Occlusive Properties of Transrenal Ureteral Occlusion Self-Expandable Metallic Stents: 3D-Printed Phantom and Ex Vivo Studies
Abstract
:1. Introduction
2. Materials and Methods
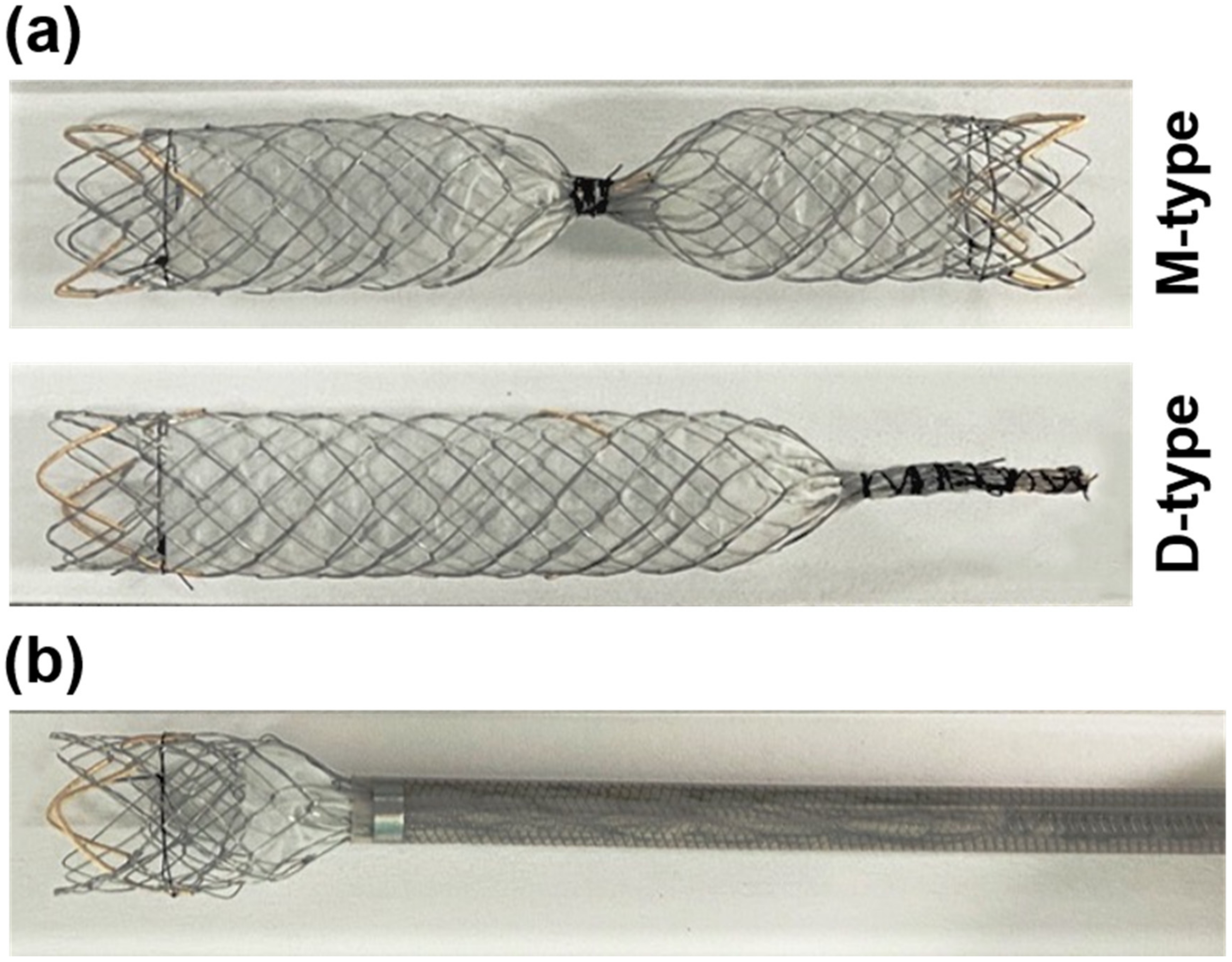
2.1. Preparation of Occlusion SEMSs
2.2. Design of the 3D-Printed Phantom
2.3. In Vitro Study Using 3D-Printed Phantom
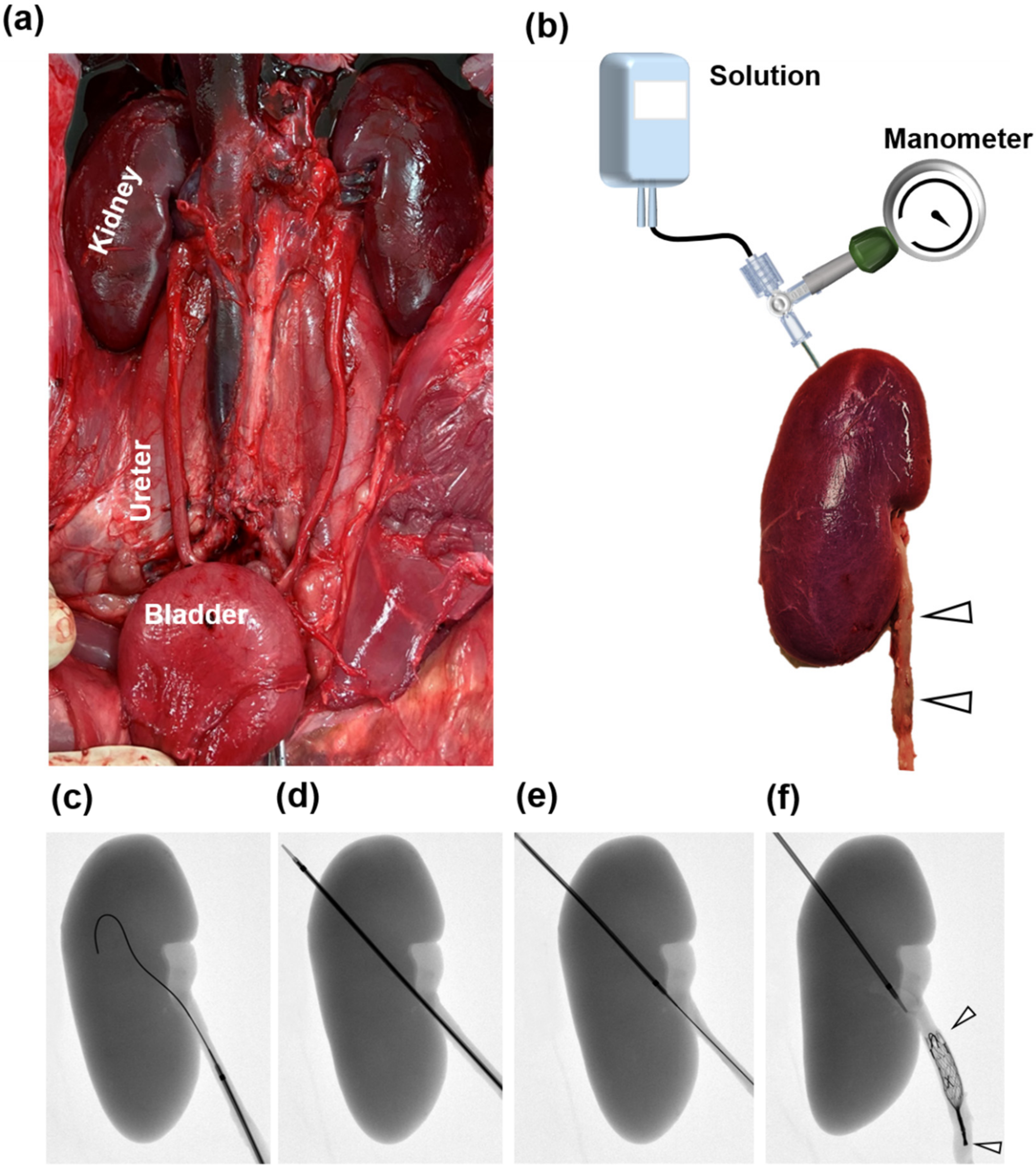
2.4. Ex Vivo Study Design
2.5. Occlusive SEMS Placement and Experimental Set-Up
2.6. Study Definitions
2.7. Statistical Analysis
3. Results
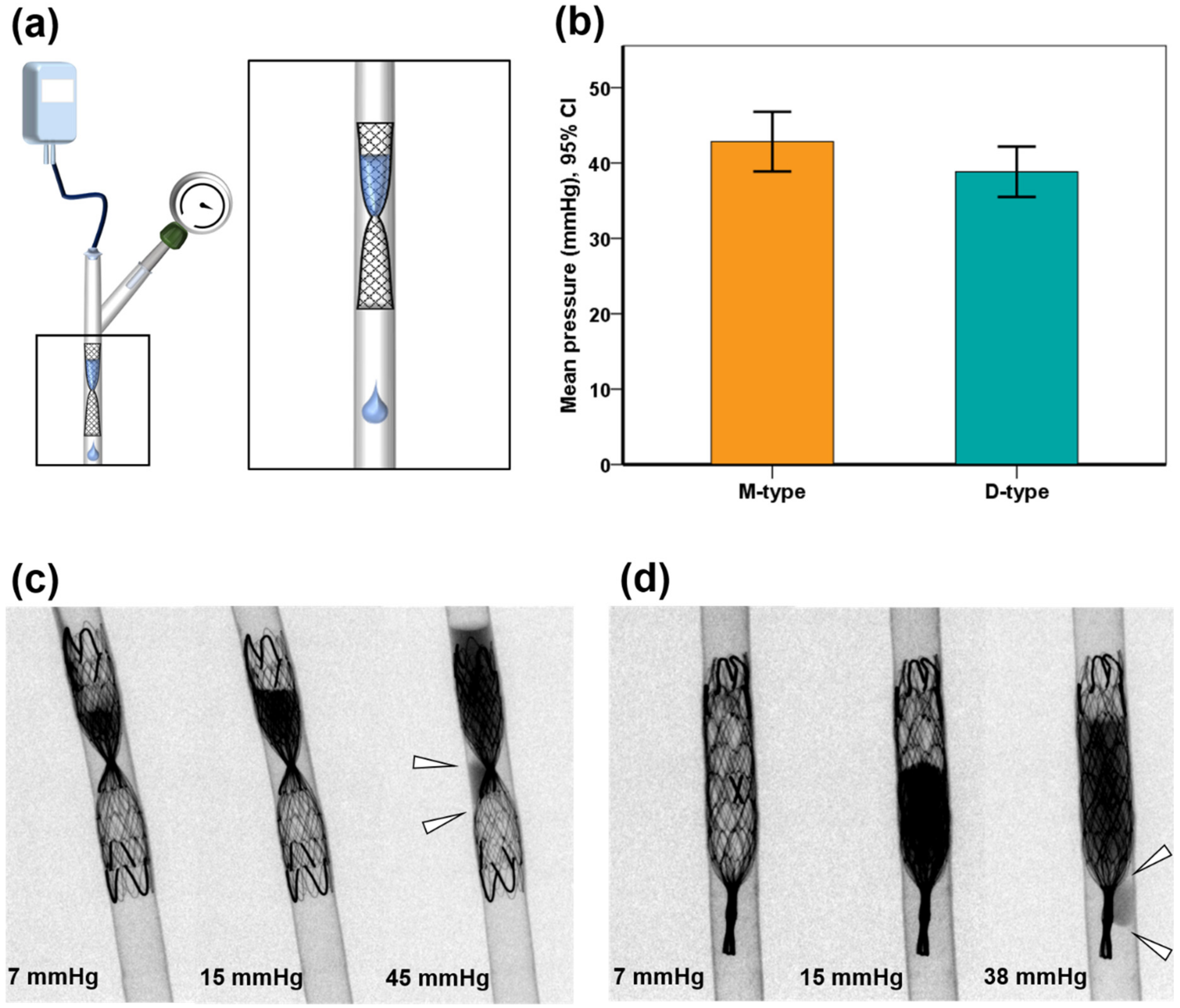
3.1. Occlusive Properties in 3D-Printed Phantom
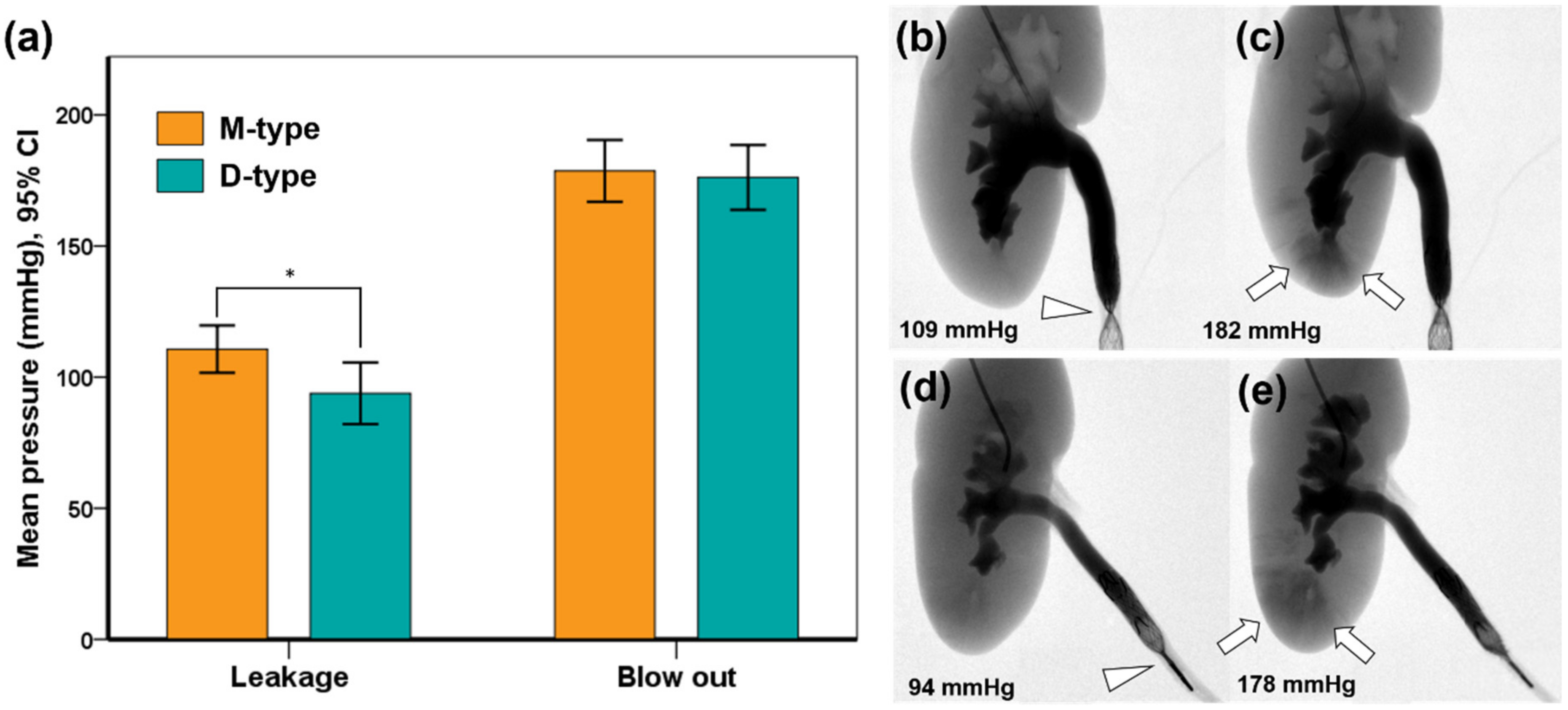
3.2. Occlusive Properties in the Extracted Porcine Urinary Tract
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuetting, D.; Meyer, C.; Schild, H.H.; Pieper, C.C. In Vitro Evaluation of the Occlusive Properties of the ArtVentive Endoluminal Occlusion System Occlusion Device for Transrenal Ureteral Occlusion. J. Endourol. 2017, 31, 1084–1089. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, Y.R.; Kyung, M.S.; Choi, J.S. Transrenal ureteral occlusion with the use of microcoils in five patients with ureterovaginal fistulas. Abdom. Imaging 2008, 33, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Günther, R.; Marberger, M.; Klose, K. Transrenal ureteral embolization. Radiology 1979, 132, 317–319. [Google Scholar] [CrossRef]
- Avritscher, R.; Madoff, D.C.; Ramirez, P.T.; Wallace, M.J.; Ahrar, K.; Morello, F.A., Jr.; Gupta, S.; Murthy, R.; Wright, K.C.; Hicks, M.E. Fistulas of the lower urinary tract: Percutaneous approaches for the management of a difficult clinical entity. Radiographics 2004, 24 (Suppl. S1), S217–S236. [Google Scholar] [CrossRef] [PubMed]
- Augustin, A.M.; Koneval, L.; Kalogirou, C.; Kocot, A.; Kickuth, R. Percutaneous transrenal ureteral plug embolization: Is there a need for tissue adhesives? Diagn. Interv. Radiol. 2021, 27, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Saad, W.E.; Kalagher, S.; Turba, U.C.; Sabri, S.S.; Park, A.W.; Stone, J.; Angle, J.F.; Matsumoto, A.H. Ureteric embolization for lower urinary tract fistulae: Use of two amplatzer vascular plugs and N-butyl cyanoacrylate employing the “sandwich” technique. Cardiovasc. Interv. Radiol. 2013, 36, 1068–1072. [Google Scholar] [CrossRef]
- Jalaeian, H.; Hicks, R.J.; Hartnell, G.G.; d’Othée, B.J. Transrenal Ureteral Occlusion for Palliation of Refractory Urine Leaks Using Vascular Plugs and Liquid Ethylene Vinyl Alcohol. J. Vasc. Interv. Radiol. 2019, 30, 1994–2001. [Google Scholar] [CrossRef] [PubMed]
- Schild, H.H.; Günther, R.; Thelen, M. Transrenal ureteral occlusion: Results and problems. J. Vasc. Interv. Radiol. 1994, 5, 321–325. [Google Scholar] [CrossRef]
- Papanicolaou, N.; Pfister, R.C.; Yoder, I.C. Percutaneous occlusion of ureteral leaks and fistulae using nondetachable balloons. Urol. Radiol. 1985, 7, 28–31. [Google Scholar] [CrossRef]
- Asvadi, N.H.; Arellano, R.S. Transrenal Antegrade Ureteral Occlusion: Clinical Assessment of Indications, Technique and Outcomes. J. Urol. 2015, 194, 1428–1432. [Google Scholar] [CrossRef]
- Schild, H.H.; Meyer, C.; Möhlenbroch, M.; Mueller, S.C.; Simon, B.; Kuhl, C.K. Transrenal ureter occlusion with an Amplatzer vascular plug. J. Vasc. Interv. Radiol. 2009, 20, 1390–1392. [Google Scholar] [CrossRef]
- Pieper, C.C.; Meyer, C.; Hauser, S.; Wilhelm, K.E.; Schild, H.H. Transrenal ureteral occlusion using the Amplatzer vascular plug II: A new interventional treatment option for lower urinary tract fistulas. Cardiovasc. Interv. Radiol. 2014, 37, 451–457. [Google Scholar] [CrossRef]
- Horenblas, S.; Kröger, R.; van Boven, E.; Meinhardt, W.; Newling, D.W. Use of balloon catheters for ureteral occlusion in urinary leakage. Eur. Urol. 2000, 38, 613–617. [Google Scholar] [CrossRef]
- Chen, C.; Kim, J.W.; Shin, J.H.; Hong, B.; Li, H.L.; Kang, C.H.; Cho, Y.J.; Chu, H.H. Therapeutic Ureteral Occlusion with Use of Occlusion Stents for Urinary Leakage or Fistula: A Bicentric Study. Cardiovasc. Interv. Radiol. 2020, 43, 1492–1497. [Google Scholar] [CrossRef]
- Park, H.J.; Shin, J.H.; Kim, J.W.; Hong, B.S. Postoperative ureteral leak treated using a silicone-covered nitinol stent. Int. Neurourol. J. 2015, 19, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Shindel, A.W.; Zhu, H.; Hovsepian, D.M.; Brandes, S.B. Ureteric embolization with stainless-steel coils for managing refractory lower urinary tract fistula: A 12-year experience. BJU Int. 2007, 99, 364–368. [Google Scholar] [CrossRef]
- Lingeman, J.E.; Wong, M.Y.; Newmark, J.R. Endoscopic management of total ureteral occlusion and ureterovaginal fistula. J. Endourol. 1995, 9, 391–396. [Google Scholar] [CrossRef]
- Shabrang, C.; Kelbach, S.M.; Hsu, D.P.; Zippe, C.D.; Lie, K.T. Therapeutic ureteral occlusion with n-butyl cyanoacrylate glue and an AMPLATZER plug scaffold. J. Vasc. Interv. Radiol. 2012, 23, 428–430. [Google Scholar] [CrossRef]
- Flueckiger, F.; Lammer, J.; Klein, G.E.; Hausegger, K.; Lederer, A.; Szolar, D.; Tamussino, K. Malignant ureteral obstruction: Preliminary results of treatment with metallic self-expandable stents. Radiology 1993, 186, 169–173. [Google Scholar] [CrossRef]
- Chung, H.H.; Kim, M.D.; Won, J.Y.; Won, J.H.; Cho, S.B.; Seo, T.S.; Park, S.W.; Kang, B.C. Multicenter experience of the newly designed covered metallic ureteral stent for malignant ureteral occlusion: Comparison with double J stent insertion. Cardiovasc. Interv. Radiol. 2014, 37, 463–470. [Google Scholar] [CrossRef]
- Bratt, C.G.; Nilsson, S. Intrapelvic pressure and caliceal dilatation in the evaluation of intermittent hydronephrosis. J. Urol. 1987, 137, 845–848. [Google Scholar] [CrossRef]
- Pope, J.C.T.; Showalter, P.R.; Milam, D.F.; Brock, J.W., 3rd. Intrapelvic pressure monitoring in the partially obstructed porcine kidney. Urology 1994, 44, 565–571. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.W.; Chu, H.H.; Won, D.-S.; Zeng, C.H.; Kim, S.H.; Park, Y.; Kang, J.M.; Ryu, D.S.; Shin, J.H.; Park, J.-H. Occlusive Properties of Transrenal Ureteral Occlusion Self-Expandable Metallic Stents: 3D-Printed Phantom and Ex Vivo Studies. Appl. Sci. 2022, 12, 1516. https://doi.org/10.3390/app12031516
Kim JW, Chu HH, Won D-S, Zeng CH, Kim SH, Park Y, Kang JM, Ryu DS, Shin JH, Park J-H. Occlusive Properties of Transrenal Ureteral Occlusion Self-Expandable Metallic Stents: 3D-Printed Phantom and Ex Vivo Studies. Applied Sciences. 2022; 12(3):1516. https://doi.org/10.3390/app12031516
Chicago/Turabian StyleKim, Ji Won, Hee Ho Chu, Dong-Sung Won, Chu Hui Zeng, Song Hee Kim, Yubeen Park, Jeon Min Kang, Dae Sung Ryu, Ji Hoon Shin, and Jung-Hoon Park. 2022. "Occlusive Properties of Transrenal Ureteral Occlusion Self-Expandable Metallic Stents: 3D-Printed Phantom and Ex Vivo Studies" Applied Sciences 12, no. 3: 1516. https://doi.org/10.3390/app12031516






