Multi-Modality Cardiovascular Imaging Assessment in Fabry Disease
Abstract
:1. Introduction
2. Diagnosis: Maximal Wall Thickness and LV Hypertrophy
2.1. Transthoracic Echocardiography
2.2. Cardiac MRI
- LV maximal wall thickness and LVH are important triggers to treatment in FD;
- Measurement of MWT is highly variable in expert hands-on 2D echocardiography and CMR;
- Further work should be addressed at validating AI-based analysis of 3D echocardiography and CMR stacks in this population with the aim for this to eventually become the gold standard.
3. Diagnosis: Tissue Characterization in Early Disease
3.1. Transthoracic Echocardiography
3.2. Cardiac MRI
- Impairment of TDI velocity, Doppler and speckle tracking strain and strain rate are early signs of the functional impact of sphingolipid storage on the heart;
- T1 lowering is a sensitive sign of FD cardiomyopathy that precedes LVH and late gadolinium enhancement but is not yet an accepted trigger for therapy;
- Late gadolinium enhancement is an accepted trigger for initiation of therapy but there remain questions regarding efficacy once identified.
4. Diagnosis: Tissue Characterization in Later Disease
- Staging disease requires a combination of echocardiography and CMR data to define a pre-hypertrophy stage when both structural and functional abnormalities can be subtle;
- Following hypertrophy, there is increasing recognition from biomarker and imaging studies that inflammation plays a role in progression to fibrosis and scar, that may coincide with altered perfusion detectable on CMR and nuclear medicine techniques.
5. Prognosis
5.1. Transthoracic Echocardiography
5.2. Cardiac MRI
- Standard imaging parameters of MWT, LVH and diastolic function are predictive of cardiovascular outcome in FD;
- Advanced parameters, including GLS and MW, may independently predict risk of MACE in FD, consistent with data from other non-ischaemic cardiomyopathies but further work is required;
- Data on prognosis in FD based on CMR are limited but presence of LGE is associated with risk of malignant ventricular arrhythmia.
6. Managing Complications
6.1. Chest Pain
6.2. Breathlessness
6.3. Arrhythmia
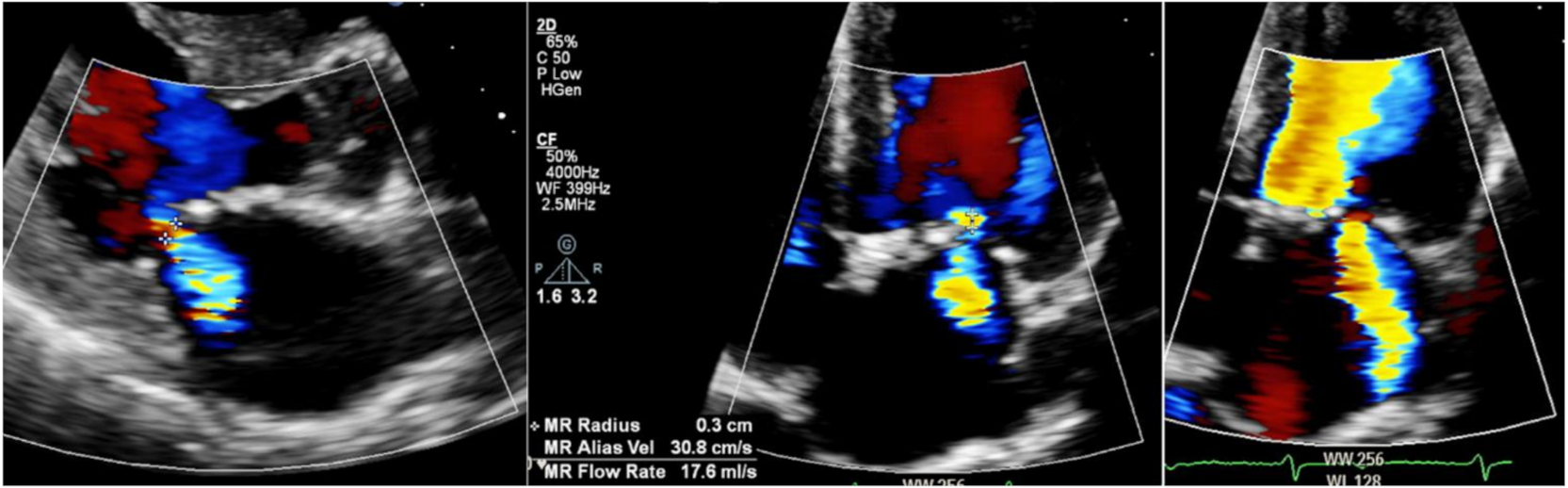
6.4. Valve Disease
7. Future Imaging
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desnick, R.J.; Brady, R.; Barranger, J.; Collins, A.J.; Germain, D.P.; Goldman, M. Fabry disease, an under-recognized multisystemic disorder: Expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann. Intern Med. 2003, 138, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Sweeley, C.C.; Klionsky, B. Fabry’s disease: Classification as a sphingolipidosis and partial characterization of a novel glycolipid. J. Biol. Chem. 1963, 238, 3148–3150. [Google Scholar] [CrossRef]
- Mehta, A.; Beck, M.; Eyskens, F.; Feliciani, C.; Kantola, I.; Ramaswami, U. Fabry disease: A review of current management strategies. QJM Int. J. Med. 2010, 103, 641–659. [Google Scholar] [CrossRef] [Green Version]
- Linhart, A.; Germain, D.P.; Olivotto, I.; Akhtar, M.M.; Anastasakis, A.; Hughes, D. An expert consensus document on the management of cardiovascular manifestations of Fabry disease. Eur. J. Heart Fail. 2020, 22, 1076–1096. [Google Scholar] [CrossRef]
- Hiwot, T.; Hughes, D.; Ramaswami, U. Guidelines for the treatment of Fabry Disease. Br. Inherit. Metab. Dis. Group 2020, 2, 1–10. [Google Scholar]
- Yeung, D.F.; Sirrs, S.; Tsang, M.Y.; Gin, K.; Luong, C.; Jue, J.; Nair, P.; Lee, P.K.; Tsang, T.S. Echocardiographic Assessment of Patients with Fabry Disease. J. Am. Soc. Echocardiogr. 2018, 31, 639–649. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Captur, G.; Manisty, C.H.; Raman, B.; Marchi, A.; Wong, T.C.; Ariga, R. Maximal Wall Thickness Measurement in Hypertrophic Cardiomyopathy: Biomarker Variability and its Impact on Clinical Care. JACC Cardiovasc. Imaging. 2021, 14, 2123–2134. [Google Scholar] [CrossRef]
- Park, S.H.; Shub, C.; Nobrega, T.P.; Bailey, K.R.; Seward, J.B. Two-dimensional echocardiographic calculation of left ventricular mass as recommended by the American Society of Echocardiography: Correlation with autopsy and M-mode echocardiography. J. Am. Soc. Echocardiogr. 1996, 9, 119–128. [Google Scholar] [CrossRef]
- Mor-Avi, V.; Sugeng, L.; Weinert, L.; MacEneaney, P.; Caiani, E.G.; Koch, R. Fast measurement of left ventricular mass with real-time three-dimensional echocardiography: Comparison with magnetic resonance imaging. Circulation 2004, 110, 1814–1818. [Google Scholar] [CrossRef] [Green Version]
- Volpato, V.; Mor-Avi, V.; Narang, A.; Prater, D.; Gonçalves, A.; Tamborini, G.; Fusini, L.; Pepi, M.; Patel, A.R.; Lang, R.M. Automated, machine learning-based, 3D echocardiographic quantification of left ventricular mass. Echocardiography 2019, 36, 312–319. [Google Scholar] [CrossRef]
- O’Brien, C.; Britton, I.; Karur, G.R.; Iwanochko, R.M.; Morel, C.F.; Nguyen, E.T. Left Ventricular Mass and Wall Thickness Measurements Using Echocardiography and Cardiac MRI in Patients with Fabry Disease: Clinical Significance of Discrepant Findings. Radiol. Cardiothorac. Imaging 2020, 2, e190149. [Google Scholar] [CrossRef] [PubMed]
- Hazari, H.; Belenkie, I.; Kryski, A.; White, J.A.; Oudit, G.Y.; Thompson, R. Comparison of Cardiac Magnetic Resonance Imaging and Echocardiography in Assessment of Left Ventricular Hypertrophy in Fabry Disease. Can. J. Cardiol. 2018, 34, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Maceira, A.M.; Prasad, S.K.; Khan, M.; Pennell, D. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2006, 8, 417–426. [Google Scholar] [CrossRef]
- Petersen, S.E.; Aung, N.; Sanghvi, M.; Zemrak, F.; Fung, K.; Paiva, J.M.; Francis, J.M.; Khanji, M.Y.; Lukaschuk, E.; Lee, A.; et al. Reference ranges for cardiac structure and function using cardiovascular magnetic resonance (CMR) in Caucasians from the UK Biobank population cohort. J. Cardiovasc. Magn. Reson. 2017, 19, 18. [Google Scholar] [CrossRef] [Green Version]
- Moody, W.E.; Hudsmith, L.E.; Holloway, B.; Treibel, T.A.; Davies, R.; Kozor, R. Variation in cardiovascular magnetic resonance myocardial contouring: Insights from an international survey. J. Magn. Reson. Imaging 2019, 50, 1336–1338. [Google Scholar] [CrossRef] [Green Version]
- Augusto, J.B.; Davies, R.H.; Bhuva, A.N.; Knott, K.D.; Seraphim, A.; Alfarih, M. Diagnosis and risk stratification in hypertrophic cardiomyopathy using machine learning wall thickness measurement: A comparison with human test-retest performance. Lancet Digit. Health 2021, 3, e20–e28. [Google Scholar] [CrossRef]
- Arends, M.; Wanner, C.; Hughes, D.; Mehta, A.; Oder, D.; Watkinson, O.T.; Elliott, P.M.; Linthorst, G.E.; Wijburg, F.A.; Biegstraaten, M.; et al. Characterization of Classical and Nonclassical Fabry Disease: A Multicenter Study. J. Am. Soc. Nephrol. 2017, 28, 1631–1641. [Google Scholar] [CrossRef] [Green Version]
- Vijapurapu, R.; Baig, S.; Nordin, S.; Augusto, J.; Price, A.M.; Wheeldon, N.; Lewis, N.; Kozor, R.; Kotecha, D.; Hodson, J.; et al. Longitudinal Assessment of Cardiac Involvement in Fabry Disease Using Cardiovascular Magnetic Resonance Imaging. JACC Cardiovasc. Imaging 2020, 13, 1850–1852. [Google Scholar] [CrossRef]
- Nordin, S.; Kozor, R.; Vijapurapu, R.; Augusto, J.; Knott, K.D.; Captur, G.; Treibel, T.; Ramaswami, U.; Tchan, M.; Geberhiwot, T.; et al. Myocardial Storage, Inflammation, and Cardiac Phenotype in Fabry Disease After One Year of Enzyme Replacement Therapy. Circ. Cardiovasc. Imaging 2019, 12, e009430. [Google Scholar] [CrossRef]
- Ho, C.; Sweitzer, N.K.; McDonough, B.; Maron, B.J.; Casey, S.A.; Seidman, J.; Seidman, C.E.; Solomon, S.D. Assessment of diastolic function with Doppler tissue imaging to predict genotype in preclinical hypertrophic cardiomyopathy. Circulation 2002, 105, 2992–2997. [Google Scholar] [CrossRef] [Green Version]
- Pieroni, M.; Chimenti, C.; Ricci, R.; Sale, P.; Russo, M.A.; Frustaci, A. Early detection of Fabry cardiomyopathy by tissue Doppler imaging. Circulation 2003, 107, 1978–1984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamorano, J.; Serra, V.; de Isla, L.P.; Feltes, G.; Calli, A.; Barbado, F.J.; Torras, J.; Hernandez, S.; Herrera, J.; Herrero, J.A.; et al. Usefulness of tissue Doppler on early detection of cardiac disease in Fabry patients and potential role of enzyme replacement therapy (ERT) for avoiding progression of disease. Eur. J. Echocardiogr. 2011, 12, 671–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weidemann, F.; Breunig, F.; Beer, M.; Knoll, A.; Turschner, O.; Wanner, C.; Sandstede, J.; Voelker, W.; Ertl, G.; Strotmann, J. Improvement of cardiac function during enzyme replacement therapy in patients with Fabry disease: A prospective strain rate imaging study. Circulation 2003, 108, 1299–1301. [Google Scholar] [CrossRef] [Green Version]
- Weidemann, F.; Niemann, M.; Breunig, F.; Herrmann, S.; Beer, M.; Störk, S. Long-term effects of enzyme replacement therapy on fabry cardiomyopathy: Evidence for a better outcome with early treatment. Circulation 2009, 119, 524–529. [Google Scholar] [CrossRef] [Green Version]
- Shanks, M.; Thompson, R.; Paterson, D.I.; Putko, B.; Khan, A.; Chan, A.; Becher, H.; Oudit, G.Y. Systolic and diastolic function assessment in fabry disease patients using speckle-tracking imaging and comparison with conventional echocardiographic measurements. J. Am. Soc. Echocardiogr. 2013, 26, 1407–1414. [Google Scholar] [CrossRef]
- Faggiano, A.; Avallone, C.; Gentile, D.; Provenzale, G.; Toriello, F.; Merlo, M.; Sinagra, G.; Carugo, S. Echocardiographic Advances in Di-lated Cariomyopathy. J. Clin. Med. 2021, 10, 5518. [Google Scholar] [CrossRef]
- Sado, D.M.; White, S.K.; Piechnik, S.K.; Banypersad, S.M.; Treibel, T.; Captur, G. Identification and assessment of Anderson-Fabry disease by cardiovascular magnetic resonance noncontrast myocardial T1 mapping. Circ. Cardiovasc. Imaging 2013, 6, 392–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordin, S.; Kozor, R.; Baig, S.; Abdel-Gadir, A.; Medina-Menacho, K.; Rosmini, S.; Captur, G.; Tchan, M.; Geberhiwot, T.; Murphy, E.; et al. Cardiac Phenotype of Prehypertrophic Fabry Disease. Circ. Cardiovasc. Imaging 2018, 11, e007168. [Google Scholar] [CrossRef] [Green Version]
- Pica, S.; Sado, D.M.; Maestrini, V.; Fontana, M.; White, S.K.; Treibel, T.; Captur, G.; Anderson, S.; Piechnik, S.K.; Robson, M.D.; et al. Reproducibility of native myocardial T1 mapping in the assessment of Fabry disease and its role in early detection of cardiac involvement by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2014, 16, 1–9. [Google Scholar] [CrossRef]
- Camporeale, A.; Pieroni, M.; Pieruzzi, F.; Lusardi, P.; Pica, S.; Spada, M. Predictors of Clinical Evolution in Prehypertrophic Fabry Disease. Circ. Cardiovasc. Imaging 2019, 12, e008424. [Google Scholar] [CrossRef] [PubMed]
- Knott, K.D.; Augusto, J.B.; Nordin, S.; Kozor, R.; Camaioni, C.; Xue, H.; Hughes, R.K.; Manisty, C.; Brown, L.A.E.; Kellman, P.; et al. Quantitative Myocardial Perfusion in Fabry Disease. Circ. Cardiovasc. Imaging 2019, 12, e008872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dass, S.; Suttie, J.J.; Piechnik, S.K.; Ferreira, V.M.; Holloway, C.J.; Banerjee, R.; Mahmod, M.; Cochlin, L.; Karamitsos, T.D.; Robson, M.D.; et al. Myocardial tissue characterization using magnetic resonance noncontract t1 mapping in hypertrophic and dilated cardiomyopathy. Circ. Cardiovasc. Imaging 2012, 5, 726–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seydelmann, N.; Liu, D.; Krämer, J.; Drechsler, C.; Hu, K.; Nordbeck, P. High-Sensitivity Troponin: A Clinical Blood Biomarker for Staging Cardiomyopathy in Fabry Disease. J. Am. Heart Assoc. 2016, 5, e002839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, D.A.; Aguiar, P.; Deegan, P.B.; Ezgu, F.; Frustaci, A.; Lidove, O. Early indicators of disease progression in Fabry disease that may indicate the need for disease-specific treatment initiation: Findings from the opinion-based PREDICT-FD modified Delphi consensus initiative. BMJ Open 2020, 10, e035182. [Google Scholar] [CrossRef]
- Nordin, S.; Kozor, R.; Menacho, K.; Abdel-Gadir, A.; Baig, S.; Sado, D.M.; Lobascio, I.; Murphy, E.; Lachmann, R.H.; Mehta, A.; et al. Proposed Stages of Myocardial Phenotype Development in Fabry Disease. JACC Cardiovasc. Imaging. 2019, 12, 1673–1683. [Google Scholar] [CrossRef]
- Moon, J.; Sachdev, B.; Elkington, A.G.; McKenna, W.J.; Mehta, A.; Pennell, D.; Leed, P.J.; Elliott, P. Gadolinium enhanced cardiovascular magnetic resonance in Anderson-Fabry disease. Evidence for a disease specific abnormality of the myocardial interstitium. Eur. Heart J. 2003, 24, 2151–2155. [Google Scholar] [CrossRef] [Green Version]
- Augusto, J.; Nordin, S.; Vijapurapu, R.; Baig, S.; Bulluck, H.; Castelletti, S.; Alfarih, M.; Knott, K.; Captur, G.; Kotecha, T.; et al. Myocardial Edema, Myocyte Injury, and Disease Severity in Fabry Disease. Circ. Cardiovasc. Imaging 2020, 13, e010171. [Google Scholar] [CrossRef]
- Imbriaco, M.; Nappi, C.; Ponsiglione, A.; Pisani, A.; Dell’Aversana, S.; Nicolai, E. Hybrid positron emission tomography-magnetic resonance imaging for assessing different stages of cardiac impairment in patients with Anderson-Fabry disease: AFFINITY study group. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1004–1011. [Google Scholar] [CrossRef]
- Spinelli, L.; Imbriaco, M.; Nappi, C.; Nicolai, E.; Giugliano, G.; Ponsiglione, A. Early Cardiac Involvement Affects Left Ventricular Longitudinal Function in Females Carrying α-Galactosidase A Mutation: Role of Hybrid Positron Emission Tomography and Magnetic Resonance Imaging and Speckle-Tracking Echocardiography. Circ. Cardiovasc. Imaging 2018, 11, e007019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yogasundaram, H.; Nikhanj, A.; Putko, B.N.; Boutin, M.; Jain-Ghai, S.; Khan, A.; Auray-Blais, C.; West, M.L.; Oudit, G.Y. Elevated Inflammatory Plasma Biomarkers in Patients with Fabry Disease: A Critical Link to Heart Failure with Preserved Ejection Fraction. J. Am. Heart Assoc. 2018, 7, e009098. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.R.; Cecchi, F.; Cizmarik, M.; Kantola, I.; Linhart, A.; Nicholls, K.; Strotmann, J.; Tallaj, J.; Tran, T.C.; West, M.L.; et al. Cardiovascular events in patients with fabry disease natural history data from the fabry registry. J. Am. Coll. Cardiol. 2011, 57, 1093–1099. [Google Scholar] [CrossRef] [Green Version]
- Feriozzi, S.; Linhart, A.; Ramaswami, U.; Kalampoki, V.; Gurevich, A.; Hughes, D. Effects of Baseline Left Ventricular Hypertrophy and Decreased Renal Function on Cardiovascular and Renal Outcomes in Patients with Fabry Disease Treated with Agalsidase Alfa: A Fabry Outcome Survey Study. Clin. Ther. 2020, 42, 2321–2330. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, L.; Giugliano, G.; Pisani, A.; Imbriaco, M.; Imbriaco, M.; Riccio, E.; Russo, C.; Cuocolo, A.; Trimarco, B.; Esposito, G. Does left ven-tricular function predict cardiac outcome in Anderson-Fabry disease. Int. J. Cardiovasc. Imaging 2021, 27, 1225–1236. [Google Scholar] [CrossRef]
- Hopkin, R.J.; Cabrera, G.; Charrow, J.; Lemay, R.; Martins, A.M.; Mauer, M.; Ortiz, A.; Patel, M.R.; Sims, K.; Waldek, S.; et al. Risk factors for severe clinical events in male and female patients with Fabry disease treated with agalsidase beta enzyme replacement therapy: Data from the Fabry Registry. Mol. Genet. Metab. 2016, 119, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Krämer, J.; Niemann, M.; Störk, S.; Frantz, S.; Beer, M.; Ertl, G. Relation of burden of myocardial fibrosis to malignant ventricular arrhythmias and outcomes in Fabry disease. Am. J. Cardiol. 2014, 114, 895–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zegard, A.; Okafor, O.; de Bono, J.; Kalla, M.; Lencioni, M.; Marshall, H.; Hudsmith, L.; Qiu, T.; Steeds, R.; Stegemann, B.; et al. Myocardial Fibrosis as a Predictor of Sudden Death in Patients with Coronary Artery Disease. J. Am. Coll. Cardiol. 2021, 77, 29–41. [Google Scholar] [CrossRef]
- Di Marco, A.; Anguera, I.; Schmitt, M.; Klem, I.; Neilan, T.G.; White, J.A. Late Gadolinium Enhancement and the Risk for Ventricular Arrhythmias or Sudden Death in Dilated Cardiomyopathy: Systematic Review and Meta-Analysis. JACC Heart Fail. 2017, 5, 28–38. [Google Scholar] [CrossRef]
- Tomberli, B.; Cecchi, F.; Sciagrà, R.; Berti, V.; Lisi, F.; Torricelli, F.; Morrone, A.; Castelli, G.; Yacoub, M.H.; Olivotto, I. Coronary microvascular dysfunction is an early feature of cardiac involvement in patients with Anderson-Fabry disease. Eur. J. Heart Fail. 2013, 15, 1363–1373. [Google Scholar] [CrossRef] [Green Version]
- Spinelli, L.; Imbriaco, M.; Giugliano, G.; Nappi, C.; Gaudieri, V.; Riccio, E.; Pisani, A.; Trimarco, B.; Cuocolo, A. Focal reduction in left ventricular. J. Nucl. Cardiol. 2021, 28, 641–649. [Google Scholar] [CrossRef]
- Chimenti, C.; Morgante, E.; Tanzilli, G.; Mangieri, E.; Critelli, G.; Gaudio, C.; Russo, M.A.; Frustaci, A. Angina in fabry disease reflects coronary small vessel disease. Circ. Heart Fail. 2008, 1, 161–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, A.; Umar, H.; Ochoa-Ferraro, A.; Warfield, A.; Lewis, N.; Geberhiwot, T. Atherosclerosis in Fabry Disease—A Contemporary Review. J. Clin. Med. 2021, 10, 4422. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.K.; Miller, A.; Bhuptani, A.; Sloane, M.F.; I Zimmerman, M.; Schilero, G.; Eng, C.M.; Desnick, R.J. Pulmonary involvement in Fabry disease. Am. J. Respir. Crit. Care Med. 1997, 155, 1004–1010. [Google Scholar] [CrossRef]
- Franzen, D.; Krayenbuehl, P.A.; Lidove, O.; Aubert, J.D.; Barbey, F. Pulmonary involvement in Fabry disease: Overview and perspectives. Eur. J. Intern. Med. 2013, 24, 707–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.Y.; Abe, J.T.; Cohen, A.H.; Wilcox, W.R. Enzyme replacement therapy stabilizes obstructive pulmonary Fabry disease associated with respiratory globotriaosylceramide storage. J. Inherit. Metab. Dis. 2008, 31 (Suppl. S2), S369–S374. [Google Scholar] [CrossRef]
- Wang, R.Y.; Lelis, A.; Mirocha, J.; Wilcox, W.R. Heterozygous Fabry women are not just carriers, but have a significant burden of disease and impaired quality of life. Genet. Med. 2007, 9, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Koskenvuo, J.W.; Kantola, I.M.; Nuutila, P.; Knuuti, J.; Parkkola, R.; Mononen, I.; Hurme, S.; Kalliokoski, R.; Viikari, J.S.; Wendelin-Saarenhovi, M.; et al. Cardiopulmonary involvement in Fabry’s disease. Acta Cardiol. 2010, 65, 185–192. [Google Scholar] [CrossRef]
- Vilcant, V.; Hai, O. Left Ventricular Outflow Tract Obstruction; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Wu, J.C.; Ho, C.Y.; Skali, H.; Abichandani, R.; Wilcox, W.R.; Banikazemi, M.; Packman, S.; Sims, K.; Solomon, S.D. Cardiovascular manifestations of Fabry disease: Relationships between left ventricular hypertrophy, disease severity, and alpha-galactosidase A activity. Eur. Heart J. 2010, 31, 1088–1097. [Google Scholar] [CrossRef] [Green Version]
- Calcagnino, M.; O’Mahony, C.; Coats, C.; Cardona, M.; Garcia, A.; Janagarajan, K.; Mehta, A.; Hughes, D.; Murphy, E.; Lachmann, R.; et al. Exercise-induced left ventricular outflow tract obstruction in symptomatic patients with Anderson-Fabry disease. J. Am. Coll. Cardiol. 2011, 58, 88–89. [Google Scholar] [CrossRef] [Green Version]
- Baig, S.; Edward, N.C.; Kotecha, D.; Liu, B.; Nordin, S.; Kozor, R.; Moon, J.C.; Geberhiwot, T.; Steeds, R.P. Ventricular arrhythmia and sudden cardiac death in Fabry disease: A systematic review of risk factors in clinical practice. Europace 2018, 20, F153–F161. [Google Scholar] [CrossRef]
- Issac, T.T.; Dokainish, H.; Lakkis, N.M. Role of inflammation in initiation and perpetuation of atrial fibrillation: A systematic review of the published data. J. Am. Coll. Cardiol. 2007, 50, 2021–2028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seko, Y.; Kato, T.; Haruna, T.; Izumi, T.; Miyamoto, S.; Nakane, E.; Inoko, M. Association between atrial fibrillation, atrial enlargement, and left ventricular geometric remodeling. Sci. Rep. 2018, 8, 6366. [Google Scholar] [CrossRef] [PubMed]
- Donal, E.; Lip, G.Y.H.; Galderisi, M.; Goette, A.; Shah, D.; Marwan, M.; Lederlin, M.; Mondillo, S.; Edvardsen, T.; Sitges, M.; et al. EACVI/EHRA Expert Consensus Document on the role of multi-modality imaging for the evaluation of patients with atrial fibrillation. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 355–383. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.C.; Lo, Q.; Devine, K.; Tchan, M.C.; Sillence, D.O.; Sadick, N.; Richards, D.A.; Thomas, L. Left atrial enlargement and reduced atrial compliance occurs early in Fabry cardiomyopathy. J. Am. Soc. Echocardiogr. 2013, 26, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Pichette, M.; Serri, K.; Pagé, M.; Di, L.Z.; Bichet, D.G.; Poulin, F. Impaired Left Atrial Function in Fabry Disease: A Longitudinal Speckle-Tracking Echocardiography Study. J. Am. Soc. Echocardiogr. 2017, 30, 170–179.e2. [Google Scholar] [CrossRef] [PubMed]
- Esposito, R.; Russo, C.; Santoro, C.; Cocozza, S.; Riccio, E.; Sorrentino, R.; Pontillo, G.; Luciano, F.; Imbriaco, M.; Brunetti, A.; et al. Association between Left Atrial Deformation and Brain Involvement in Patients with Anderson-Fabry Disease at Diagnosis. J. Clin. Med. 2020, 9, 2741. [Google Scholar] [CrossRef]
- Weidemann, F.; Strotmann, J.M.; Niemann, M.; Herrmann, S.; Wilke, M.; Beer, M. Heart valve involvement in Fabry cardiomyopathy. Ultrasound Med. Biol. 2009, 35, 730–735. [Google Scholar] [CrossRef]
- Linhart, A.; Kampmann, C.; Zamorano, J.L.; Sunder-Plassmann, G.; Beck, M.; Mehta, A. Cardiac manifestations of Anderson-Fabry disease: Results from the international Fabry outcome survey. Eur. Heart J. 2007, 28, 1228–1235. [Google Scholar] [CrossRef] [Green Version]
- Barbey, F.; Qanadli, S.D.; Juli, C.; Brakch, N.; Palaček, T.; Rizzo, E.; Jeanrenaud, X.; Eckhardt, B.; Linhart, A. Aortic remodelling in Fabry disease. Eur. Heart J. 2010, 31, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Yogasundaram, H.; Nikhanj, A.; Chatur, S.; Qi, A.; Hagen, L.; Bailey, L.; Khan, A.; Hopkin, R.J.; Fine, N.M.; Jefferies, J.L.; et al. Burden of Valvular Heart Disease in Patients with Fabry Disease. J. Am. Soc. Echocardiogr. 2021, 21, 762–768. [Google Scholar] [CrossRef]
- Shiina, T.; Nightingale, K.R.; Palmeri, M.L.; Hall, T.J.; Bamber, J.C.; Barr, R.G.; Castera, L.; Choi, B.I.; Chou, Y.-H.; Cosgrove, D.; et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: Basic principles and terminology. Ultrasound Med. Biol. 2015, 41, 1126–1147. [Google Scholar] [CrossRef] [Green Version]
- Brekke, B.; Nilsen, L.C.; Lund, J.; Torp, H.; Bjastad, T.; Amundsen, B.H.; Stoylen, A.; Aase, S.A. Ultra-high frame rate tissue Doppler imaging. Ultrasound Med. Biol. 2014, 40, 222–231. [Google Scholar] [CrossRef]
- Strachinaru, M.; Bosch, J.G.; van Dalen, B.M.; van Gils, L.; van der Steen, A.F.W.; de Jong, N. Cardiac Shear Wave Elastography Using a Clinical Ultrasound System. Ultrasound Med. Biol. 2017, 43, 1596–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, F.; Cafezeiro, C. NCT04456582 Noninvasive Assessment of Myocardial Stiffness by 2D-SWE Ultrasound Technique (Two-Dimensional Shear Wave Elastography) in Patients with Amyloidosis and Fabry Disease (FABRY); U.S. National Library of Medicine: Bethesda, MD, USA, 2021. [Google Scholar]
- Khalique, Z.; Ferreira, P.F.; Scott, A.D.; Nielles-Vallespin, S.; Firmin, D.N.; Pennell, D.J. Diffusion Tensor Cardiovascular Magnetic Resonance Imaging: A Clinical Perspective. JACC Cardiovasc. Imaging 2020, 13, 1235–1255. [Google Scholar] [CrossRef]
- Lewis, A.J.M.; Rider, O.J. The use of cardiovascular magnetic resonance for the assessment of left ventricular hypertrophy. Cardiovasc. Diagn. Ther. 2020, 10, 568–582. [Google Scholar] [CrossRef] [PubMed]
- Caredda, G.; Bassareo, P.P.; Cherchi, M.V.; Pontone, G.; Suri, J.S.; Saba, L. Anderson-fabry disease: Role of traditional and new cardiac MRI techniques. Br. J. Radiol. 2021, 94, 20210020. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.-Y.; Siontis, K.C.; Attia, Z.I.; Carter, R.E.; Kapa, S.; Ommen, S.R.; Demuth, S.J.; Ackerman, M.J.; Gersh, B.J.; Arruda-Olson, A.M.; et al. Detection of Hypertrophic Cardiomyopathy Using a Convolutional Neural Network-Enabled Electrocardiogram. J. Am. Coll. Cardiol. 2020, 75, 722–733. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, A.; Mansour, M.; Oxborough, D.; Geberhiwot, T.; Steeds, R. Multi-Modality Cardiovascular Imaging Assessment in Fabry Disease. Appl. Sci. 2022, 12, 1605. https://doi.org/10.3390/app12031605
Roy A, Mansour M, Oxborough D, Geberhiwot T, Steeds R. Multi-Modality Cardiovascular Imaging Assessment in Fabry Disease. Applied Sciences. 2022; 12(3):1605. https://doi.org/10.3390/app12031605
Chicago/Turabian StyleRoy, Ashwin, Mohamed Mansour, David Oxborough, Tarekegn Geberhiwot, and Richard Steeds. 2022. "Multi-Modality Cardiovascular Imaging Assessment in Fabry Disease" Applied Sciences 12, no. 3: 1605. https://doi.org/10.3390/app12031605






