Collection of Data Variation Using a High-Throughput Image-Based Assay Platform Facilitates Data-Driven Understanding of TRPA1 Agonist Diversity
Abstract
:1. Introduction
2. Materials and Methods
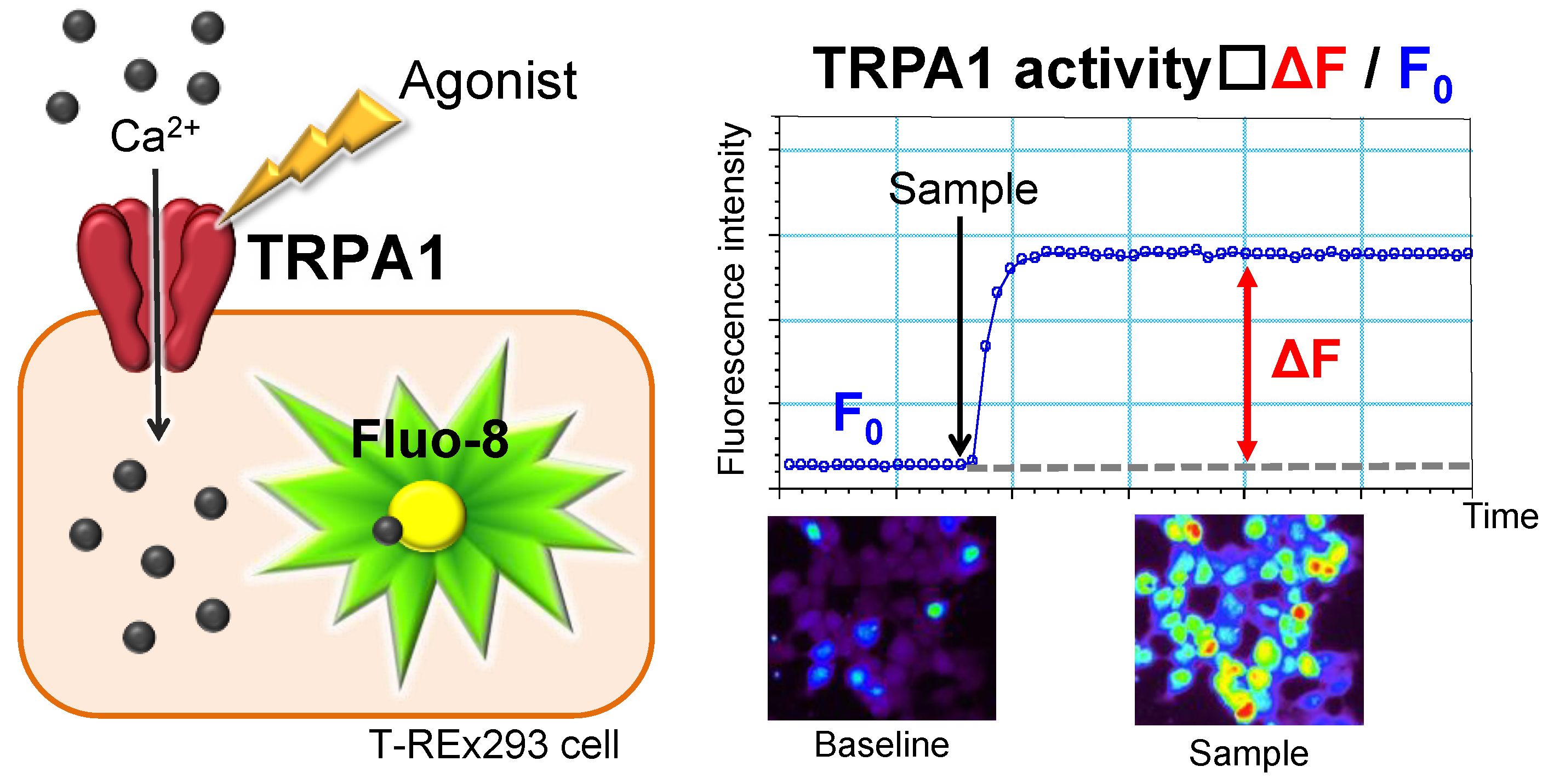
2.1. Image-Based Human TRPA1 Assay System
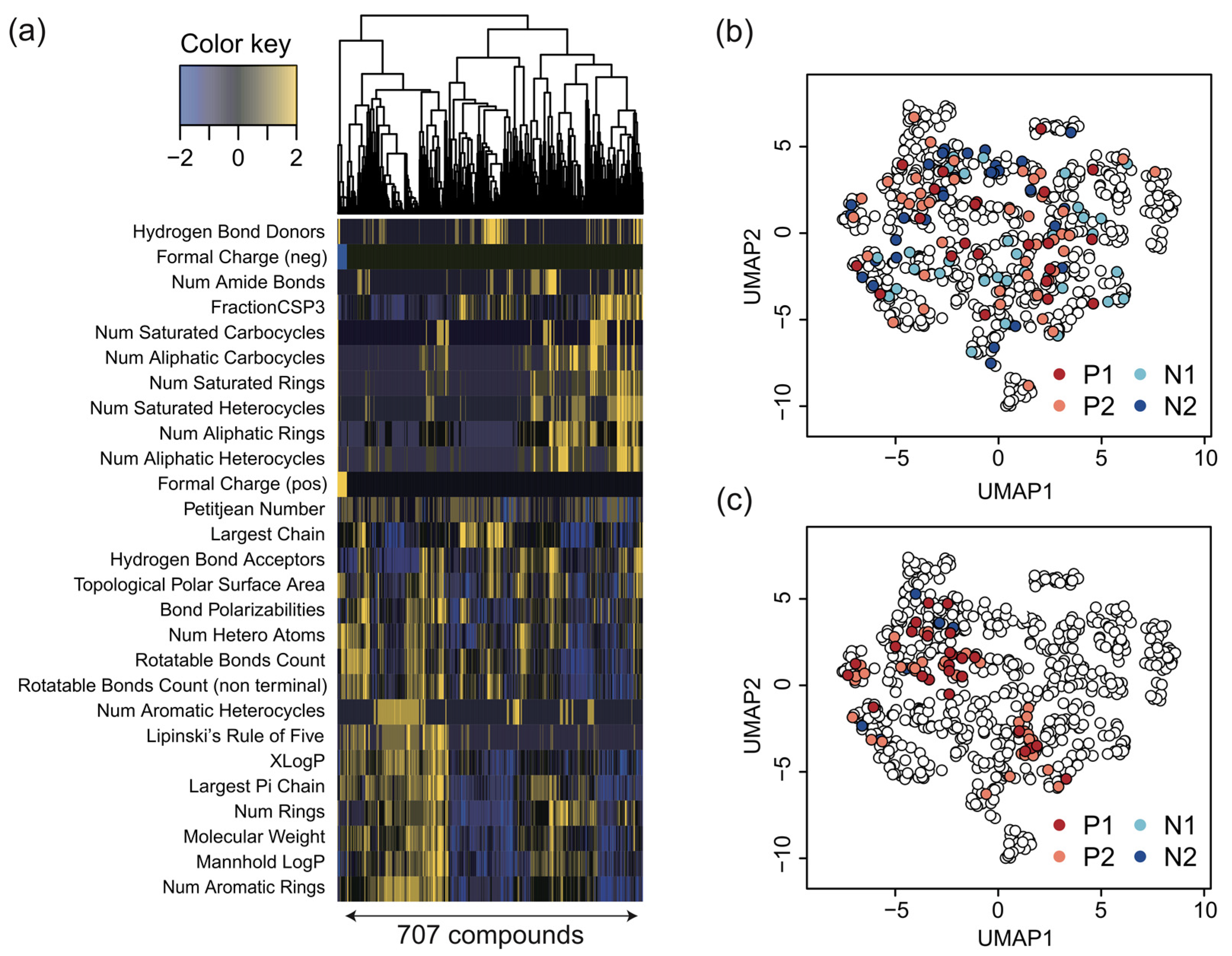
2.2. Generating Physicochemical Descriptors
2.3. Chemical Library (Library707) Information
2.4. Hierarchical Clustering and Compounds Grouping
3. Results and Discussion
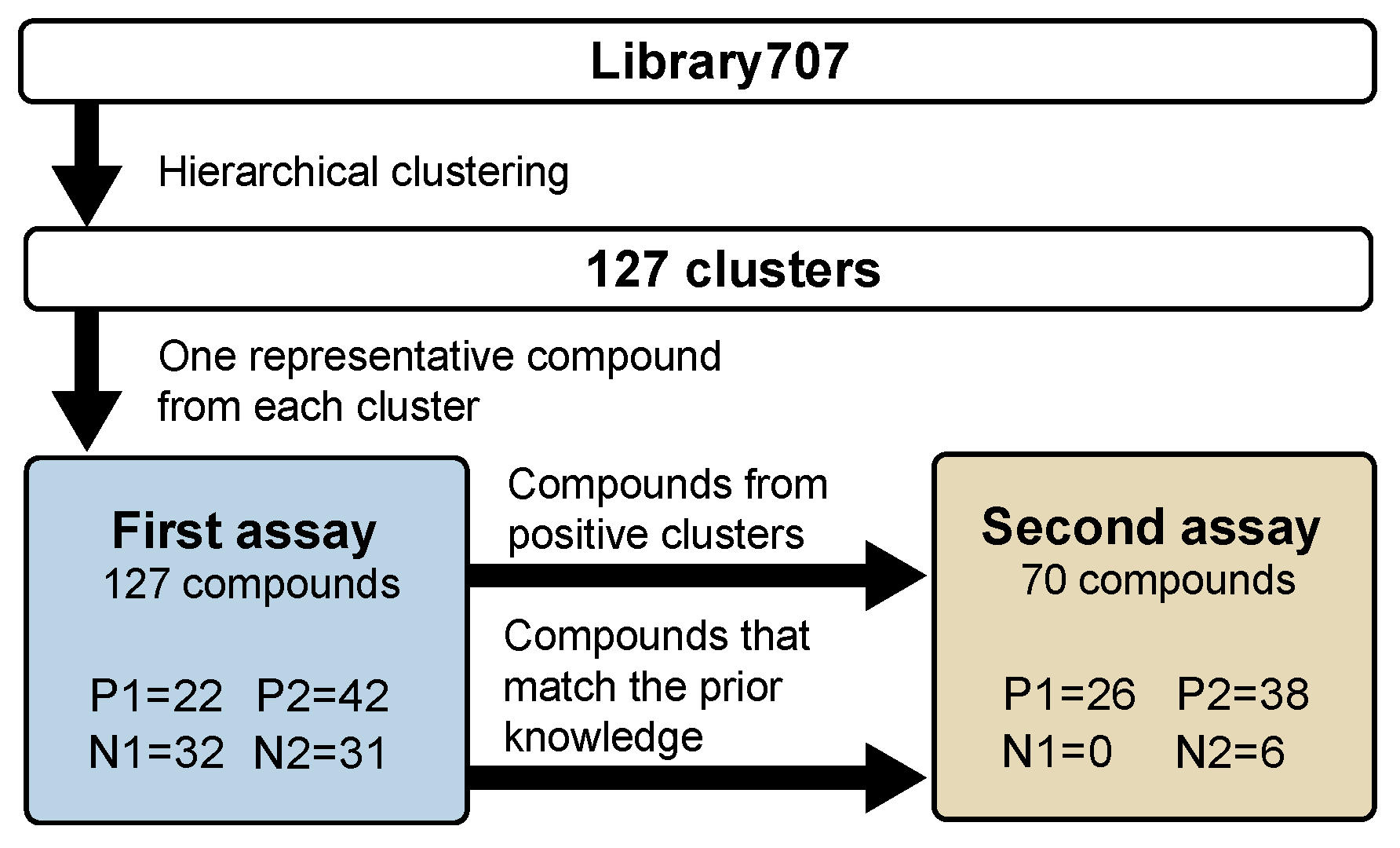
3.1. Design of Efficient Assay Platform for Collecting TRPA1 Agonist Variations
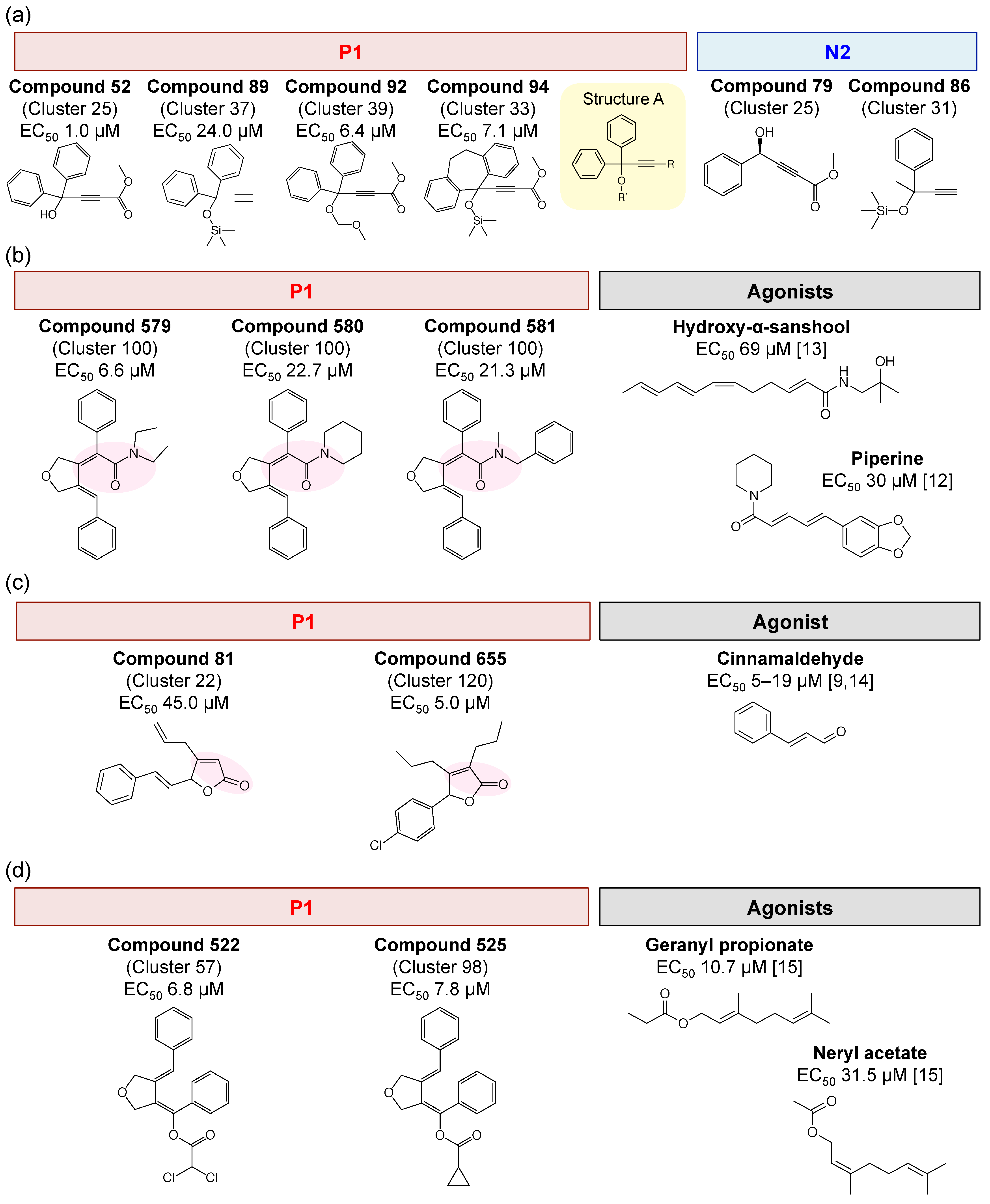
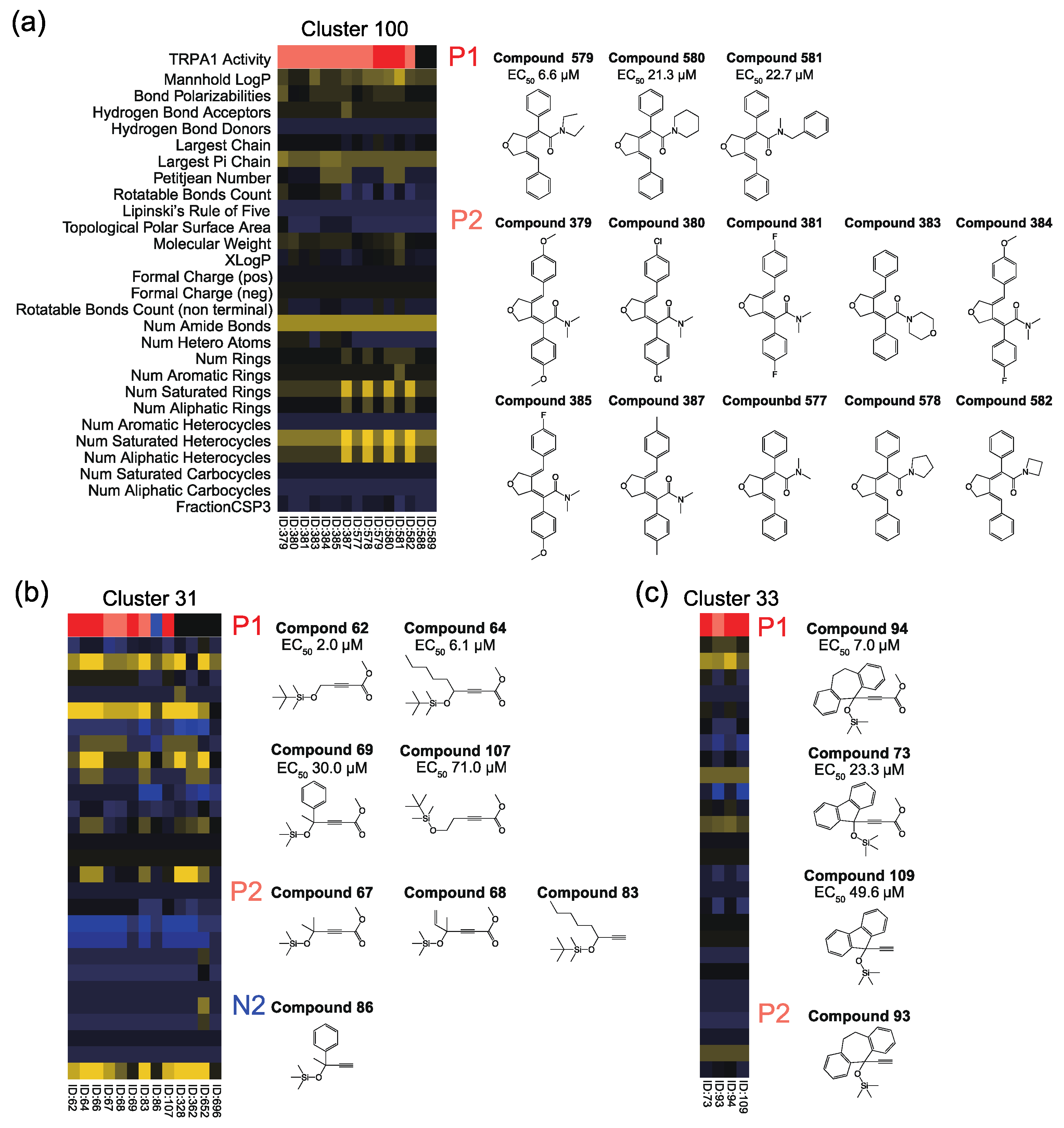
3.2. The First Assay (127 Compounds) Results and Obtained TRPA1 Agonists
3.3. The Second Assay (70 Compounds) Results and Obtained TRPA1 Agonists
3.4. Interpretation of Assay Results from Previous Agonist Structures
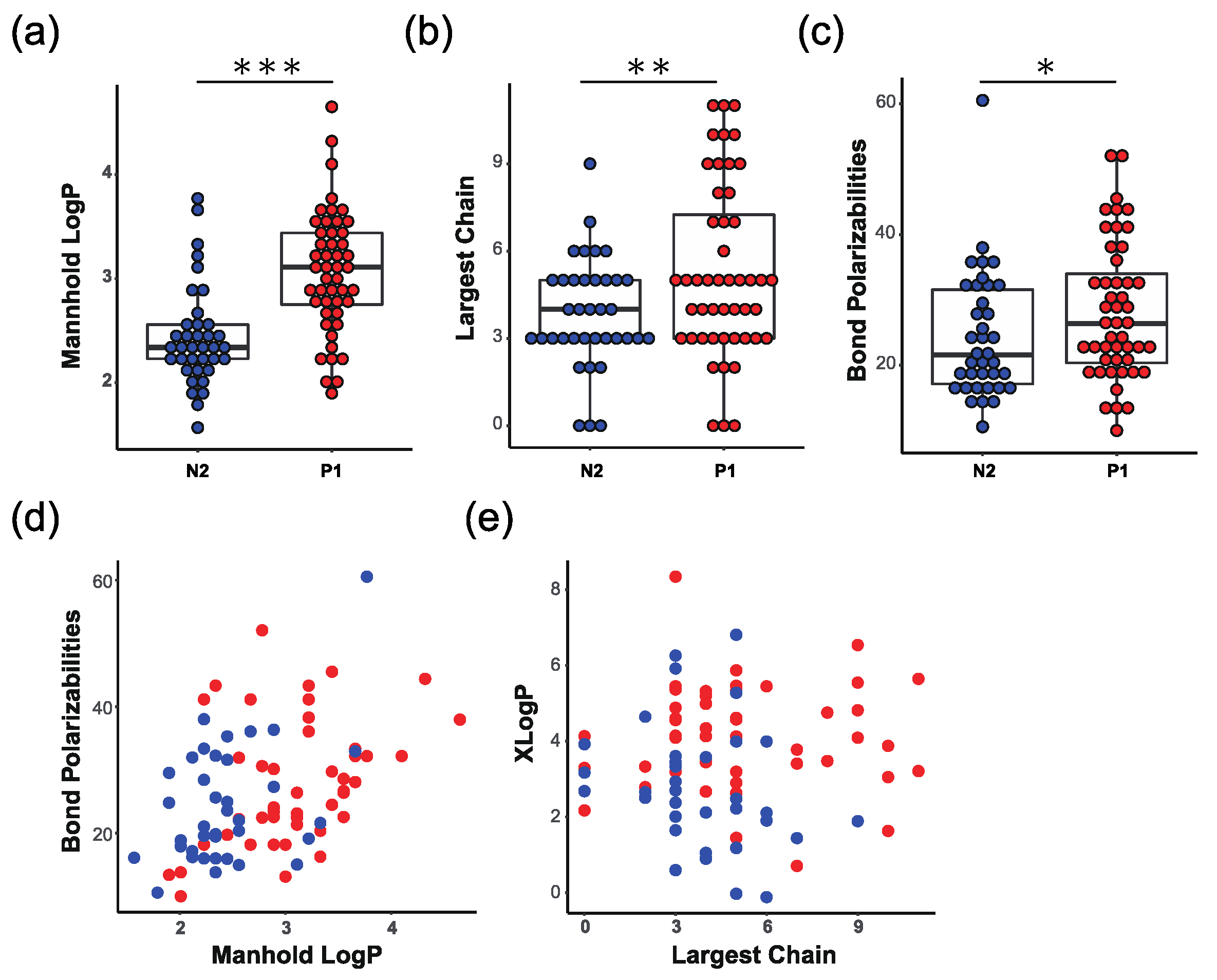
3.5. Interpretation of Assay Results from Physicochemical Descriptors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahajan, N.; Khare, P.; Kondepudi, K.K.; Bishnoi, M. TRPA1: Pharmacology, natural activators and role in obesity prevention. Eur. J. Pharmacol. 2021, 912, 174553. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ye, D.; Ye, J.; Wang, M.; Liu, J.; Jiang, H.; Xu, Y.; Zhang, J.; Chen, J.; Wan, J. The TRPA1 channel in the cardiovascular system: Promising features and challenges. Front. Pharmacol. 2019, 10, 1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talavera, K.; Startek, J.B.; Alvarez-Collazo, J.; Boonen, B.; Alpizar, Y.A.; Sanchez, A.; Naert, R.; Nilius, B. Mammalian transient receptor potential TRPA1 channels: From structure to disease. Phys. Rev. 2020, 100, 725–803. [Google Scholar] [CrossRef] [PubMed]
- Terada, Y.; Horie, S.; Takayama, H.; Uchida, K.; Tominaga, M.; Watanabe, T. Activation and inhibition of thermosensitive TRP channels by voacangine, an alkaloid present in Voacanga africana, an African tree. J. Nat. Prod. 2014, 77, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Mori, S.; Shibuya, M. A combined transition-metal-catalyzed and photopromoted process: Synthesis of 2,3-fused 4-phenylnaphthalen-1-yl carboxylates from 1,7-diaryl-1,6-diynes. Chem. Eur. J. 2015, 21, 9093–9100. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Shibuya, M.; Yamamoto, Y. Ruthenium-catalyzed hydrocarbamoylative cyclization of 1,6-diynes with formamides. Chem. Lett. 2016, 46, 207–210. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Shibano, S.; Kurohara, T.; Shibuya, M. Synthesis of β-allylbutenolides via one-pot copper-catalyzed hydroallylation/cyclization of γ-hydroxybutynoate derivatives. J. Org. Chem. 2014, 79, 4503–4511. [Google Scholar] [CrossRef] [PubMed]
- Hinman, A.; Chuang, H.H.; Bautista, D.M.; Julius, D. TRP channel activation by reversible covalent modification. Proc. Natl. Acad. Sci. USA 2006, 103, 19564–19568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macpherson, L.J.; Dubin, A.E.; Evans, M.J.; Marr, F.; Schultz, P.G.; Cravatt, B.F.; Patapoutian, A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 2007, 445, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Terada, Y.; Masuda, H.; Watanabe, T. Structure-activity relationship study on isothiocyanates: Comparison of TRPA1-activating ability between allyl isothiocyanate and specific flavor components of wasabi, horseradish, and white mustard. J. Nat. Prod. 2015, 78, 1937–1941. [Google Scholar] [CrossRef] [PubMed]
- Riera, C.E.; Menozzi-Smarrito, C.; Affolter, M.; Michlig, S.; Munari, C.; Robert, F.; Vogel, H.; Simon, S.A.; Le Coutre, J. Compounds from sichuan and melegueta peppers activate, covalently and non-covalently, TRPA1 and TRPV1 channels. Br. J. Pharmacol. 2009, 157, 1398–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okumura, Y.; Narukawa, M.; Iwasaki, Y.; Ishikawa, A.; Matsuda, H.; Yoshikawa, M.; Watanabe, T. Activation of TRPV1 and TRPA1 by black pepper components. Biosci. Biotechnol. Biochem. 2010, 74, 1068–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menozzi-Smarrito, C.; Riera, C.E.; Munari, C.; Le Coutre, J.; Robert, F. Synthesis and evaluation of new alkylamides derived from alpha-hydroxysanshool, the pungent molecule in szechuan pepper. J. Agric. Food Chem. 2009, 57, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Tanabe, M.; Kayama, Y.; Abe, M.; Kashio, M.; Koizumi, K.; Okumura, Y.; Morimitsu, Y.; Tominaga, M.; Ozawa, Y.; et al. Miogadial and miogatrial with alpha, beta-unsaturated 1,4-dialdehyde moieties—Novel and potent TRPA1 agonists. Life Sci. 2009, 85, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Terada, Y.; Yamashita, R.; Ihara, N.; Yamazaki-Ito, T.; Takahashi, Y.; Masuda, H.; Sakuragawa, S.; Ito, S.; Ito, K.; Watanabe, T. Human TRPA1 activation by terpenes derived from the essential oil of daidai, Citrus aurantium L. var. daidai Makino. Biosci. Biotechnol. Biochem. 2019, 83, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Meents, J.E.; Ciotu, C.I.; Fischer, M. TRPA1: A molecular view. J. Neurophysiol. 2019, 121, 427–443. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terada, Y.; Tanaka, K.; Matsuyama, M.; Fujitani, M.; Shibuya, M.; Yamamoto, Y.; Kato, R.; Ito, K. Collection of Data Variation Using a High-Throughput Image-Based Assay Platform Facilitates Data-Driven Understanding of TRPA1 Agonist Diversity. Appl. Sci. 2022, 12, 1622. https://doi.org/10.3390/app12031622
Terada Y, Tanaka K, Matsuyama M, Fujitani M, Shibuya M, Yamamoto Y, Kato R, Ito K. Collection of Data Variation Using a High-Throughput Image-Based Assay Platform Facilitates Data-Driven Understanding of TRPA1 Agonist Diversity. Applied Sciences. 2022; 12(3):1622. https://doi.org/10.3390/app12031622
Chicago/Turabian StyleTerada, Yuko, Kenjiro Tanaka, Minami Matsuyama, Masaya Fujitani, Masatoshi Shibuya, Yoshihiko Yamamoto, Ryuji Kato, and Keisuke Ito. 2022. "Collection of Data Variation Using a High-Throughput Image-Based Assay Platform Facilitates Data-Driven Understanding of TRPA1 Agonist Diversity" Applied Sciences 12, no. 3: 1622. https://doi.org/10.3390/app12031622






