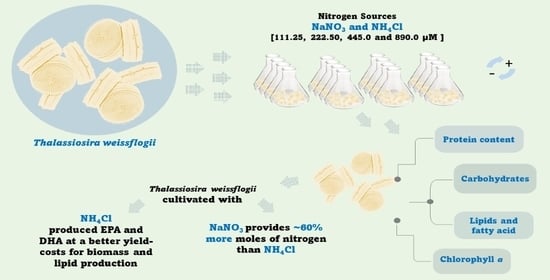
Effects on Cell Growth, Lipid and Biochemical Composition of Thalassiosira weissflogii (Bacillariophyceae) Cultured under Two Nitrogen Sources
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Culture
2.2. Experimental Design
2.3. Growth and Biomass Production
2.4. Biochemical Composition
2.4.1. Total Protein
2.4.2. Total Carbohydrates
2.4.3. Chlorophyll a
2.4.4. Total Lipids
2.4.5. Fatty Acid Composition and Statistical Analyses
3. Results
3.1. Growth and Biomass Production of Microalgae
3.2. Biochemical Composition
3.2.1. Chlorophyll a
3.2.2. Proteins, Carbohydrates and Lipids
3.3. Fatty Acids
4. Discussion
4.1. Microalgae Growth and Biomass Production
4.2. Biochemical Composition
4.3. Fatty Acids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO-Food and Agriculture Organization of the United Nations. The State of World Fisheries Aquaculture; FAO Fisheries and Aquaculture Department: Rome, Italy, 2010; p. 197. ISBN 978-92-5-106675-1. [Google Scholar]
- Lavens, P.; Sorgeloos, P. Manual on the Production and Use of Live Food for Aquaculture; FAO Fisheries Technical: Rome, Italy, 1996; p. 295, Paper No. 361. [Google Scholar]
- Knauer, J.; Southgate, P.C.A. Review of the Nutritional Requirements of Bivalves and the Development of Alternative and Artificial Diets for Bivalve Aquaculture. Rev. Fish. Sci. 1999, 7, 241–280. [Google Scholar] [CrossRef]
- Aji, L.P. The Use of Algae Concentrates Dried Algae and Algal Substitutes to Feed Bivalves. Makara Sains 2011, 15, 1–8. [Google Scholar]
- Sukenik, A.; Zmora, O.; Carmeli, Y. Biochemical Quality of Marine Unicellular Algae with Special Emphasis on Lipid Composition. II Nannochloropsis sp. Aquaculture 1993, 117, 313–326. [Google Scholar] [CrossRef]
- Volkman, J.K.; Jeffrey, S.W.; Nichols, P.D.; Rogers, G.I.; Garland, C.D. Fatty Acid and Lipid Composition of 10 Species of Microalgae Used in Mariculture. J. Exp. Mar. Biol. Ecol. 1989, 128, 219–240. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a Source of Protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Patil, V.; Källqvist, T.; Olsen, E.; Vogt, G.; Gislerød, H.R. Fatty Acid Composition of 12 Microalgae for Possible Use in Aquaculture Feed. Aquacult. Int. 2007, 15, 1–9. [Google Scholar] [CrossRef]
- Ehteshami, F.; Christianus, A.; Rameshi, H.; Harmin, S.A.; Saad, C.R. Proximate and Fatty Acid Composition of the Gonads of Wild versus Hatchery-Conditioned Pinctada margaritifera Broodstock. Aquacul. Nut. 2011, 17, 675–682. [Google Scholar] [CrossRef]
- Kiatmetha, P.; Siangdang, W.; Bunnag, B.; Senapin, S.; Withyachumnarnkul, B. Enhancement of Survival and Metamorphosis Rates of Penaeus monodon Larvae by Feeding with the Diatom Thalassiosira weissflogii. Aquacul. Int. 2011, 19, 599–609. [Google Scholar] [CrossRef]
- Kiron, V.; Phromkunthong, W.; Huntley, M.; Archibald, I.; De Scheemaker, G. Marine Microalgae from Biorefinery as a Potential Feed Protein Source for Atlantic Salmon, Common Carp and Whiteleg Shrimp. Aquacult. Nutr. 2012, 18, 521–531. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, G.C.; Zhou, B.C. Effect of Iron on Growth and Lipid Accumulation in Chlorella vulgaris. Bioresour. Technol. 2008, 99, 4717–4722. [Google Scholar] [CrossRef] [PubMed]
- Costard, G.S.; Machado, R.R.; Barbarino, E.R.; Martino, C.; Lourenço, S.O. Chemical Composition of Five Marine Microalgae that Occur on the Brazilian Coast. Int. J. Fish. Aquacult. 2012, 4, 191–201. [Google Scholar]
- Garcia, N.; Lopez-Elias, J.A.; Miranda, A.; Martinez-Porchas, M.; Huerta, N.; Garcia, A. Effect of Salinity on Growth and Chemical Composition of the Diatom Thalassiosira weissflogii at Three Culture Phases. Lat. Am. J. Aquat. Res. 2012, 40, 435–440. [Google Scholar] [CrossRef]
- Moronta, R.; Mora, R.; Morales, E. Respuesta de la Microalga Chlorella sorokiniana al pH, Salinidad y Temperatura en Condiciones Axénicas y no Axénicas. La Rev.Fac.Agron. 2006, 23, 27–41. [Google Scholar]
- Pacheco, J.M.; Cadena, M.A.; Sánchez, M.P.; Tovar, R.D.; Rangel, D.C. Effect of Culture Medium and Nutrient Concentration on Fatty Acid Content of Chaetoceros muelleri. Revista Latinoam. Biotecnol. Ambient. Algal. 2010, 1, 6–15. [Google Scholar]
- Renaud, S.M.; Thinh, L.V.; Parry, D.L. The Gross Chemical Composition and Fatty Acid Composition of 18 Species of Tropical Australian Microalgae for Possible Use in Mariculture. Aquaculture 1999, 170, 147–159. [Google Scholar] [CrossRef]
- Gouveia, L.; Oliveira, A.C. Microalgae as a Raw Material for Biofuels Production. J. Ind. Microbiol. Biotechnol. 2009, 36, 269–274. [Google Scholar] [CrossRef]
- Pruvost, J.; Vooren, G.; Gouic, B.; Mossion, A.; Legrand, J. Systematic Investigation of Biomass and Lipid Productivity by Microalgae in Photobioreactors for Biodiesel Application. Bioresour. Technol. 2011, 102, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Lourenco, S.O.; Barbarino, E.; Mancini-Filho, J.; Schinke, K.P.; Aldar, E. Effects of Different Nitrogen Sources on the Growth and Biochemical Profile of 10 Marine Microalgae in Batch Culture: An Evaluation for Aquaculture. Phycologia 2002, 41, 158–168. [Google Scholar] [CrossRef]
- Guillard, R.R. Culture of Phytoplankton for Feeding Marine Invertebrate Animals. Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 29–60. [Google Scholar]
- Verhulst, P.F. Recherches mathématiques sur la loi d’accroissement de la population [Mathematical Researches into the Law of Population Growth Increase]. Nouv. Mém. L’académie R. Sci. Belles-Lett. Brux. 1845, 18, 1–42. [Google Scholar]
- McKendrick, A.; Pai, M. The Rate of Multiplication of Micro-organisms: A Mathematical Study. Proc. R. Soc. Edinb. 1912, 31, 649–655. [Google Scholar] [CrossRef] [Green Version]
- Pearl, R.; Reed, L. On the Rate of Growth of the Population of the United States. Proc. Natl. Acad. Sci. USA 1920, 6, 275–288. [Google Scholar] [CrossRef] [Green Version]
- Guillard, R.R.L.; John, H.; Ryther, J.H. Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Levasseur, M.; Thompson, P.A.; Harrison, P.J. Physiological acclimation of marine phytoplankton to different nitrogen sources. J. Phycol. 1993, 29, 587–595. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, Y.; Yang, S.T. Modes of Culture/Animal Cells. In Comprehensive Biotechnology, 2nd ed.; Murray, M.Y., Ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 285–302. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin-Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.D.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Vidussi, F.; Claustre, H.; Bustillos, G.J.; Cailiau, C.; Marty, J.C. A Rapid HPLC Method for the Determination of Phytoplankton Pigments in Natural Waters Including the Separation of Chlorophyll a from Divinyl-chlorophyll a and Zeaxathin from Lutein. J. Plankton Res. 1996, 18, 2377–2382. [Google Scholar] [CrossRef] [Green Version]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Marsh, J.B.; Weinstein, D.B. Simple Charring Method for Determination of Lipids. J. Lip. Res. 1966, 7, 574–576. [Google Scholar] [CrossRef]
- Sato, N.; Murata, N. Membrane Lipids. Methods Enzymol. 1988, 167, 251–259. [Google Scholar]
- Zar, J. Biostatitical Analysis; Prentice Hall: Englewood Cliffs, NJ, USA, 1999; p. 663. [Google Scholar]
- Huertas, E.; Montero, O.; Lubian, L.M. Effects of Dissolved Inorganic Carbon Availability on Growth, Nutrient Uptake and Chlorophyll Fluorescence of two Marine Microalgae. Aquac. Eng. 2000, 22, 181–197. [Google Scholar] [CrossRef]
- Chitra, R.S.; Sumitra, V.C.; Yash, S. Effect of Different Nitrogen Sources and Plant Growth Regulators on Glutamine Synthetase and Glutamate Synthase Activities of Radish cotyledons. Bul. J. Plant Physiol. 2002, 28, 46–56. [Google Scholar]
- Rees, T.A. Metabolic and Ecological Constraints Imposed by Similar Rates of Ammonium and Nitrate Uptake per Unit Surface Area at Low Substrate Concentrations in Marine Phytoplankton and Macroalgae. J. Phycol. 2007, 43, 197–207. [Google Scholar]
- Available online: https://www.made-in-china.com/products-search/hot-china-products/nano3_price.html (accessed on 6 November 2021).
- Available online: https://www.made-in-china.com/products-search/hot-china-products/nh4cl_price.html (accessed on 6 November 2021).
- Fu, W.; Wichuk, K.; Brynjólfsson, S. Developing diatoms for value-added products: Challenges and opportunities. N. Biotechnol. 2015, 32, 547–551. [Google Scholar] [CrossRef]
- Vinayak, V.; Manoylov, K.M.; Gateau, H.; Blanckaert, V.; Hérault, J.; Pencréac’h, G.; Marchand, J.; Gordon, R.; Schoefs, B. Diatom Milking: A Review and New Approaches. Mar. Drugs 2015, 13, 2629–2665. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, A.; Cerón, M.; García, F.; Molina, E.; Chisti, Y. Growth and Biochemical Characterization of Microalgal Biomass Produced in Bubble Column and Airlift Photobioreactors: Studies in Fed-Batch Culture. Enzyme Microb. Technol. 2002, 31, 1015–1023. [Google Scholar] [CrossRef]
- Lee, Y.C.; Kim, B.; Farooq, W.; Chung, J.; Han, H.; Shin, J.; Jeong, H.; Park, J.; Lee, S.; Oh, K. Harvesting of Oleaginous Chlorella sp. by Organoclays. Bioresour. Technol. 2013, 132, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, C.M.; Orcutt, D.M.; Kreeger, D.A.; Parker, B.C.; Jones, V.A.; Neves, R.J. Biochemical Composition of Three Algal Species Proposed as Food for Captive Freshwater Mussels. J. Appl. Phycol. 2003, 5, 1–11. [Google Scholar] [CrossRef]
- Yi, Z.; Xu, M.; Di, X.; Brynjolfsson, S.; Fu, W. Exploring Valuable Lipids in Diatoms. Front. Mar. Sci. 2017, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of Nitrogen Sources on Cell Growth and Lipid Accumulation of Green Algae Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Sabia, A.; Clavero, E.; Pancaldi, S.; Salvadó, R.J. Effect of different CO2 concentrations on biomass, pigment content, and lipid production of the marine diatom Thalassiosira pseudonana. Appl. Microbiol. Biotechnol. 2018, 102, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Kangsen, M. Effect of Growth Phase on the Fatty Acid Compositions of Four Species of Marine Diatoms. J. Ocean Univ. China. 2005, 4, 157–162. [Google Scholar] [CrossRef]
- Fryxell, G.A.; Hasle, G.R. Taxonomy of diatoms. In Manual on Harmful Marine Microalgae; Hallegraeff, G.M., Andersen, D.M., Cembella, A.D., Eds.; UNESCO: Paris, France, 2003; pp. 465–510. [Google Scholar]
- Wang, X.; Huo, Y.; Yang, F.; Wang, Y. Induced Allelopathic Effects of Thalassiosira weissflogii on Colony Formation in Phaeocystis globosa. Water 2021, 13, 581. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from Microalgae—A Review of Technologies for Production, Processing, and Extractions of Biofuels and Co-Products. Renew. Sust. Energ. Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Ördöget, V.; Stirk, W.A.; Bálint, P.; Staden, J.; Lovász, C. Changes in Lipid, Protein and Pigment Concentrations in Nitrogen-Stressed Chlorella minutissima Cultures. J. Appl. Phycol. 2012, 24, 907–914. [Google Scholar] [CrossRef]
- Silva, A.F.; Lourenco, S.O.; Chaloub, R.M. Effects of Nitrogen Starvation on the Photosynthetic Physiology of a Tropical Marine Microalgae Rhodomonas sp. (Cryptophyceae). Aquat. Bot. 2009, 91, 291–297. [Google Scholar] [CrossRef]
- Makarevičienė, V.; Andrulevičiūt, V.; Skorupskaitė, V.; Kasperovičienė, J. Cultivation of Microalgae Chlorella sp. and Scenedesmus sp. as a Potentional Biofuel Feedstock. Environ. Res. Eng. Manage. 2011, 3, 21–27. [Google Scholar]
- Navarro, P.A.; Rebolloso, M.M.; Ramos, J.J.; Guil, J.L. Biomass Nutrient Profiles of the Microalgae Phaeodactylum tricornutum. J. Food Biochem. 2001, 25, 57–76. [Google Scholar]
- Lv, J.M.; Cheng, L.H.; Xu, X.H.; Zhang, L.; Chen, H.L. Enhanced Lipid Production of Chlorella vulgaris by Adjustment of Cultivation Conditions. Bioresour. Technol. 2010, 101, 6797–6804. [Google Scholar] [CrossRef]
- Marella, T.K.; Tiwari, A. Marine diatom Thalassiosira weisflogii based biorefinery for co-production of eicosapentaenoic acid and fucoxanthin. Bioresour. Technol. 2020, 307, 123245. [Google Scholar] [CrossRef] [PubMed]
- d´Ippolito, G.; Sardo, A.; Paris, D.; Vella, F.M.; Adelfi, M.G.; Botte, P.; Gallo, C.; Fontana, A. Potential of lipid metabolism in marine diatoms for biofuel production. Biotechnol. Biofuels. 2015, 8, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryckebosch, E.; Bruneel, C.; Termote-Verhalle, R.; Goiris, K.; Muylaert, K.; Foubert, I. Nutritional evaluation of microalgae oils in omega-3 chain polyunsaturated fatty acid as an alternative for fish oil. Food Chem. 2014, 160, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Kumar, B.R.; Deviram, G.; Mathimani, T.; Duc, P.A.; Pugazhendhi, A. Microalgae as rich source of polyunsaturated fatty acids. Biocatal. Agric. Biotechnol. 2019, 17, 583–588. [Google Scholar] [CrossRef]
| Concentration (µM) | Treatments (NH4Cl) | Treatments (NaNO3) |
|---|---|---|
| 111.25 | C1 | N1 |
| 222.5 | C2 | N2 |
| 445 | C3 | N3 |
| 890 | C4 | N4 |
| NaNO3 µmol | NH4Cl µmol | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | N1 | N2 | N3 | N4 | C1 | C2 | C3 | C4 |
| µmol L−1 [as Salt] | 111.25 | 222.50 | 445.00 | 890.00 | 111.25 | 222.50 | 445.00 | 890.00 |
| µg L−1 [N] | 18.33 | 36.67 | 73.33 | 146.67 | 29.13 | 58.26 | 116.52 | 233.05 |
| Parameter estimation of the sigmoidal curve via least squares (SSQ) | ||||||||
| N0 (cells mL−1) | 507,498 | 416,176 | 505,885 | 457,116 | 473,424 | 439,717 | 485,535 | 426,034 |
| r (h−1) | 0.0304 | 0.0221 | 0.0351 | 0.0275 | 0.0358 | 0.0294 | 0.0367 | 0.0385 |
| k or Nmax (cells mL−1) | 5,378,478 | 8,360,922 | 9,553,601 | 12,580,306 | 5,589,747 | 7,700,407 | 7,921,201 | 11,852,018 |
| % max k | 43 | 66 | 76 | 100 | 44 | 61 | 63 | 94 |
| k/2 (cells mL−1) | 2,689,239 | 4,180,461 | 4,776,801 | 6,290,153 | 2,794,873 | 3,850,203 | 3,960,600 | 5,926,009 |
| tk/2 (hours) | 74 | 134 | 82 | 119 | 67 | 95 | 75 | 86 |
| Formula estimation of µ and PDT | ||||||||
| µmax (h−1) | 0.0111 | 0.0125 | 0.0133 | 0.0114 | 0.0117 | 0.0130 | 0.0129 | 0.0118 |
| PDT (hours) | 62.2 | 55.3 | 52.2 | 60.8 | 59.4 | 53.3 | 53.9 | 58.8 |
| Parameter estimation of an exponential rise to maximum model via non-linear regression (Note t (days) | ||||||||
| b | 0.343 | 0.114 | 0.241 | 0.122 | 0.277 | 0.173 | 0.295 | 0.145 |
| 95% CI b | 0.264 | 0.064 | 0.087 | 0.040 | 0.173 | 0.080 | 0.135 | 0.044 |
| a | 4,349,873 | 9,604,878 | 9,306,673 | 15,151,914 | 5,246,430 | 8,220,818 | 7,207,902 | 14,152,484 |
| 95% CI a | 851,406 | 2,393,787 | 1,012,272 | 2,163,974 | 931,908 | 1,363,124 | 902,546 | 1,691,236 |
| Time (Days) | |||||
|---|---|---|---|---|---|
| NaNO3 µmol | 3 | 9 | 15 | 18 | |
| Protein (µg mg−1) | 111.25 | 74.7 ± 9.5 | 88.5 ± 7.1 | 77.5 ± 12.7 | 45.2 ± 7.7 |
| 222.50 | 158.9 ± 12.0 | 123.7 ± 43.4 | 116.6 ± 19.1 | 96.27 ± 2.5 | |
| 445 | 325.1 ± 63.4 | 200.0 ± 24.4 | 183.4 ± 2.2 | 144.7 ± 7.5 | |
| 890 | 351.4 ± 22.8 | 239.7 ± 36.5 | 232.9 ± 3.6 | 185.6 ± 10.5 | |
| Carbohydrates (µg mg−1) | 111.25 | 178.0 ± 27.7 | 300.4 ± 9.7 | 399.4 ± 50.3 | 290.2 ± 9.9 |
| 222.50 | 188.8 ± 70.5 | 386.7 ± 10.9 | 312.8 ± 48.7 | 436.6 ± 5.3 | |
| 445 | 216.5 ± 66.7 | 274.1 ± 36.7 | 334.1 ± 43.0 | 272.1 ± 64.7 | |
| 890 | 215.8 ± 15.4 | 253.7 ± 10.0 | 238.5 ± 7.4 | 211.5 ± 5.8 | |
| Lipids (µg mg−1) | 111.25 | 318.6 ± 22.0 | 494.5 ± 10.5 | 447.1 ± 20.9 | 370.1 ± 8.7 |
| 222.50 | 338.9 ± 31.2 | 460.4 ± 51.1 | 388.2 ± 22.4 | 356.4 ± 40.2 | |
| 445 | 244.8 ± 5.7 | 349.1 ± 44.2 | 340.3 ± 38.7 | 268.5 ± 32.8 | |
| 890 | 240.0 ± 10.1 | 281.9 ± 61.9 | 335.5 ± 12.5 | 289.1 ± 13.3 | |
| Time (Days) | |||||
|---|---|---|---|---|---|
| NH4Cl µmol | 3 | 9 | 15 | 18 | |
| Protein (µg mg−1) | 111.25 | 126.5 ± 10.5 | 72.21 ± 20.9 | 124.4 ± 16.6 | 102.9 ± 11.8 |
| 222.50 | 143.4 ± 22.8 | 146.8 ± 11.8 | 165.8 ± 5.8 | 128.5 ± 23.3 | |
| 445 | 230.2 ± 16.0 | 238.3 ± 52.1 | 171.2 ± 43.7 | 183.8 ± 56.7 | |
| 890 | 393.1 ± 68.5 | 242.3 ± 12.5 | 218.7 ± 6.9 | 199.4 ± 11.5 | |
| Carbohydrates (µg mg−1) | 111.25 | 350.9 ± 45.9 | 354.3 ± 22.2 | 279.9 ± 45.6 | 279.1 ± 22.9 |
| 222.50 | 351.7 ± 106.9 | 385.6 ± 13.6 | 380.9 ± 29.1 | 266.6 ± 21.4 | |
| 445 | 205.1 ± 77.4 | 348.6 ± 20.3 | 284.2 ± 20.6 | 248.1 ± 16.9 | |
| 890 | 142.3 ± 50.6 | 353.0 ± 54.3 | 271.6 ± 34.8 | 267.9 ± 30.0 | |
| Lipids (µg mg−1) | 111.25 | 378.6 ± 10.5 | 497.7 ± 11.1 | 404.7 ± 17.7 | 387.6 ± 11.1 |
| 222.50 | 48.6 ± 28.3 | 450.9 ± 28.2 | 404.8 ± 0.08 | 425 ± 14.4 | |
| 445 | 263.1 ± 10.8 | 328.6 ± 24.3 | 414.2 ± 12.0 | 309.4 ± 7.2 | |
| 890 | 235.0 ± 9.4 | 319.8 ± 6.54 | 318.8 ± 9.0 | 309.2 ± 14.9 | |
| NaNO3 (µM) | NH4Cl (µM) | |||||||
|---|---|---|---|---|---|---|---|---|
| PUFA | 111.25 | 222.5 | 445 | 890 | 111.25 | 222.5 | 445 | 890 |
| 18:2 | 1.20 ± 0.53 | 1.59 ± 0.46 | 1.32 ± 0.31 | 2.96 ± 0.83 | 0.63 ± 0.21 | 0.97 ± 0.38 | 1.38 ± 0.68 | 1.52 ± 0.37 |
| 18:3 | 3.03 ± 1.13 | 2.82 ± 1.17 | 2.58 ± 1.91 | 4.08 ± 2.69 | 1.33 ± 0.29 | 2.57 ± 1.99 | 3.53 ± 1.29 | 4.08 ± 2.69 |
| 18:4 | 3.11 ± 1.05 | 4.86 ± 1.82 | 3.53 ± 2.13 | 5.95 ± 4.67 | 3.61 ± 1.43 | 3.14 ± 1.90 | 5.83 ± 3.61 | 1.89 ± 1.45 |
| 20:4 | 0.014 ± 0.04 | 0.026 ± 0.01 | 0.059 ± 0.04 | 0.043 ± 0.02 | 0.01 ± 0.0009 | 0.01 ± 0.006 | 0.01 ± 0.007 | 0.02 ± 0.009 |
| 20:5n3 (EPA) | 16.67 ± 4.35 | 7.58 ± 2.48 | 14.12 ± 3.09 | 7.29 ± 1.14 | 11.94 ± 9.47 | 16.91 ± 4.73 | 10.24 ± 3.36 | 11.04 ± 6.67 |
| 22:6n3 (DHA) | 7.22 ± 1.45 | 4.37 ± 3.02 | 6.45 ± 2.76 | 9.24 ± 3.69 | 4.82 ± 2.06 | 8.38 ± 2.01 | 5.25 ± 2.44 | 6.77 ± 4.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Sandoval, F.E.; Del Ángel-Rodríguez, J.A.; Núñez-Vázquez, E.J.; Band-Schmidt, C.J.; Arredondo-Vega, B.O.; Campa-Córdova, Á.I.; Moreno-Legorreta, M.; Fernández-Herrera, L.J.; López-Cortés, D.J. Effects on Cell Growth, Lipid and Biochemical Composition of Thalassiosira weissflogii (Bacillariophyceae) Cultured under Two Nitrogen Sources. Appl. Sci. 2022, 12, 961. https://doi.org/10.3390/app12030961
Hernández-Sandoval FE, Del Ángel-Rodríguez JA, Núñez-Vázquez EJ, Band-Schmidt CJ, Arredondo-Vega BO, Campa-Córdova ÁI, Moreno-Legorreta M, Fernández-Herrera LJ, López-Cortés DJ. Effects on Cell Growth, Lipid and Biochemical Composition of Thalassiosira weissflogii (Bacillariophyceae) Cultured under Two Nitrogen Sources. Applied Sciences. 2022; 12(3):961. https://doi.org/10.3390/app12030961
Chicago/Turabian StyleHernández-Sandoval, Francisco Eduardo, Jorge Arturo Del Ángel-Rodríguez, Erick Julian Núñez-Vázquez, Christine Johanna Band-Schmidt, Bertha Olivia Arredondo-Vega, Ángel Isidro Campa-Córdova, Manuel Moreno-Legorreta, Leyberth José Fernández-Herrera, and David Javier López-Cortés. 2022. "Effects on Cell Growth, Lipid and Biochemical Composition of Thalassiosira weissflogii (Bacillariophyceae) Cultured under Two Nitrogen Sources" Applied Sciences 12, no. 3: 961. https://doi.org/10.3390/app12030961
APA StyleHernández-Sandoval, F. E., Del Ángel-Rodríguez, J. A., Núñez-Vázquez, E. J., Band-Schmidt, C. J., Arredondo-Vega, B. O., Campa-Córdova, Á. I., Moreno-Legorreta, M., Fernández-Herrera, L. J., & López-Cortés, D. J. (2022). Effects on Cell Growth, Lipid and Biochemical Composition of Thalassiosira weissflogii (Bacillariophyceae) Cultured under Two Nitrogen Sources. Applied Sciences, 12(3), 961. https://doi.org/10.3390/app12030961