An Evaluation of Sporicidal Treatments against Blown Pack Spoilage Associated Clostridium estertheticum and Clostridium gasigenes Spores
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Preparation of Spore Inoculum
2.2. Sporicidal Treatments
2.3. Suspension Test
2.4. Determination of Dipicolinic acid (DPA) Release from Spores
2.5. Statistical Analysis
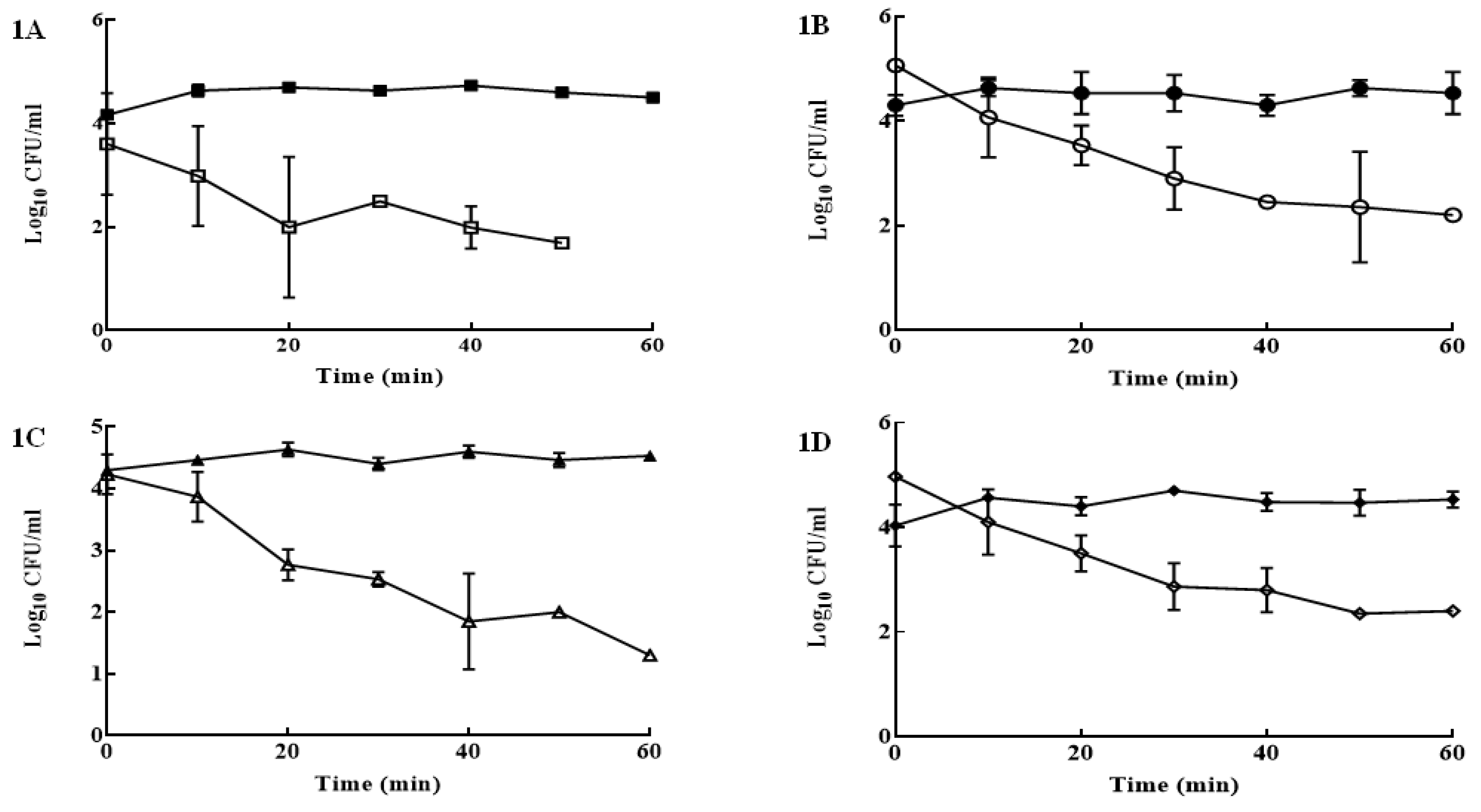
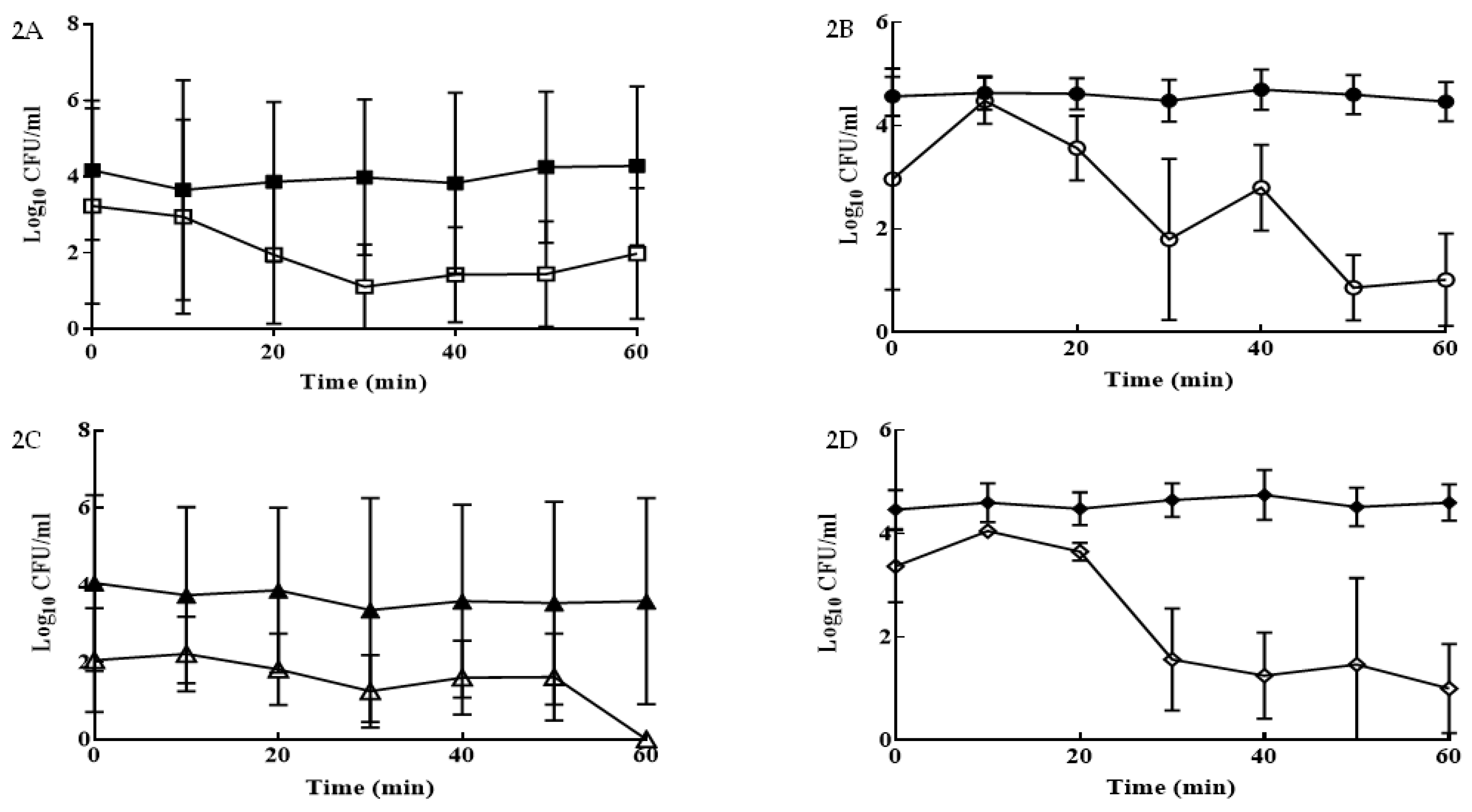
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalchayanand, N.; Ray, B.; Field, R.A.; Johnson, M.C. Spoilage of Vacuum-Packaged Refrigerated Beef by Clostridium. J. Food Prot. 1989, 52, 424–426. [Google Scholar] [CrossRef]
- Broda, D.M. Psychrotrophic Clostridium spp. associated with ‘blown pack’ spoilage of chilled vacuum-packed red meats and dog rolls in gas-impermeable plastic casings. Int. J. Food Microbiol. 2002, 29, 335–352. [Google Scholar] [CrossRef]
- Cavill, L.; Renteria-Monterrubio, A.L.; Helps, C.R.; Corry, J.E. Detection of cold-tolerant clostridia other than Clostridium estertheticum in raw vacuum-packed chill-stored meat. Food Microbiol. 2011, 28, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Broda, D.M.; De Lacy, K.M.; Cook, L.; Bell, G.R. Prevalence of cold-tolerant clostridia associated with vacuum-packed beef and lamb stored at abusive and chill temperatures. N. Z. J. Agric. Res. 1997, 40, 93–98. [Google Scholar] [CrossRef]
- Broda, D.M.; Bell, R.G.; Boerema, J.A.; Musgrave, D.R. The abattoir source of culturable psychrophilic Clostridium spp. causing ‘blown pack’ spoilage of vacuum-packed chilled venison. J. Appl. Microbiol. 2002, 93, 817–824. [Google Scholar] [CrossRef]
- Moschonas, G.; Bolton, D.J.; Sheridan, J.J.; McDowell, D.A. Isolation and sources of blown pack spoilage clostridia in beef abattoirs. J. Appl. Microbiol. 2009, 107, 616–624. [Google Scholar] [CrossRef]
- Moschonas, G.; Bolton, D.J.; McDowell, D.A.; Sheridan, J.J. Diversity of culturable psychrophilic and psychrotrophic anaerobic bacteria isolated from beef abattoirs and their environments. Appl. Environ. Microbiol. 2011, 77, 4280–4284. [Google Scholar] [CrossRef] [Green Version]
- Esteves, E.; Whyte, P.; Gupta, T.B.; Bolton, D.J. An investigation of the ecological niches and seasonal nature of Clostridium estertheticum and Clostridium gasigenes in the Irish beef farm environment. Lett. Appl. Microbiol. 2020, 71, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Maillard, J.-Y. Innate resistance to sporicides and potential failure to decontaminate. J. Hosp. Infect. 2011, 77, 204–209. [Google Scholar] [CrossRef]
- Boerema, J.A.; Broda, D.M.; Penney, N.; Brightwell, G. Influence of Peryoxyacetic Acid-Based Carcass Rinse on the Onset of ‘Blown Pack’ Spoilage in Artificially Inoculated Vacuum-Packed Chilled Beef. J. Food Prot. 2007, 70, 1434–1439. [Google Scholar] [CrossRef]
- Broda, D.M. The effect of peroxyacetic acid-based sanitizer, heat and ultrasonic waves on the survival of Clostridium estertheticum spores in vitro. Lett. Appl. Microbiol. 2007, 45, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Brightwell, G.; Clemens, R.; Adam, K.; Urlich, S.; Boerema, J. Comparison of culture-dependent and independent techniques for characterisation of the microflora of peryoxyacetic acid treated, vacuum-packaged beef. Food Microbiol. 2009, 26, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.; Horváth, K.M.; Brightwell, G. Antimicrobial effect of different peroxyacetic acid and hydrogen peroxide formats against spores of Clostridium estertheticum. Meat Sci. 2018, 143, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D. Bacterial Spores and Chemical Sporicidal Agents. Clin. Microbiol. Rev. 1990, 3, 99–119. [Google Scholar] [CrossRef]
- Alasri, A.; Valverde, M.; Roques, C.; Michel, G.; Cabassud, C.; Aptel, P. Sporocidal properties of peracetic acid and hydrogen peroxide, alone and in combination, in comparison with chlorine and formaldehyde for ultrafiltration membrane disinfection. Can. J. Microbiol. 1993, 39, 52–60. [Google Scholar] [CrossRef]
- Melly, E.; Cowan, A.E.; Setlow, P. Studies on the mechanism of killing of Bacillus subtilis spores by hydrogen peroxide. J. Appl. Microbiol. 2002, 93, 316–325. [Google Scholar] [CrossRef] [Green Version]
- Leggett, M.J.; Schwarz, J.S.; Burke, P.A.; McDonnell, G.; Denyer, S.P.; Maillard, J.Y. Mechanism of Sporicidal Activity for the Synergistic Combination of Peracetic Acid and Hydrogen Peroxide. Appl. Environ. Microbiol. 2016, 82, 1035–1039. [Google Scholar] [CrossRef] [Green Version]
- Sisti, M.; Brandi, G.; De Santi, M.; Rinaldi, L.; Schiavano, G.F. Disinfection efficacy of chlorine and peracetic acid alone or in combination against Aspergillus spp. and Candida albicans in drinking water. J. Water Health 2012, 10, 11–19. [Google Scholar] [CrossRef]
- Straus, D.L.; Meinelt, T.; Farmer, B.D.; Mitchell, A.J. Peracetic acid is effective for controlling fungus on channel catfish eggs. J. Fish Dis. 2012, 35, 505–511. [Google Scholar] [CrossRef]
- Zhang, C.; Brown, P.J.B.; Miles, R.J.; White, T.A.; Grant, D.G.; Stalla, D.; Hu, Z. Inhibition of regrowth of planktonic and biofilm bacteria after peracetic acid disinfection. Water Res. 2019, 149, 640–649. [Google Scholar] [CrossRef]
- Middleton, A. Disinfection of bronchoscopes, contaminated in vitro with Mycobacterium tuberculosis, Mycobacterium avium-intracellulare and Mycobacterium chelonae in sputum, using stabilized, buffered peracetic acid solution (‘Nu-Cidex’). J. Hosp. Infect. 1997, 37, 137–143. [Google Scholar] [CrossRef]
- Marquis, R.; Rutherford, G.; Faraci, M.; Shin, S. Sporicidal action of peracetic acid and protective effects of transition metal ions. J. Ind. Microbiol. Biotechnol. 1995, 15, 486–492. [Google Scholar] [CrossRef] [PubMed]
- André, S.; Hédin, S.; Remize, F.; Zuber, F. Evaluation of Peracetic Acid Sanitizers Efficiency against Spores Isolated from Spoiled Cans in Suspension and on Stainless Steel Surfaces. J. Food Prot. 2012, 75, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Uwamahoro, M.; Massicotte, R.; Hurtubise, Y.; Gagné-Bourque, F.; Mafu, A.; Yahia, L. Evaluating the Sporicidal Activity of Disinfectants against Clostridium difficile and Bacillus amyloliquefaciens Spores by Using the Improved Methods Based on ASTM E2197-11. Front. Public Health 2018, 6, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatemi, P.; Frank, J.F. Inactivation of Listeria monocytogenes/Pseudomonas Biofilms by Peracid Sanitizers. J. Food Prot. 1999, 62, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Gram, L.; Bagge-Ravn, D.; Yin Ng, Y.; Gymoese, P.; Fonnesbech, B. Influence of food soiling matrix on cleaning and disinfection efficiency on surface attached Listeria monocytogenes. Food Control 2007, 18, 1165–1171. [Google Scholar] [CrossRef]
- Ibusquiza, P.S.; Herrera, J.J.R.; Cabo, M.L. Resistance to benzalkonium chloride, peracetic acid and nisin during formation of mature biofilms by Listeria monocytogenes. Food Microbiol. 2011, 28, 418–425. [Google Scholar] [CrossRef]
- Urano, H.; Fukuzaki, S. The mode of action of sodium hypochlorite in the cleaning process. Biocontrol Sci. 2005, 10, 21–29. [Google Scholar] [CrossRef]
- Omidbakhsh, N. Evaluation of sporicidal activities of selected environmental surface disinfectants: Carrier tests with the spores of Clostridium difficile and its surrogates. Am. J. Infect. Control 2010, 38, 718–722. [Google Scholar] [CrossRef]
- Hilgren, J.; Swanson, K.; Diez-Gonzalez, F.; Cords, B. Inactivation of Bacillus anthracis Spores by Liquid Biocides in the Presence of Food Residue. Appl. Environ. Microbiol. 2007, 73, 6370–6377. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Ran, Z.; Wen, G.; Liang, Z.; Wan, Q.; Chen, Z.; Lin, Y.; Li, K.; Wang, J.; Huang, T. Efficient inactivation of bacteria in ballast water by adding potassium peroxymonosulfate alone: Role of halide ions. Chemosphere 2020, 253, 126656. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Cao, S.; Jin, W.; Zhou, X.; Ding, W.; Tu, R.; Han, S.; Wang, C.; Jiang, Q.; Huang, H.; et al. Inactivation of chlorine-resistant bacterial spores in drinking water using UV irradiation, UV/Hydrogen peroxide and UV/Peroxymonosulfate: Efficiency and mechanism. J. Clean. Prod. 2020, 243, 118666. [Google Scholar] [CrossRef]
- Saklou, N.; Burgess, B.; Van Metre, D.; Hornig, K.; Morley, P.; Byers, S. Comparison of disinfectant efficacy when using high-volume directed mist application of accelerated hydrogen peroxide and peroxymonosulfate disinfectants in a large animal hospital. Equine Vet. J. 2015, 48, 485–489. [Google Scholar] [CrossRef]
- Nerandzic, M.; Sunkesula, V.; Thriveen Sankar, C.; Setlow, P.; Donskey, C. Unlocking the Sporicidal Potential of Ethanol: Induced Sporicidal Activity of Ethanol against Clostridium difficile and Bacillus Spores under Altered Physical and Chemical Conditions. PLoS ONE 2015, 10, e0132805. [Google Scholar] [CrossRef]
- Reid, R.; Bolton, D.; Tiuftin, A.A.; Kerry, J.P.; Fanning, S.; Whyte, P. Controlling Blown Pack Spoilage Using Anti-Microbial Packaging. Foods 2017, 6, 67. [Google Scholar] [CrossRef] [Green Version]
- Reid, R.; Burgess, C.M.; McCabe, E.; Fanning, S.; Whyte, P.; Kerry, J.; Bolton, D. Real-time PCR methods for the detection of blown pack spoilage causing Clostridium species; C. estertheticum, C. gasigenes and C. ruminantium. Meat Sci. 2017, 133, 56–60. [Google Scholar] [CrossRef]
- Jamroskovic, J.; Chromikova, Z.; List, C.; Bartova, B.; Barak, I.; Bernier-Latmani, R. Variability in DPA and Calcium Content in the Spores of Clostridium Species. Front. Microbiol. 2016, 7, 1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajagopal, S.; McMullen, L.M.; Gill, C.O.; Yang, X. Characterization of germination of spores of Clostridium estertheticum, the primary causative agent of blown pack spoilage of vacuum packaged beef. Food Res. Int. 2016, 87, 109–114. [Google Scholar] [CrossRef]
- Boerema, J.A.; Broda, D.M.; Bell, R.G. Abattoir sources of psychrophilic clostridia causing blown pack spoilage of vacuum-packed chilled meats determined by culture-based and molecular detection procedures. Lett. Appl. Microbiol. 2003, 36, 406–411. [Google Scholar] [CrossRef]
- Bolton, D.J.; Carroll, J.; Walsh, D. A four-year survey of blown pack spoilage Clostridium estertheticum and Clostridium gasigenes on beef primal cuts. Lett. Appl. Microbiol. 2015, 61, 153–157. [Google Scholar] [CrossRef]
- McSharry, S.; Koolman, L.; Whyte, P.; Bolton, D. Investigation of the Effectiveness of Disinfectants Used in Meat-Processing Facilities to Control Clostridium sporogenes and Clostridioides difficile Spores. Foods 2021, 10, 1436. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, M.; Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.Y. Mode of action of hydrogen peroxide and other oxidizing agents: Differences between liquid and gas forms. J. Antimicrob. Chemother. 2010, 65, 2108–2115. [Google Scholar] [CrossRef] [Green Version]
- Blakistone, B.; Chuyate, R.; Kautter, D., Jr.; Charbonneau, J.; Suit, K. Efficacy of Oxonia Active Against Selected Spore Formers. J. Food Prot. 1999, 62, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Shetty, N.; Srinivasan, S.; Holton, J.; Ridgway, G.L. Evaluation of microbicidal activity of a new disinfectant: Sterilox® 2500 against Clostridium difficile spores, Helicobacter pylori, vancomycin resistant Enterococcus species, Candida albicans and several Mycobacterium species. J. Hosp. Infect. 1999, 41, 101–105. [Google Scholar] [CrossRef]
- Dawson, L.F.; Valiente, E.; Donahue, E.H.; Birchenough, G.; Wren, B.W. Hypervirulent Clostridium difficile PCR-Ribotypes Exhibit Resistance to Widely Used Disinfectants. PLoS ONE 2011, 6, e25754. [Google Scholar] [CrossRef] [Green Version]
- Cho, W.-I.; Chung, M.-S. Sporicidal activities and mechanism of surfactant components against Clostridium sporogenes spores. J. Food Sci. Technol. 2018, 55, 4675–4680. [Google Scholar] [CrossRef]
- Harrison, J.; Hand, R. The effect of dilution and organic matter on the antibacterial property of 5.25% sodium hypochlorite. J. Endod. 1981, 7, 128–132. [Google Scholar] [CrossRef]
- Ulrich, J. Antimicrobial Efficacy in the Presence of Organic Matter. In Skin Microbiology; Springer: New York, NY, USA, 1981; pp. 149–157. [Google Scholar] [CrossRef]
- Lourenço, C.; Macdonald, T.J.; Gavriilidis, A.; Allan, E.; MacRobert, A.J.; Parkin, I.P. Effects of bovine serum albumin on light activated antimicrobial surfaces. RSC Adv. 2018, 8, 34252–34258. [Google Scholar] [CrossRef] [Green Version]
- Kunanusont, N.; Punyadarsaniya, D.; Jantafong, T.; Pojprasath, T.; Takehara, K.; Ruenphet, S. Bactericidal efficacy of potassium peroxymonosul fate under various concentrations, organic material conditions, exposure timing and its application on various surface carriers. J. Vet. Med. Sci. 2020, 82, 320–324. [Google Scholar] [CrossRef] [Green Version]
- Korukluoglu, M.; Sahan, Y.; Yigit, A. The fungicidal efficacy of various commercial disinfectants used in the food industry. Ann. Microbiol. 2006, 56, 325. [Google Scholar] [CrossRef]
- Hasan, T.; Ali Kadhum, H.; Alasedi, K. The Using of Ethanol and Isopropyl Alcohol as a disinfectant: Review. Int. J. Pharm. Res. 2020, 13, 2150–2152. [Google Scholar] [CrossRef]
- Sanderson, H.; van Compernolle, R.; Dyer, S.; Price, B.; Nielsen, A.; Selby, M.; Ferrer, D.; Stanton, K. Occurrence and risk screening of alcohol ethoxylate surfactants in three U.S. river sediments associated with wastewater treatment plants. Sci. Total Environ. 2013, 463–464, 600–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias-Moliz, M.; Ferrer-Luque, C.; Espigares-Rodríguez, E.; Liébana-Ureña, J.; Espigares-García, M. Bactericidal activity of phosphoric acid, citric acid, and EDTA solutions against Enterococcus faecalis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 106, e84–e89. [Google Scholar] [CrossRef]
- Scott, B.; Yang, X.; Geornaras, I.; Delmore, R.; Woerner, D.; Reagan, J.; Morgan, J.; Belk, K. Antimicrobial Efficacy of a Sulfuric Acid and Sodium Sulfate Blend, Peroxyacetic Acid, and Cetylpyridinium Chloride against Salmonella on Inoculated Chicken Wings. J. Food Prot. 2015, 78, 1967–1972. [Google Scholar] [CrossRef]
- Prada, I.; Micó-Muñoz, P.; Giner-Lluesma, T.; Micó-Martínez, P.; Muwaquet-Rodríguez, S.; Albero-Monteagudo, A. Update of the therapeutic planning of irrigation and intracanal medication in root canal treatment. A literature review. J. Clin. Exp. Dent. 2019, 11, e185–e193. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, G.; Russell, A. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [Green Version]
- Ruano, M.; El-Attrache, J.; Villegas, P. Efficacy Comparisons of Disinfectants Used by the Commercial Poultry Industry. Avian Dis. 2001, 45, 972. [Google Scholar] [CrossRef]
- Tennen, R.; Setlow, B.; Davis, K.; Loshon, C.; Setlow, P. Mechanisms of killing of spores of Bacillus subtilis by iodine, glutaraldehyde and nitrous acid. J. Appl. Microbiol. 2000, 89, 330–338. [Google Scholar] [CrossRef]
- Pennell, K.; Naunovic, Z.; Blatchley, E. Sequential Inactivation of Bacillus Subtilis Spores with Ultraviolet Radiation and Iodine. J. Environ. Eng. 2008, 134, 513–520. [Google Scholar] [CrossRef]
- Setlow, P. Spore germination. Curr. Opin. Microbiol. 2003, 6, 550–556. [Google Scholar] [CrossRef]
- Huang, S.S.; Chen, D.; Pelczar, P.L.; Vepachedu, V.R.; Setlow, P.; Li, Y.Q. Levels of Ca2+-Dipicolinic Acid in Individual Bacillus Spores Determined Using Microfluidic Raman Tweezers. J. Bacteriol. 2007, 189, 4681–4687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leggett, M.J.; McDonnell, G.; Denyer, S.P.; Setlow, P.; Maillard, J.Y. Bacterial spore structures and their protective role in biocide resistance. J. Appl. Microbiol. 2012, 113, 485–498. [Google Scholar] [CrossRef] [PubMed]



| Disinfectant/Treatment | Chemical Composition | Recommended Concentration |
|---|---|---|
| 1 | Hydrogen Peroxide (10–30%), Acetic acid (1–10%), Peracetic acid (1–10%) | 10% |
| 2 | Sodium hypochlorite (5.2–10%), Sodium hydroxide (5–10%), Alkylamine oxide (3–5%) | 5% |
| 3 | Peroxymonosulphate (30–50%), Sulphamic Acid (1–10%), Troclosene Sodium (1–10%) | 4% |
| 4 | Alcohols, C9–11, Ethoxylate (10–30%), Orthophosphoric acid (10–30%), Sulphuric acid (1–10%), Iodine (1–10%) | 2% |
| Sporicidal Product | Treatment Conditions | D-Value (Minutes) | SE 1 |
|---|---|---|---|
| Clostridium estertheticum | |||
| 1 | SDW | 26.8 A/B | 0.009 |
| BSA | 20.0 B/A | 0.006 | |
| 2 | SDW | 39.4 A/C | 0.012 |
| BSA | 18.5 B/A | 0.018 | |
| 3 | SDW | 12.7 A/A | 0.009 |
| BSA | 25.9 B/B | 0.003 | |
| 4 | SDW | 23.3 A/B | 0.006 |
| BSA | 33.3 B/C | 0.004 | |
| Clostridium gasigenes | |||
| 1 | SDW | 20.5 A/B | 0.006 |
| BSA | 22.6 B/B | 0.006 | |
| 2 | SDW | 30.4 A/C | 0.009 |
| BSA | 17.1 B/A | 0.013 | |
| 3 | SDW | 16.1 A/A | 0.007 |
| BSA | 24.8 B/B | 0.006 | |
| 4 | SDW | 19.9 A/B | 0.007 |
| BSA | 31.5 B/C | 0.003 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteves, E.; Koolman, L.; Whyte, P.; Gupta, T.B.; Bolton, D. An Evaluation of Sporicidal Treatments against Blown Pack Spoilage Associated Clostridium estertheticum and Clostridium gasigenes Spores. Appl. Sci. 2022, 12, 1663. https://doi.org/10.3390/app12031663
Esteves E, Koolman L, Whyte P, Gupta TB, Bolton D. An Evaluation of Sporicidal Treatments against Blown Pack Spoilage Associated Clostridium estertheticum and Clostridium gasigenes Spores. Applied Sciences. 2022; 12(3):1663. https://doi.org/10.3390/app12031663
Chicago/Turabian StyleEsteves, Eden, Leonard Koolman, Paul Whyte, Tanushree B. Gupta, and Declan Bolton. 2022. "An Evaluation of Sporicidal Treatments against Blown Pack Spoilage Associated Clostridium estertheticum and Clostridium gasigenes Spores" Applied Sciences 12, no. 3: 1663. https://doi.org/10.3390/app12031663
APA StyleEsteves, E., Koolman, L., Whyte, P., Gupta, T. B., & Bolton, D. (2022). An Evaluation of Sporicidal Treatments against Blown Pack Spoilage Associated Clostridium estertheticum and Clostridium gasigenes Spores. Applied Sciences, 12(3), 1663. https://doi.org/10.3390/app12031663








