Insights of Raceway Bioreactor Scale-Up: Effect of Agitation on Microalgae Culture and Reduction of the Liquid Medium Speed
Abstract
:1. Introduction
2. Materials and Methods
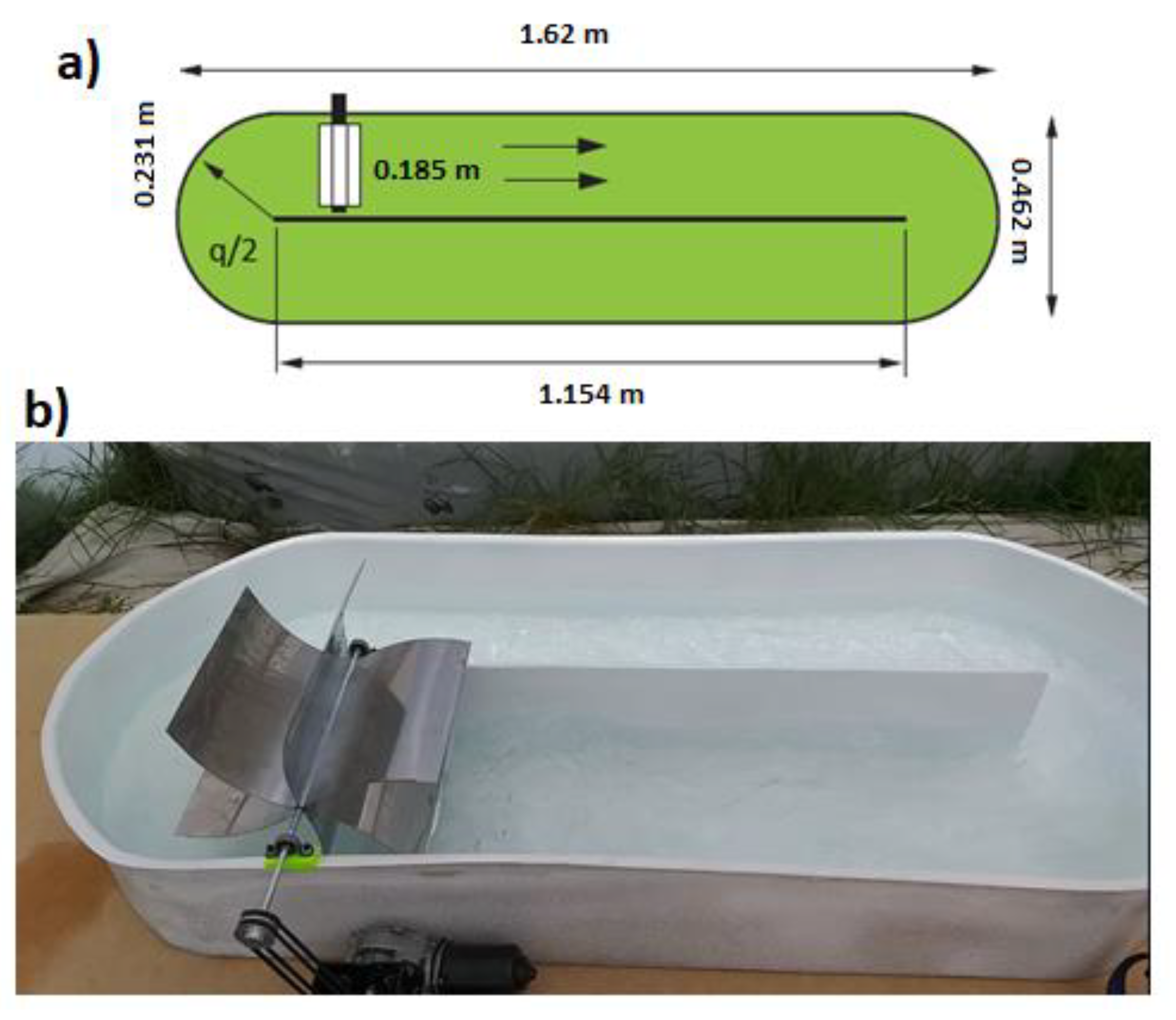
2.1. Bioreactor Scaling-Up
2.2. Bioreactor and System Conditions
2.2.1. Abiotic Media
2.2.2. Flow Patterns and Dead Zones
2.2.3. Speed Adjustment in Both Reactors
2.2.4. Biotic Media
3. Results
3.1. Raceway Bioreactor Scaling-Up
3.2. Speed Correlation
3.3. Flow Patterns and Dead Agitation Zones
3.4. Effect of Stirring Speed on Microalgae Growth
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michels, M.H.; van der Goot, A.J.; Norsker, N.-H.; Wijffels, R.H. Effects of shear stress on the microalgae Chaetoceros muelleri. Bioprocess Biosyst. Eng. 2010, 33, 921–927. [Google Scholar] [CrossRef] [Green Version]
- Pires, J.C.; Alvim-Ferraz, M.C.; Artins, F.G. Photobioreactor design for microalgae production through computational fluid dynamics: A review. Renew. Sustain. Energy Rev. 2017, 79, 248–254. [Google Scholar] [CrossRef]
- Slegers, P.M. Scenario Studies for Algae Production. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, March 2014. [Google Scholar]
- Chisti, Y. Large-Scale Production of algal biomass: Raceway ponds. In Algae Biotechnology. Green Energy and Technology; Bux, F., Chisti, Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 21–40. [Google Scholar] [CrossRef]
- Kanhaiya, K.; Sanjiv, M.; Anupama, S.; Min, S.P.; Won Yang, J. Recent trends in the mass cultivation of algae in raceway ponds. Renew. Sustain. Energy Rev. 2015, 51, 875–885. [Google Scholar] [CrossRef]
- Slegers, P.M.; Lösing, M.B.; Wijiffels, R.H.; van Straten, G.; van Boxtel, A.J. Scenario evaluation of open pond microalgae productio. Algal. Res. 2013, 2, 358–368. [Google Scholar] [CrossRef]
- Sompech, K.; Chisti, Y.; Srinophakun, T. Design of raceway ponds for producing microalgae. Biofuels 2012, 3, 387–397. [Google Scholar] [CrossRef]
- Cadena-Ramírez, A.; Bautista-Monroy, S.S.; Monsalvo Licona, F.; Téllez Jurado, A.; Salgado Ramírez, J.C.; Gómez Aldapa, C.A. Dispositivo de Agitación Mécanica de Mezcla Completa y Bajo Consumo de Energía Para el Cultivo de Microorganismos Fotosintéticos. Mexican Patent No. MX2017011071, 7 May 2021. Instituto Mexicano de la Propiedad Industrial (Patent Office). Available online: https://patentscope.wipo.int/search/es/detail.jsf?docId=MX242521807&_cid=P12-KZ09M4-19990-1 (accessed on 10 January 2022).
- Leupold, M.; Hindersin, S.; Gust, G.; Martín, K.; Hanelt, D. Influence of mixing and shear stress on Chlorella vulgaris, Scenedesmus obliquus, and Chlamydomonas reinhardtii. J. Appl. Phycol. 2013, 25, 485–495. [Google Scholar] [CrossRef]
- Scarsella, M.; Torzillo, G.; Cicci, A.; Belotti, G.; de Filippis, P.; Bravi, M. Mechanical stress tolerance of two microalgae. Process Biochem. 2012, 47, 1603–1611. [Google Scholar] [CrossRef]
- Fadlallah, H.; Jarrahi, M.; Herbert, E.; Ferrari, R.; Mejean, A.; Peerhossaini, H. Effects of shear stress on the growth rate of microorganisms in agitated reactors. In Proceedings of the ASME 2016 Fluids Engineering Division Summer Meeting, FEDSM2016, Washington, DC, USA, 10–14 July 2016. [Google Scholar] [CrossRef]
- Michels, M.H.; van der Goot, A.J.; Vermuë, M.H.; Wijffels, R.H. Cultivation of shear stress sensitive and tolerant microalgal species in a tubular photobioreactor equipped with a centrifugal pump. J. Appl. Phycol. 2016, 28, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, Q.; Wang, Z.; Wu, X.; Cong, W. Evaluation of power consumption of paddle wheel in an open raceway pond. Bioprocess Biosyst. Eng. 2014, 37, 1325–1336. [Google Scholar] [CrossRef]
- Zeng, F.; Huang, J.; Meng, C.; Zhu, F.; Chen, J.; Li, Y. Investigation on novel Raceway pondwith inclined paddle wheels through simulationand microalgae culture experiments. Bioprocess Biosyst. Eng. 2015, 39, 169–180. [Google Scholar] [CrossRef]
- Bautista-Monroy, S.S.; Salgado-Ramírez, J.C.; Téllez-Jurado, A.; Ramírez-Vargas, M.D.R.; Gómez-Aldapa, C.A.; Pérez-Viveros, K.J.; Medina-Moreno, S.A.; Cadena-Ramírez, A. Hydrodynamic characterization in a raceway bioreactor with diferent stirrers. Rev. Mex. Ing. Quím. 2019, 18, 605–619. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Elmore, S.; Van Gerven, T.; Stankiewicz, A. Hydrodynamic evaluations in high rate algae pond (HRAP) design. Chem. Eng. J. 2013, 217, 231–239. [Google Scholar] [CrossRef]
- Marín Palacio, L.D. El papel de la Potencia Volumétrica en la Producción y Glicolisación de Proteinas Recombinantes en Bacterias Filamentosas: APA (Proteínas de 45/47 KDa) de Mycobacterium Tuberculosis en Streptomyces Lividans. Ph.D. Thesis, Universidad Nacional Autónoma de México, Mexico City, Mexico, February 2016. [Google Scholar]
- Mayorga, J.; Chávez, R.; Mayorga, O.; Delgado-Linares, J.; Sánchez, R.; Delgado-Linares, G. Escalamiento del reactor del proceso de coquización retardada. Cienc. Ing. 2014, 35, 147–155. [Google Scholar]
- Ruiz Colorado, A.A. Factores de Escala para la Producción Biotecnológica de Etanol Carburante. Ph.D. Thesis, Universidad Nacional de Colombia, Facultad de Minas, Escuela de procesos y energía, Medellin, Colombia, March 2009. [Google Scholar]
- Radmann, E.M.; Reinehr, C.O.; Costa, J.A. Optimization of the repeated batch cultivation of microalga Spirulina platensis in open raceway ponds. Aquaculture 2007, 265, 118–126. [Google Scholar] [CrossRef]
- García Cruz, E.L. Thesis Evaluación del Crecimiento de Consorcio de Microalgas Alcalófilas en Fotobiorreactores con Potencial de Aplicación en el Enriquecimiento de Biogás. Facultad de Estudios Superiores Zaragoza, Institúto de Ingeniería, Mexico City, Mexico, 2015. [Google Scholar]
- De los Cobos-Vasconcelos, D.; García-Cruz, E.L.; Franco-Morgado, M.; González-Sánchez, A. Short-term evaluation of the photosynthetic activity of an alkaliphilic microalgae consortium in a novel tubular closed photobioreactor. J. Appl. Phycol. 2016, 28, 795–802. [Google Scholar] [CrossRef]
- Bermúdez, J.L.; Lodeiros, C.; Morales, E. Producción de biomasa de la microalga marina Chroomonas sp., en función del pH, intencidad luminosa y salinidad. Boletín Investig. Mar. Costeras 2002, 31, 167–185. [Google Scholar]
- Ruiz, Á.A.; Álvarez, H. Escalamiento de Procesos Químicos y Bioquímicos basado en un Modelo Fenomenológico. Inf. Tecnol. 2011, 22, 33–52. [Google Scholar] [CrossRef]
- Muñoz Equihua, E.S. Escalamiento del Proceso de Obtención de Fructooligosacáridos a Partir de Jugo de Caña Mediante Síntesis Enzimática con Células Permeabilizadas de Candida Apicola. Master’s Thesis, Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco, Guadalajara, Mexico, June 2016. [Google Scholar]
- Garcia-Ochoa, F.; Gomez, E. Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview. Biotech. Adv. 2009, 27, 153–176. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Prussi, M.; Casini, D.; Tredici, M.R.; Basi, N.; Chini Zittelli, G.; Bondioli, P. Review of energy balance in raceway ponds for microalgae cultivation: Re-thinking a traditional system is possible. Appl. Energ. 2013, 102, 101–111. [Google Scholar] [CrossRef]
- Escobar Parra, S. Estudio del Transporte y Consumo de Oxígeno en Cultivos Bacterianos: Estrés Hidrodinámico. Ph.D. Thesis, Universidad complutense de Madrid, Facultad de ciencias químicas, Departamento de Ingeniería Química Madrid, Madrid, Spain, 2014. [Google Scholar]
- García-Ochoa, F.; Gomez, E.; Alcon, A.; Santos, V.E. The effect of hydrodynamic stress on the growth of Xanthomonas campestris cultures in a stirred and sparged tank bioreactor. Bioprocess. Biosyst. Eng. 2012, 36, 911–925. [Google Scholar] [CrossRef]
- Mazzuca Sobczuk, T. Influencia de las Condiciones Hidrodinámicas y de la Fracción Molar de CO2 en la Fase Gaseosa Sobre el Crecimiento Celular en Cultivos de Microalgas. Ph.D. Thesis, Universidad de Almeria, Almeria, Spain, 2003. [Google Scholar]
- Olivero Novillo, M.L.; Aguirre Pe, J. Esfuerzo de corte en canales lisos. Ing. Hidraul. Mex. 2001, 16, 39–45. [Google Scholar]
- Wang, C.; Land, C.Q. Effects of shear stress on microalgae—A review. Biotechnol. Adv. 2018, 36, 986–1002. [Google Scholar] [CrossRef]
- Dimar-CIOH. Catálogo de Fitoplancton de la Bahía de Cartagena, Bahía Portete y Agua de Lastre; Dirección General Marítima-Centro de Investigaciones Oceanográficas e Hidrográficas del Caribe, Ed Dimar, Serie de Publicaciones Especiales CIOH: Cartagena de Indias, Colombia, 2011; Volume 5, p. 135. [Google Scholar]
- Guamán Burneo, M.C.; González Romero, N.P. Catálogo de Microalagas y Cianobacterias de Agua Dulce del Ecuador; Corporación para la Investigación Energética, Laboratorio de Biotecnología Energética: Quito, Ecuador, 2016; p. 122. ISBN 978-9942-14-874-2. [Google Scholar]
 ) Experimental Data, (
) Experimental Data, (  ) Fitted model.
) Fitted model.
 ) Experimental Data, (
) Experimental Data, (  ) Fitted model.
) Fitted model.
 ) Experimental pdf, (
) Experimental pdf, (  ) Theoretical pdf.
) Theoretical pdf.
 ) Experimental pdf, (
) Experimental pdf, (  ) Theoretical pdf.
) Theoretical pdf.




| ω (rpm) | V (m/s) | Re | Fr | Re′ | τ (Pa) |
|---|---|---|---|---|---|
| 20 | 0.2452 | 99.008 | 0.2858 | 205.251 | 0.1344 |
| 25 | 0.2870 | 115.887 | 0.3345 | 256.564 | 0.1856 |
| 30 | 0.3515 | 141.952 | 0.4098 | 307.877 | 0.2430 |
| 35 | 0.4058 | 163.881 | 0.4731 | 359.190 | 0.3047 |
| 40 | 0.4563 | 184.275 | 0.532 | 410.502 | 0.3707 |
| 45 | 0.5068 | 201.669 | 0.5908 | 461.815 | 0.4408 |
| ω (rpm) | Vlab (m/s) | Vpil (m/s) | V′ (pil/lab) | V″ (lab/pil) |
|---|---|---|---|---|
| 30 | 0.3485 | 0.3515 | 1.0086 | 0.9915 |
| 35 | 0.3949 | 0.4058 | 1.0276 | 0.9731 |
| 40 | 0.4494 | 0.4563 | 1.0154 | 0.9849 |
| 45 | 0.4903 | 0.5068 | 1.0337 | 0.9674 |
| ω (rpm) | Eca (J) | Rest | Rech | Fr | P (W) | Np | τ (Pa) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 35 | 0.3949 | 0.3115 | 1.6618 × 10−5 | 21,325 | 35,900 | 0.7974 | 0.4743 | 7.1489 | 0.3576 | 13.48 |
| 40 | 0.4494 | 0.356 | 2.1705 × 10−5 | 24,371 | 40,855 | 0.9075 | 0.542 | 5.4734 | 0.3834 | 7.93 |
| 45 | 0.4903 | 0.4006 | 2.7470 × 10−5 | 27,418 | 44,573 | 0.9901 | 0.6098 | 4.3247 | 0.4072 | 4.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bautista-Monroy, S.S.; Chávez-Urbiola, E.A.; Ortega-Palacios, R.; González-Sánchez, A.; Gómez-Aldapa, C.A.; Rodríguez-Nava, O.; Salgado-Ramírez, J.C.; Cadena-Ramírez, A. Insights of Raceway Bioreactor Scale-Up: Effect of Agitation on Microalgae Culture and Reduction of the Liquid Medium Speed. Appl. Sci. 2022, 12, 1513. https://doi.org/10.3390/app12031513
Bautista-Monroy SS, Chávez-Urbiola EA, Ortega-Palacios R, González-Sánchez A, Gómez-Aldapa CA, Rodríguez-Nava O, Salgado-Ramírez JC, Cadena-Ramírez A. Insights of Raceway Bioreactor Scale-Up: Effect of Agitation on Microalgae Culture and Reduction of the Liquid Medium Speed. Applied Sciences. 2022; 12(3):1513. https://doi.org/10.3390/app12031513
Chicago/Turabian StyleBautista-Monroy, Suri Sadai, Edgar Arturo Chávez-Urbiola, Rocío Ortega-Palacios, Armando González-Sánchez, Carlos Alberto Gómez-Aldapa, Odín Rodríguez-Nava, Julio Cesar Salgado-Ramírez, and Arturo Cadena-Ramírez. 2022. "Insights of Raceway Bioreactor Scale-Up: Effect of Agitation on Microalgae Culture and Reduction of the Liquid Medium Speed" Applied Sciences 12, no. 3: 1513. https://doi.org/10.3390/app12031513








