Liposomes as Carriers for the Delivery of Efavirenz in Combination with Glutathione—An Approach to Combat Opportunistic Infections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
2.2.1. Formulation of Liposomes
2.2.2. Characterization of Liposomes
2.3. HPLC Analysis
2.3.1. Chromatographic System for Efavirenz Analysis
2.3.2. Chromatographic System for Glutathione Analysis
2.4. Intracellular Uptake of EFA Liposomes in Mo/Mac Cells (THP-1 Cells)
2.4.1. THP-1 Cell Culture
2.4.2. Quantification of Efavirenz Intracellular Uptake
2.4.3. Rhodamine 123 (R123) Loaded EFA Liposomes and EFA Aqueous Dispersions
2.4.4. Confocal Laser Scanning Microscopy (CLSM)
2.5. MTS Cytotoxicity Assay
2.6. Measurement of ROS-Induced Fluorescence by Flow Cytometry
2.7. Statistical Analysis
3. Results
3.1. Formulation and Characterization of Liposomes
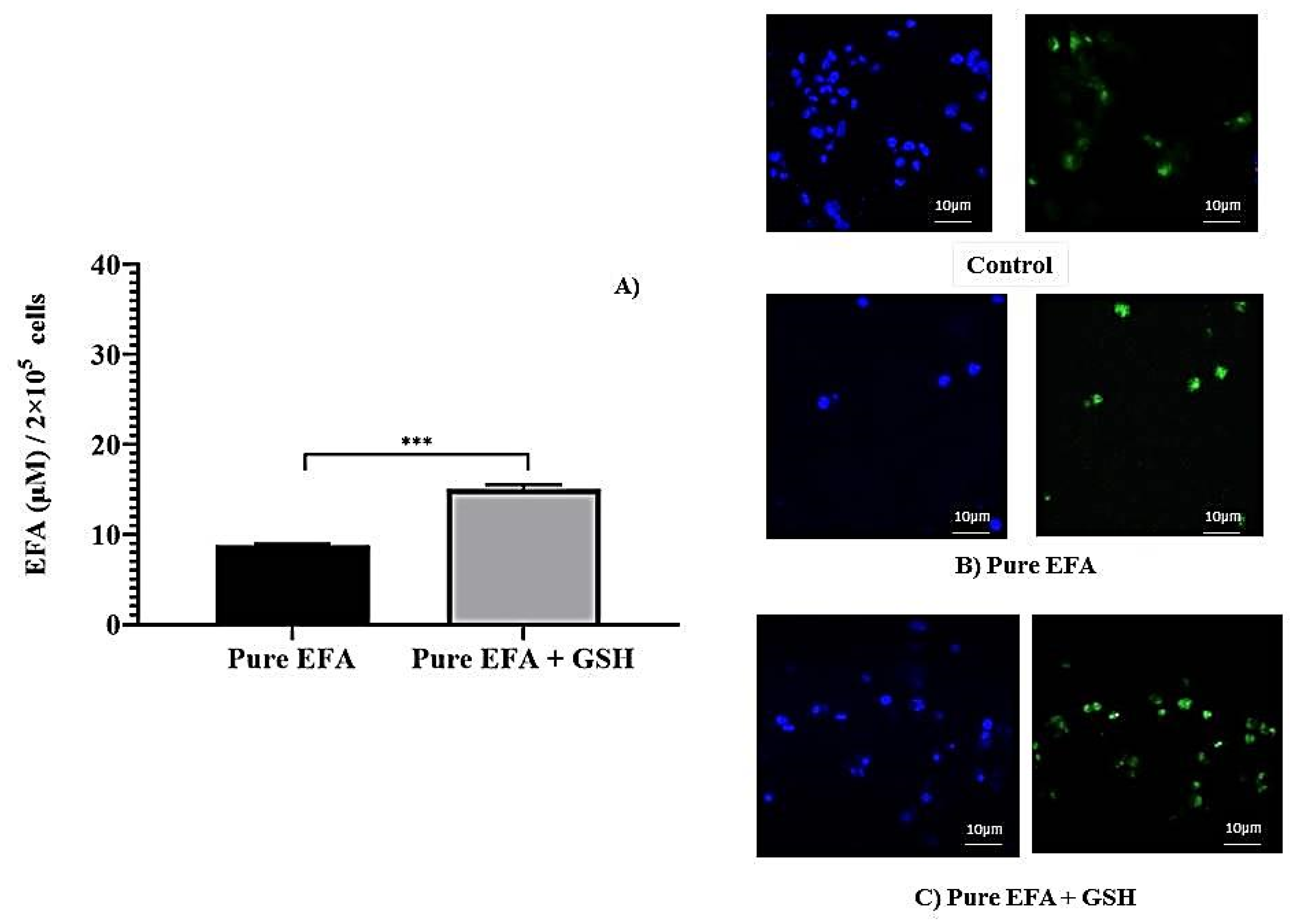
3.2. Intracellular Uptake by Macrophages
3.3. Cytotoxicity Study
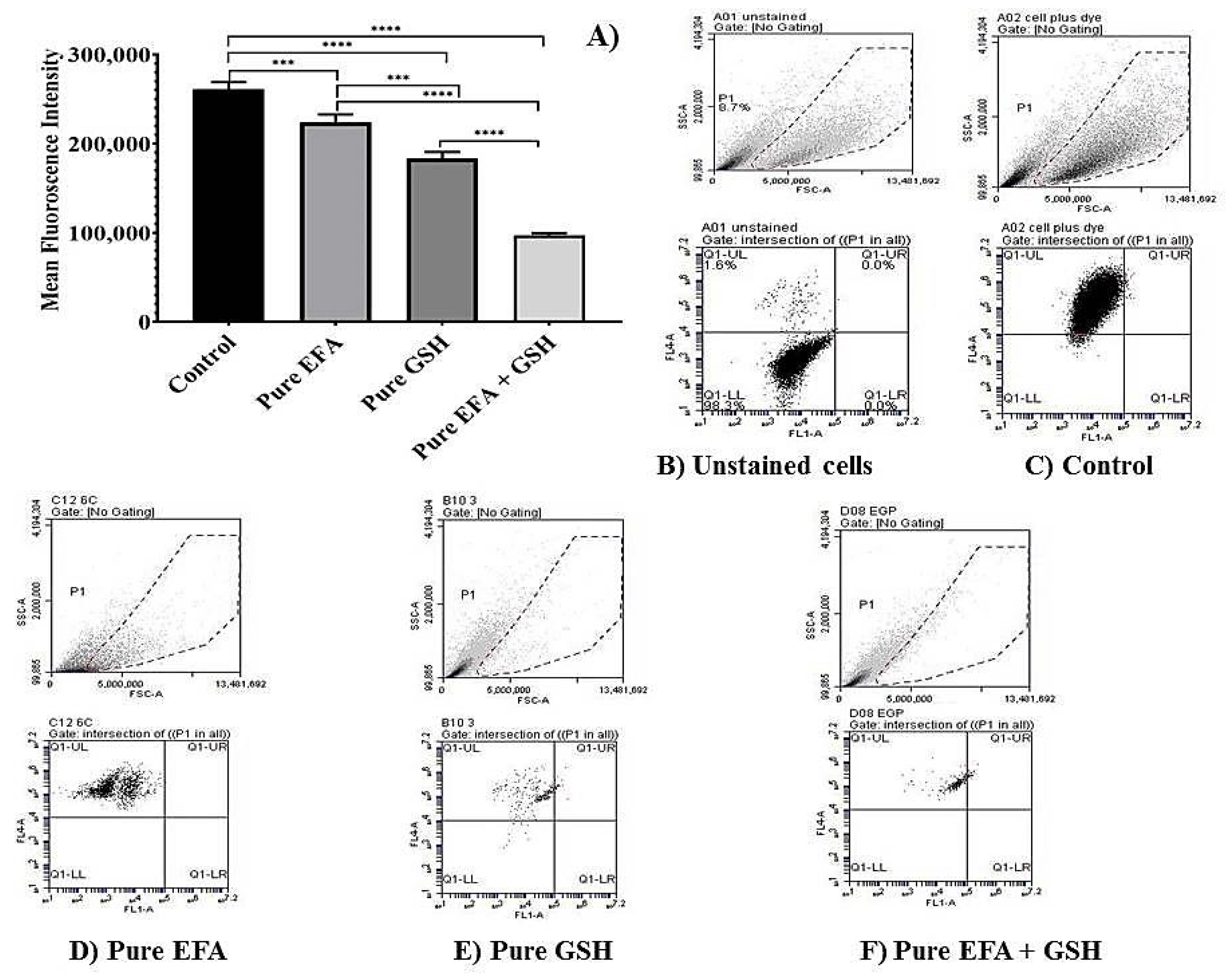
3.4. Measurement of ROS-Induced Fluorescence by Flow Cytometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. 2020 04232020. Available online: https://www.who.int/hiv/data/en/ (accessed on 15 September 2021).
- Morris, D.; Guerra, C.; Khurasany, M.; Guilford, F.; Saviola, B.; Huang, Y.; Venketaraman, V. Glutathione supplementation improves macrophage functions in HIV. J. Interferon Cytokine Res. 2013, 33, 270–279. [Google Scholar] [CrossRef]
- Douek, D.C.; Brenchley, J.M.; Betts, M.R.; Ambrozak, D.R.; Hill, B.J.; Okamoto, Y.; Casazza, J.P.; Kuruppu, J.; Kunstman, K.; Wolinsky, S.; et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature 2002, 417, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Yew, W.W.; Chan, D.P.; Chang, K.C.; Zhang, Y. Does oxidative stress contribute to antituberculosis drug resistance? J. Thorac. Dis. 2019, 11, E100–E102. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.A.; Handy, J.; Levander, O.A. The role of oxidative stress in viral infections. Ann. N. Y. Acad. Sci. 2000, 917, 906–912. [Google Scholar] [CrossRef]
- Venketaraman, V.; Dayaram, Y.K.; Amin, A.G.; Ngo, R.; Green, R.M.; Talaue, M.T.; Mann, J.; Connell, N.D. Role of glutathione in macrophage control of mycobacteria. Infect. Immun. 2003, 71, 1864–1871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venketaraman, V.; Rodgers, T.; Linares, R.; Reilly, N.; Swaminathan, S.; Hom, D.; Millman, A.C.; Wallis, R.; Connell, N.D. Glutathione and growth inhibition of Mycobacterium tuberculosis in healthy and HIV infected subjects. AIDS Res. Ther. 2006, 3, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Scaling up Antiretroviral Therapy in Resource-Limited Settings: Treatment Guidelines for a Public Health Approach; Accessed August 2; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Apostolova, N.; Blas-Garcia, A.; Galindo, M.J.; Esplugues, J.V. Efavirenz: What is known about the cellular mechanisms responsible for its adverse effects. Eur. J. Pharmacol. 2017, 812, 163–173. [Google Scholar] [CrossRef]
- Guerra, C.; Morris, D.; Sipin, A.; Kung, S.; Franklin, M.; Gray, D.; Tanzil, M.; Guilford, F.; Khasawneh, F.T.; Venketaraman, V. Glutathione and adaptive immune responses against Mycobacterium tuberculosis infection in healthy and HIV infected individuals. PLoS ONE 2011, 6, e28378. [Google Scholar] [CrossRef]
- Morris, D.; Khurasany, M.; Nguyen, T.; Kim, J.; Guilford, F.; Mehta, R.; Gray, D.; Saviola, B.; Venketaraman, V. Glutathione and infection. Biochim. Biophys Acta 2013, 1830, 3329–3349. [Google Scholar] [CrossRef]
- Wendel, A.; Cikryt, P. The level and half-life of glutathione in human plasma. FEBS Lett. 1980, 120, 209–211. [Google Scholar] [CrossRef] [Green Version]
- Lagman, M.; Ly, J.; Saing, T.; Kaur Singh, M.; Vera Tudela, E.; Morris, D.; Chi, P.T.; Ochoa, C.; Sathananthan, A.; Venketaraman, V. Investigating the causes for decreased levels of glutathione in individuals with type II diabetes. PLoS ONE 2015, 10, e0118436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ly, J.; Lagman, M.; Saing, T.; Singh, M.K.; Tudela, E.V.; Morris, D.; Anderson, J.; Daliva, J.; Ochoa, C.; Patel, N.; et al. Liposomal Glutathione Supplementation Restores TH1 Cytokine Response to Mycobacterium tuberculosis Infection in HIV-Infected Individuals. J. Interferon Cytokine Res. 2015, 35, 875–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraternale, A.; Paoletti, M.F.; Dominici, S.; Caputo, A.; Castaldello, A.; Millo, E.; Brocca-Cofano, E.; Smietana, M.; Clayette, P.; Oiry, J.; et al. The increase in intra-macrophage thiols induced by new pro-GSH molecules directs the Th1 skewing in ovalbumin immunized mice. Vaccine 2010, 28, 7676–7682. [Google Scholar] [CrossRef] [PubMed]
- Fraternale, A.; Paoletti, M.F.; Casabianca, A.; Orlandi, C.; Schiavano, G.F.; Chiarantini, L.; Clayette, P.; Oiry, J.; Vogel, J.U.; Cinatl, J., Jr.; et al. Inhibition of murine AIDS by pro-glutathione (GSH) molecules. Antivir. Res. 2008, 77, 120–127. [Google Scholar] [CrossRef]
- Fraternale, A.; Paoletti, M.F.; Casabianca, A.; Oiry, J.; Clayette, P.; Vogel, J.U.; Cinatl, J., Jr.; Palamara, A.T.; Sgarbanti, R.; Garaci, E.; et al. Antiviral and immunomodulatory properties of new pro-glutathione (GSH) molecules. Curr. Med. Chem. 2006, 13, 1749–1755. [Google Scholar] [CrossRef]
- Felts, R.L.; Narayan, K.; Estes, J.D.; Shi, D.; Trubey, C.M.; Fu, J.; Hartnell, L.M.; Ruthel, G.T.; Schneider, D.K.; Nagashima, K.; et al. 3D visualization of HIV transfer at the virological synapse between dendritic cells and T cells. Proc. Natl. Acad. Sci. USA 2010, 107, 13336–13341. [Google Scholar] [CrossRef] [Green Version]
- Kelly, C.; Jefferies, C.; Cryan, S.-A. Targeted liposomal drug delivery to monocytes and macrophages. J. Drug Deliv. 2011, 2011, 727241. [Google Scholar] [CrossRef]
- Weissig, V.; Pettinger, T.K.; Murdock, N. Nanopharmaceuticals (part 1): Products on the market. Int. J. Nanomed. 2014, 9, 4357–4373. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, E.; Soares, T.B.; Gonçalves, H.; Lúcio, M. Spectroscopic Studies as a Toolbox for Biophysical and Chemical Characterization of Lipid-Based Nanotherapeutics. Front. Chem. 2018, 6, 323. [Google Scholar] [CrossRef] [Green Version]
- Faria, M.J.; Lopes, C.M.; das Neves, J.; Lúcio, M. Lipid Nanocarriers for Anti-HIV Therapeutics: A Focus on Physicochemical Properties and Biotechnological Advances. Pharmaceutics 2021, 13, 1294. [Google Scholar] [CrossRef]
- Qi, J.; Zhuang, J.; Lu, Y.; Dong, X.; Zhao, W.; Wu, W. In vivo fate of lipid-based nanoparticles. Drug Discov. Today 2017, 22, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Katragadda, A.B.R.; Betageri, G. Effect of liposome composition and cholesterol on the cellular uptake of stavudine by human monocyte/macrophages. Cell Mol. Biol. Lett. 2000, 5, 483–494. [Google Scholar]
- Levy, J.A.; Shimabukuro, J.; McHugh, T.; Casavant, C.; Stites, D.; Oshiro, L. AIDS-associated retroviruses (ARV) can productively infect other cells besides human T helper cells. Virology 1985, 147, 441–448. [Google Scholar] [CrossRef]
- Chopra, S.; Venkatesan, N.; Betageri, G.V. Liposomes as nanocarriers for anti-HIV therapy. Drug Deliv. Transl. Res. 2013, 3, 471–478. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Guo, C.L.; Yao, W.T.; Cai, Q.Q.; Wang, Y.S.; Wang, J.Q. Vitamin E TPGS based liposomal delivery of doxorubicin in osteosarcoma cancer cells. Biomed. Res. 2017, 28, 1344–1349. [Google Scholar]
- Vanaja, K.; Rani, R.H.S.; Sacchidananda, S. Formulation and Clinical Evaluation Of Ultradeformable Liposomes in the Topical Treatment of Psoriasis. Clin. Res. Regul. Aff. 2008, 25, 41–52. [Google Scholar] [CrossRef]
- Si, F.; Shin, S.H.; Biedermann, A.; Ross, G.M. Estimation of PC12 cell numbers with acid phosphatase assay and mitochondrial dehydrogenase assay: Dopamine interferes with assay based on tetrazolium. Exp. Brain Res. 1999, 124, 145–150. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; The Lipid Bilayer; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Makabi-Panzu, B.; Gourde, P.; Désormeaux, A.; Bergeron, M.G. Intracellular and serum stability of liposomal 2’,3’-dideoxycytidine. Effect of lipid composition. Cell Mol. Biol. 1998, 44, 277–284. [Google Scholar]
- Wang, Y.; de Clercq, E.; Li, G. Current and emerging non-nucleoside reverse transcriptase inhibitors (NNRTIs) for HIV-1 treatment. Expert Opin. Drug Metab. Toxicol. 2019, 15, 813–829. [Google Scholar] [CrossRef]
- Jin, J.; Grimmig, B.; Izzo, J.; Brown, L.A.M.; Hudson, C.; Smith, A.J.; Tan, J.; Bickford, P.C.; Giunta, B. HIV Non-Nucleoside Reverse Transcriptase Inhibitor Efavirenz Reduces Neural Stem Cell Proliferation In Vitro and In Vivo. Cell Transplant. 2016, 25, 1967–1977. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, A.V.; Valuev-Elliston, V.T.; Ivanova, O.N.; Kochetkov, S.N.; Starodubova, E.S.; Bartosch, B.; Isaguliants, M.G. Oxidative Stress during HIV Infection: Mechanisms and Consequences. Oxid. Med. Cell Longev. 2016, 2016, 8910396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendich, A. 15—Role of Antioxidants in the Maintenance of Immune Functions. In Natural Antioxidants in Human Health and Disease; Frei, B., Ed.; Academic Press: San Diego, CA, USA, 1994; pp. 447–467. [Google Scholar]
- Singh, G.; Pai, R.S. Dawn of antioxidants and immune modulators to stop HIV-progression and boost the immune system in HIV/AIDS patients: An updated comprehensive and critical review. Pharmacol. Rep. 2015, 67, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Bressler, P.; Kinter, A.; Duh, E.; Timmer, W.C.; Rabson, A.; Justement, J.S.; Stanley, S.; Fauci, A.S. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor alpha by transcriptional and post-transcriptional mechanisms. J. Exp. Med. 1990, 172, 151–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venketaraman, V.; Kaushal, D.; Saviola, B. Mycobacterium tuberculosis. J. Immunol. Res. 2015, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anddre Valdivia, J.L.; Gonzalez, L.; Hussain, P.; Saing, T.; Islamoglu, H.; Pearce, D.; Ochoa, C.; Venketaraman, V. Restoring Cytokine Balance in HIV-Positive Individuals with Low CD4 T Cell Counts. AIDS Res. Hum. Retrovir. 2017, 33, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.E. Prodrug approaches for enhancing the bioavailability of drugs with low solubility. Chem. Biodivers 2009, 6, 2071–2083. [Google Scholar]
- Makoni, P.A.; Kasongo, K.W.; Walker, R.B. Short Term Stability Testing of Efavirenz-Loaded Solid Lipid Nanoparticle (SLN) and Nanostructured Lipid Carrier (NLC) Dispersions. Pharmaceutics 2019, 11, 397. [Google Scholar] [CrossRef] [Green Version]
- Varshosaz, J.; Taymouri, S.; Jahanian-Najafabadi, A.; Alizadeh, A. Efavirenz oral delivery via lipid nanocapsules: Formulation, optimisation, and ex-vivo gut permeation study. IET Nanobiotechnol 2018, 12, 795–806. [Google Scholar] [CrossRef]
- Kamboj, S.; Sethi, S.; Rana, V. Lipid based delivery of Efavirenz: An answer to its erratic absorption and food effect. Eur. J. Pharm. Sci. 2018, 123, 199–216. [Google Scholar] [CrossRef]
- Pokharkar, V.; Patil-Gadhe, A.; Palla, P. Efavirenz loaded nanostructured lipid carrier engineered for brain targeting through intranasal route: In-vivo pharmacokinetic and toxicity study. Biomed. Pharm. 2017, 94, 150–164. [Google Scholar] [CrossRef]
- Senapati, P.C.; Sahoo, S.K.; Sahu, A.N. Mixed surfactant based (SNEDDS) self-nanoemulsifying drug delivery system presenting efavirenz for enhancement of oral bioavailability. Biomed. Pharm. 2016, 80, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Ramana, L.N.; Sethuraman, S.; Ranga, U.; Krishnan, U.M. Development of a liposomal nanodelivery system for nevirapine. J. Biomed. Sci. 2010, 17, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cagdas, F.M.; Ertugral, N.; Bucak, S.; Atay, N.Z. Effect of preparation method and cholesterol on drug encapsulation studies by phospholipid liposomes. Pharm. Dev. Technol. 2011, 16, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Vanaja, K.; Wahl, M.A.; Bukarica, L.; Heinle, H. Liposomes as carriers of the lipid soluble antioxidant resveratrol: Evaluation of amelioration of oxidative stress by additional antioxidant vitamin. Life Sci. 2013, 93, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.A.; Ehrlich, L.S. Cell biology of HIV-1 infection of macrophages. Annu. Rev. Microbiol. 2008, 62, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Nasr, N.; Maddocks, S.; Turville, S.G.; Harman, A.N.; Woolger, N.; Helbig, K.J.; Wilkinson, J.; Bye, C.R.; Wright, T.K.; Rambukwelle, D.; et al. HIV-1 infection of human macrophages directly induces viperin which inhibits viral production. Blood 2012, 120, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; McGrath, M.S.; Xu, H. Inhibition of HIV Expression and Integration in Macrophages by Methylglyoxal-Bis-Guanylhydrazone. J. Virol. 2015, 89, 11176–11189. [Google Scholar] [CrossRef] [Green Version]
- Teskey, G.; Abrahem, R.; Cao, R.; Gyurjian, K.; Islamoglu, H.; Lucero, M.; Martinez, A.; Paredes, E.; Salaiz, O.; Robinson, B.; et al. Glutathione as a Marker for Human Disease. Adv. Clin. Chem. 2018, 87, 141–159. [Google Scholar]
- Paromov, V.; Kumari, S.; Brannon, M.; Kanaparthy, N.S.; Yang, H.; Smith, M.G.; Stone, W.L. Protective Effect of Liposome-Encapsulated Glutathione in a Human Epidermal Model Exposed to a Mustard Gas Analog. J. Toxicol. 2011, 2011, 109516. [Google Scholar] [CrossRef]
- Magnani, M.; Fraternale, A.; Casabianca, A.; Schiavano, G.F.; Chiarantini, L.; Palamara, A.T.; Ciriolo, M.R.; Rotilio, G.; Garaci, E. Antiretroviral effect of combined zidovudine and reduced glutathione therapy in murine AIDS. AIDS Res. Hum. Retrovir. 1997, 13, 1093–1099. [Google Scholar] [CrossRef]
- Fraternale, A.; Casabianca, A.; Tonelli, A.; Chiarantini, L.; Brandi, G.; Magnani, M. New drug combinations for the treatment of murine AIDS and macrophage protection. Eur. J. Clin. Investig. 2001, 31, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, F.; Rivas, I.P.; Khan, M.A.; Torres Suarez, A.I. Targeting to macrophages: Role of physicochemical properties of particulate carriers--liposomes and microspheres--on the phagocytosis by macrophages. J. Control Release 2002, 79, 29–40. [Google Scholar] [CrossRef]
- Caddeo, C.; Teskac, K.; Sinico, C.; Kristl, J. Effect of resveratrol incorporated in liposomes on proliferation and UV-B protection of cells. Int. J. Pharm. 2008, 363, 183–191. [Google Scholar] [CrossRef]
- Honeycutt, J.B.; Liao, B.; Nixon, C.C.; Cleary, R.A.; Thayer, W.O.; Birath, S.L.; Swanson, M.D.; Sheridan, P.; Zakharova, O.; Prince, F.; et al. T cells establish and maintain CNS viral infection in HIV-infected humanized mice. J. Clin. Investig. 2018, 128, 2862–2876. [Google Scholar] [CrossRef] [PubMed]
- Honeycutt, J.B.; Wahl, A.; Baker, C.; Spagnuolo, R.A.; Foster, J.; Zakharova, O.; Wietgrefe, S.; Caro-Vegas, C.; Madden, V.; Sharpe, G.; et al. Macrophages sustain HIV replication in vivo independently of T cells. J. Clin. Investig. 2016, 126, 1353–1366. [Google Scholar] [CrossRef] [PubMed]
- Honeycutt, J.B.; Thayer, W.O.; Baker, C.E.; Ribeiro, R.M.; Lada, S.M.; Cao, Y.; Cleary, R.A.; Hudgens, M.G.; Richman, D.D.; Garcia, J.V. HIV persistence in tissue macrophages of humanized myeloid-only mice during antiretroviral therapy. Nat. Med. 2017, 23, 638–643. [Google Scholar] [CrossRef] [PubMed]



| Formula Code | Lipid | Drug: Lipid: Sterol: T-80 (w/v) | Physical Appearance (Post 24 h) |
|---|---|---|---|
| E1 | SPC | 1: 2:0.5:0.1 | Coagulation |
| E2 | DMPC | 1: 2:0.5:0.1 | Coagulation |
| E3 | DSPC | 1: 2:0.5:0.1 | Coagulation |
| F | DMPC/DMPG | 1:5/15: 0.1:0 | Milky suspension (stable) |
| Formula Code | EFA: GSH (mM) | Size (nm) | Zeta Potential (mV) | Encapsulation Efficiency (%) | |
|---|---|---|---|---|---|
| EFA | GSH | ||||
| F1 | 3.17: 0.0 | 127.7 ± 12.98 | −93.8 ± 3.56 | 95.63 ± 3.91 | - |
| F2 | 3.17: 0.95 | 205.03 ± 11.59 | −89.2 ± 2.58 | 96.23 ± 3.45 | 43.5 ± 4.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenchappa, V.; Cao, R.; Venketaraman, V.; Betageri, G.V. Liposomes as Carriers for the Delivery of Efavirenz in Combination with Glutathione—An Approach to Combat Opportunistic Infections. Appl. Sci. 2022, 12, 1468. https://doi.org/10.3390/app12031468
Kenchappa V, Cao R, Venketaraman V, Betageri GV. Liposomes as Carriers for the Delivery of Efavirenz in Combination with Glutathione—An Approach to Combat Opportunistic Infections. Applied Sciences. 2022; 12(3):1468. https://doi.org/10.3390/app12031468
Chicago/Turabian StyleKenchappa, Vanaja, Ruoqiong Cao, Vishwanath Venketaraman, and Guru V. Betageri. 2022. "Liposomes as Carriers for the Delivery of Efavirenz in Combination with Glutathione—An Approach to Combat Opportunistic Infections" Applied Sciences 12, no. 3: 1468. https://doi.org/10.3390/app12031468







