Mediterranean Coast. Egypt. J. Aquat. Res. 2018, 44, 89–99. [Google Scholar] [CrossRef]
- Abdel-Razek, A.; El-Sheikh, H.; Suleiman, W.; Taha, T.H.; Mohamed, M. Bioelimination of phenanthrene using degrading bacteria isolated from petroleum soil: Safe approach. Desalin. Water Treat. 2020, 181, 131–140. [Google Scholar] [CrossRef]
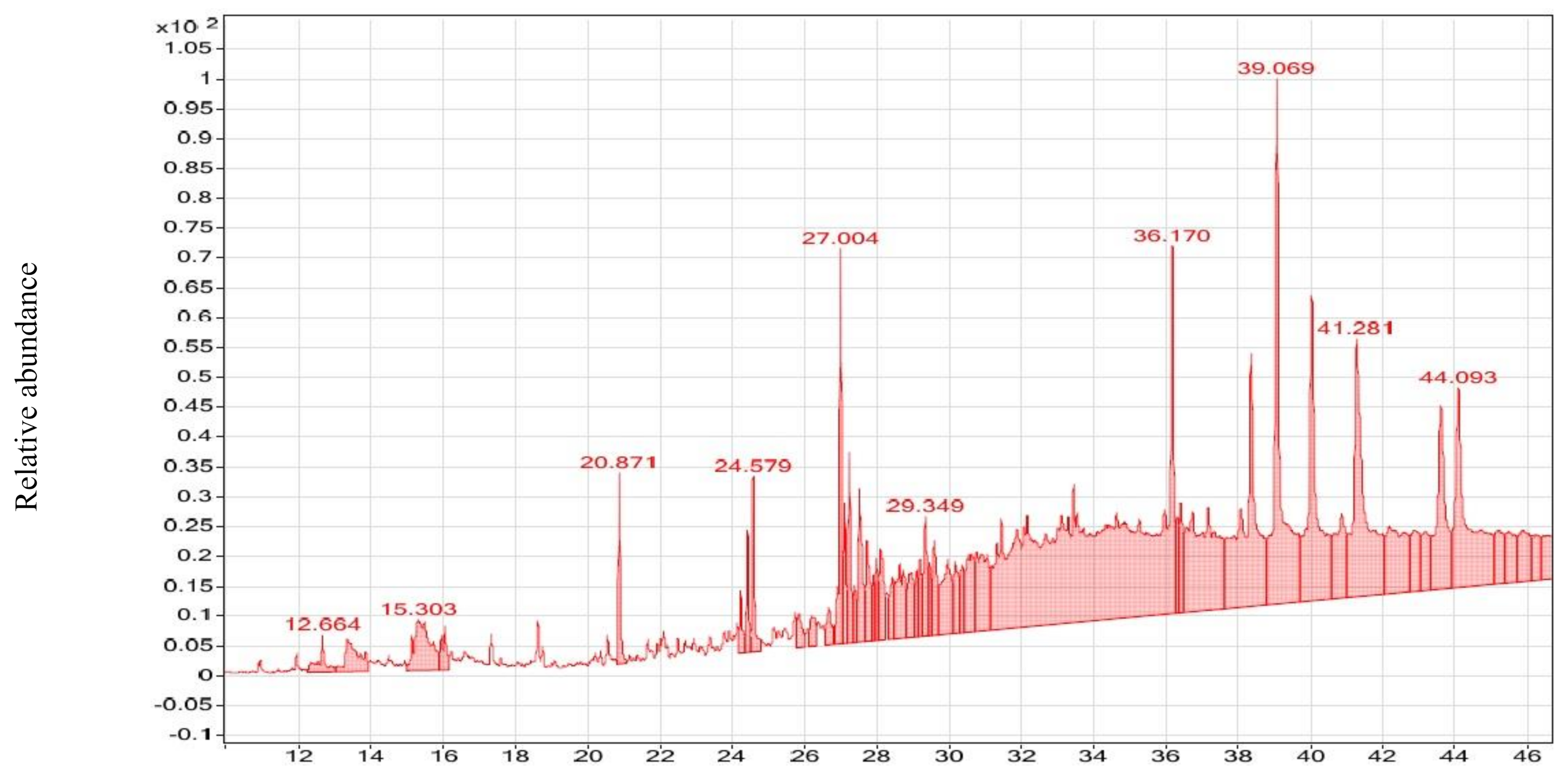
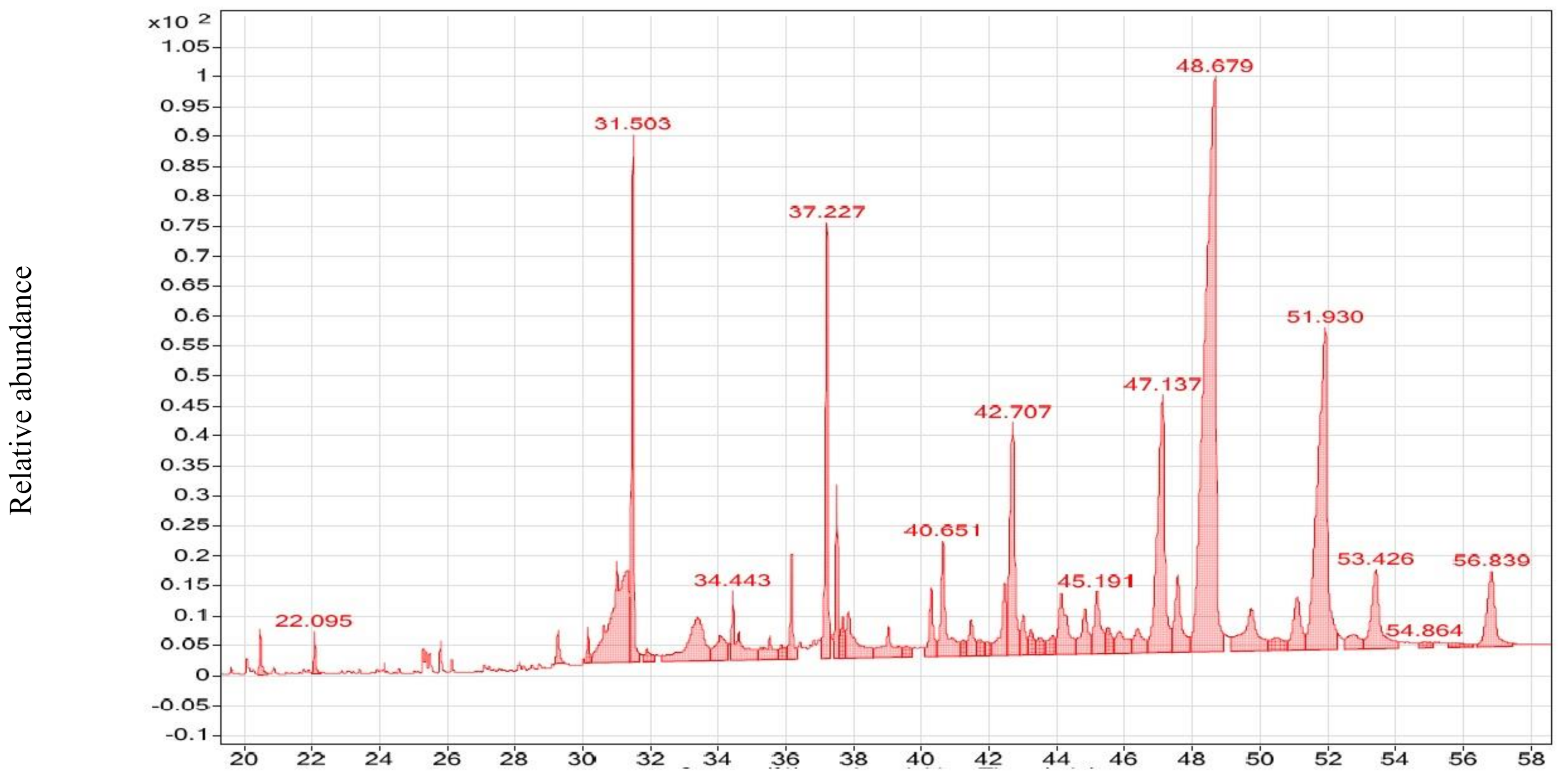




| Compound Name | Molecular Formula | RT (min) | MF | RMF | Prob (%) | CAS # | Lib | ID | MW (g/M) | Ext | Biological Activity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| d-Mannose | C6H12O6 | 15.298 | 705 | 777 | 22.3% | 3458-28-4 | ML | 37454 | 180 | NmE | Anticancer [57] antimicrobial [58] |
| Cyclohexane,1-ethenyl-1-methyl-2-(1- methyleth-enyl)-4-(1-methylethylidene)- | C15H24 | 12.670 | 736 | 891 | 7.65% | 3242-08-8 | RL | 17769 | 204 | NmE | Anticancer [59] |
| γ-Elemene | C15H24 | 12.670 | 748 | 944 | 11.5% | 29873-99-2 | ML, RL | 91836 | 204 | NmE | Antimicrobial [60] |
| β-Guaiene | C15H24 | 15.956 | 701 | 763 | 6.59% | 88-84-6 | ML | 133155 | 204 | NmE | Antimicrobial [61] |
| (-)-Spathulenol | C15H24O | 18.751 | 776 | 813 | 10.1% | 77171-55-2 | ML | 5991 | 220 | NmE | Antiproliferative [62] |
| Isoaromadendrene epoxide | C15H24O | 18.751 | 780 | 841 | 11.9% | N/A | ML | 2203 | 220 | NmE | Anticancer [63] |
| Undecanoic acid, 11-bromo-, methyl ester | C12H23BrO2 | 17.336 | 666 | 715 | 4.16% | 6287-90-7 | RL | 9817 | 278 | NmE | Antimicrobial [64] |
| Desulphosinigrin | C10H17NO6S | 15.725 | 704, | 776 | 27.4% | 5115-81-1 | ML | 28432 | 279 | NmE | Anticancer [65] |
| Androstan-17-one, 3-ethyl-3-hydroxy-, (5α)- | C21H34O2 | 18.751 | 722 | 746 | 5.12% | 57344-99-7 | ML | 55206 | 318 | NmE | Antimicrobial [66] |
| Propanoic acid, 2-methyl-,(dodecahydro -6a-hydroxy-9a-methyl-3-methylene-2,9-dioxoazuleno [4,5-b]furan-6-yl)m | C19H26O6 | 16.609 | 699 | 733 | 4.32% | 33649-17-1 | ML | 7019 | 350 | NmE | Anticancer [67] |
| 9,10-Secochola-5,7,10(19)-trien-24-al, 3-hydroxy-, (3.beta.,5Z,7E)- | C24H36O2 | 16.609 | 723 | 804 | 10.5% | 40013-88-5 | ML | 88071 | 356 | NmE | Anticancer [68] |
| Mannofuranoside | C21H36O6 | 15.956 | 693 | 745 | 4.70% | N/A | ML | 31505 | 384 | NmE | Anticancer [69] |
| Docosahexaenoic acid | C25H40O2Si | 15.500 | 645 | 658 | 8.94% | N/A | ML | 37920 | 400 | NmE | Anticancer [70] |
| Digitoxin | C41H64O13 | 13.351 | 603 | 641 | 9.74% | 71-63-6 | RL | 8938 | 764 | NmE | Antiviral [71], anticancer [72] |
| 2-Tridecanone | C13H26O | 20.455 | 746 | 827 | 5.68% | 593-08-8 | RL | 6452 | 198 | CsE | Antimicrobial [73] |
| 2-Pentadecanone | C15H30O | 20.455 | 752 | 834 | 7.22% | 2345-28-0 | ML | 25959 | 226 | CsE | Anticancer [74] |
| tert-Hexadecanethiol | C16H34S | 29.303 | 711 | 761 | 10.7% | 25360-09-2 | ML | 23376 | 258 | CsE | Anticancer [75] |
| 9,12-Octadecadienoic acid (Z,Z)- | C18H32O2 | 37.203 | 709 | 751 | 3.03% | 60-33-3 | RL | 7667 | 280 | CsE | Anticancer [74] |
| 2-Nonadecanone | C19H38O | 20.455 | 771 | 906 | 14.2% | 629-66-3 | ML | 25712 | 282 | CsE | Anticancer [76] |
| cis-Vaccenic acid | C18H34O2 | 25.353 | 763 | 791 | 5.10% | 506-17-2 | ML | 18782 | 282 | CsE | Antimicrobial [77] |
| trans-13-Octadecenoic acid | C18H34O2 | 25.353 | 760 | 792 | 4.53% | 693-71-0 | ML | 18062 | 282 | CsE | Antimicrobial [78] |
| Hexadecanoic acid, ethyl ester | C18H36O2 | 22.095 | 789 | 812 | 17.2% | 628-97-7 | ML, RL | 52733 | 284 | CsE | Antibacterial [79] |
| Octadecanoic acid, ethyl ester | C20H40O2 | 22.095 | 778 | 834 | 8.68% | 111-61-5 | RL | 12005 | 312 | CsE | Anticancer [80] |
| Octadecane, 3-ethyl-5-(2-ethylbutyl)- | C26H54 | 44.128 | 681 | 686 | 3.65% | 55282-12-7 | ML | 7471 | 366 | CsE | Antibacterial [81] |
| Fenretinide | C26H33NO2 | 53.432 | 655 | 713 | 6.97% | 65646-68-6 | ML | 77816 | 391 | CsE | Antiviral (anti-COVID-19) [11] and anticancer [82] |
| Ursodeoxycholic acid | C24H40O4 | 44.128 | 668 | 680 | 3.63% | 128-13-2 | ML | 19201 | 392 | CsE | Anticancer [83] |
| Campesterol | C28H48O | 47.068 | 726 | 776 | 14.8% | 474-62-4 | ML | 6713 | 400 | CsE | Anticancer [84] |
| Ergost | C28H48O | 47.068 | 680 | 821 | 1.92% | 4651-51-8 | ML | 6865 | 400 | CsE | Anticancer [85] |
| 25-Hydroxycholesterol | C27H46O2 | 44.128 | 670 | 703 | 5.61% | 2140-46-7 | ML | 27474 | 402 | CsE | Anti-viral [86] anticancer [87] |
| Stigmasterol | C29H48O | 44.128 | 683 | 730 | 8.43% | 83-48-7 | RL, ML | 19475 | 412 | CsE | Anticancer [88], antibiotic [89] |
| β-Sitosterol | C29H50O | 47.068 | 743 | 777 | 27.6% | 83-46-5 | RL | 1982 | 414 | CsE | Anticancer [90] |
| γ-Sitosterol | C29H50O | 47.068 | 738 | 752 | 22.2% | 83-47-6 | ML | 6839 | 414 | CsE | Anticancer [91] |
| Ethyl iso-allocholate | C26H44O5 | 40.316 | 658 | 666 | 4.38% | N/A | ML | 6654 | 436 | CsE | Anticancer [92] and antiviral (anti-COVID-19) [11] |
| Retinoyl-β-glucuronide | C26H34O7 | 51.087 | 614 | 654 | 6.30% | 101470-87-5 | ML | 132135 | 458 | CsE | Antibacterial [93] |
| Oleic acid, eicosyl ester | C38H74O2 | 34.443 | 656 | 665 | 2.87% | 22393-88-0 | ML | 25265 | 562 | CsE | Cancer preventive [94,95] |
| Oleic acid, 3-(octadecyl-oxy)propyl ester | C39H76O3 | 34.443 | 657 | 689 | 2.99% | 17367-41-8 | ML | 22460 | 592 | CsE | Antifungal [96] |
| Investigated Cancer Cells | Investigated Extract | |
|---|---|---|
| NmE | CsE | |
| HepG2 | 10.33 | 271.48 |
| MCF-7 | 12.48 | 467.19 |
| Caco-2 | 10.52 | 1570.82 |
| Tested Microorganisms | AU by the Crude Extracts | Positive Standard | ||
|---|---|---|---|---|
| CsE | NmE | |||
| Fungi * Ketoconazole 100 µg | Aspergillus flavus | 2.42 | -ve | 3.16 |
| Penicillium expansum | -ve | -ve | 2.42 | |
| Candida lipolytica | 2.78 | -ve | 4.0 | |
| Cryptococcus neoformans | 2.09 | -ve | 2.42 | |
| Gram-negative bacteria * gentamycin 4 µg | Escherichia coli | -ve | 2.42 | 4.46 |
| Salmonella typhimurium | 4.46 | -ve | 3.57 | |
| Klebsiella pneumonia | 2.09 | -ve | - | |
| Gram-positive bacteria * gentamycin 4 µg | Micrococcus sp. | -ve | -ve | 5.98 |
| Streptococcus mutants | 3.16 | -ve | 4.94 | |
| MRSA | -ve | 3.57 | 11.11 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Naggar, H.A.; Bashar, M.A.E.; Rady, I.; El-Wetidy, M.S.; Suleiman, W.B.; Al-Otibi, F.O.; Al-Rashed, S.A.; Abd El-Maoula, L.M.; Salem, E.-S.S.; Attia, E.M.H.; et al. Two Red Sea Sponge Extracts (Negombata magnifica and Callyspongia siphonella) Induced Anticancer and Antimicrobial Activity. Appl. Sci. 2022, 12, 1400. https://doi.org/10.3390/app12031400
El-Naggar HA, Bashar MAE, Rady I, El-Wetidy MS, Suleiman WB, Al-Otibi FO, Al-Rashed SA, Abd El-Maoula LM, Salem E-SS, Attia EMH, et al. Two Red Sea Sponge Extracts (Negombata magnifica and Callyspongia siphonella) Induced Anticancer and Antimicrobial Activity. Applied Sciences. 2022; 12(3):1400. https://doi.org/10.3390/app12031400
Chicago/Turabian StyleEl-Naggar, Hussein A., Mansour A. E. Bashar, Islam Rady, Mohammad S. El-Wetidy, Waleed B. Suleiman, Fatimah O. Al-Otibi, Sara A. Al-Rashed, Lamiaa M. Abd El-Maoula, El-Sayed S. Salem, Enas M. H. Attia, and et al. 2022. "Two Red Sea Sponge Extracts (Negombata magnifica and Callyspongia siphonella) Induced Anticancer and Antimicrobial Activity" Applied Sciences 12, no. 3: 1400. https://doi.org/10.3390/app12031400
APA StyleEl-Naggar, H. A., Bashar, M. A. E., Rady, I., El-Wetidy, M. S., Suleiman, W. B., Al-Otibi, F. O., Al-Rashed, S. A., Abd El-Maoula, L. M., Salem, E.-S. S., Attia, E. M. H., & Bakry, S. (2022). Two Red Sea Sponge Extracts (Negombata magnifica and Callyspongia siphonella) Induced Anticancer and Antimicrobial Activity. Applied Sciences, 12(3), 1400. https://doi.org/10.3390/app12031400







