Conditions Influencing Mould Growth for Effective Prevention of Wood Deterioration Indoors
Abstract
:1. Introduction
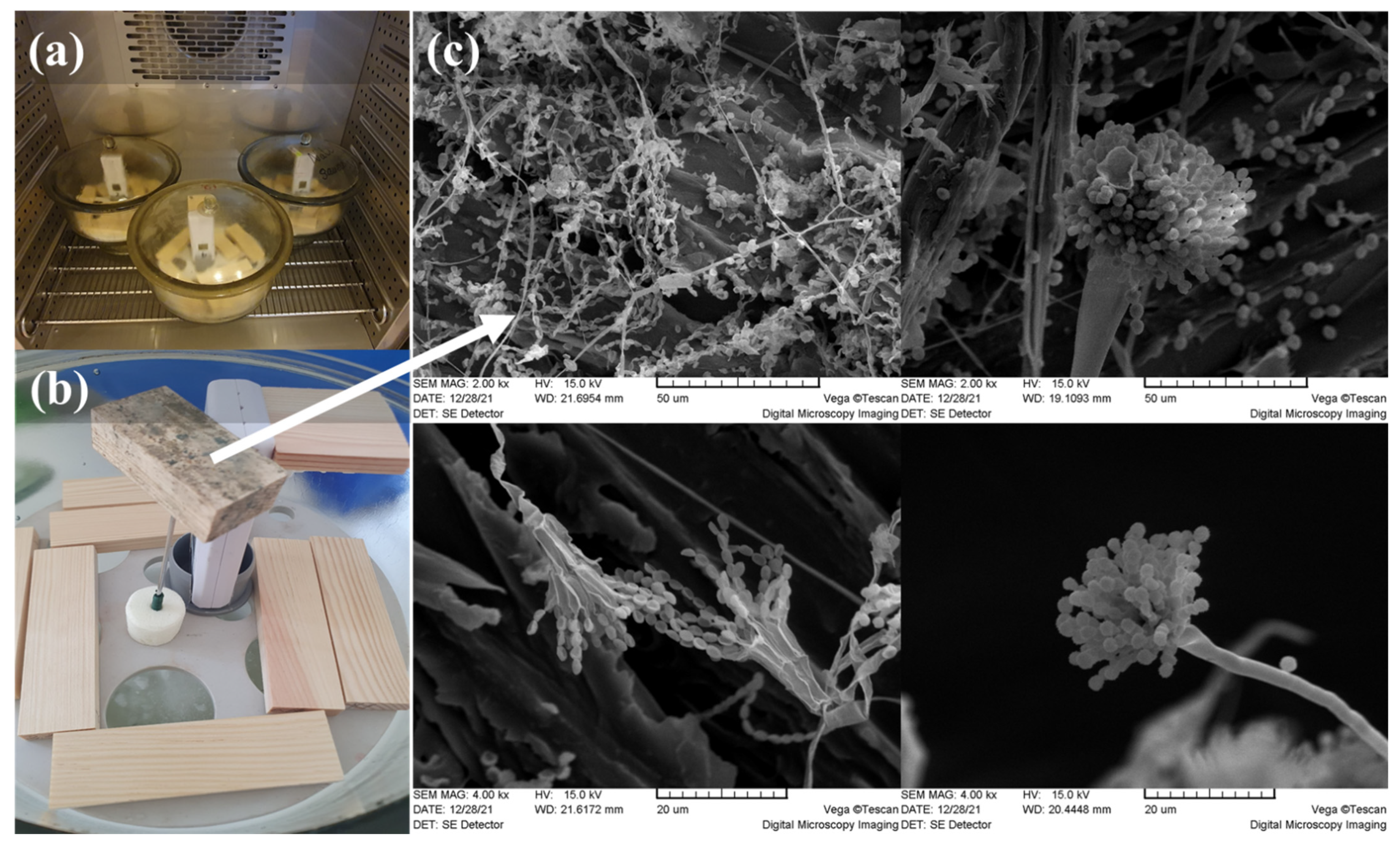
2. Materials and Methods
3. Results and Discussion
3.1. Effect of Constant RH and Temperature on Mould Growth Development at ‘Clean’ Conditions (Experiment I)
3.2. Effect of Accidental Wetting of Wood on the Mould Growth Development at ‘Clean’ Conditions (Experiment II)
3.3. Effect of Accidental Wetting of Wood on the Mould Growth Development at ‘Clean’ Conditions (Experiment II)
3.4. Effect of the MC of Wood on Mould Growth Development Exposed to Environment with Mould Contaminator (Experiment IV)
4. Conclusions
- The rate of mould growth is considerably affected by the presence of spore contamination at the temperatures of 10 and 20 °C for both RH (91% and 97%); however, a relatively small or even insignificant difference was observed at a temperature of 30 °C.
- The VTT model can predict mould growth with relatively good accuracy when wood is exposed to conditions of mould-infected objects.
- No significant difference was detected in mould growth development between ‘Dry’ and ‘Wet’ wood specimens exposed to ‘clean’ conditions at temperatures of 20 and 30 °C for both RH (91% and 97%), while a significantly higher rate of mould growth was observed for the ‘Wet’ specimens at a temperature of 10 °C.
- No significant difference was detected in mould growth development between ‘Dry’ and ‘At EMC’ specimens exposed to a mould-infected object at a temperature of 30 °C for both RH (91% and 97%) and at 20 °C for RH 97%. The rate of mould growth is significantly higher for the ‘At EMC’ than for the ‘Dry’ specimens at a temperature of 20 °C for RH 91%.
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gradeci, K.; Labonnote, N.; Time, B.; Köhler, J. Mould growth criteria and design avoidance approaches in wood-based ma-terials—A systematic review. Constr. Build. Mater. 2017, 150, 77–88. [Google Scholar] [CrossRef]
- Heseltine, E.; Rosen, J. WHO Guidelines for Indoor Air Quality: Dampness and Mould; World Health Organization: Copenhagen, Denmark, 2009.
- Nielsen, K.F.; Holm, G.; Uttrup, L.; Nielsen, P. Mould growth on building materials under low water activities. Influence of humidity and temperature on fungal growth and secondary metabolism. Int. Biodeterior. Biodegrad. 2004, 54, 325–336. [Google Scholar] [CrossRef]
- Hunter, C.; Grant, C.; Flannigan, B.; Bravery, A. Mould in buildings: The air spora of domestic dwellings. Int. Biodeterior. 1988, 24, 81–101. [Google Scholar] [CrossRef]
- Pasanen, A.-L.; Juutinen, T.; Jantunen, M.; Kalliokoski, P. Occurrence and moisture requirements of microbial growth in building materials. Int. Biodeterior. Biodegrad. 1992, 30, 273–283. [Google Scholar] [CrossRef]
- Andersen, B.; Frisvad, J.C.; Søndergaard, I.; Rasmussen, I.S.; Larsen, L.S. Associations between fungal species and wa-ter-damaged building materials. Appl. Environ. Microbiol. 2011, 77, 4180–4188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsongas, G.A.; Rioroan, F. Minimum conditions for visible mold growth. ASHRAE J. 2016, 58, 32–43. [Google Scholar]
- Nielsen, K.F. Mycotoxin production by indoor molds. Fungal Genet. Biol. 2003, 39, 103–117. [Google Scholar] [CrossRef]
- Guild, S.; MacDonald, M. Mould Prevention and Collection Recovery: Guidelines for Heritage Collections—Technical Bulletin 26; Canadian Conservation Institute: Ottawa, ON, Canada, 2004. [Google Scholar]
- Sterflinger, K. Fungi: Their role in deterioration of cultural heritage. Fungal Biol. Rev. 2010, 24, 47–55. [Google Scholar] [CrossRef]
- Kim, M.-J.; Choi, Y.-S.; Oh, J.-J.; Kim, G.-H. Experimental investigation of the humidity effect on wood discoloration by selected mold and stain fungi for a proper conservation of wooden cultural heritages. J. Wood Sci. 2020, 66, 1–5. [Google Scholar] [CrossRef]
- Broda, M. Natural Compounds for Wood Protection against Fungi—A Review. Molecules 2020, 25, 3538. [Google Scholar] [CrossRef]
- Adan, O.C.G.; Samson, R.A. Fundamentals of Mold Growth in Indoor Environments and Strategies for Healthy Living; Wageningen Academic Publishers: Wageningen, The Netherlands, 2011. [Google Scholar]
- Feng, J.; Li, C.; Chen, J.; Chen, M.; Shu, X.; Shi, Q. Evaluation of the association between natural mold resistance and chemical components of nine wood species. BioResources 2019, 13, 6524–6541. [Google Scholar] [CrossRef]
- Arango, R.; Yang, V.; Lebow, S.; Lebow, P.; Wiemann, M.; Grejczyk, M.; DeWald, P. Variation in mold susceptibility among hardwood species under laboratory conditions. Int. Biodeterior. Biodegrad. 2020, 154, 105082. [Google Scholar] [CrossRef]
- Brischke, C.; Alfredsen, G. Wood-water relationships and their role for wood susceptibility to fungal decay. Appl. Microbiol. Biotechnol. 2020, 104, 3781–3795. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Gartner, B.L.; Morrell, J.J. Heartwood formation and natural durability—A review. Wood Fiber Sci. 2002, 34, 587–611. [Google Scholar]
- Gobakken, L.R.; Westin, M. Surface mould growth on five modified wood substrates coated with three different coating systems when exposed outdoors. Int. Biodeterior. Biodegrad. 2008, 62, 397–402. [Google Scholar] [CrossRef]
- Imken, A.A.P.; Brischke, C.; Kögel, S.; Krause, K.C.; Mai, C. Resistance of different wood-based materials against mould fungi: A comparison of methods. Eur. J. Wood Wood Prod. 2020, 78, 661–671. [Google Scholar] [CrossRef]
- Yinodotlgör, N.; Kartal, S.N. Heat Modification of Wood: Chemical Properties and Resistance to Mold and Decay Fungi. For. Prod. J. 2010, 60, 357–361. [Google Scholar] [CrossRef]
- Kamperidou, V. The Biological Durability of Thermally- and Chemically-Modified Black Pine and Poplar Wood Against Basidiomycetes and Mold Action. Forests 2019, 10, 1111. [Google Scholar] [CrossRef] [Green Version]
- Andersone, I.; Dobele, G.; Andersons, B.; Kurnosova, N.; Kuka, E.; Volperts, A.; Grinins, J. A study of thermo-hydro-treated (THT) birch wood by chemical analysis and Py-GC/MS. Holzforschung 2019, 73, 653–661. [Google Scholar] [CrossRef]
- Hens, H. IEA Annex 14: Condensation and Energy. J. Therm. Insul. 1992, 15, 261–273. [Google Scholar] [CrossRef]
- Hukka, A.; Viitanen, H.A. A mathematical model of mould growth on wooden material. Wood Sci. Technol. 1999, 33, 475–485. [Google Scholar] [CrossRef]
- Lie, S.K.; Vestøl, G.I.; Høibø, O.; Gobakken, L.R. Surface mould growth on wood: A comparison of laboratory screening tests and outdoor performance. Holz als Roh- und Werkst. 2019, 77, 1137–1150. [Google Scholar] [CrossRef]
- Anagnost, S. Wood in the built environment—conditions for mold and decay. In Sampling and Analysis of Indoor Microorganisms; Yang, C.S., Heinsohn, P.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 155–178. [Google Scholar]
- Ferdyn-Grygierek, J.; Kaczmarczyk, J.; Blaszczok, M.; Lubina, P.; Koper, P.; Bulińska, A. Hygrothermal Risk in Museum Buildings Located in Moderate Climate. Energies 2020, 13, 344. [Google Scholar] [CrossRef] [Green Version]
- Martens, M.H.J. Climate risk assessment in museums: Degradation risks determined from temperature and relative humidity data. Build. Environ. 2012, 2005, 41–44. [Google Scholar]
- Sharif-Askari, H.; Abu-Hijleh, B. Review of museums’ indoor environment conditions studies and guidelines and their impact on the museums’ artifacts and energy consumption. Build. Environ. 2018, 143, 186–195. [Google Scholar] [CrossRef]
- Ayerst, G. The effects of moisture and temperature on growth and spore germination in some fungi. J. Stored Prod. Res. 1969, 5, 127–141. [Google Scholar] [CrossRef]
- Viitanen, H.A. Modelling the Time Factor in the Development of Mould Fungi—The Effect of Critical Humidity and Temperature Conditions on Pine and Spruce Sapwood. Holzforschung 1997, 51, 6–14. [Google Scholar] [CrossRef]
- Sedlbauer, K. Prediction of Mould Fungus Formation on the Surface of/and inside Building Components. Ph.D. Thesis, Universi-tät Stuttgart, Stuttgart, Germany, 2001. [Google Scholar]
- Møller, E.B.; Andersen, B.; Rode, C.; Peuhkuri, R. Conditions for mould growth on typical interior surfaces. Energy Procedia 2017, 132, 171–176. [Google Scholar] [CrossRef]
- Johansson, P.; Wamming, T.; Bok, G.; Edlund, M.-L. Mould growth on kiln-dried and air-dried timber. Eur. J. Wood Wood Prod. 2013, 71, 473–481. [Google Scholar] [CrossRef]
- Myronycheva, O.; Sidorova, E.; Hagman, O.; Sehlstedt-Persson, M.; Karlsson, O.; Sandberg, D. Hyperspectral Imaging Surface Analysis for Dried and Thermally Modified Wood: An Exploratory Study. J. Spectrosc. 2018, 2018, 7423501. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.A.; Sehlstedt-Persson, M.; Morén, T. Development of a new rapid method for mould testing in a climate chamber: Preliminary tests. Eur. J. Wood Wood Prod. 2013, 71, 451–461. [Google Scholar] [CrossRef]
- Doran, W.L. Effect of External and Internal Factors on the Germination of Fungous Spores. Bull. Torrey Bot. Club 1922, 49, 313. [Google Scholar] [CrossRef]
- Northolt, M.D.; Bullerman, L.B. Prevention of Mold Growth and Toxin Production through Control of Environmental Condi-tions. J. Food Prot. 1982, 45, 519–526. [Google Scholar] [CrossRef]
- Banks, W.B. Water uptake by scots pine sapwood, and its restriction by the use of water repellents. Wood Sci. Technol. 1973, 7, 271–284. [Google Scholar] [CrossRef]
- Sandberg, K.; Salin, J.-G. Liquid water absorption in dried Norway spruce timber measured with CT scanning and viewed as a percolation process. Wood Sci. Technol. 2012, 46, 207–219. [Google Scholar] [CrossRef]
- Fakhouri, B.; Mounji, H.; Vergnaud, J.M. Comparison of the absorption and desorption of water betweenscots pine and spruce after submersion in water. Holzforschung 1993, 47, 271–277. [Google Scholar] [CrossRef]
- de Meijer, M.; Militz, H. Moisture transport in coated wood. Part 1: Analysis of sorption rates and moisture content profiles in spruce during liquid water uptake. Holz als Roh- und Werkst. 2000, 58, 354–362. [Google Scholar] [CrossRef]
- Metsä-Kortelainen, S.; Antikainen, T.; Viitaniemi, P. The water absorption of sapwood and heartwood of Scots pine and Norway spruce heat-treated at 170 °C, 190 °C, 210 °C and 230 °C. Holz als Roh- und Werkst. 2006, 64, 192–197. [Google Scholar] [CrossRef]
- Salin, J.-G. Modelling water absorption in wood. Wood Mater. Sci. Eng. 2008, 3, 102–108. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Chun, S.K.; Miller, R.B.; Chong, S.H.; Kim, A.J. Liquid penetration in different cells of two hardwood species. J. Wood Sci. 2011, 57, 179–188. [Google Scholar] [CrossRef]
- Sedighi-Gilani, M.; Griffa, M.; Mannes, D.; Lehmann, E.; Carmeliet, J.; Derome, D. Visualization and quantification of liquid water transport in softwood by means of neutron radiography. Int. J. Heat Mass Transf. 2012, 55, 6211–6221. [Google Scholar] [CrossRef]
- Gezici-Koç, Ö.; Erich, S.J.F.; Huinink, H.P.; Van Der Ven, L.G.J.; Adan, O.C.G. Bound and free water distribution in wood during water uptake and drying as measured by 1D magnetic resonance imaging. Cellulose 2017, 24, 535–553. [Google Scholar] [CrossRef] [Green Version]
- Antons, A.; Cirule, D.; Verovkins, A.; Kuka, E. Effect of thermal treatment on physical and mechanical properties of birch and pine wood. In Proceedings of the Research for Rural Development, 24th Annual International Scientific Conference on Research for Rural Development, Jelgava, Latvia, 16–18 May 2018; pp. 78–85. [Google Scholar] [CrossRef]
- Cirule, D.; Verovkins, A.; Andersone, I.; Kuka, E.; Andersons, B. Thermally modified birch wood interaction with liquids. Holzals Roh- und Werkst. 2020, 78, 1–9. [Google Scholar] [CrossRef]
- Kuka, E.; Andersons, B.; Cirule, D.; Andersone, I.; Kajaks, J.; Militz, H.; Bicke, S. Weathering properties of wood-plastic composites based on heat-treated wood and polypropylene. Compos. Part. A Appl. Sci. Manuf. 2020, 139, 106102. [Google Scholar] [CrossRef]
- El Kouali, M.; Bouzon, J.; Vergnaud, J.M. Process of absorption and desorption of water in a wood board, with 3-dimensional transport beyond the FSP. Wood Sci. Technol. 1992, 26, 307–321. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Zhang, M. An Investigation of the Drying Rate of Water in Wood at Different Relative Humidities Studied by Time Domain Nuclear Magnetic Resonance. Bioresour. 2017, 12, 2991–3000. [Google Scholar] [CrossRef] [Green Version]
- Engelund, E.T.; Thygesen, L.G.; Svensson, S.; Hill, C.A.S. A critical discussion of the physics of wood–water interactions. Wood Sci. Technol. 2013, 47, 141–161. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.A.S.; Norton, A.J.; Newman, G. The water vapour sorption properties of Sitka spruce determined using a dynamic vapour sorption apparatus. Wood Sci. Technol. 2010, 44, 497–514. [Google Scholar] [CrossRef]
- Johansson, P.; Ekstrand-Tobin, A.; Svensson, T.; Bok, G. Laboratory study to determine the critical moisture level for mould growth on building materials. Int. Biodeterior. Biodegrad. 2012, 73, 23–32. [Google Scholar] [CrossRef]
- Suchorab, Z.; Frąc, M.; Guz, Ł.; Oszust, K.; Łagód, G.; Gryta, A.; Bilińska-Wielgus, N.; Czerwiński, J. A method for early de-tection and identification of fungal contamination of building materials using e-nose. PLoS ONE 2019, 14, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Vereecken, E.; Vanoirbeek, K.; Roels, S. Towards a more thoughtful use of mould prediction models: A critical view on experimental mould growth research. J. Build. Phys. 2015, 39, 102–123. [Google Scholar] [CrossRef]
- Thybring, E.E.; Glass, S.V.; Zelinka, S.L. Kinetics of water vapor sorption in wood cell walls: State of the art and research needs. Forests 2019, 10, 704. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuka, E.; Cirule, D.; Andersone, I.; Andersons, B.; Fridrihsone, V. Conditions Influencing Mould Growth for Effective Prevention of Wood Deterioration Indoors. Appl. Sci. 2022, 12, 975. https://doi.org/10.3390/app12030975
Kuka E, Cirule D, Andersone I, Andersons B, Fridrihsone V. Conditions Influencing Mould Growth for Effective Prevention of Wood Deterioration Indoors. Applied Sciences. 2022; 12(3):975. https://doi.org/10.3390/app12030975
Chicago/Turabian StyleKuka, Edgars, Dace Cirule, Ingeborga Andersone, Bruno Andersons, and Velta Fridrihsone. 2022. "Conditions Influencing Mould Growth for Effective Prevention of Wood Deterioration Indoors" Applied Sciences 12, no. 3: 975. https://doi.org/10.3390/app12030975
APA StyleKuka, E., Cirule, D., Andersone, I., Andersons, B., & Fridrihsone, V. (2022). Conditions Influencing Mould Growth for Effective Prevention of Wood Deterioration Indoors. Applied Sciences, 12(3), 975. https://doi.org/10.3390/app12030975





