A Pelvic Reconstruction Procedure for Custom-Made Prosthesis Design of Bone Tumor Surgical Treatments
Abstract
:1. Introduction
2. Methods
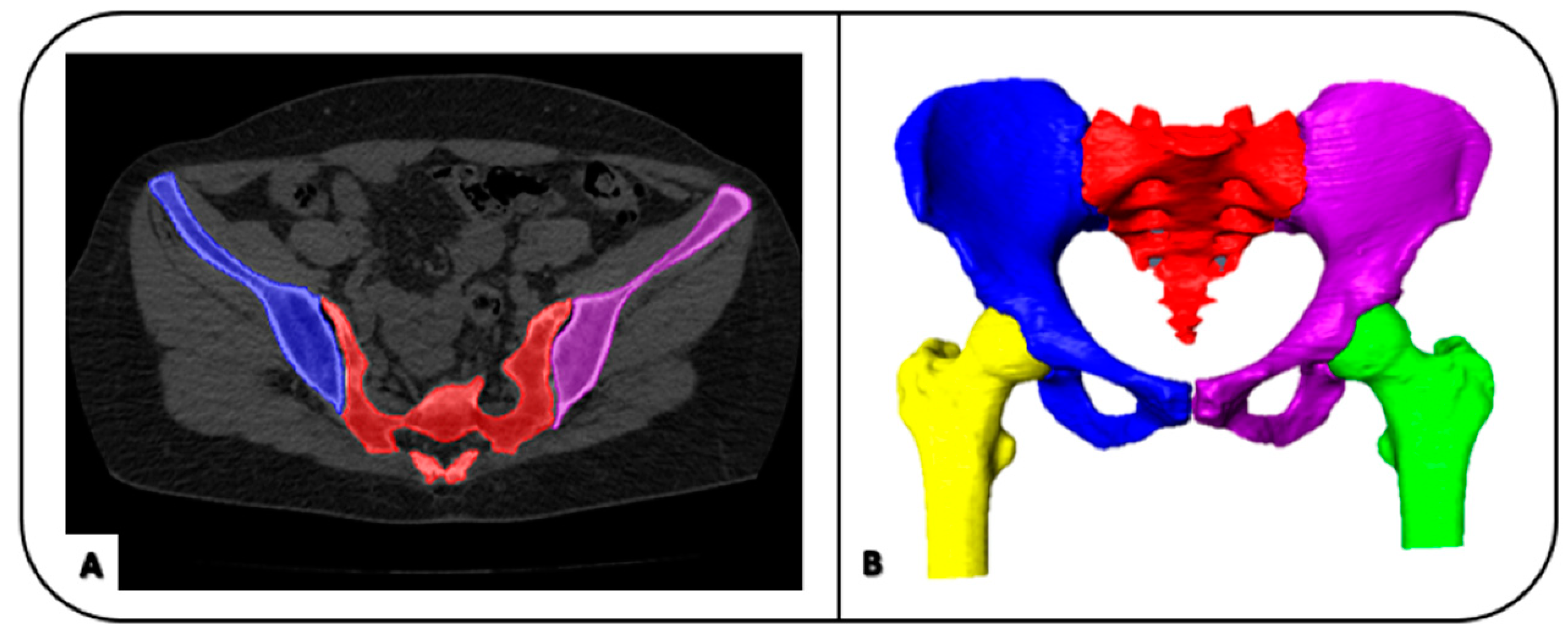
2.1. Image Processing: Image Segmentation and Geometrical Modelling
2.2. Registrations of 3D Models
2.3. Design of Bone Resection Planes
2.4. Creation of the Models for the Final Surgery
2.5. Virtual Planning Analysis and Post-Operative Evaluations
3. Results
3.1. Registration of 3D Models
3.2. Creation of Final Models for Implant Design
3.3. Virtual Planning Analysis and Post-Operative Evaluations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, J.W.; Kang, H.G.; Lim, K.M.; Park, D.W.; Kim, J.H.; Kim, H.S. Bone tumor resection guide using three-dimensional printing for limb salvage surgery. J. Surg. Oncol. 2018, 118, 898–905. [Google Scholar] [CrossRef]
- Enneking, W.F.; Dunham, W.K. Resection and reconstruction for primary neoplasms involving the innominate bone. J. Bone Jt. Surg. Am. 1978, 60, 731–746. [Google Scholar] [CrossRef]
- Zhu, D.; Fu, J.; Wang, L.; Guo, Z.; Wang, Z.; Fan, H. Reconstruction with customized, 3D-printed prosthesis after resection of periacetabular Ewing’s sarcoma in children using “triradiate cartilage-based” surgical strategy:a technical note. J. Orthop. Translat. 2021, 28, 108–117. [Google Scholar] [CrossRef]
- Angelini, A.; Calabro, T.; Pala, E.; Trovarelli, G.; Maraldi, M.; Ruggieri, P. Resection and reconstruction of pelvic bone tumors. Orthopedics 2015, 38, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennessy, D.W.; Santiago, M.E.A.; Lozano-Calderón, A. Complex Pelvic Reconstruction using Patient-Specific Instrumentation and a 3D-Printed Custom Implant following Tumor Resection. J. Hip Surg. 2017, 2, 061–067. [Google Scholar] [CrossRef]
- Matar, H.E.; Selvaratnam, V.; Shah, N.; Wynn Jones, H. Custom triflange revision acetabular components for significant bone defects and pelvic discontinuity: Early UK experience. J. Orthop. 2020, 21, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Dong, E.; Wang, L.; Iqbal, T.; Li, D.; Liu, Y.; He, J.; Zhao, B.; Li, Y. Finite Element Analysis of the Pelvis after Customized Prosthesis Reconstruction. J. Bionic Eng. 2018, 15, 443–451. [Google Scholar] [CrossRef]
- Fujiwara, T.; Medellin Rincon, M.R.; Sambri, A.; Tsuda, Y.; Clark, R.; Stevenson, J.; Parry, M.C.; Grimer, R.J.; Jeys, L. Limb-salvage reconstruction following resection of pelvic bone sarcomas involving the acetabulum. Bone Jt. J. 2021, 103-B, 795–803. [Google Scholar] [CrossRef]
- Ozaki, T.; Hillmann, A.; Bettin, D.; Wuisman, P.; Winkelmann, W. High complication rates with pelvic allografts. Experience of 22 sarcoma resections. Acta Orthop. Scand. 1996, 67, 333–338. [Google Scholar] [CrossRef] [Green Version]
- Abudu, A.; Grimer, R.J.; Cannon, S.R.; Carter, S.R.; Sneath, R.S. Reconstruction of the hemipelvis after the excision of malignant tumours. Complications and functional outcome of prostheses. J. Bone Jt. Surg. Br. 1997, 79, 773–779. [Google Scholar] [CrossRef]
- Gebert, C.; Wessling, M.; Hoffmann, C.; Roedl, R.; Winkelmann, W.; Gosheger, G.; Hardes, J. Hip transposition as a limb salvage procedure following the resection of periacetabular tumors. J. Surg. Oncol. 2011, 103, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.A.; van de Sande, M.A.; Dijkstra, P.D. Poor long-term clinical results of saddle prosthesis after resection of periacetabular tumors. Clin. Orthop. Relat. Res. 2013, 471, 324–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelini, A.; Trovarelli, G.; Berizzi, A.; Pala, E.; Breda, A.; Ruggieri, P. Three-dimension-printed custom-made prosthetic reconstructions: From revision surgery to oncologic reconstructions. Int. Orthop. 2019, 43, 123–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belvedere, C.; Siegler, S.; Fortunato, A.; Caravaggi, P.; Liverani, E.; Durante, S.; Ensini, A.; Konow, T.; Leardini, A. New comprehensive procedure for custom-made total ankle replacements: Medical imaging, joint modeling, prosthesis design, and 3D printing. J. Orthop. Res. 2019, 37, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Kang, H.G.; Kim, J.H.; Kim, H.S. The application of 3D-printing technology in pelvic bone tumor surgery. J. Orthop. Sci. 2021, 26, 276–283. [Google Scholar] [CrossRef]
- Sun, W.; Li, J.; Li, Q.; Li, G.; Cai, Z. Clinical effectiveness of hemipelvic reconstruction using computer-aided custom-made prostheses after resection of malignant pelvic tumors. J. Arthroplast. 2011, 26, 1508–1513. [Google Scholar] [CrossRef]
- Liang, H.; Ji, T.; Zhang, Y.; Wang, Y.; Guo, W. Reconstruction with 3D-printed pelvic endoprostheses after resection of a pelvic tumour. Bone Jt. J. 2017, 99-B, 267–275. [Google Scholar] [CrossRef]
- Iqbal, T.; Shi, L.; Wang, L.; Liu, Y.; Li, D.; Qin, M.; Jin, Z. Development of finite element model for customized prostheses design for patient with pelvic bone tumor. Proc. Inst. Mech. Eng. H 2017, 231, 525–533. [Google Scholar] [CrossRef]
- Colen, S.; Dalemans, A.; Schouwenaars, A.; Mulier, M. Outcome of custom-made IMP femoral components of total hip arthroplasty: A follow-up of 15 to 22 years. J. Arthroplast. 2014, 29, 397–400. [Google Scholar] [CrossRef]
- Fan, H.; Fu, J.; Li, X.; Pei, Y.; Li, X.; Pei, G.; Guo, Z. Implantation of customized 3-D printed titanium prosthesis in limb salvage surgery: A case series and review of the literature. World J. Surg. Oncol. 2015, 13, 308. [Google Scholar] [CrossRef] [Green Version]
- Xiu, P.; Jia, Z.; Lv, J.; Yin, C.; Cheng, Y.; Zhang, K.; Song, C.; Leng, H.; Zheng, Y.; Cai, H.; et al. Tailored Surface Treatment of 3D Printed Porous Ti6Al4V by Microarc Oxidation for Enhanced Osseointegration via Optimized Bone In-Growth Patterns and Interlocked Bone/Implant Interface. ACS Appl. Mater. Interfaces 2016, 8, 17964–17975. [Google Scholar] [CrossRef] [PubMed]
- Sing, S.L.; An, J.; Yeong, W.Y.; Wiria, F.E. Laser and electron-beam powder-bed additive manufacturing of metallic implants: A review on processes, materials and designs. J. Orthop. Res. 2016, 34, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Snis, A.; Matic, A.; Thomsen, P.; Palmquist, A. 3D printed Ti6Al4V implant surface promotes bone maturation and retains a higher density of less aged osteocytes at the bone-implant interface. Acta Biomater. 2016, 30, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Angelini, A.; Kotrych, D.; Trovarelli, G.; Szafranski, A.; Bohatyrewicz, A.; Ruggieri, P. Analysis of principles inspiring design of three-dimensional-printed custom-made prostheses in two referral centres. Int. Orthop. 2020, 44, 829–837. [Google Scholar] [CrossRef]
- Attarilar, S.; Ebrahimi, M.; Djavanroodi, F.; Fu, Y.; Wang, L.; Yang, J. 3D Printing Technologies in Metallic Implants: A Thematic Review on the Techniques and Procedures. Int. J. Bioprint 2021, 7, 306. [Google Scholar] [CrossRef]
- Wang, B.; Hao, Y.; Pu, F.; Jiang, W.; Shao, Z. Computer-aided designed, three dimensional-printed hemipelvic prosthesis for peri-acetabular malignant bone tumour. Int. Orthop. 2018, 42, 687–694. [Google Scholar] [CrossRef]
- Fang, C.; Cai, H.; Kuong, E.; Chui, E.; Siu, Y.C.; Ji, T.; Drstvensek, I. Surgical applications of three-dimensional printing in the pelvis and acetabulum: From models and tools to implants. Unfallchirurg 2019, 122, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Sohling, N.; Neijhoft, J.; Nienhaus, V.; Acker, V.; Harbig, J.; Menz, F.; Ochs, J.; Verboket, R.D.; Ritz, U.; Blaeser, A.; et al. 3D-Printing of Hierarchically Designed and Osteoconductive Bone Tissue Engineering Scaffolds. Materials 2020, 13, 1836. [Google Scholar] [CrossRef]
- Pei, X.; Ma, L.; Zhang, B.; Sun, J.; Sun, Y.; Fan, Y.; Gou, Z.; Zhou, C.; Zhang, X. Creating hierarchical porosity hydroxyapatite scaffolds with osteoinduction by three-dimensional printing and microwave sintering. Biofabrication 2017, 9, 045008. [Google Scholar] [CrossRef]
- Wong, K.C.; Kumta, S.M.; Geel, N.V.; Demol, J. One-step reconstruction with a 3D-printed, biomechanically evaluated custom implant after complex pelvic tumor resection. Comput. Aided Surg. 2015, 20, 14–23. [Google Scholar] [CrossRef]
- Chen, X.; Xu, L.; Wang, Y.; Hao, Y.; Wang, L. Image-guided installation of 3D-printed patient-specific implant and its application in pelvic tumor resection and reconstruction surgery. Comput. Methods Programs Biomed. 2016, 125, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Gouin, F.; Paul, L.; Odri, G.A.; Cartiaux, O. Computer-Assisted Planning and Patient-Specific Instruments for Bone Tumor Resection within the Pelvis: A Series of 11 Patients. Sarcoma 2014, 2014, 842709. [Google Scholar] [CrossRef]
- Cartiaux, O.; Paul, L.; Francq, B.G.; Banse, X.; Docquier, P.L. Improved accuracy with 3D planning and patient-specific instruments during simulated pelvic bone tumor surgery. Ann. Biomed. Eng. 2014, 42, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C.; Sze, K.Y.; Wong, I.O.; Wong, C.M.; Kumta, S.M. Patient-specific instrument can achieve same accuracy with less resection time than navigation assistance in periacetabular pelvic tumor surgery: A cadaveric study. Int. J. Comput. Assist. Radiol. Surg. 2016, 11, 307–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, K.C.; Kumta, S.M.; Sze, K.Y.; Wong, C.M. Use of a patient-specific CAD/CAM surgical jig in extremity bone tumor resection and custom prosthetic reconstruction. Comput. Aided Surg. 2012, 17, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, T.; Wang, L.; Li, D.; Dong, E.; Fan, H.; Fu, J.; Hu, C. A general multi-objective topology optimization methodology developed for customized design of pelvic prostheses. Med. Eng. Phys. 2019, 69, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Park, D.W.L.A.; Park, J.W.; Lim, K.M.; Kang, H.G. Biomechanical Evaluation of a New Fixation Type in 3D-Printed Periacetabular Implants using a Finite Element Simulation. Appl. Sci. 2019, 9, 820. [Google Scholar] [CrossRef] [Green Version]
- Durastanti, G.; Leardini, A.; Siegler, S.; Durante, S.; Bazzocchi, A.; Belvedere, C. Comparison of cartilage and bone morphological models of the ankle joint derived from different medical imaging technologies. Quant. Imaging Med. Surg. 2019, 9, 1368–1382. [Google Scholar] [CrossRef]
- De Paolis, M.; Sambri, A.; Zucchini, R.; Frisoni, T.; Spazzoli, B.; Taddei, F.; Donati, D.M. Custom made 3D-printed prosthesis in periacetabular resections through a novel ileoadductor approach. Orthopedics 2021. [Google Scholar] [CrossRef]
- Van Eijnatten, M.; van Dijk, R.; Dobbe, J.; Streekstra, G.; Koivisto, J.; Wolff, J. CT image segmentation methods for bone used in medical additive manufacturing. Med. Eng. Phys. 2018, 51, 6–16. [Google Scholar] [CrossRef]
- Besl, P.J.; McKay, N.D. A method for registration of 3-D shapes. IEEE Trans. Pattern Anal. Mach. Intell. 1992, 14, 239–256. [Google Scholar] [CrossRef]
- Bohme, J.; Shim, V.; Hoch, A.; Mutze, M.; Muller, C.; Josten, C. Clinical implementation of finite element models in pelvic ring surgery for prediction of implant behavior: A case report. Clin. Biomech. 2012, 27, 872–878. [Google Scholar] [CrossRef] [PubMed]






| FIRST MODEL - Affected Hemipelvis Meant to Host the Implant | SECOND MODEL - Hemipelvis Section Containing the Tumor | THIRD MODEL - Model of the First Shape of the Implant | |
|---|---|---|---|
| SECTION AREA (mm2) | 2454.8 | 2454.8 | 2489.6 |
| VOLUME (mm3) | 178,601.5 | 103,961.6 | 135,535.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durastanti, G.; Belvedere, C.; Ruggeri, M.; Donati, D.M.; Spazzoli, B.; Leardini, A. A Pelvic Reconstruction Procedure for Custom-Made Prosthesis Design of Bone Tumor Surgical Treatments. Appl. Sci. 2022, 12, 1654. https://doi.org/10.3390/app12031654
Durastanti G, Belvedere C, Ruggeri M, Donati DM, Spazzoli B, Leardini A. A Pelvic Reconstruction Procedure for Custom-Made Prosthesis Design of Bone Tumor Surgical Treatments. Applied Sciences. 2022; 12(3):1654. https://doi.org/10.3390/app12031654
Chicago/Turabian StyleDurastanti, Gilda, Claudio Belvedere, Miriana Ruggeri, Davide Maria Donati, Benedetta Spazzoli, and Alberto Leardini. 2022. "A Pelvic Reconstruction Procedure for Custom-Made Prosthesis Design of Bone Tumor Surgical Treatments" Applied Sciences 12, no. 3: 1654. https://doi.org/10.3390/app12031654
APA StyleDurastanti, G., Belvedere, C., Ruggeri, M., Donati, D. M., Spazzoli, B., & Leardini, A. (2022). A Pelvic Reconstruction Procedure for Custom-Made Prosthesis Design of Bone Tumor Surgical Treatments. Applied Sciences, 12(3), 1654. https://doi.org/10.3390/app12031654








