Abstract
Cardiovascular and metabolic diseases are a leading cause of death worldwide. Epidemiological studies strongly highlight various benefits of consuming colorful fruits and vegetables in everyday life. In this review, we aimed to revisit previous studies conducted in the last few decades regarding green-colored foods and their bioactive compounds in consideration of treating and/or preventing cardiovascular and metabolic diseases. This review draws a comprehensive summary and assessment of research on the physiological effects of various bioactive compounds, mainly polyphenols, derived from green-colored fruits and vegetables. In particular, their health-beneficial effects, including antioxidant, anti-inflammatory, anti-diabetic, anti-obesity, cardioprotective, and lipid-lowering properties, will be discussed. Furthermore, the bioavailability and significance of action of these bioactive compounds on cardiovascular and metabolic diseases will be discussed in detail.
Keywords:
green; colorful; fruits; vegetables; phytonutrient; bioactive compounds; metabolic; cardiovascular 1. Introduction
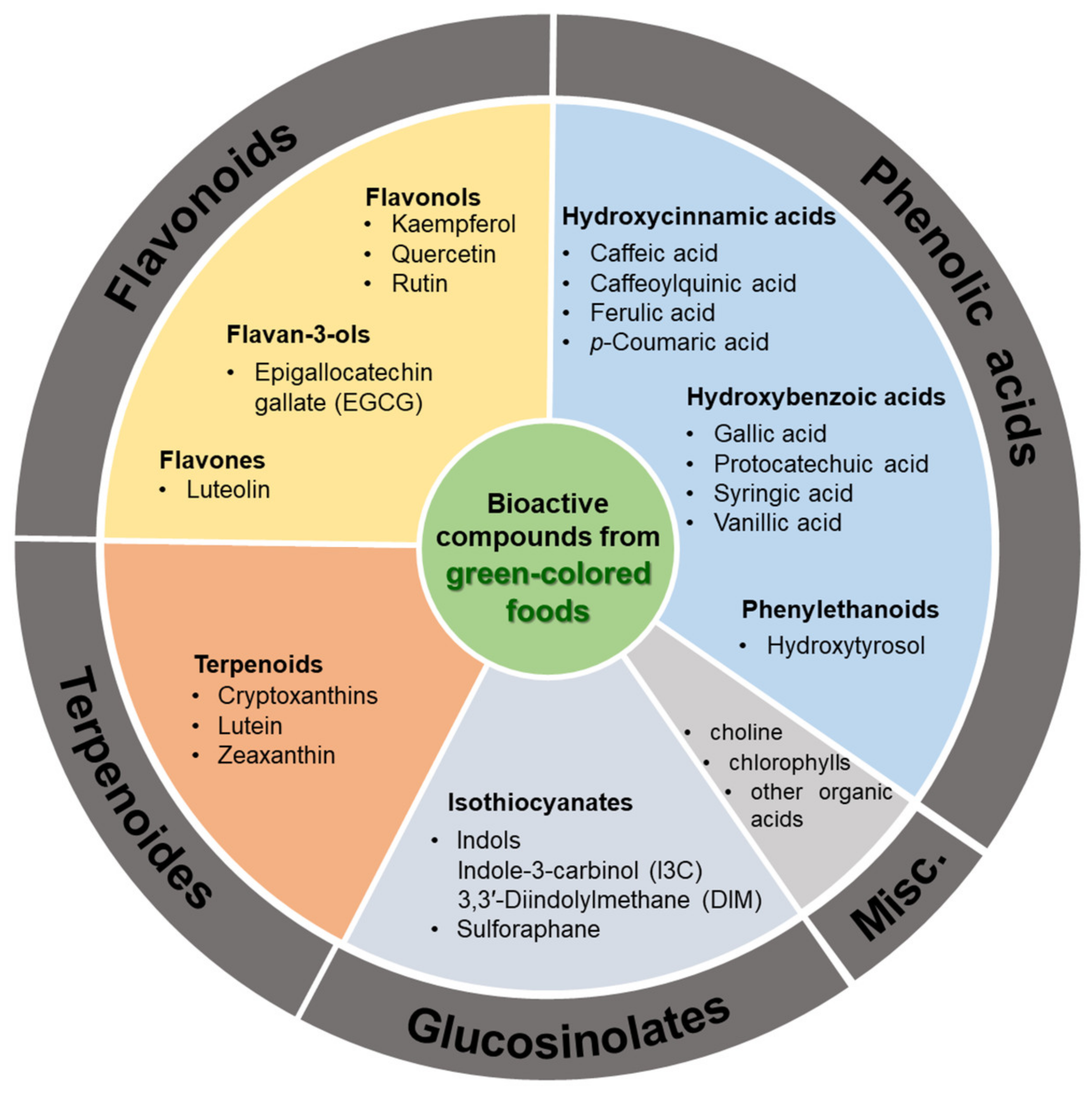
‘Eating the rainbow’ is a concept that highlights the need to consume plant-based foods, especially fruits and vegetables of different colors, for physiological and psychological health. Emerging studies have documented how a variety of bioactive compounds contained in fruits and vegetables, also called phytochemicals, are important contributors to their color formation and health-improving biofunctions. Likewise, epidemiological studies have shown a strong relationship between the intake of abundant color fruits and vegetables and reduced risks on chronic diseases [1,2]. Green-colored foods, owing to their constituents, such as flavonoids, terpenoids, glucosinolates, and phenolic acid (Figure 1), exhibit antioxidant, anti-inflammatory, and other biological effects, which contribute to improving human health and preventing a variety of diseases. In particular, the consumption of green-colored food performed unparalleled therapeutic and preventive effects on cardiovascular and metabolic diseases [3].

Figure 1.
List of bioactive compounds derived from green-colored foods. This figure was adapted from references [4,5,6,7].
Therefore, achieving a better understanding of the bioactivities and underlying mechanisms of how green-colored food improves human health should facilitate the development of green-colored food, and their phytochemicals, as therapeutic agents or functional food. However, the vast majority of studies on these biological effects were performed with preparations whose biochemical features were largely uncharacterized, in part because identifying the bioactive components of green-colored food is a formidable challenge. In recent years, green-colored food and their phytochemicals attracted increasing attention from academic and producer communities owing to their superior modulation in improving human health and treating a variety of diseases.
Polyphenols are one of the main components of green-colored foods and are present in plants as aglycones, glycosides, esters, or polymers. How they are metabolized in one’s body is different. For instance, aglycones can be absorbed from the small intestine, while glycosides, esters, and polymers are hydrolyzed by enzymes or by the gut microflora to be absorbed [8,9]. Certain chemical characteristics such as molecular weight, lipophilicity, stereochemistry, and the presence of a group capable of hydrogen bonding, influence the metabolism of the polyphenols in the gastrointestinal tract (GIT). Low-molecular weight compounds, such as gallic acid and isoflavones, which can be absorbed from the gastrointestinal tract, account for 0.3–43% of the polyphenols, whereas the metabolite content circulating in the plasma can be low. Hence, various factors may interfere with the direct bioavailability of the phenolic compounds present in the food.
The antioxidant potential of natural compounds confers their therapeutic activities for a wide variety of diseases such as cardiovascular disease (CVD), cancer, liver diseases, diabetes, and neurodegenerative disorders [10]. The antioxidant capacity of dietary phenolic compounds may explain their health benefits because several in vitro studies have demonstrated that these compounds protect low-density lipoprotein (LDL) from oxidative modifications [4,5,6]. However, studies analyzing the antioxidant capacity of phenolic compounds in vivo are controversial, since many of the phenolic compounds are poorly absorbed and metabolized into inactive forms, resulting in low blood concentrations [7]. The health beneficial effects of phenolic acids can be affected by several factors such as poor stability, restricted bioavailability, and absorption [5,7,8]. It is well established that phenolic compounds are absorbed in a dose-dependent manner in the intestine.
Regarding phytochemical bioactivity, the characteristics of biphasic regulation and dose-dependent biological effects have often been observed, and bioavailability is an important factor that confers bioactivities. Bioavailability is defined as the amount of an ingested nutrient that is absorbed and available for physiological functions, depending on digestion, release from the food matrix, assimilation into blood, and transportation to body cells [11]. Several factors, such as molecular structure, chemical bonding, host digestibility, and gut microbiota species, impact the bioavailability of natural phytochemicals.
In general, owing to the complex molecular structure of dietary phytochemicals, their absorption in the upper GIT of the host is low, thus enabling only small quantities of these compounds to enter the blood and be transported to different tissues. Some of these compounds can directly interact with tissue cells by activating cellular signaling pathways and regulating metabolic processes. In contrast, more indigestible compounds reach the hindgut almost unaltered, interact with resident gut microbiota, and get metabolized into many small molecular compounds, which can either interact directly with intestinal cells or are absorbed and transported to different tissues and organs, exerting multi-target biofunctions in different tissues and organs and regulating body metabolism. The interaction between phytochemicals and gut microbiota is an important way of exerting biological effects [12,13].
One of the mechanisms by which food phytochemicals exert multi-target therapeutic or preventive effects on human cardiovascular and metabolic diseases is their capacity to shape the healthy gut microbial community and modulate health-improving metabolic pathways. Several approaches, such as fermentation, different delivery systems, complexation with other compounds, can be used to not only increase the bioavailability of phytochemicals but also to improve the capacity of their bioactivities [14,15]. It is considered a feasible strategy to develop a personalized dietary program based on the green-colored food for preventing and remedying cardiovascular and metabolic diseases in humans.
Thus, it is important to elucidate the molecular structure, bioavailability, and bioactivity of phytochemicals for expanding their application as therapeutic agents or functional foods in improving human health.
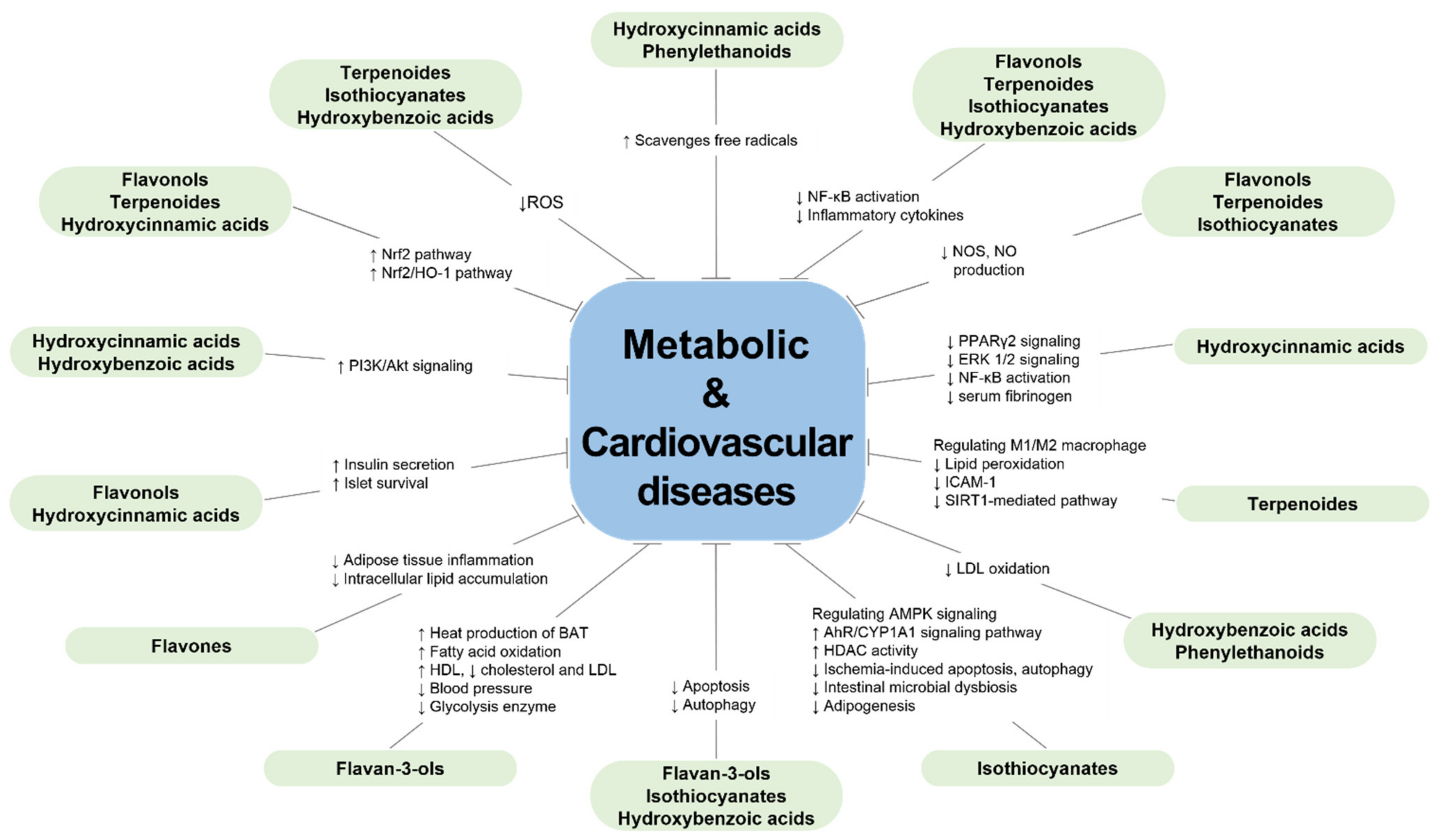
In the present review, a search of the literature was conducted using the PubMed, ScienceDirect, google scholar, and the Web of Science databases taking the green-colored food, cardiovascular, and metabolic diseases as the MeSH terms. Between 2000 to 2021, a total of 8896 articles have focused on the molecular structure, biological activities and beneficial effects of green-colored foods and/or their active compounds on cardiovascular and metabolic diseases. A systematic summary was performed for those prior studies: the most common green-colored fruits and vegetables and their active ingredient are listed in Table 1, the primary functional components contained in green-colored food are classified in Figure 1, the detailed names and molecular formulas of those compounds are listed in Table 2, and potential health effects, prevention and/or therapeutic mechanisms to cardiovascular and metabolic diseases are summarized in Figure 2.

Table 1.
Green-colored fruits and vegetables and bioactive compounds.

Table 2.
The chemical structure of bioactive compounds.
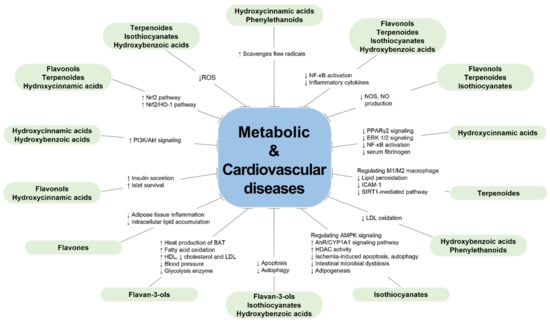
Figure 2.
Protective effects of the bioactive compounds from green-colored foods on metabolic diseases and cardiovascular diseases.
The aims of this review are to discuss the bioactive compounds derived from green-colored foods and their bioavailabilities and bioactivities, thus providing a scientific reference for the application of green-colored food in the management of cardiovascular and metabolic diseases.
2. Bioactive Compounds Commonly Found in Green-Colored Fruits and Vegetables
The main biologically-active substances in green vegetables and fruits include flavonoids, terpenoids, phenolic acids, and glucosinolates, which play a pivotal role in color formation and biological functions. Here, we listed the bioactive compounds derived from green-colored fruits and vegetables and summarized their general characteristics, food sources, bioavailability, and reported health beneficial effects. However, it should be noted that the “potential health benefits” of these compounds summarized from previous studies/literatures are partly derived from clinical trial observations in humans and partly from mechanism studies in model animals (mice & rabbits), which will provide scientific evidence for expanding the applications of bioactive compounds from green-colored food as functional foods or therapeutic agents.
2.1. Flavonoids
2.1.1. Flavonols: Kaempferol, Quercetin, and Rutin
General Characteristics and Food Sources
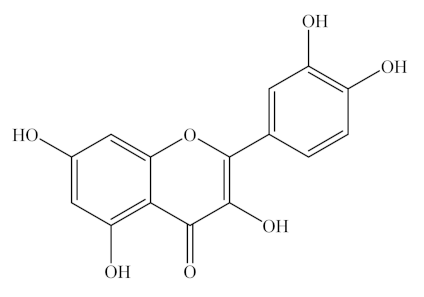
Kaempferol, quercetin, and rutin are natural flavonols, a type of flavonoid, found in a variety of plants and plant-derived foods (Figure 1). Kaempferol can be easily found in beans, broccoli, kale, spinach, and tea [33], whereas quercetin is found in onion, asparagus, and berries, as listed in Table 1 [34]. Lastly, rutin is predominantly found in apples, buckwheat, citrus fruits, cranberries, mulberry, red wine, and tea [35,36]. In addition, rutin derivatives have been tested as a common dietary flavonoid that is consumed in fruits, vegetables, and plant-derived beverages and is metabolized by gut microflora to a range of phenolic compounds [37].
As listed in Table 2, kaempferol is a tetrahydroxyflavone in which four hydroxy groups are located at positions 3, 5, 7, and 4′, C15H10O6 (MW 286.23 g/mol). Quercetin belongs to flavonols, one of the six subclasses of flavonoid compounds with the molecular formula C15H10O7 (MW 302.24 g/mol). Rutin, generally termed vitamin P, with the molecular formula of C27H30O6, is considered to be one of the most therapeutically active phytochemicals. The difference between quercetin and kaempferol is that the latter lacks an OH group at the 3′ position. Quercetin, an aglycone with no attached sugar, exists as a brilliant citron yellow needle crystal, poorly soluble in water but soluble in alcohol and lipids. Quercetin glycosides are formed by attaching a glycosyl group, such as glucose, rhamnose, or rutinose, by replacing one of the OH groups, usually at position 3. Thus, dietary quercetin is present mainly as various O-glycosidic forms, including quercetin-3-O-rutinoside (rutin), quercetin-3-O-glucoside (isoquercetin), and quercetin-3,4′-O-diglucoside.
Bioavailability
Pharmacokinetic studies of kaempferol, a highly polar glycoside, have shown that this polyphenol is poorly absorbed compared with the moderately polar aglycone [38]. The plasma concentrations of quercetin and kaempferol were quite similar. These results may be due to better clearance of plasma kaempferol conjugates from the kidney [39]. In general, kaempferol is metabolized to the methyl, sulfate, or glucuronide forms, but the oral bioavailability of kaempferol is extremely low and absorption is poor [40,41]. The absorbed kaempferol undergoes metabolic transformation by intestinal conjugation enzymes to yield the glucurono/sulfoconjugates (methylated or nonmethylated) forms in the liver [42] and small intestine [39]. Kaempferol and its glycosides are metabolized by the bacterial microflora that releases aglycones and broken aglycone C3 ring to form compounds, such as 4-methylphenol, phloroglucinol, and 4-hydroxyphenylacetic acid, which are either absorbed and can reach systemic circulation and tissues or be excreted in feces and urine.
Moreover, quercetin has low bioavailability. The expected absorption rate of quercetin glucoside, the naturally occurring form of quercetin, ranges between 3% and 17% of healthy individuals consuming 100 mg, and this low bioavailability may be due to low absorption, extensive metabolism and/or rapid clearance [43]. Quercetin normally exists as quercetin glycoside in plants and contains a quercetin aglycone conjugated to a sugar such as glucose or rutinose. Quercetin glycosides show different absorption rates depending on the type of attached sugar [44]. Furthermore, quercetin glucoside is absorbed more efficiently than rutin [43]. In human studies, the requirement of the colonic microbiota for the hydrolysis of rutinoside may explain the low bioavailability of rutin compared with quercetin-3-glucoside [45].
Potential Health Benefits
Kaempferol has a wide range of therapeutic properties, such as antioxidant, anti-inflammatory, and anti-obesity, so it can act as a chemotherapeutic agent in the treatment of inflammatory disease, obesity, diabetes, and CVD [46]. In addition, various in vitro and in vivo studies in human tissue cells or model animals have attributed the potential cardioprotective effects of kaempferol to its anti-inflammatory activity [47,48,49]. Ischemic heart disease mortality tended to be lower at higher quercetin and kaempferol intakes [50]. Although kaempferol belongs to the flavonol family, like quercetin, it exhibits different gut metabolism. Unlike quercetin, it does not have a catechol group, and this structural difference may be related to changes in mice gut metabolism [51].
Quercetin has also been shown to have antioxidant and anti-inflammatory, cardioprotective, and antihypertensive effects in both in vitro and in vivo studies. It exhibits the biphasic immuno-stimulatory or anti-inflammatory capacity. For example, a study in animal model revealed that supplement with Quercetin at nanomolar doses demonstrated not only the activation of basophils, but also the prevention of allergic inflammation [52]. Additionally, Quercetin influences immunity and inflammation by affecting leukocytes and regulating many intracellular signaling proteins. Additionally, it has been shown to ameliorate tumor necrosis factor-α (TNF-α) and nitric oxide (NO) production in the visceral adipose tissue and downregulate nitric oxide synthase (NOS) expression in obese Zucker rats [53].
Rutin is considered a promising agent for its antidiabetic, anti-inflammatory, antitumor, antibacterial, and antioxidant effects in the pharmaceutical and cosmetic industries [54,55,56]. It is oxidized to a quinone that helps reduce the risk of CVD by up-regulating the nuclear factor erythroid 2-related factor 2 (Nrf2)-mediated endogenous antioxidant response. Thus, rutin is considered a modulator of lipid metabolic and signaling pathways, and it normalizes UV-induced Nrf2 expression [57]. Huang et al. documented how rutin presented antidiabetic physiological activity in ApoEknockout mice [58]. Additionally, rutin could significantly suppress the increases in inflammatory cells and cytokines in the bronchoalveolar lavage fluid of mice [52]. The anti-diabetic effects of rutin on humans include the reduction of carbohydrate absorption from the small intestine, the improvement of glucose uptake, the suppression of gluconeogenesis, and the activation of insulin secretion from pancreatic β-cells [59].
2.1.2. Flavan-3-ols: Epigallocatechin Gallate; EGCG
General Characteristics and Food Sources
Green tea is one of the most popular natural beverages that is produced from the leaves of Camellia sinensis L. through a series of processing steps. In recent years, green tea has received much extensive attention for its putative bioactivities in preventing metabolic diseases and improving human health. The chemical composition of green tea includes chlorophyll, polyphenols, flavonoid, gallic acid, catechins, and caffeine, with chlorophyll and catechins being the primary contributors for its green appearance (Table 1). The catechins are a kind of polyphenol compounds and consist of (−)-epicatechin-3-gallate (ECG), (−)-epicatechin (EC), (−)-epigallocatechin (EGC), and (−)-epigallocatechin-3-gallate (EGCG) [60]. Of these, EGCG is the most abundant catechin present in green tea and accounts for approximately 30%~50% of the catechin content. Its chemical formula is C22H18O11, and its molar mass is 458.37 g/mol (Table 2).
Bioavailability
In spite of its numerous health benefits, the green tea catechin, EGCG, has poor bioavailability mainly because of the intensive deterioration during digestion, especially in the small intestine [60,61]. In addition, low transcellular transport and rapid biliary excretion of EGCG partially contributes to its poor bioavailability [62]. For example, maximum plasma EGCG concentrations of up to 1~2 mol/L were achieved within 2 h after consumption, followed by rapid clearance, restoring plasma concentrations to the baseline levels within 24 h of initial consumption in humans [61]. Additionally, it has previously been reported that long-term treatment of rats and mice with dietary green tea resulted in an initial increase in plasma EGCG levels over the first four days of the treatment [63]. Over the subsequent 10 days of treatment, the plasma levels of EGCG decreased and eventually restored to the baseline levels. Thus, these results indicate that EGCG had low absorption and rapid clearance rates in blood, resulting in its poor bioavailability.
Therefore, it is important to determine a proper strategy to improve the bioavailability of EGCG, and thus better develop it as a therapeutic agent or functional food in improving human health and preventing metabolic diseases. Alteration of the delivery system for EGCG by oral administration was believed to be a feasible strategy to improve its bioavailability. Recently, EGCG was reported to display increased stability, and thus improved bioavailability and therapeutic efficacy, when formulated as dual drug-loaded PEGylated poly(lactic-co-glycolic acid) nanoparticles [64]. Likewise, Han et al. found that the complexation of royal jelly proteins with the green tea extract enriched with EGCG would enhance its bioavailability [65].
Potential Health Benefits
Numerous studies have shown that EGCG has a wide range of biological activities in anti-obesity, anti-cancer, anti-bacteriostatic, anti-oxidation, anti-aging, cardiovascular protection, and pro-reproductive capacities [4,5].
EGCG supplementation prevented high-fat diet (HFD)-induced body weight increase by partially inhibiting subcutaneous and epididymal adipose tissue weight gain in rodents [66]. In a parallel study, Zhu et al. found that green tea consumption by lean C57BL/6J mice could prevent HFD-induced-obesity after stopping green tea drinking, while the mice with pre-existing obesity did not receive such a benefit [13]. On the other hand, another study reported that the dietary supplementation of EGCG reversed the established obesity in Sprague-Dawley rats [60]. Thus, the inconsistent results regarding the anti-obesity effects of EGCG might be attributed to the differences in animal species, purity of green tea, dosage of EGCG, and duration of the study.
In addition, a study conducted in HFD-fed mice demonstrated that EGCG played a role in inhibiting intestinal lipid absorption and promoting lipid excretion, which might contribute to its anti-obesity effects [60]. In addition, it could reduce the levels of hepatic triglyceride (TG) and possibly visceral fat deposition by reducing the activities of enzymes related to hepatic fatty acid synthesis [6]. Furthermore, EGCG enhances the expression of genes related to fatty acid oxidation and heat production of brown adipose tissue (BAT), thereby promoting fat oxidation and BAT heat production, which in turn lead to body energy expenditure to prevent obesity of mice [67].
EGCG protected cardio-cerebrovascular cells by inhibiting the apoptosis of hypertrophic cardiomyocytes induced by overload. Furthermore, it could reduce diastolic and systolic blood pressure to regulate blood pressure and inhibit glycolysis enzyme to control blood glucose in the HFD-fed mice [60,68]. EGCG exerts an anti-atherosclerosis effect in rats by decreasing blood cholesterol and LDL and increasing high-density lipoprotein (HDL) levels [69].
2.1.3. Flavones: Luteolin
General Characteristics, Structure, and Food Sources
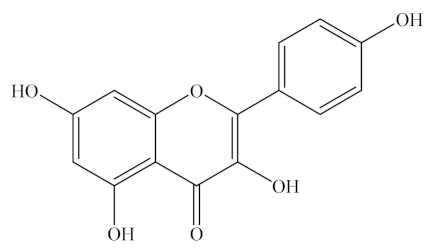
Luteolin, a dietary flavone, occurs naturally in the plant kingdom as pure yellow crystals and is abundant in some green-colored vegetables and fruits, such as artichoke, broccoli, cabbage, celery, green apple (skin), onion leaves, and parsley, as listed in Table 1 [70]. Dietary luteolin is mostly present in its glycosylated form. Luteolin (C15H10O6) is a 3′,4′,5,7-tetra hydroxyflavone with a C6-C3-C6 structure and possesses two benzene rings, one oxygen containing ring, and a 2–3 carbon double bond (Table 2). The hydroxyl moieties and 2–3 double bonds are important structural features associated with its biochemical and biological activities [70,71,72].
Bioavailability
According to many studies, it is known that the oral absorption and bioavailability of flavonoids, including luteolin, is very low [73,74]. In fact, luteolin has poor stabilization and a low absorption rate; thus, its efficacy is low because of its relatively low in vivo bioavailability. A mice study showed that the oral bioavailability of luteolin (approximately 26 ± 6%) was higher than that of luteolin-7-O-glucoside (approximately 10 ± 2%) [75].
Shimoi et al. observed that luteolin and its glycosides can be converted to glucuronides when they cross the intestinal mucosa, and that free luteolin, its conjugates and methylated conjugates can be found in rat and human plasma after administration [76]. According to the findings of Zhou et al., luteolin was passively absorbed in the small intestine of rats and was more efficiently absorbed in the duodenum and jejunum than in the colon and ileum, and luteolin in peanut hull extract was absorbed more efficiently than pure luteolin [77].
Potential Health Benefits
Luteolin is a widely used flavone. According to preclinical studies, the ingestion of luteolin alone has been reported to have various biological and pharmacological antioxidant, anti-inflammatory, anti-diabetic, anti-cancer, and cardioprotective properties [70,78]. Although many previous studies of luteolin have focused on cancer and inflammation, reports of its cardioprotective effects have steadily increased since the 1950s [79]. Accumulated evidence suggests that luteolin plays a pivotal role in regulating glucose production and adipogenesis, and thus may act as a modulator of insulin resistance and diabetes. A study of obese mice treated with luteolin or its glucoside (i.e., luteolin-enriched plant extracts) improved blood glucose levels and hepatic steatosis [80]. Furthermore, luteolin exhibits anti-obesity and anti-diabetic effects, in part through altering adipose tissue physiology. The mechanism of luteolin in these potential health benefits is as follows; (1) regulated adipose tissue inflammation, (2) reduced intracellular lipid accumulation, and (3) improved insulin sensitivity and glucose utilization.
2.2. Terpenoides
Tetraterpenoids/Carotennoids: Cryptoxanthins, Lutein, and Zeaxanthin
General Characteristics, Structure, and Food Sources
Cryptoxanthin, also known as β-cryptoxanthin, is a natural pigment that imparts bright colors to vegetables and fruits. It is an oxidized carotenoid containing hydroxyl and other oxygen groups. It belongs to the lutein group and is yellow-orange in solution and free state. Its molecular formula is C40H56O, and its relative molecular weight is 552.88 g/mol (Table 2). Cryptoxanthin is widely found in oranges, papaya, persimmon, avocado, grapefruit, red bell pepper, coriander, pumpkin, corn, leafy vegetables, and some yellow animal foods, such as egg yolks and butter [81]. It is a major dietary provitamin A carotenoid mostly provided by citrus fruits.
Lutein and zeaxanthin belong to the family of oxygenated carotenoids classified as xanthophylls, which are found in high quantities in green leafy vegetables and fruits, such as avocado, kale, parsley, spinach, broccoli, green peas, green beans, kiwi, green lettuce, cucumber, green pepper, celery, green grapes, brussels sprouts, scallions, watercress, romaine lettuce, and zucchini (Table 1) [82,83]. Lutein and its isomer zeaxanthin are the dihydroxy derivatives of α-carotene and β-carotene, respectively [84]. They are termed macular pigments. Lutein (C40H56O2) is a lipid-soluble molecule with a melting point of 190 °C and a molecular mass of 568.87 g/mol (Table 2). In addition, lutein contains several secondary metabolite compounds such as steroid/triterpenoid, alkaloid, flavonoid, tannin, and saponin [85].
Bioavailability
Food as well as host-related factors contribute to the bioavailability and bioaccessibility of carotenoids. Previous studies have demonstrated that the bioavailability of carotenoids (i.e., β-cryptoxanthin) under in vitro conditions ranged from 0.1% to almost 100% [86,87,88]. In spite of much attention on lutein, owing to its essential effects such as its antioxidant properties, little information is available regarding its pharmacokinetic properties. Sato et al., calculated the bioavailability of lutein to be 5.20% and showed that a large amount of lutein accumulated in the intestinal mucosa [89]. In addition, the absorption of lutein is improved significantly by food intake. The ability to respond to lutein and blood lutein status are related in humans [90]. Lutein can either exist in the free form or be esterified to fatty acids, and the bioavailability of lutein diester formulation is higher than the free lutein formulation [82]. It is necessary that dietary lutein and zeaxanthin esters be hydrolyzed by enzymes in the gastrointestinal tract before their uptake, since only free forms of xanthophylls can be absorbed into the circulation [91].
Potential Health Benefits
Dietary cryptoxanthin has several bioactive anti-oxidant, anti-atherosclerosis, and anti-aging properties and can be converted into vitamin A. It can reduce oxidative damage, and protein loss and improve acute nephritis and immunity [81]. In general, there is evidence to suggest that β-cryptoxanthin has beneficial effects in animal models of both non-alcoholic fatty liver disease (NAFLD) and alcoholic liver disease [92].
In addition, previous studies have documented the use of β-cryptoxanthin as a nutritional regulators of adipose tissue to be a feasible strategy for preventing obesity and obesity-related metabolic diseases of humans [93]. Specific carotenoids and carotenoid derivatives restrain adipogenesis and adipocyte hypertrophy, while they enhance the fat oxidation and energy dissipation in brown and white adipocytes, and counteract obesity in animal models, indicating a beneficial effect of β-cryptoxanthin supplementation. Moreover, β-cryptoxanthin alleviated myocardial ischemia/reperfusion injury by inhibiting nuclear factor-kappa B (NF-κB)-mediated inflammatory signaling in rats [94]. The dietary administration of β-cryptoxanthin not only prevents but also reverses nonalcoholic steatohepatitis progression by regulating M1/M2 macrophage polarization in mice [95]. Three possible modes of action can be proposed to explain these benefits; (1) enhanced hepatic antioxidative status widely attributed to carotenoids in general; (2) production of vitamin A from β-carotene and β-cryptoxanthin, leading to improved hepatic retinoid signaling; and (3) production of apocarotenoid metabolites from β-carotene and lycopene that may play a role in hepatic signaling pathways [92].
Lutein is not a vitamin A precursor and has been found to play important roles in improving visual functions such as improving red-green color discrimination sensitivity, protecting retinal epithelial cells from reactive oxygen species (ROS), and preventing age-related eye diseases [96,97,98]. In addition, lutein acts as a strong antioxidant by scavenging superoxide radicals, hydroxyl radicals, and NO and by inhibiting lipid peroxidation both in vitro and in vivo [99]. A vast amount of research in clinics and mice indicates that lutein may also reduce the risk of CVDs and coronary artery diseases. Furthermore, lutein appeared to have multiple anti-inflammatory functions in vivo and in vitro models. Lutein decreased lipopolysaccharides (LPS)-induced secretion of inflammatory cytokines and increased antioxidant enzymes in a dose-dependent manner [100,101]. The mechanism for the anti-inflammatory property of lutein might be partially due to the modulatory effects of NF-κB, intercellular adhesion molecule (ICAM)-1, and Nrf-2 activation [102,103,104,105]. A recent study has shown that lutein inhibits lipid accumulation in differentiated 3T3-L1 cells and abdominal adipose tissue of rats by the sirtuin 1 (SIRT1)-mediated pathway [106].
2.3. Glucosinolates
2.3.1. Isothiocyanates: Indoles
General Characteristics, Structure, and Food Sources
Isothiocyanates (ITCs) are a class of small molecular compounds widely found in cruciferous plants such as broccoli, cabbage, horseradish seed, kale, radish, mustard, and watercress. Their molecular formula is N=C=S, and their structures and varieties in plants are tightly related to the precursors of glucosinolates. ITCs are the main metabolites of glucosinolates hydrolyzed by plant myrosinase in vitro or degraded in human and animal digestive tract by enzymes or microbiota [107]. In general, they exist as oily or low-melting-point solids (Table 1) [108].
Indoles, one of the major unstable ITCs, are planar heteroaromatic molecules. Among indoles, only indole-3-carbinol (I3C) is commercially available as an off-white solid [109]. It is a naturally occurring hydrolysis product of glucobrassicin found in vegetables of the genus Brassica (family Cruciferae), such as broccoli, brussels sprouts, cauliflower, cabbages, kale, mustard greens, bok choy, collard greens, arugula, and kohlrabi, as listed in Table 1 [110,111,112]. The molecular formula of I3C is C9H9NO and its chemical structure is shown in Table 2. Under acidic conditions, I3C is unstable and gets converted to a series of oligomeric products in vivo, such as 3,3′-diindoylmethane (DIM, C17H14N2) [35,112,113,114], a dimer transformed from indole-3-methanol, which belongs to the indoleosinolates family. It is a kind of pharmaceutical intermediate with a white crystalline powder appearance. DIM is also found in many cruciferous plants such as brussels sprouts, broccoli, cabbage, collards, cabbage, shepherd’s purse, radish, turnip, and watercress (Table 1).
Bioavailability
After oral administration, I3C is converted to a series of oligomeric products (mainly DIM) under the acidic conditions of the stomach. It is rapidly absorbed, distributed, and eliminated from the plasma and tissues, and its concentrations are the highest in the liver and the least in the brain [21,115]. Owing to their lipid solubility characteristic, the concentrations of I3C, DIM, and other derivatives are approximately 6-fold higher in the tissues than in the plasma. Furthermore, the absorption, distribution, metabolism and, elimination of I3C occur more rapidly than its derivatives, such as DIM [21,115], which is eliminated through the renal system in humans [116]. Interestingly, DIM is the only oligomer found in human plasma. Researchers assume that the production of these oligomers is insufficient or they may not be effectively absorbed in the human gut [112].
Potential Health Benefits
ITC has a wide range of biological anti-bacterial, antioxidant, anti-inflammatory, anti-hypertensive, and cardio-protective effects in human and model animals [114,117]. Dietary supplementation of ITC improved the relaxation of the aorta, reduced the number of infiltrating activated macrophages, and decreased the blood pressure. In an ischemia/reperfusion animal model, feeding broccoli extracts improved postischemic ventricular function, reduced myocardial infarct size, and decreased cardiomyocyte apoptosis [118]. One of the potential mechanisms by which ITC prevents and/or treats cardiovascular and metabolic diseases might be its capacity to remove metabolic waste and ROS [119].
I3C also has various biological anti-inflammatory, immunoregulatory, anti-hypertensive, anti-osteoporosis, and anti-diabetic properties [109,120,121]. I3C inhibited NO, TNF-α, and interleukin-10 (IL-10) production in LPS-activated macrophage cells and rat models [122,123], suggesting its immunoregulatory effects. In addition, I3C immunoregulatory effects on polarization of monocyte-derived macrophages in systemic lupus erythematosus patients were observed as downregulated expression of pro-inflammatory genes and overexpression of anti-inflammatory cytokines [121]. I3C and its derivative DIM have been shown to prevent ethanol-induced liver injury or drug-induced liver injury in mice by oxidative stress regulatory, antioxidant, anti-inflammatory, and anti-apoptotic mechanisms [124,125].
Moreover, I3C exerts anti-obesity effects by reducing body weight and fat accumulation in epididymal adipose tissue in HFD-induced obese mice and thereby improves hyperglycemia and hyperinsulinemia [126]. In an in vitro trial, I3C prevented the differentiation of 3T3-L1 preadipocytes into adipocytes, inhibits adipogenesis by regulating 5′-adenosine monophosphate-activated protein kinase (AMPK) signaling [127,128,129].
DIM exerts a wide range of biological effects, especially in treating metabolic, cardiovascular, and neurodegenerative diseases. Emerging studies have indicated that DIM increases the structural and functional integrity of the intestinal barrier and thus improves the pathophysiological metabolism [130]. Similarly, Choi and Yoo [131] have demonstrated that DIM can improve insulin-dependent diabetes and nephropathy in streptozotocin (STZ)-induced mice and relieve some symptoms of diabetes such as hunger and thirst. Additionally, DIM has a therapeutic effect on CVDs.
The anti-diabetic effects of DIM on hyperglycemia and diabetic nephropathy of mice were thought to be associated with the obstruction of protein kinase C (PKC)-α and transforming growth factor (TGF)-β1 signaling pathways [131]. Similarly, by depressing the expression of interleukin-6 (IL-6) and chemokine (C-X-C motif) ligand 1 (CXCL1), DIM exhibited a beneficial impact on the treatment of CVDs, thus protecting the impaired neurological function in a mice model [132]. Meanwhile, other studies have also revealed that DIM protected the hematopoietic function of irradiated mice by inhibiting the increase of ROS generation in hematopoietic stem cells (HSCs) induced by whole-body irradiation and phosphorylation of histone H2AX (γ-H2AX). DIM significantly improved bone marrow HSC frequency, hematopoietic progenitor cell cloning function and multifamily transplantation after transplantation [133]. DIM has a neuroprotective mechanism, which has been proved to be achieved by inhibiting ischemia-induced apoptosis and autophagy, while suppressing the aryl hydrocarbon receptor/cytochrome P4501A1 (AhR/CYP1A1) signaling pathway and increasing histone deacetylases (HDAC) activity [134]. The radiation protection effect of DIM may be due to its anti-DNA damage effect on irradiated human intestinal epithelial cell line HIEC-6. These changes are related to the decrease in ROS levels and increase in antioxidant enzyme activity in human intestinal epithelial cell line-6 (HIEC-6) cells after irradiation. In addition, the radiation protection effect of DIM on the intestine leads to the recovery of intestinal microbial community composition, alleviates intestinal radiation damage, and thus improves body metabolism in mice treated with withaferin-A [130].
2.3.2. Isothiocyanates: Sulforaphane
General Characteristics, Structure, and Food Sources
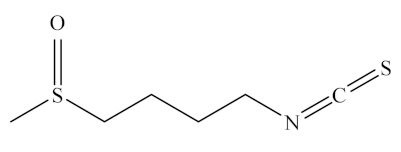
Sulforaphane is one of the most promising diet-derived chemopreventive agents [135] belonging to the ITC group of organosulfur compounds. It exists as its biologically inactive precursor, glucoraphanin, a storage form in cruciferous vegetables, being the most abundant in broccoli (Table 1). This precursor belongs to the group of phytochemicals called glucosinolates, which contain a sugar moiety in their structure, and is rapidly converted to sulforaphane by the enzyme myrosinase [136]. The chemical name of sulforaphane is 4-methylsulfinylbutyl isothiocyanate or 1-isothiocyanate-4-methylsulfinylbutane, and its chemical structure is shown in Table 2.
Bioavailability
Sulforaphane has the highest bioavailability among phytochemicals, with its absolute bioavailability being approximately 80% [137]. It is converted from its inactive precursor glucoraphanin to its active form by the enzyme myrosinase, whose amount and activity are closely related to its own bioavailability. When plant cells are damaged, the enzyme myrosinase is released, which interacts with glucoraphanin to produce sulforaphane, which is metabolized in vivo via the mercapturic acid pathway, triggered by a spontaneous reaction between the electrophilic -N=C-S group and glutathione. The end products of sulforaphane metabolism are sulforaphane-cysteine and sulforaphane-N-acetyl-l-cysteine, and the main metabolite is sulforaphane-N-acetyl-l-cysteine [138].
Potential Health Benefits
Sulforaphane is a dietary phytochemical (a secondary plant metabolite) with low toxicity and is widely consumed in cruciferous vegetables and many dietary nutraceuticals. It has been extensively studied in the past several years for its health benefits in various in vitro and in vivo studies, which have reported its anti-diabetic [139,140,141], anti-inflammatory [138], and anti-obesity [142] effects in human and model animals. For example, it exerts cytoprotective effects on mice by increasing the expression of several antioxidant proteins [143]. A clinical study has found that consuming broccoli sprouts, which are rich in glucoraphanin, for just one week improved cholesterol metabolism and reduced oxidative stress markers [144]. A study suggested that the potential cardioprotective action of sulforaphane could be attributed to decreased ROS production and DNA fragmentation as well as increased cell viability [135]. Thus, sulforaphane may protect against various types of metabolic diseases and decrease the risk of CVDs.
2.4. Phenolic Acids
2.4.1. Hydroxycinnamic Acids: Caffeic Acid, Caffeoliquinic Acid, Ferulic Acids, and p-Coumaric Acid
The primary phenolic acids in green fruits and vegetables include caffeic acid (CA), caffeoliquinic acid (CQA), ferulic acid (FA), and p-coumaric acid (p-CA), which belong to hydroxycinnamic acids (HAs) and have similar molecular structures. They play an important role in the antioxidant, anti-inflammatory, anti-hypertensive, and other biological functions of green fruits and vegetables.
General Characteristics, Structure, and Food Sources
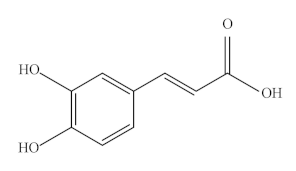
Caffeic acid (CA), a natural polyphenolic compound, is widely found in cabbage, buckwheat, dandelion, artichoke, olive, mint, and other green plants. It is a typical representative of HA and has phenolic hydroxyl and acrylic acid functional group structures. In addition, it is a significant micronutrient in the human body and the main source of HA in human diet. It has good biological activity [145]. It is also known as 3,4-dihydroxy cinnamic acid and exists as yellow crystals at room temperature. It is slightly soluble in cold water and easily soluble in hot water and organic solvents. The chemical formula of CA is C9H8O4, and its molecular weight is 180.15 Da. Its melting point is 223~225 °C.
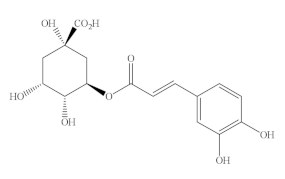
Caffeoylquinic acids (CQA), a natural phenolic compound widespread in the plant kingdom, is a combination of quinic acid (QA) and CA via an esteratic linkage. In recent years, CQA has received great attention because of its high content in coffee, tea, certain fruits, and vegetables. In nature, CQA includes monocaffeic, dicaffeic, tricaffeic, and polycaffeic quinic acids, of which monocaffeic and dicaffeic quinic acids are widely present in plants. Another vital derivate of CQA is 3(5)-O-caffeoylquinic acid (5-CQA) or 3-CQA (pre-IUPAC nomenclature), generally termed chlorogenic acid (CGA), which is one of the most abundant HAs and available phenolic acid compounds present in foods, such as green bean coffee, avocado, broccoli, artichoke, green tea, and Angelica officinalis L. fruits [146,147,148,149,150].
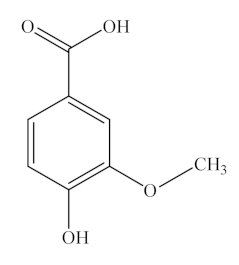
Ferulic acid (FA) and feruloylquinic acid (FQA). Ferulic acid (FA, also known as 4-hydroxy-3-methoxycinnamic acid), is a ubiquitous phenolic compound found in numerous fruits and vegetables, including bananas, citrus fruits, eggplant, and cabbage, as well as in seeds, leaves, and wheat brans [151,152]. It is also considered one of the most effective agents in the Chinese medicinal plant Angelica sinensis and has been approved as a food additive in many European countries. Feruloylquinic acid (FQA) has an additional quinyl ester of HA, which is synthesized by the condensation of FA and QA [153]. It has a wide range of sources, including kiwi fruit, tomato, avocado, Angelica sinensis and green coffee beans, as well as in the roots of nutrient-deprived Brachiaria species [154]. Although FQA has been reported to have anti-tumor properties, its biological functions have not been studied thus far.
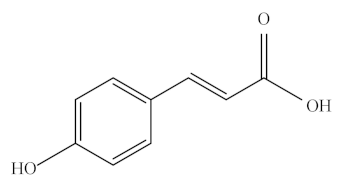
p-Coumaric acid (p-CA), also known as 4-hydroxycinnamic acid, is a phenolic acid that functions as a precursor for other phenolic compounds [155]. It belongs to the HA family, which is synthesized from phenylalanine and tyrosine via the shikimate pathway. It plays a critical role in secondary metabolism because it can be subsequently transformed to other phenolic acids, such as CA, FA, CGA, and sinapic acid, and other secondary metabolites [156]. p-CA is found ubiquitously in fruits (e.g., apples, pears, grapes, oranges, tomatoes, and berries), vegetables (e.g., beans, potatoes, and onions), and cereals (e.g., maize, oats, and wheat), as listed in Table 1 [155]. In addition, it can be also found in green-colored foods including avocado, kiwi, green grape, basil, and spinach.
Bioavailability
Hydroxycinnamic acids (HAs) are one of the major classes of phenolic compounds found in green-colored foods and cereal grains. However, the majority of phenolic compounds in foods are present in bound forms with low bioaccessibility which affect their poor bioavailability [157]. The structure of the fiber matrix and the functions of bioactive phenolic compounds found in green-colored vegetables or cereals strongly affect their physiological functions [158]. A low proportion of ingested conjugated HAs was shown to be absorbed in the small intestine, and the remainder reached the larger intestine and were modified or degraded by the gut microbiota [159]. The conjugated HA is inaccessible to the digestive enzymes and thus presents poor bioaccessibility and bioavailability.
Most of the metabolized HAs are excreted in urine instead of feces. Recently, Kishida and Matsumoto reported that the ingested HAs, except for CGA, were primarily excreted as free or conjugated forms in the urine within 0~6 h [159]. The total urinary excretion rate (% of the dose) at 48 h followed the order FA (73.2%) > CA (61.6%) > p-CA (54.1%) > CGA (4.9%). The percentages of the conjugates in the urine differed among the rats gavage-fed with individual HAs (74% for CGA, 83% for CA, 68% for p-CA, and 96% for FA), which may be explained by their distinct bioactivities. The urinary excretion rate of various HAs, including p-CA, in non-fasted rats under normal physiological conditions was 54.1% of the dose at 48 h [159]. Among the four HAs (i.e., CGA, CA, p-CA, and FA), CA, p-CA, and FA are much more bioavailable than CGA, although they are excreted more rapidly than CGA. FA and p-CA are absorbed by the monocarboxylic acid transporter (MCT) in Caco-2 cells, although gallic acid (GA) is not [160].
Low bioavailability of HAs limits the exhibition of their beneficial bioactive effects. Therefore, many studies have focused on developing the appropriate food processing technologies that can facilitate the release and/or increase the accessibility of conjugated phenolic compounds in green-colored foods, primarily through fermentation, breakdown of cereal matrices, or degradation of fiber polymers.
Potential Health Benefits
HAs, including CA, CQA, GA, and FA, exert a wide variety of biological antioxidant, anti-inflammatory, antimicrobial, antiallergic, hepatoprotective, anticarcinogenic, antithrombotic, and antiviral activities. Studies in rats and piglets suggest that HAs play a role in increasing sperm viability, vasodilatory actions, enzyme activity modulation, transcriptional factor activation, gene expression, and signal transduction [161,162,163,164].
CA has a wide range of pharmacological activities similar to most phenolic compounds. It exerts its antioxidant effect by scavenging free radicals, such as 2,2′-azino-bis3-ethylbenzothiazoline-6-sulphonic acid (ABTS) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) [165], reducing iron ions and chelating metal ions [166]. Additionally, CA exerts potential cardiovascular benefits through antioxidant mechanisms associated with LDL oxidation, NO bioavailability, and blood pressure lowering. Daily use of CA in moderate amounts can reduce the incidence of CVDs such as atherosclerosis and coronary heart disease of humans [167]. Furthermore, CA could fight diabetes and hyperglycemia through multiple mechanisms, including the antibiotic effect of CA in animal models, promotion of islet survival, reduction of serum fibrinogen levels in diabetic animals, and regulation of β cell and adipocyte glucose transporter type 4 (GLUT4) performance [168]. Furthermore, consuming CA every day is beneficial in the treatment of CVDs because of its anti-hypertensive properties and its ability to lower blood pressure throughout the body of rats [169]. Furthermore, it could increase the number of platelets by increasing the content of granulocytes and megakaryocytes, thus speeding up coagulation to achieve hemostasis [167]. Additionally, some human and animal bioactivities of CA are closely related to gut microbial community. Studies have shown that dietary CA interacts with human and/or animal intestinal microbes and thus produces various metabolites that affect host metabolism and cardiovascular health [170]. In anaerobic conditions, bacteria possessing tyrosine decarboxylase could induce the decarboxylation of CA, generating [3-(3-hydroxyphenyl)-propionic acid], a product with higher antioxidant activity compared with CA [169].
CQA has a lipid-lowering activity that improves metabolic dysregulation in diet-induced obesity models partially by regulating peroxisome proliferator-activated receptor (PPAR)γ2 expression. In addition, CQA ameliorates obesity and obesity-related metabolic disorders through the PPARγ2 and NF-κB signaling pathways in rat adipose tissue [171]. Both CQA and CGA have antioxidant properties in humans and animals [172,173,174], which contribute to excellent functions in treating cardiovascular and metabolic diseases. In human bioavailability, one-third of the CGA from foods is absorbed in the small intestine, and a part enters into the blood circulation, while most reaches the colon [175]. The pathways of CGA are as follows: in humans, a part of the 5-CQA (approximately 33%) from food is absorbed intact in the upper GIT without hydrolysis and enters into circulation; a small amount of the 5-CQA (approximately 7%) is absorbed throughout the small intestine involving hydrolysis into CA and QA; colonic microbiota-mediated 5-CQA metabolism with the absorption of metabolites occurs in the colon; after the intact 5-CQA and its metabolites enter the bloodstream, they are absorbed and/or metabolized in the liver (reviewed in ref. [172]). One should point out that the small molecular metabolites produced by gut microbes degrading CGA and its derivatives also play a vital role in regulating physiological and pathological status of host.
FA plays an antioxidant role by inhibiting ROS production and aldose reductase activity and by activating the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathway. The antioxidant property of FA can be further enhanced by the nuclear shift of heme oxygenase-1 (HO-1) via activation of the Nrf2/HO-1 pathway. In addition, FA can inhibit the extracellular signal-regulated kinase (ERK)1/2 signal, regulate the expression of NO/endothelin-1 (ET-1) and vascular endothelial factors, protect endothelial cells, and enhance angiogenesis to maintain the normal function of the vascular endothelium [176]. Moreover, FA can significantly eliminate the negative effects of antibiotics, including upregulation of a variety of antioxidant genes, increase the total antioxidant levels of mice compared with the antibiotic group, reduce the lipid oxidation levels in vivo, regulate NO synthesis through NOS, and stimulate intestinal contraction and peristalsis to promote digestion. Moreover, FA can regulate the intestinal metabolism, intestinal microecological balance, and inflammatory response of piglets by increasing the abundance ratio of Proteobacteria and Firmicutes, significantly increasing the abundance of Clostridium and decreasing the abundance of Diplococcus and Enterobacter [162].
Various investigations have shown that p-CA and its conjugates exhibit various bioactivities, including antioxidant, anti-inflammatory, cardioprotective, and anti-diabetic activities. In animal models, p-CA decreased basal oxidative stress levels more effectively than vitamin E, as assessed by DNA damage in rat colonic mucosa [177]. p-CA showed anti-inflammatory effects in adjuvant-induced arthritic rats, reducing the levels of TNF-α and macrophage phagocytic index, while increasing serum immunoglobulin levels [155].
2.4.2. Hydroxybenzoic Acids: Gallic Acid, Protocatechuic Acid, Syringic Acid and Vanillic Acid
General Characteristics, Structure, and Food Sources
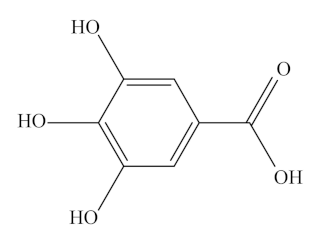
Gallic acid (GA), 5,4,3-trihydroxybenzoic acid, is one of the most important and abundant phenolic acids. Gallic acid and its derivatives are present in fruits and vegetables such as grapes, mango, avocado, and green tea [178]. It is an organic acid containing one benzene ring structure with three adjacent -OH groups at the positions 3,4,5 and one -COOH group at position 1. Its molecular formula is C7H6O5, and its molecular weight is 170.12 g/mol [179]. It is soluble in water, alcohol, ether, and glycerol. In nature, it exists predominantly as hydroxybenzoic acids and in different forms of ester and catechin derivatives [180]. The most common ester derivatives of GA are alkyl esters such as methyl gallate, propyl gallate, octyl gallate, dodecyl gallate, tetradecyl gallate, and hexadecyl gallate. Some of the main catechin derivatives of GA are EC, ECG, EGC, EGCG, and gallocatechin gallate [181].
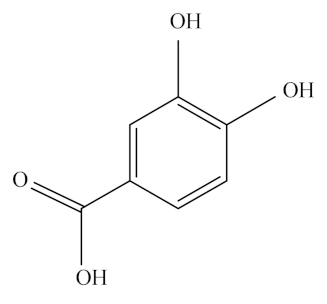
Protocatechuic acid (PCA), 3,4-dihydroxybenzoic acid, is a phenolic compound found in various green food plants such as calamondin, olives, roselle, and white wine grapes [182]. It is the main secondary metabolite of antioxidant polyphenols and the main metabolite of anthocyanins [182,183]. The molecular formula of protocatechuic acid is (HO)2C6H3COOH. It exists as white-to-brown crystalline powder that changes color in the air.
Syringic acid (SA) is a phenolic compound readily found in fruits and vegetables such as olives, dates, pumpkin, and grapes, spices, acai palm, honey, and red wine [184]. It is a white powder with high solubility in ethanol, methanol, and ethyl ether and poor solubility in water. Like other phenolic compounds from plants, such as PCA, gallic acid, and QA, SA is also synthesized by the shikimic acid pathway [185]. SA consists of a single benzene ring linked with two OCH3 groups, one OH group, and one COOH group, and its molecular formula is C9H10O5 (Table 2) [184].
Vanillic acid (VA), 4-hydroxy-3-methoxybenzoic acid, is a benzoic acid derivative, and its molecular formula is C8H8O4. The solution process of VA in the solvents is endothermic and spontaneous [186]. It has a pleasant and creamy odor; therefore, its widely used as a flavoring agent, preservative, and food additive in the food industry. It is a phenolic compound that is oxidized from vanillin, which is obtained from several foods, such as avocado, green olive, Angelica sinensis, green tea and many other plants [187,188,189,190].
Bioavailability
Many beneficial biological effects of hydroxybenzoic acids, including GA, PCA, SA, and VA, on the treatment and prevention of cardiovascular and metabolic diseases have been documented [157,191]. Meanwhile, the health beneficial properties of phenolic acids can be influenced by various factors such as poor stability, restricted bioavailability, and absorption [9,15,179]. The bioavailability of different phenolic acids varies and does not reconcile with their ubiquity.
Previous animal and human trials have shown that GA can be absorbed in the body, and its effectiveness is hindered because of its rapid metabolism and elimination from the body [180]. After oral administration, approximately 70% of GA is absorbed and then excreted in the urine as 4-O-methylgallic acid [15,160,192]. Thus, the usage of GA is restricted because of its low bioavailability and rapid elimination in both animals and humans. Therefore, efforts have been made to enhance its bioavailability. For example, repeated dosing, use of structural analogs or derivative compounds, phospholipid complexation, and microencapsulation have been tested to improve the plasma levels of GA [15].
The water-insolubility of SA could result in a low systemic bioavailability as with other water-insoluble drugs such as other conventional clinical drugs (e.g., simvastatin and fenofibrate) [193]. Despite its promising biological effects, the poor solubility, fast elimination, and concomitant low bioavailability of SA are the major hurdles for its application. Therefore, efforts have been made to improve its bioavailability by employing emerging technologies, such as incorporation of SA into liposome or micelles and utilization of self-microemulsifying drug delivery systems [194,195]. Recently, Liu et al. demonstrated that the incorporation of SA in liposomes enhanced its oral bioavailability and antioxidant properties in rats [30]. In addition, another study reports that a nano system for the oral delivery of SA improved its bioavailability and enhanced its hypolipidemic effect in diet-induced hyperlipidemic mice compared with an SA suspension-treated group [194]. Lastly, VA has significantly increased plasma concentrations and urinary excretions after oral administration [196]. The oral bioavailability of VA was calculated to be 25.3~36.2% in rat plasma, and it was quickly absorbed into the system circulation and slowly discharged from the blood [197]. It is worth noting that different physiological and/or pathophysiological conditions of animals and humans can also have a great impact on the absorption, distribution, metabolism, and excretion of such compounds. Future studies are warranted to evaluate the pharmacokinetic profile of GA in different pathological conditions.
Potential Health Benefits
Among numerous polyphenols, GA is a low-molecular-weight phenolic compound with outstanding anti-antioxidant, inflammatory, antimicrobial, anticancer, cardioprotective, gastroprotective, and neuroprotective properties in humans [198]. In metabolic syndrome and obesity mice, GA appeared to improve the plasma concentrations of TG and glucose as well as the recovery of glucose tolerance and lipid metabolism [180,199,200]. In addition, it has been shown to specifically target adipose tissue to inhibit lipogenesis, promote insulin signaling, and concomitantly combat raised proinflammatory response and oxidative stress in mice [180]. The anti-obesity effects of GA can be harnessed either directly by inhibiting the generation of lipid droplets in the liver or adipose tissue, or indirectly by ameliorating the serum levels of TG and LDL of rats [201,202]. GA could exert its protective effects on the cardiovascular system under oxidative stress [203] by restoring a series of enzymic and nonenzymic antioxidants and attenuating cardiotoxic content and ROS generation. Thus, the preclinical data support the beneficial effects of GA in preventing obesity-associated complication as well as CVDs. In a clinical trial, a placebo-controlled pilot study with 19 patients with type 2 diabetes showed that GA supplementation (15 mg/day for 7 days which was in the range of daily consumption in Europeans) prevented oxidative DNA damage and reduced the levels of inflammatory biomarkers, including C-reactive protein [204]. However, in order to provide meaningful data in determining the efficacy and safety of a given GA treatment, well-designed clinical trials should be necessary.
PCA is the main metabolite of anthocyanins and presents a variety of beneficial physiological activities for potential health benefits in humans such as cardiovascular-protective, anti-diabetic, and anti-obesity effects [205,206]. For example, a previous study has shown that PCA can reduce the risk of CVDs and protect myocardial injury [207]. The mechanism of this rests on the ability of PCA to significantly reduce myocardial infarction size, serum TNF-α levels, and platelet aggregation and significantly inhibit the apoptosis rate and cleaved caspase-3 expression of damaged myocardial cells. In addition, PCA exerts positive effects on insulin resistance and other metabolic and vascular disorders related to type 2 diabetes, partially by enhancing the insulin reaction to manage blood glucose stability of rats [208] as well as by upregulating the expression of phosphorylated Akt [207]. Some studies have proposed a possible anti-diabetes mechanism of PCA, which may improve hepatic insulin resistance and vascular oxidation state by regulating insulin signaling pathway and age-rage-NOX (NADPH oxidase) 4 pathways, respectively, thus exerting its hypoglycemic effect in rats [208].
SA has a wide range of therapeutic applications in preventing diabetes and CVDs, besides having antioxidant, antimicrobial, neuroprotective, and hepatoprotective activities [184]. In addition, SA exerts health-promoting effects on hyperlipidemia and NAFLD. Briefly, the SA treatment (0.05%, wt/wt) not only lowers the serum glucose and insulin levels, but also increases the adiponectin levels of HFD-fed rats [209]. Moreover, SA appeared to reduce the expression of inflammatory and lipogenic genes and increase the expression of fatty acid oxidation-related genes in the liver of an NAFLD mouse model [209]. The oral administration of SA (50 mg/kg body weight) for 30 days significantly improved the glycemic status of alloxan-induced diabetic Wistar rats, as indicated by the decreased plasma glucose and glycoprotein levels of the liver and kidney [210].
Extensive investigations have shown that VA exhibits various bioactivities, such as anti-obesity, anti-diabetes, antioxidant, anti-inflammatory, hepatoprotective, cardioprotective, and hypolipidemic effects [211]. It has been shown to significantly reduce fasting plasma glucose, insulin, blood pressure, and lipid peroxidation marker levels in Wistar rat diabetes models [212]. Moreover, it strongly regulates inflammatory mediators. For example, it has been shown to suppress leukocyte recruitment, oxidative stress, cytokine production, and NF-κB activation in mouse models [213,214] and to have anti-obesity effects by improving energy metabolism in vitro and in vivo [215]. It exerts its anti-diabetic effects either directly by reducing methylglyoxal-induced Neuro-2A cell apoptosis or by inhibiting the metabolic enzymes such as acetylcholinesterase enzymes, α-glycosidase, and α-amylase [216,217]. Thus, VA could be used as a natural health promoter and potential drug candidate but should be investigated further.
2.4.3. Phenylethanoids (Hydroxytyrosol)
General Characteristics, Structure, and Food Sources
Hydroxytyrosol (HXT) is an amphipathic phenolic compound derived from olives, olive oil, and olive tree leaves [218]. It is also called 3,4-dihydroxyphenylethanol or 3,4-dihydroxyphenolethanol, or 4-(2-hydroxyethyl)-1,2-benzenediol according to the IUPAC system [219]. In nature, HXT is generated by the hydrolysis of oleuropein by the enzyme β-glucosidase during olive ripening. In its chemical structure, HXT consists of a benzene ring with three adjacent -OH groups, and its molecular formula is C8H10O3.
Bioavailability
Hydrophilic phenolic compounds are known to be absorbed in a dose-dependent manner and excreted in the urine mainly as glucuronide conjugates in animals and humans [220,221]. Similar to other phenolic compounds, HXT undergoes an intense and rapid metabolism first inside enterocytes and then in the liver [222,223]. Although the absorption process varies depending on the administration vehicle employed, HXT is mainly absorbed in the small intestine and colon by passive transport from 75% to 100% [221]. It reaches its maximum concentration in plasma approximately 7 min after intake and then becomes undetectable after 4 h [224]. HXT and its metabolites have excellent distribution capacities in tissues, such as the muscle, testis, liver, and brain, which may contribute to their health-beneficial properties [225]. It is noteworthy that phenolic bioavailability is influenced by different factors such as age, hormonal status, or gender (reviewed in [219]). In the case of HXT, the food matrix in which it is incorporated plays an important role in its metabolism. Extra virgin olive oil has been identified as the best matrix for HXT [226,227,228].
Potential Health Benefits
Mediterranean countries have lower rates of mortality from CVDs and cancer than Northern European or other Western countries [219,229]. Olive oil, the major fat source of the Mediterranean diet, has been associated with the lower incidence of coronary heart disease in these countries. Especially HXT, one of the main compounds present in olive oil, inhibits radical-induced LDL oxidation and scavenges free radicals, and exerts other biological activities such as the inhibition of platelet aggregation and potentiation of immune response [218]. Thus, HXT features outstanding antioxidant, anti-inflammatory, and neuroprotective activities, which confer HXT with a critical role in the prevention of cardiovascular and metabolic syndromes of humans [230,231,232,233]. Although the cardioprotective effects of HXT remain unclear, its administration has been related to the decline in atheroma plaque in cadmium-intoxicated rats [234].
3. Conclusions
In this review, we discussed various bioactive compounds commonly found in green-colored fruits and vegetables and their health beneficial effects on metabolic and cardiovascular diseases (Figure 2). The color ‘green’ can be made up with various types of color-contributing polyphenols and pigments, many of which co-exist in different fruits and vegetables that we consume every day. Green-colored food-derived bioactive compounds have potent antioxidant, anti-inflammatory, anti-diabetic, anti-obesity, and cardioprotective effects that may help to offset an increased risk of metabolic diseases. Although the poor bioavailability of these bioactive compounds limits their wide application in the field of food products and supplements, it is not doubtful that they can benefit health when consumed as part of a healthy and well-balanced diet.
Author Contributions
Conceptualization, Y.L. and G.Z.; data curation, E.K. and J.C.; writing—original draft preparation, E.K., J.C., G.Z. and Y.L.; writing—review and editing, Y.L. and G.Z.; supervision, Y.L. and G.Z.; funding acquisition, Y.L. and G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key R&D Program of China-Korea Cooperative Project (2019YFE0107700, NRF-2019K1A3A1A20081146), the National Research Foundation Grant of Korea (NRF-2017R1D1A3B03031665, NRF-2020R1A2C2004144), Basic Science Research Program through the NRF funded by the Ministry of Education (NRF-2021R1A6A3A13046827), major application technology innovation projects (SD2019XM001), the Forage Industrial Innovation Team Project (SDAIT-23-05), and the Excellent Seed Project (LZ201712080160, 2019LZGC012) of China.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AhR | Aryl hydrocarbon receptor |
| Akt | Protein kinase B |
| BAT | Brown adipose tissue |
| CA | Caffeic acid |
| CGA | Chlorogenic acid |
| CQA | Caffeoylquinic acid |
| CVD | Cardiovascular disease |
| CXCL1 | Chemokine (C-X-C motif) ligand 1 |
| CYP1A1 | Cytochrome P4501A1 |
| DIM | 3,3′-diindoylmethane |
| EC | Epicatechin |
| ECG | Epicatechin-3-gallate |
| EGC | Epigallocatechin |
| EGCG | Epigallocatechin-3-gallate |
| ERK | Extracellular signal-regulated kinase |
| ET-1 | Endothelin-1 |
| FA | Ferulic acid |
| FQA | Feruloylquinic acid |
| GA | Gallic acid |
| GIT | Gastrointestinal tract |
| GLUT4 | Glucose transporter type 4 |
| HA | Hydroxylcinnamic acid |
| HDAC | Histone deacetylases |
| HDL | High-density lipoprotein |
| HFD | High-fat diet |
| HIEC-6 | Human intestinal epithelial cell line-6 |
| HO-1 | Heme oxygenase-1 |
| HSC | Hematopoietic stem cell |
| HXT | Hydroxytyrosol |
| I3C | Indole-3-carbinol |
| ICAM | Intercellular adhesion molecule |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| ITC | Isothiocyanate |
| IUPAC | International Union of Pure and Applied Chemistry |
| LDL | Low-density lipoprotein |
| LPS | Lipopolysaccharides |
| NAFLD | Non-alcoholic fatty liver disease |
| NF-κB | Nuclear factor-kappa B |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| Nrf-2 | Nuclear factor erythroid 2-related factor 2 |
| p-CA | p-Coumaric acid |
| PCA | Proteocatechuic acid |
| PI3K | Phosphatidylinositol 3-kinase |
| PKC | Protein kinase C |
| PPAR | Peroxisome proliferator-activated receptor |
| QA | Quinic acid |
| ROS | Reactive oxygen species |
| SA | Syringic acid |
| SIRT1 | Sirtuin 1 |
| TG | Triglyceride |
| TGF-β1 | Transforming growth factor-beta 1 |
| TNF-α | Tumor necrosis factor-α |
| VA | Vanillic acid |
References
- Joshipura, K.J.; Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Rimm, E.B.; Speizer, F.E.; Colditz, G.; Ascherio, A.; Rosner, B.; Spiegelman, D. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann. Intern. Med. 2001, 134, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Mazur, A.; Scalbert, A. Polyphenols and prevention of cardiovascular diseases. Curr. Opin. Lipidol. 2005, 16, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Blekkenhorst, L.C.; Sim, M.; Bondonno, C.P.; Bondonno, N.P.; Ward, N.C.; Prince, R.L.; Devine, A.; Lewis, J.R.; Hodgson, J.M. Cardiovascular health benefits of specific vegetable types: A narrative review. Nutrients 2018, 10, 595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, K.B.; Mackert, J.D.; McIntosh, M.K. Polyphenols and intestinal health. In Nutrition and Functional Foods for Healthy Aging; Elsevier: Amsterdam, The Netherlands, 2017; pp. 191–210. [Google Scholar]
- Cano-Lamadrid, M.; Artés-Hernández, F. By-Products Revalorization with Non-Thermal Treatments to Enhance Phytochemical Compounds of Fruit and Vegetables Derived Products: A Review. Foods 2022, 11, 59. [Google Scholar] [CrossRef]
- Devappa, R.K.; Rakshit, S.K.; Dekker, R.F. Forest biorefinery: Potential of poplar phytochemicals as value-added co-products. Biotechnol. Adv. 2015, 33, 681–716. [Google Scholar] [CrossRef]
- Esteve Ràfols, M. Mechanisms underlying biological effects of cruciferous glucosinolate-derived isothiocyanates/indoles: A focus on metabolic syndrome. Front. Nutr. 2020, 7, 111. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Karakaya, S. Bioavailability of phenolic compounds. Crit. Rev. Food Sci. Nutr. 2004, 44, 453–464. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Kaliora, A.C.; Kanellos, P.T.; Kalogeropoulos, N. Gallic acid bioavailability in humans. In Anonymous Handbook on Gallic Acid: Natural Occurrences, Antioxidant Properties and Health Implications; Nova Science Publishers: Huntington, NY, USA, 2013; pp. 301–312. [Google Scholar]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly) phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef]
- Zhu, J.; Cai, R.; Tan, Y.; Wu, X.; Wen, Q.; Liu, Z.; Ouyang, S.-H.; Yin, Z.; Yang, H. Preventive consumption of green tea modifies the gut microbiota and provides persistent protection from high-fat diet-induced obesity. J. Funct. Foods 2020, 64, 103621. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, Y.; Yang, N.; Jiang, T.; Xu, H.; Lei, H. Fermentation of kiwifruit juice from two cultivars by probiotic bacteria: Bioactive phenolics, antioxidant activities and flavor volatiles. Food Chem. 2022, 373, 131455. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Ahammed, S.M.; Saha, B.P.; Mukherjee, P.K. The gallic acid–phospholipid complex improved the antioxidant potential of gallic acid by enhancing its bioavailability. AAPS PharmSciTech 2013, 14, 1025–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siriwong, S.; Teethaisong, Y.; Thumanu, K.; Dunkhunthod, B.; Eumkeb, G. The synergy and mode of action of quercetin plus amoxicillin against amoxicillin-resistant Staphylococcus epidermidis. BMC Pharmacol. Toxicol. 2016, 17, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ashokkumar, K.; Selvaraj, K.; KM, S.D. Reverse phase-high performance liquid chromatography-diode array detector (RP-HPLC-DAD) analysis of flavonoids profile from curry leaf (Murraya koenigii. L). J. Med. Plants Res. 2013, 7, 3393–3399. [Google Scholar]
- Ou, S.-F.; Tsao, Y.-L.; Lin, W.-C.; Wang, Y.-T.; Wang, L.; Fan, F.-Y. Novel epigallocatechin-3-gallate (Egcg)-loaded mesoporous bioglass scaffolds for bone recruitment applications. Appl. Sci. 2021, 11, 243. [Google Scholar] [CrossRef]
- Casetti, F.; Jung, W.; Wölfle, U.; Reuter, J.; Neumann, K.; Gilb, B.; Wähling, A.; Wagner, S.; Merfort, I.; Schempp, C. Topical application of solubilized Reseda luteola extract reduces ultraviolet B-induced inflammation in vivo. J. Photochem. Photobiol. B Biol. 2009, 96, 260–265. [Google Scholar] [CrossRef]
- Jiao, Y.; Reuss, L.; Wang, Y. β-Cryptoxanthin: Chemistry, occurrence, and potential health benefits. Curr. Pharmacol. Rep. 2019, 5, 20–34. [Google Scholar] [CrossRef]
- Anderton, M.J.; Manson, M.M.; Verschoyle, R.D.; Gescher, A.; Lamb, J.H.; Farmer, P.B.; Steward, W.P.; Williams, M.L. Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin. Cancer Res. 2004, 10, 5233–5241. [Google Scholar] [CrossRef] [Green Version]
- Shirai, Y.; Fujita, Y.; Hashimoto, K. Effects of the antioxidant sulforaphane on hyperlocomotion and prepulse inhibition deficits in mice after phencyclidine administration. Clin. Psychopharmacol. Neurosci. 2012, 10, 94. [Google Scholar] [CrossRef]
- Toyama, D.O.; Ferreira, M.J.; Romoff, P.; Fávero, O.A.; Gaeta, H.H.; Toyama, M.H. Effect of chlorogenic acid (5-caffeoylquinic acid) isolated from Baccharis oxyodonta on the structure and pharmacological activities of secretory phospholipase A2 from Crotalus durissus terrificus. BioMed Res. Int. 2014, 2014, 726585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad Abdul Kadar, N.N.; Ahmad, F.; Teoh, S.L.; Yahaya, M.F. Caffeic Acid on Metabolic Syndrome: A Review. Molecules 2021, 26, 5490. [Google Scholar] [CrossRef] [PubMed]
- Kitagaki, H. Medical Application of Substances Derived from Non-Pathogenic Fungi Aspergillus oryzae and A. luchuensis-Containing Koji. J. Fungi 2021, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Saad, W.M.M.; Ridwan, R.; Lasim, N.S.M.; Rapi, N.L.M.; Salim, F. Determination and Quantification of p-Coumaric Acid in Pineapples (Ananas comosus) Extracts using Gradient Mode RP-HPLC. Pharmacogn. Res. 2019, 11, 78–82. [Google Scholar] [CrossRef]
- Rinaldi, D.E.; Ontiveros, M.Q.; Saffioti, N.A.; Vigil, M.A.; Mangialavori, I.C.; Rossi, R.C.; Rossi, J.P.; Espelt, M.V.; Ferreira-Gomes, M.S. Epigallocatechin 3-gallate inhibits the plasma membrane Ca2+-ATPase: Effects on calcium homeostasis. Heliyon 2021, 7, e06337. [Google Scholar] [CrossRef]
- Álvarez-Rodríguez, M.L.; Belloch, C.; Villa, M.; Uruburu, F.; Larriba, G.; Coque, J.-J.R. Degradation of vanillic acid and production of guaiacol by microorganisms isolated from cork samples. FEMS Microbiol. Lett. 2003, 220, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Capitain, C.; Wagner, S.; Hummel, J.; Tippkötter, N. Investigation of C–N formation between catechols and chitosan for the formation of a strong, novel adhesive mimicking mussel adhesion. Waste Biomass Valorization 2021, 12, 1761–1779. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, C.; Li, W.; Adu-Frimpong, M.; Wang, Q.; Yu, J.; Xu, X. Preparation and characterization of Syringic acid–loaded TPGS liposome with enhanced oral bioavailability and in vivo antioxidant efficiency. AAPS PharmSciTech 2019, 20, 98. [Google Scholar] [CrossRef]
- Sirangelo, I.; Borriello, M.; Liccardo, M.; Scafuro, M.; Russo, P.; Iannuzzi, C. Hydroxytyrosol Selectively Affects Non-Enzymatic Glycation in Human Insulin and Protects by AGEs Cytotoxicity. Antioxidants 2021, 10, 1127. [Google Scholar] [CrossRef]
- Wisniewska, A.; Widomska, J.; Subczynski, W.K. Carotenoid-membrane interactions in liposomes: Effect of dipolar, monopolar, and nonpolar carotenoids. Acta Biochim. Pol. 2006, 53, 475–484. [Google Scholar] [CrossRef]
- Panche, A.; Diwan, A.; Chandra, S. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haytowitz, D.B.; Wu, X.; Bhagwat, S. USDA Database for the Flavonoid Content of Selected Foods, Release 3.3; US Department of Agriculture: Beltsville, MD, USA, 2018; Volume 173.
- Lins, T.L.B.G.; Gouveia, B.B.; Barberino, R.S.; Silva, R.L.S.; Monte, A.P.O.; Pinto, J.G.C.; Campinho, D.S.P.; Palheta Jr, R.C.; Matos, M.H.T. Rutin prevents cisplatin-induced ovarian damage via antioxidant activity and regulation of PTEN and FOXO3a phosphorylation in mouse model. Reprod. Toxicol. 2020, 98, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Semwal, R.; Joshi, S.K.; Semwal, R.B.; Semwal, D.K. Health benefits and limitations of rutin—A natural flavonoid with high nutraceutical value. Phytochem. Lett. 2021, 46, 119–128. [Google Scholar] [CrossRef]
- Pashikanti, S.; de Alba, D.R.; Boissonneault, G.A.; Cervantes-Laurean, D. Rutin metabolites: Novel inhibitors of nonoxidative advanced glycation end products. Free Radic. Biol. Med. 2010, 48, 656–663. [Google Scholar] [CrossRef]
- Lehtonen, H.-M.; Lehtinen, O.; Suomela, J.-P.; Viitanen, M.; Kallio, H. Flavonol glycosides of sea buckthorn (Hippophae rhamnoides ssp. sinensis) and lingonberry (Vaccinium vitis-idaea) are bioavailable in humans and monoglucuronidated for excretion. J. Agric. Food Chem. 2010, 58, 620–627. [Google Scholar] [CrossRef]
- Crespy, V.; Morand, C.; Besson, C.; Cotelle, N.; Vézin, H.; Demigné, C.; Rémésy, C. The splanchnic metabolism of flavonoids highly differed according to the nature of the compound. Am. J. Physiol.-Gastrointest. Liver Physiol. 2003, 284, G980–G988. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.-P.; Sun, J.; Chen, H.-X.; Xiao, Y.-Y.; Liu, D.; Chen, J.; Cai, H.; Cai, B.-C. Comparative pharmacokinetics and bioavailability studies of quercetin, kaempferol and isorhamnetin after oral administration of Ginkgo biloba extracts, Ginkgo biloba extract phospholipid complexes and Ginkgo biloba extract solid dispersions in rats. Fitoterapia 2010, 81, 1045–1052. [Google Scholar] [CrossRef]
- Barve, A.; Chen, C.; Hebbar, V.; Desiderio, J.; Saw, C.L.L.; Kong, A.N. Metabolism, oral bioavailability and pharmacokinetics of chemopreventive kaempferol in rats. Biopharm. Drug Dispos. 2009, 30, 356–365. [Google Scholar] [CrossRef] [Green Version]
- Yodogawa, S.; Arakawa, T.; Sugihara, N.; Furuno, K. Glucurono-and sulfo-conjugation of kaempferol in rat liver subcellular preparations and cultured hepatocytes. Biol. Pharm. Bull. 2003, 26, 1120–1124. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Scholz. Interactions affecting the bioavailability of dietary polyphenols in vivo. Int. J. Vitam. Nutr. Res. 2007, 77, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; Hollman, P.C.; Vree, T.B.; Katan, M.B. Bioavailabilities of quercetin-3-glucoside and quercetin-4′-glucoside do not differ in humans. J. Nutr. 2000, 130, 1200–1203. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rauf, A.; Shah, Z.A.; Saeed, F.; Imran, A.; Arshad, M.U.; Ahmad, B.; Bawazeer, S.; Atif, M.; Peters, D.G. Chemo-preventive and therapeutic effect of the dietary flavonoid kaempferol: A comprehensive review. Phytother. Res. 2019, 33, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Luo, C.; Li, X.; Zhou, Y.; He, H. The anti-inflammatory effect of kaempferol on early atherosclerosis in high cholesterol fed rabbits. Lipids Health Dis. 2013, 12, 115. [Google Scholar] [CrossRef] [Green Version]
- Crespo, I.; Garcia-Mediavilla, M.V.; Gutiérrez, B.; Sánchez-Campos, S.; Tunon, M.J.; González-Gallego, J. A comparison of the effects of kaempferol and quercetin on cytokine-induced pro-inflammatory status of cultured human endothelial cells. Br. J. Nutr. 2008, 100, 968–976. [Google Scholar] [CrossRef] [Green Version]
- Suchal, K.; Malik, S.; Khan, S.I.; Malhotra, R.K.; Goyal, S.N.; Bhatia, J.; Ojha, S.; Arya, D.S. Molecular pathways involved in the amelioration of myocardial injury in diabetic rats by kaempferol. Int. J. Mol. Sci. 2017, 18, 1001. [Google Scholar] [CrossRef] [Green Version]
- Knekt, P.; Kumpulainen, J.; Järvinen, R.; Rissanen, H.; Heliövaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [CrossRef] [Green Version]
- Bian, Y.; Lei, J.; Zhong, J.; Wang, B.; Wan, Y.; Li, J.; Liao, C.; He, Y.; Liu, Z.; Ito, K. Kaempferol reduces obesity, prevents intestinal inflammation, and modulates gut microbiota in high-fat diet mice. J. Nutr. Biochem. 2022, 99, 108840. [Google Scholar] [CrossRef]
- Chen, L.-W.; Ko, W.-C. Suppressive effects of rutin, quercitrin, and isoquercitrin on atypical allergic asthma in an animal model. Med. Drug Discov. 2021, 12, 100106. [Google Scholar] [CrossRef]
- Mzhelskaya, K.V.; Shipelin, V.A.; Shumakova, A.A.; Musaeva, A.D.; Soto, J.S.; Riger, N.A.; Trusov, N.V.; Kirbaeva, N.V.; Apryatin, S.A.; Gmoshinski, I.V. Effects of quercetin on the neuromotor function and behavioral responses of Wistar and Zucker rats fed a high-fat and high-carbohydrate diet. Behav. Brain Res. 2020, 378, 112270. [Google Scholar] [CrossRef]
- Amjadi, S.; Shahnaz, F.; Shokouhi, B.; Azarmi, Y.; Siahi-Shadbad, M.; Ghanbarzadeh, S.; Kouhsoltani, M.; Ebrahimi, A.; Hamishehkar, H. Nanophytosomes for enhancement of rutin efficacy in oral administration for diabetes treatment in streptozotocin-induced diabetic rats. Int. J. Pharm. 2021, 610, 121208. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.R.; Wadhwa, R.; Tew, X.N.; Lau, N.J.X.; Madheswaran, T.; Panneerselvam, J.; Zeeshan, F.; Kumar, P.; Gupta, G.; Anand, K.; et al. Rutin loaded liquid crystalline nanoparticles inhibit non-small cell lung cancer proliferation and migration in vitro. Life Sci. 2021, 276, 119436. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ding, Z.; Li, Z.; Ding, Y.; Jiang, F.; Liu, J. Antioxidant and antibacterial study of 10 flavonoids revealed rutin as a potential antibiofilm agent in Klebsiella pneumoniae strains isolated from hospitalized patients. Microb. Pathog. 2021, 159, 105121. [Google Scholar] [CrossRef] [PubMed]
- Oluranti, O.I.; Alabi, B.A.; Michael, O.S.; Ojo, A.O.; Fatokun, B.P. Rutin prevents cardiac oxidative stress and inflammation induced by bisphenol A and dibutyl phthalate exposure via NRF-2/NF-κB pathway. Life Sci. 2021, 284, 119878. [Google Scholar] [CrossRef]
- Huang, R.; Shi, Z.; Chen, L.; Zhang, Y.; Li, J.; An, Y. Rutin alleviates diabetic cardiomyopathy and improves cardiac function in diabetic ApoEknockout mice. Eur. J. Pharmacol. 2017, 814, 151–160. [Google Scholar] [CrossRef]
- AL-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Busselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Bose, M.; Lambert, J.D.; Ju, J.; Reuhl, K.R.; Shapses, S.A.; Yang, C.S. The Major Green Tea Polyphenol, (-)-Epigallocatechin-3-Gallate, Inhibits Obesity, Metabolic Syndrome, and Fatty Liver Disease in High-Fat–Fed Mice. J. Nutr. 2008, 138, 1677–1683. [Google Scholar] [CrossRef]
- Peters, C.M.; Green, R.J.; Janle, E.M.; Ferruzzi, M.G. Formulation with ascorbic acid and sucrose modulates catechin bioavailability from green tea. Food Res. Int. 2010, 43, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Zagury, Y.; Kazir, M.; Livney, Y.D. Improved antioxidant activity, bioaccessibility and bioavailability of EGCG by delivery in β-lactoglobulin particles. J. Funct. Foods 2019, 52, 121–130. [Google Scholar] [CrossRef]
- Kim, S.; Lee, M.-J.; Hong, J.; Li, C.; Smith, T.J.; Yang, G.-Y.; Seril, D.N.; Yang, C.S. Plasma and tissue levels of tea catechins in rats and mice during chronic consumption of green tea polyphenols. Nutr. Cancer 2000, 37, 41–48. [Google Scholar] [CrossRef]
- Cano, A.; Ettcheto, M.; Chang, J.-H.; Barroso, E.; Espina, M.; Kühne, B.A.; Barenys, M.; Auladell, C.; Folch, J.; Souto, E.B. Dual-drug loaded nanoparticles of Epigallocatechin-3-gallate (EGCG)/Ascorbic acid enhance therapeutic efficacy of EGCG in a APPswe/PS1dE9 Alzheimer’s disease mice model. J. Control. Release 2019, 301, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.C.; Chiu, H.F.; Ho, Y.T.; Venkatakrishnan, K.; Wang, C.K. Improved bioavailability of EGCG after complexation with royal jelly protein. J. Food Biochem. 2020, 44, e13372. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, S.; Raederstorff, D.; Wang, Y.; Teixeira, S.R.; Elste, V.; Weber, P. TEAVIGOTM (epigallocatechin gallate) supplementation prevents obesity in rodents by reducing adipose tissue mass. Ann. Nutr. Metab. 2005, 49, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, I.; Hamamoto, R.; Uzu, K.; Imaizumi, K.; Nagao, K.; Yanagita, T.; Suzuki, Y.; Kobayashi, M.; Kakuda, T. Dietary gallate esters of tea catechins reduce deposition of visceral fat, hepatic triacylglycerol, and activities of hepatic enzymes related to fatty acid synthesis in rats. Biosci. Biotechnol. Biochem. 2005, 69, 1049–1053. [Google Scholar] [CrossRef] [Green Version]
- Klaus, S.; Pültz, S.; Thöne-Reineke, C.; Wolfram, S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int. J. Obes. 2005, 29, 615–623. [Google Scholar] [CrossRef] [Green Version]
- Raederstorff, D.G.; Schlachter, M.F.; Elste, V.; Weber, P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J. Nutr. Biochem. 2003, 14, 326–332. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Esa, N.M. Luteolin, a bioflavonoid inhibits colorectal cancer through modulation of multiple signaling pathways: A review. Asian Pac. J. Cancer Prev. 2014, 15, 5501–5508. [Google Scholar] [CrossRef]
- Chan, T.S.; Galati, G.; Pannala, A.S.; Rice-Evans, C.; O’Brien, P.J. Simultaneous detection of the antioxidant and pro-oxidant activity of dietary polyphenolics in a peroxidase system. Free Radic. Res. 2003, 37, 787–794. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Gortzi, O.; Sobarzo-Sanchez, E.; Daglia, M.; Skalicka-Woźniak, K.; Nabavi, S.M. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res. Bull. 2015, 119, 1–11. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ahmad, N.; Ahmed, Z.; Siddique, R.; Zeng, X.A.; Rahaman, A.; Muhammad Aadil, R.; Wahab, A. Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J. Food Biochem. 2019, 43, e12974. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-C.; Pai, Y.-F.; Tsai, T.-H. Isolation of luteolin and luteolin-7-O-glucoside from Dendranthema morifolium Ramat Tzvel and their pharmacokinetics in rats. J. Agric. Food Chem. 2015, 63, 7700–7706. [Google Scholar] [CrossRef] [PubMed]
- Shimoi, K.; Okada, H.; Furugori, M.; Goda, T.; Takase, S.; Suzuki, M.; Hara, Y.; Yamamoto, H.; Kinae, N. Intestinal absorption of luteolin and luteolin 7-O-β-glucoside in rats and humans. FEBS Lett. 1998, 438, 220–224. [Google Scholar] [CrossRef]
- Zhou, P.; Li, L.-P.; Luo, S.-Q.; Jiang, H.-D.; Zeng, S. Intestinal absorption of luteolin from peanut hull extract is more efficient than that from individual pure luteolin. J. Agric. Food Chem. 2008, 56, 296–300. [Google Scholar] [CrossRef]
- Xu, T.; Li, D.; Jiang, D. Targeting cell signaling and apoptotic pathways by luteolin: Cardioprotective role in rat cardiomyocytes following ischemia/reperfusion. Nutrients 2012, 4, 2008–2019. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Shang, P.; Li, D. Luteolin: A flavonoid that has multiple cardio-protective effects and its molecular mechanisms. Front. Pharmacol. 2017, 8, 692. [Google Scholar] [CrossRef] [Green Version]
- Zang, Y.; Igarashi, K.; Li, Y. Anti-diabetic effects of luteolin and luteolin-7-O-glucoside on KK-A y mice. Biosci. Biotechnol. Biochem. 2016, 80, 1580–1586. [Google Scholar] [CrossRef] [Green Version]
- Burri, B.J. Beta-cryptoxanthin as a source of vitamin A. J. Sci. Food Agric. 2015, 95, 1786–1794. [Google Scholar] [CrossRef]
- Tsao, R.; Wang, M.; Deng, Z. Lutein: Separation, Antioxidant Activity, and Potential Health Benefits; ACS Publications: Washington, DC, USA, 2007. [Google Scholar]
- Abdel-Aal, E.-S.M.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients 2013, 5, 1169–1185. [Google Scholar] [CrossRef] [Green Version]
- Granado, F.; Olmedilla, B.; Blanco, I. Nutritional and clinical relevance of lutein in human health. Br. J. Nutr. 2003, 90, 487–502. [Google Scholar] [CrossRef] [Green Version]
- Kusmiati; Ningsih, E.B.; Ramadhani, I.; Amir, M. Antibacterial and antioxidant activity test of crude lutein extracted from sunflower (Helianthus annuus L.). AIP Conf. Proc. 2021, 2331, 050001. [Google Scholar]
- Granado-Lorencio, F.; Donoso-Navarro, E.; Sánchez-Siles, L.M.; Blanco-Navarro, I.; Pérez-Sacristán, B. Bioavailability of β-cryptoxanthin in the presence of phytosterols: In Vitro and in vivo studies. J. Agric. Food Chem. 2011, 59, 11819–11824. [Google Scholar] [CrossRef] [PubMed]
- Granado-Lorencio, F.; Olmedilla-Alonso, B.; Herrero-Barbudo, C.; Pérez-Sacristán, B.; Blanco-Navarro, I.; Blázquez-García, S. Comparative in vitro bioaccessibility of carotenoids from relevant contributors to carotenoid intake. J. Agric. Food Chem. 2007, 55, 6387–6394. [Google Scholar] [CrossRef] [PubMed]
- Dhuique-Mayer, C.; Borel, P.; Reboul, E.; Caporiccio, B.; Besancon, P.; Amiot, M.-J. β-Cryptoxanthin from citrus juices: Assessment of bioaccessibility using an in vitro digestion/Caco-2 cell culture model. Br. J. Nutr. 2007, 97, 883–890. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Kobayashi, M.; Itagaki, S.; Hirano, T.; Noda, T.; Mizuno, S.; Sugawara, M.; Iseki, K. Pharmacokinetic properties of lutein emulsion after oral administration to rats and effect of food intake on plasma concentration of lutein. Biopharm. Drug Dispos. 2011, 32, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Desmarchelier, C.; Nowicki, M.; Bott, R.; Morange, S.; Lesavre, N. Interindividual variability of lutein bioavailability in healthy men: Characterization, genetic variants involved, and relation with fasting plasma lutein concentration. Am. J. Clin. Nutr. 2014, 100, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Nwachukwu, I.D.; Udenigwe, C.C.; Aluko, R.E. Lutein and zeaxanthin: Production technology, bioavailability, mechanisms of action, visual function, and health claim status. Trends Food Sci. Technol. 2016, 49, 74–84. [Google Scholar] [CrossRef]
- Clugston, R.D. Carotenoids and fatty liver disease: Current knowledge and research gaps. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158597. [Google Scholar] [CrossRef]
- Bonet, M.L.; Canas, J.A.; Ribot, J.; Palou, A. Carotenoids in adipose tissue biology and obesity. Carotenoids Nat. 2016, 377–414. [Google Scholar]
- Zhang, F.; Shi, D.; Wang, X.; Zhang, Y.; Duan, W.; Li, Y. β-cryptoxanthin alleviates myocardial ischaemia/reperfusion injury by inhibiting NF-κB-mediated inflammatory signalling in rats. Arch. Physiol. Biochem. 2020, 1–8. [Google Scholar] [CrossRef]
- Ni, Y.; Zhuge, F.; Nagashimada, M.; Ota, T. Novel action of carotenoids on non-alcoholic fatty liver disease: Macrophage polarization and liver homeostasis. Nutrients 2016, 8, 391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Lin, X.M. Effects of lutein and zeaxanthin on aspects of eye health. J. Sci. Food Agric. 2010, 90, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Carmona, M.; Kvansakul, J.; Alister Harlow, J.; Köpcke, W.; Schalch, W.; Barbur, J.L. The effects of supplementation with lutein and/or zeaxanthin on human macular pigment density and colour vision. Ophthalmic Physiol. Opt. 2006, 26, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Hammond, B.; Renzi, L. The characteristics and function of lutein and zeaxanthin within the human retina. In Phytochemicals: Aging and Health; CRC Press: Boca Raton, FL, USA, 2008; pp. 89–106. [Google Scholar]
- Sindhu, E.R.; Preethi, K.C.; Kuttan, R. Antioxidant Activity of Carotenoid lutein In Vitro and In Vivo. Indian J. Exp. Biol. 2010, 48, 843–848. [Google Scholar]
- Chung, R.W.; Leanderson, P.; Lundberg, A.K.; Jonasson, L. Lutein exerts anti-inflammatory effects in patients with coronary artery disease. Atherosclerosis 2017, 262, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Nidhi, B.; Sharavana, G.; Ramaprasad, T.R.; Vallikannan, B. Lutein derived fragments exhibit higher antioxidant and anti-inflammatory properties than lutein in lipopolysaccharide induced inflammation in rats. Food Funct. 2015, 6, 450–460. [Google Scholar] [CrossRef]
- Hadad, N.; Levy, R. The synergistic anti-inflammatory effects of lycopene, lutein, β-carotene, and carnosic acid combinations via redox-based inhibition of NF-κB signaling. Free Radic. Biol. Med. 2012, 53, 1381–1391. [Google Scholar] [CrossRef]
- Qiao, Y.-Q.; Jiang, P.-F.; Gao, Y.-Z. Lutein prevents osteoarthritis through Nrf2 activation and downregulation of inflammation. Arch. Med. Sci. AMS 2018, 14, 617–624. [Google Scholar] [CrossRef] [Green Version]
- Armoza, A.; Haim, Y.; Basiri, A.; Wolak, T.; Paran, E. Tomato extract and the carotenoids lycopene and lutein improve endothelial function and attenuate inflammatory NF-κB signaling in endothelial cells. J. Hypertens. 2013, 31, 521–529. [Google Scholar] [CrossRef]
- Tan, D.; Yu, X.; Chen, M.; Chen, J.; Xu, J. Lutein protects against severe traumatic brain injury through anti-inflammation and antioxidative effects via ICAM-1/Nrf-2. Mol. Med. Rep. 2017, 16, 4235–4240. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Wang, D.; Luo, G.; Zhou, J.; Tan, Z.; Du, Y.; Xie, H.; Liu, L.; Yang, X.; Hao, L. Lutein attenuates excessive lipid accumulation in differentiated 3T3-L1 cells and abdominal adipose tissue of rats by the SIRT1-mediated pathway. Int. J. Biochem. Cell Biol. 2021, 133, 105932. [Google Scholar] [CrossRef] [PubMed]
- Rungapamestry, V.; Rabot, S.; Fuller, Z.; Ratcliffe, B.; Duncan, A.J. Influence of cooking duration of cabbage and presence of colonic microbiota on the excretion of N-acetylcysteine conjugates of allyl isothiocyanate and bioactivity of phase 2 enzymes in F344 rats. Br. J. Nutr. 2008, 99, 773–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palliyaguru, D.L.; Yuan, J.M.; Kensler, T.W.; Fahey, J.W. Isothiocyanates: Translating the Power of Plants to People. Mol. Nutr. Food Res. 2018, 62, e1700965. [Google Scholar] [CrossRef] [PubMed]
- Licznerska, B.; Baer-Dubowska, W. Indole-3-carbinol and its role in chronic diseases. Anti-Inflamm. Nutraceuticals Chronic Dis. 2016, 928, 131–154. [Google Scholar]
- Wang, T.T.; Schoene, N.W.; Milner, J.A.; Kim, Y.S. Broccoli-derived phytochemicals indole-3-carbinol and 3,3′-diindolylmethane exerts concentration-dependent pleiotropic effects on prostate cancer cells: Comparison with other cancer preventive phytochemicals. Mol. Carcinog. 2012, 51, 244–256. [Google Scholar] [CrossRef]
- Kapusta-Duch, J.; Kopec, A.; Piatkowska, E.; Borczak, B.; Leszczynska, T. The beneficial effects of Brassica vegetables on human health. Rocz. Państwowego Zakładu Hig. 2012, 63, 389–395. [Google Scholar]
- Aggarwal, B.B.; Ichikawa, H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle 2005, 4, 1201–1215. [Google Scholar] [CrossRef] [Green Version]
- Weng, J.-R.; Tsai, C.-H.; Kulp, S.K.; Chen, C.-S. Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Lett. 2008, 262, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Anderton, M.J.; Jukes, R.; Lamb, J.H.; Manson, M.M.; Gescher, A.; Steward, W.P.; Williams, M.L. Liquid chromatographic assay for the simultaneous determination of indole-3-carbinol and its acid condensation products in plasma. J. Chromatogr. B 2003, 787, 281–291. [Google Scholar] [CrossRef]
- Wang, S.-Q.; Cheng, L.-S.; Liu, Y.; Wang, J.-Y.; Jiang, W. Indole-3-Carbinol (I3C) and its major derivatives: Their pharmacokinetics and important roles in hepatic protection. Curr. Drug Metab. 2016, 17, 401–409. [Google Scholar] [CrossRef]
- Reed, G.A.; Sunega, J.M.; Sullivan, D.K.; Gray, J.C.; Mayo, M.S.; Crowell, J.A.; Hurwitz, A. Single-dose pharmacokinetics and tolerability of absorption-enhanced 3,3′-diindolylmethane in healthy subjects. Cancer Epidemiol. Prev. Biomark. 2008, 17, 2619–2624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hongbo, L.; Xujia, M.; Dan, X.; Haizhen, M.; Zhenbin, L.; Liangbin, H.; Xiaohui, Z. Transcriptome Analysis and Weighted Gene Co-expression Network Reveal Multitarget-Directed Antibacterial Mechanisms of Benzyl Isothiocyanate against Staphylococcus aureus. J. Agric. Food Chem. 2021, 69, 39. [Google Scholar]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Subramani, R.; Gonzalez, E.; Arumugam, A.; Nandy, S.; Gonzalez, V.; Medel, J.; Camacho, F.; Ortega, A.; Bonkoungou, S.; Narayan, M.; et al. Nimbolide inhibits pancreatic cancer growth and metastasis through ROS-mediated apoptosis and inhibition of epithelial-to-mesenchymal transition. Sci. Rep. 2016, 6, 19819. [Google Scholar] [CrossRef]
- Sravanthi, T.; Manju, S. Indoles—A promising scaffold for drug development. Eur. J. Pharm. Sci. 2016, 91, 1–10. [Google Scholar] [CrossRef]
- Mohammadi, S.; Memarian, A.; Sedighi, S.; Behnampour, N.; Yazdani, Y. Immunoregulatory effects of indole-3-carbinol on monocyte-derived macrophages in systemic lupus erythematosus: A crucial role for aryl hydrocarbon receptor. Autoimmunity 2018, 51, 199–209. [Google Scholar] [CrossRef]
- Tsai, J.-T.; Liu, H.-C.; Chen, Y.-H. Suppression of inflammatory mediators by cruciferous vegetable-derived indole-3-carbinol and phenylethyl isothiocyanate in lipopolysaccharide-activated macrophages. Mediat. Inflamm. 2010, 2010, 293642. [Google Scholar] [CrossRef]
- Rostoka, E.; Isajevs, S.; Baumane, L.; Line, A.; Silina, K.; Dzintare, M.; Sharipova, J.; Svirina, D.; Kalvinsh, I.; Sjakste, N. Effects of lycopene, indole-3-carbinol, and luteolin on nitric oxide production and iNOS expression are organ-specific in rats. Arh. Za Hig. Rada I Toksikol. 2010, 61, 275–284. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.; Abdelmegeed, M.A.; Song, B.-J. Preventive effects of indole-3-carbinol against alcohol-induced liver injury in mice via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms: Role of gut-liver-adipose tissue axis. J. Nutr. Biochem. 2018, 55, 12–25. [Google Scholar] [CrossRef]
- Munakarmi, S.; Chand, L.; Shin, H.B.; Jang, K.Y.; Jeong, Y.J. Indole-3-carbinol derivative DIM mitigates carbon tetrachloride-induced acute liver injury in mice by inhibiting inflammatory response, apoptosis and regulating oxidative stress. Int. J. Mol. Sci. 2020, 21, 2048. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.-P.; Wang, M.-L.; Chan, M.-H.; Chiu, Y.-S.; Chen, Y.-H. Antiobesity activities of indole-3-carbinol in high-fat-diet–induced obese mice. Nutrition 2011, 27, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Yoo, H.S. 3,3′-Diindolylmethane Enhances Glucose Uptake Through Activation of Insulin Signaling in 3T3-L1 Adipocytes. Obesity 2018, 26, 1153–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.-S.; Jeon, H.-J.; Lee, O.-H.; Lee, B.-Y. Indole-3-carbinol, a vegetable phytochemical, inhibits adipogenesis by regulating cell cycle and AMPKα signaling. Biochimie 2014, 104, 127–136. [Google Scholar] [CrossRef]
- Lee, J.; Yue, Y.; Park, Y.; Lee, S.-H. 3,3′-Diindolylmethane suppresses adipogenesis using AMPK α-dependent mechanism in 3T3-L1 adipocytes and Caenorhabditis elegans. J. Med. Food 2017, 20, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Jiang, M.; Zhu, C.; He, J.; Fan, S. Amelioration of whole abdominal irradiation-induced intestinal injury in mice with 3,3′-Diindolylmethane (DIM)—ScienceDirect. Free Radic. Biol. Med. 2019, 130, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Yoo, H.S. Amelioration of Hyperglycemia-Induced Nephropathy by 3,3′-Diindolylmethane in Diabetic Mice. Molecules 2019, 24, 4474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, K.; Kinoshita, K.; Yoshimizu, A.; Kurauchi, Y.; Katsuki, H. Laquinimod and 3,3′-diindolylemethane alleviate neuropathological events and neurological deficits in a mouse model of intracerebral hemorrhage. J. Neuroimmunol. 2020, 342, 577195. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Dong, J.; Li, D.; Zhang, J.; Fan, S. 3,3’-diindolylmethane mitigates total body irradiation-induced hematopoietic injury in mice. Free Radic. Biol. Med. Off. J. Oxyg. Soc. 2016, 99, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Rzemieniec, J.; Wnuk, A.; Lasoń, W.; Bilecki, W.; Kajta, M. The neuroprotective action of 3,3′-diindolylmethane against ischemia involves an inhibition of apoptosis and autophagy that depends on HDAC and AhR/CYP1A1 but not ERα/CYP19A1 signaling. Apoptosis 2019, 24, 435–452. [Google Scholar] [CrossRef] [Green Version]
- Angeloni, C.; Leoncini, E.; Malaguti, M.; Angelini, S.; Hrelia, P.; Hrelia, S. Modulation of phase II enzymes by sulforaphane: Implications for its cardioprotective potential. J. Agric. Food Chem. 2009, 57, 5615–5622. [Google Scholar] [CrossRef]
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from broccoli, sulforaphane, and its properties. J. Med. Food 2019, 22, 121–126. [Google Scholar] [CrossRef]
- Hanlon, N.; Coldham, N.; Gielbert, A.; Kuhnert, N.; Sauer, M.J.; King, L.J.; Ioannides, C. Absolute bioavailability and dose-dependent pharmacokinetic behaviour of dietary doses of the chemopreventive isothiocyanate sulforaphane in rat. Br. J. Nutr. 2008, 99, 559–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazarakis, N.; Snibson, K.; Licciardi, P.V.; Karagiannis, T.C. The potential use of l-sulforaphane for the treatment of chronic inflammatory diseases: A review of the clinical evidence. Clin. Nutr. 2020, 39, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Li, X.; Wang, Y.; Lu, Y. The protective effect of sulforaphane on type II diabetes induced by high-fat diet and low-dosage streptozotocin. Food Sci. Nutr. 2021, 9, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Potential efficacy of broccoli sprouts as a unique supplement for management of type 2 diabetes and its complications. J. Med. Food 2013, 16, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.C.; Babu, K.R.; Madhu, M.; Gopinath, C. In Vitro Antidiabetic Activity of Sulforaphane. Pharmacol. Toxicol. Biomed. Rep. 2017, 3, 47–49. [Google Scholar] [CrossRef] [Green Version]
- Martins, T.; Colaço, B.; Venâncio, C.; Pires, M.J.; Oliveira, P.A.; Rosa, E.; Antunes, L.M. Potential effects of sulforaphane to fight obesity. J. Sci. Food Agric. 2018, 98, 2837–2844. [Google Scholar] [CrossRef]
- Tanito, M.; Masutani, H.; Kim, Y.-C.; Nishikawa, M.; Ohira, A.; Yodoi, J. Sulforaphane induces thioredoxin through the antioxidant-responsive element and attenuates retinal light damage in mice. Investig. Ophthalmol. Vis. Sci. 2005, 46, 979–987. [Google Scholar] [CrossRef] [Green Version]
- Murashima, M.; Watanabe, S.; Zhuo, X.G.; Uehara, M.; Kurashige, A. Phase 1 study of multiple biomarkers for metabolism and oxidative stress after one-week intake of broccoli sprouts. Biofactors 2004, 22, 271–275. [Google Scholar] [CrossRef]
- Tolba, M.F.; Azab, S.S.; Khalifa, A.E.; Abdel-Rahman, S.Z.; Abdel-Naim, A.B. Caffeic acid phenethyl ester, a promising component of propolis with a plethora of biological activities: A review on its anti-inflammatory, neuroprotective, hepatoprotective, and cardioprotective effects. IUBMB Life 2013, 65, 699–709. [Google Scholar] [CrossRef]
- Macheiner, L.; Schmidt, A.; Schreiner, M.; Mayer, H.K. Green coffee infusion as a source of caffeine and chlorogenic acid. J. Food Compos. Anal. 2019, 84, 103307. [Google Scholar] [CrossRef]
- Senol, F.S.; Woźniak, K.S.; Khan, M.T.H.; Orhan, I.E.; Sener, B.; Głowniak, K. An in vitro and in silico approach to cholinesterase inhibitory and antioxidant effects of the methanol extract, furanocoumarin fraction, and major coumarins of Angelica officinalis L. fruits. Phytochem. Lett. 2011, 4, 462–467. [Google Scholar] [CrossRef]
- Tang, W.; Li, G.; Row, K.H.; Zhu, T. Preparation of hybrid molecularly imprinted polymer with double-templates for rapid simultaneous purification of theophylline and chlorogenic acid in green tea. Talanta 2016, 152, 1–8. [Google Scholar] [CrossRef]
- Saleh, I.A.; Vinatoru, M.; Mason, T.J.; Abdel-Azim, N.; Aboutabl, E.; Hammouda, F. A possible general mechanism for ultrasound-assisted extraction (UAE) suggested from the results of UAE of chlorogenic acid from Cynara scolymus L.(artichoke) leaves. Ultrason. Sonochem. 2016, 31, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J. Nutr. 2008, 138, 2309–2315. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, D.M.; Finger-Teixeira, A.; Mota, T.R.; Salvador, V.H.; Moreira-Vilar, F.C.; Molinari, H.B.C.; Mitchell, R.A.C.; Marchiosi, R.; Ferrarese, O.; dos Santos, W.D. Ferulic acid: A key component in grass lignocellulose recalcitrance to hydrolysis. Plant Biotechnol. J. 2015, 13, 1224–1232. [Google Scholar] [CrossRef]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. (Amst.) 2014, 4, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Menozzi-Smarrito, C.; Wong, C.C.; Meinl, W.; Glatt, H.; Fumeaux, R.; Munari, C.; Robert, F.; Williamson, G.; Barron, D. First chemical synthesis and in vitro characterization of the potential human metabolites 5-o-feruloylquinic acid 4’-sulfate and 4’-O-glucuronide. J. Agric. Food Chem. 2011, 59, 5671–5676. [Google Scholar] [CrossRef]
- Wenzl, P.; Chaves, A.L.; Mayer, J.E.; Rao, I.M.; Nair, M.G. Roots of nutrient-deprived Brachiaria species accumulate 1,3-di-O-trans-feruloylquinic acid. Phytochemistry 2000, 55, 389–395. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Said, A.M.; Khalifa, S.A.; Goransson, U.; Bohlin, L.; Borg-Karlson, A.-K.; Verpoorte, R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef] [PubMed]
- Angelino, D.; Cossu, M.; Marti, A.; Zanoletti, M.; Chiavaroli, L.; Brighenti, F.; Del Rio, D.; Martini, D. Bioaccessibility and bioavailability of phenolic compounds in bread: A review. Food Funct. 2017, 8, 2368–2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; He, F.; Chen, G. Improving bioaccessibility and bioavailability of phenolic compounds in cereal grains through processing technologies: A concise review. J. Funct. Foods 2014, 7, 101–111. [Google Scholar] [CrossRef]
- Kishida, K.; Matsumoto, H. Urinary excretion rate and bioavailability of chlorogenic acid, caffeic acid, p-coumaric acid, and ferulic acid in non-fasted rats maintained under physiological conditions. Heliyon 2019, 5, e02708. [Google Scholar] [CrossRef] [Green Version]
- Konishi, Y.; Hitomi, Y.; Yoshioka, E. Intestinal absorption of p-coumaric and gallic acids in rats after oral administration. J. Agric. Food Chem. 2004, 52, 2527–2532. [Google Scholar] [CrossRef]
- Elhessy, H.M.; Eltahry, H.; Erfan, O.S.; Mahdi, M.R.; Hazem, N.M.; El-Shahat, M.A. Evaluation of the modulation of nitric oxide synthase expression in the cerebellum of diabetic albino rats and the possible protective effect of ferulic acid. Acta Histochem. 2020, 122, 151633. [Google Scholar] [CrossRef]
- Hu, R.; Wu, S.; Li, B.; Tan, J.; Yan, J.; Wang, Y.; Tang, Z.; Liu, M.; Fu, C.; Zhang, H.; et al. Dietary ferulic acid and vanillic acid on inflammation, gut barrier function and growth performance in lipopolysaccharide-challenged piglets. Anim. Nutr. 2022, 144, 144–152. [Google Scholar] [CrossRef]
- Valério, R.; Serra, A.T.; Baixinho, J.; Cardeira, M.; Fernández, N.; Bronze, M.R.; Duarte, L.C.; Tavares, M.L.; Crespo, J.G.; Brazinha, C. Combined hydrothermal pre-treatment and enzymatic hydrolysis of corn fibre: Production of ferulic acid extracts and assessment of their antioxidant and antiproliferative properties. Ind. Crops Prod. 2021, 170, 113731. [Google Scholar] [CrossRef]
- Zdunska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Caigui, X.; Moting, L.; Qiukai, L.; Chen, F.; Huimin, L.; Chunlan, F.; Xiaoqian, Y.; Heng, L.; Wei, T. Blockade of TLRs-triggered macrophage activation by caffeic acid exerted protective effects on experimental ulcerative colitis. Cell. Immunol. 2021, 365, 104364. [Google Scholar]
- Tigist, K.; Whalin, W.J.; Richards, R.M.; Alayash, A.A. Caffeic acid: An antioxidant with novel antisickling properties. FEBS Open Bio 2021, 11, 3293–3303. [Google Scholar]
- Bonita, J.S.; Mandarano, M.; Shuta, D.; Vinson, J. Coffee and cardiovascular disease: In vitro, cellular, animal, and human studies. Pharm. Res 2007, 55, 187–198. [Google Scholar] [CrossRef]
- Nada, O.; Damir, S.; Dyana, O.; Goran, G.; Vedran, B.; Lidija, Š.; Maja, J.J. Efficacy of Caffeic Acid on Diabetes and Its Complications in the Mouse. Molecules 2021, 26, 3262. [Google Scholar]
- Huihui, S.; Xin, T.; Jiehong, Y.; Li, Y.; Huifen, Z.; Yu, W.; Yu, H.; Haitong, W.; Chang, L. Biotransformation of natural hydroxycinnamic acids by gut microbiota from normal and cerebral ischemia-reperfusion injured rats: A comparative study. Food Funct. 2020, 11, 5389–5395. [Google Scholar]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- Liu, S.-L.; Peng, B.-J.; Zhong, Y.-L.; Liu, Y.-L.; Song, Z.; Wang, Z. Effect of 5-caffeoylquinic acid on the NF-κB signaling pathway, peroxisome proliferator-activated receptor gamma 2, and macrophage infiltration in high-fat diet-fed Sprague–Dawley rat adipose tissue. Food Funct. 2015, 6, 2779–2786. [Google Scholar] [CrossRef]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef]
- Cho, A.-S.; Jeon, S.-M.; Kim, M.-J.; Yeo, J.; Seo, K.-I.; Choi, M.-S.; Lee, M.-K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef]
- Ma, C.; Dastmalchi, K.; Whitaker, B.D.; Kennelly, E.J. Two new antioxidant malonated caffeoylquinic acid isomers in fruits of wild eggplant relatives. J. Agric. Food Chem. 2011, 59, 9645–9651. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Rui, Y.-x.; Guo, S.-d.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- Boo, Y.C. p-Coumaric acid as an active ingredient in cosmetics: A review focusing on its antimelanogenic effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef] [Green Version]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef]
- Ow, Y.Y.; Stupans, I. Gallic acid and gallic acid derivatives: Effects on drug metabolizing enzymes. Curr. Drug Metab. 2003, 4, 241–248. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and oxidative stress in an obese state and the protective effects of gallic acid. Nutrients 2019, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Zhang, L.; Liao, P.; Xiao, Z.; Zhang, F.; Sindaye, D.; Xin, Z.; Tan, C.; Deng, J.; Yin, Y.; et al. Impact of Gallic Acid on Gut Health: Focus on the Gut Microbiome, Immune Response, and Mechanisms of Action. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Semaming, Y.; Pannengpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Pharmacological properties of protocatechuic acid and its potential roles as complementary medicine. Evid.-Based Complement. Altern. Med. 2015, 2015, 593902. [Google Scholar] [CrossRef] [Green Version]
- Kho, A.; Bo, C.; Song, L.; Hong, D.; Sang, L.; Jeong, J.; Kyoung-Ha, P.; Hong, S.; Hui, C.; Sang, S. Effects of Protocatechuic Acid (PCA) on Global Cerebral Ischemia-Induced Hippocampal Neuronal Death. Int. J. Mol. Sci. 2018, 19, 1420. [Google Scholar] [CrossRef] [Green Version]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.; Kumar, C.S. Syringic acid (SA)—a review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef]
- Tohge, T.; R Fernie, A. An overview of compounds derived from the shikimate and phenylpropanoid pathways and their medicinal importance. Mini Rev. Med. Chem. 2017, 17, 1013–1027. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, F.; Cui, Q.; Lu, M.; Song, X.; Tang, H.; Li, Q. Measurement and correlation of the solubility of vanillic acid in eight pure and water+ ethanol mixed solvents at temperatures from (293.15 to 323.15) K. J. Chem. Eng. Data 2016, 61, 420–429. [Google Scholar] [CrossRef]
- Pahua-Ramos, M.E.; Ortiz-Moreno, A.; Chamorro-Cevallos, G.; Hernández-Navarro, M.D.; Garduño-Siciliano, L.; Necoechea-Mondragón, H.; Hernández-Ortega, M. Hypolipidemic effect of avocado (Persea americana Mill) seed in a hypercholesterolemic mouse model. Plant Foods Hum. Nutr. 2012, 67, 10–16. [Google Scholar] [CrossRef]
- Salazar-López, N.J.; Domínguez-Avila, J.A.; Yahia, E.M.; Belmonte-Herrera, B.H.; Wall-Medrano, A.; Montalvo-González, E.; González-Aguilar, G. Avocado fruit and by-products as potential sources of bioactive compounds. Food Res. Int. 2020, 138, 109774. [Google Scholar] [CrossRef]
- Abdelkafi, S.; Sayadi, S.; Ali Gam, Z.B.; Casalot, L.; Labat, M. Bioconversion of ferulic acid to vanillic acid by Halomonas elongata isolated from table-olive fermentation. FEMS Microbiol. Lett. 2006, 262, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.C.H.; Kakalij, R.M.; Kshirsagar, R.P.; Kumar, B.H.; Komakula, S.S.B.; Diwan, P.V. Cognitive effects of vanillic acid against streptozotocin-induced neurodegeneration in mice. Pharm. Biol. 2015, 53, 630–636. [Google Scholar] [CrossRef] [Green Version]
- Oke, I.M.; Ramorobi, L.M.; Mashele, S.S.; Bonnet, S.L.; Makhafola, T.J.; Eze, K.C.; Noreljaleel, A.E.; Chukwuma, C.I. Vanillic acid–Zn (II) complex: A novel complex with antihyperglycaemic and anti-oxidative activity. J. Pharm. Pharmacol. 2021, 73, 1703–1714. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Lobo, J.K.; Janle, E.M.; Cooper, B.; Simon, J.E.; Wu, Q.-L.; Welch, C.; Ho, L.; Weaver, C.; Pasinetti, G.M. Bioavailability of gallic acid and catechins from grape seed polyphenol extract is improved by repeated dosing in rats: Implications for treatment in Alzheimer’s disease. J. Alzheimer’s Dis. 2009, 18, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Ćetković, Z.; Cvijić, S.; Vasiljević, D. In vitro/in silico approach in the development of simvastatin-loaded self-microemulsifying drug delivery systems. Drug Dev. Ind. Pharm. 2018, 44, 849–860. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Li, W.; Zhang, H.; Adu-Frimpong, M.; Ma, P.; Zhu, Y.; Deng, W.; Yu, J.; Xu, X. Improved Oral Bioavailability and Hypolipidemic Effect of Syringic Acid via a Self-microemulsifying Drug Delivery System. AAPS PharmSciTech 2021, 22, 45. [Google Scholar] [CrossRef]
- Sun, C.; Yuan, Y.; Omari-Siaw, E.; Tong, S.; Zhu, Y.; Wang, Q.; Wang, Y.; Wei, Q.; Yu, J.; Xu, X. An efficient HPLC method for determination of syringic acid liposome in rats plasma and mice tissues: Pharmacokinetic and biodistribution application. Curr. Pharm. Anal. 2018, 14, 41–52. [Google Scholar] [CrossRef]
- Koli, R.; Erlund, I.; Jula, A.; Marniemi, J.; Mattila, P.; Alfthan, G. Bioavailability of various polyphenols from a diet containing moderate amounts of berries. J. Agric. Food Chem. 2010, 58, 3927–3932. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ma, T.; Li, Y.; Cai, N. A rapid and sensitive LC-MS/MS method for the determination of vanillic acid in rat plasma with application to pharmacokinetic study. Biomed. Chromatogr. 2021, 36, e5248. [Google Scholar] [CrossRef] [PubMed]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Bak, E.-J.; Kim, J.; Jang, S.; Woo, G.-H.; Yoon, H.-G.; Yoo, Y.-J.; Cha, J.-H. Gallic acid improves glucose tolerance and triglyceride concentration in diet-induced obesity mice. Scand. J. Clin. Lab. Investig. 2013, 73, 607–614. [Google Scholar] [CrossRef]
- Chao, J.; Huo, T.-I.; Cheng, H.-Y.; Tsai, J.-C.; Liao, J.-W.; Lee, M.-S.; Qin, X.-M.; Hsieh, M.-T.; Pao, L.-H.; Peng, W.-H. Gallic acid ameliorated impaired glucose and lipid homeostasis in high fat diet-induced NAFLD mice. PLoS ONE 2014, 9, e96969. [Google Scholar]
- Hsu, C.-L.; Yen, G.-C. Effect of gallic acid on high fat diet-induced dyslipidaemia, hepatosteatosis and oxidative stress in rats. Br. J. Nutr. 2007, 98, 727–735. [Google Scholar] [CrossRef] [Green Version]
- Sergent, T.; Vanderstraeten, J.; Winand, J.; Beguin, P.; Schneider, Y.-J. Phenolic compounds and plant extracts as potential natural anti-obesity substances. Food Chem. 2012, 135, 68–73. [Google Scholar] [CrossRef]
- Gao, J.; Hu, J.; Hu, D.; Yang, X. A role of gallic acid in oxidative damage diseases: A comprehensive review. Nat. Prod. Commun. 2019, 14, 1934578X19874174. [Google Scholar] [CrossRef] [Green Version]
- Ferk, F.; Kundi, M.; Brath, H.; Szekeres, T.; Al-Serori, H.; Mišík, M.; Saiko, P.; Marculescu, R.; Wagner, K.H.; Knasmueller, S. Gallic Acid Improves Health-Associated Biochemical Parameters and Prevents Oxidative Damage of DNA in Type 2 Diabetes Patients: Results of a Placebo-Controlled Pilot Study. Mol. Nutr. Food Res. 2018, 62, 1700482. [Google Scholar] [CrossRef]
- Zheng, J.; Xiong, H.; Li, Q.; He, L.; Weng, H.; Ling, W.; Wang, D. Protocatechuic acid from chicory is bioavailable and undergoes partial glucuronidation and sulfation in healthy humans. Food Sci. Nutr. 2019, 7, 3071–3080. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Xue, Z.; Wang, Y.; Lu, Y.; Li, R.; Li, N.; Wang, Q.; Zhang, M.; Chen, H. Chemical structure and inhibition on α-glucosidase of polysaccharides from corn silk by fractional precipitation. Carbohydr. Polym. 2021, 252, 117185. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-L.; Liu, J.-X.; Dong, W.; Li, P.; Li, L.; Lin, C.-R.; Zheng, Y.-Q.; Cong, W.-H.; Hou, J.-C. Cardioprotective effect of protocatechuic acid on myocardial ischemia/reperfusion injury. J. Pharmacol. Sci. 2014, 125, 176–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mea, A.; Gsgsa, B.; Gms, A.; Has, A. Protocatechuic acid improves hepatic insulin resistance and restores vascular oxidative status in type-2 diabetic rats. Environ. Toxicol. Pharmacol. 2021, 83, 103577. [Google Scholar]
- Ham, J.R.; Lee, H.-I.; Choi, R.-Y.; Sim, M.-O.; Seo, K.-I.; Lee, M.-K. Anti-steatotic and anti-inflammatory roles of syringic acid in high-fat diet-induced obese mice. Food Funct. 2016, 7, 689–697. [Google Scholar] [CrossRef]
- Muthukumaran, J.; Srinivasan, S.; Venkatesan, R.S.; Ramachandran, V.; Muruganathan, U. Syringic acid, a novel natural phenolic acid, normalizes hyperglycemia with special reference to glycoprotein components in experimental diabetic rats. J. Acute Dis. 2013, 2, 304–309. [Google Scholar] [CrossRef] [Green Version]
- Sindhu, G.; Nishanthi, E.; Sharmila, R. Nephroprotective effect of vanillic acid against cisplatin induced nephrotoxicity in wistar rats: A biochemical and molecular study. Environ. Toxicol. Pharmacol. 2015, 39, 392–404. [Google Scholar] [CrossRef]
- Vinothiya, K.; Ashokkumar, N. Modulatory effect of vanillic acid on antioxidant status in high fat diet-induced changes in diabetic hypertensive rats. Biomed. Pharmacother. 2017, 87, 640–652. [Google Scholar] [CrossRef]
- Calixto-Campos, C.S.; Carvalho, T.T.; Hohmann, M.S.; Pinho-Ribeiro, F.A.; Fattori, V.; Manchope, M.F.; Zarpelon, A.C.; Baracat, M.M.; Georgetti, S.R.; Casagrande, R. Vanillic acid inhibits inflammatory pain by inhibiting neutrophil recruitment, oxidative stress, cytokine production, and NFκB activation in mice. J. Nat. Prod. 2015, 78, 1799–1808. [Google Scholar] [CrossRef]
- Kim, M.-C.; Kim, S.-J.; Kim, D.-S.; Jeon, Y.-D.; Park, S.J.; Lee, H.S.; Um, J.-Y.; Hong, S.-H. Vanillic acid inhibits inflammatory mediators by suppressing NF-κB in lipopolysaccharide-stimulated mouse peritoneal macrophages. Immunopharmacol. Immunotoxicol. 2011, 33, 525–532. [Google Scholar] [CrossRef]
- Jung, Y.; Park, J.; Kim, H.L.; Sim, J.E.; Youn, D.H.; Kang, J.; Lim, S.; Jeong, M.Y.; Yang, W.M.; Lee, S.G. Vanillic acid attenuates obesity via activation of the AMPK pathway and thermogenic factors in vivo and in vitro. FASEB J. 2018, 32, 1388–1402. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-M.; Hsu, C.-L.; Chuang, H.-C.; Shih, P.-H.; Wu, C.-H.; Yen, G.-C. Inhibitory effect of vanillic acid on methylglyoxal-mediated glycation in apoptotic Neuro-2A cells. Neurotoxicology 2008, 29, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Topal, F. Anticholinergic and antidiabetic effects of isoeugenol from clove (Eugenia caryophylata) oil. Int. J. Food Prop. 2019, 22, 583–592. [Google Scholar] [CrossRef] [Green Version]
- Martínez, L.; Ros, G.; Nieto, G. Hydroxytyrosol: Health Benefits and Use as Functional Ingredient in Meat. Medicines 2018, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C.; Bornet, F.; Mattei, A.; Patelli, R.; Galli, G.; Caruso, D. Olive oil phenolics are dose-dependently absorbed in humans. FEBS Lett. 2000, 468, 159–160. [Google Scholar] [CrossRef]
- Tuck, K.L.; Freeman, M.P.; Hayball, P.J.; Stretch, G.L.; Stupans, I. The in vivo fate of hydroxytyrosol and tyrosol, antioxidant phenolic constituents of olive oil, after intravenous and oral dosing of labeled compounds to rats. J. Nutr. 2001, 131, 1993–1996. [Google Scholar] [CrossRef] [Green Version]
- Rubió, L.; Macià, A.; Valls, R.M.; Pedret, A.; Romero, M.-P.; Solà, R.; Motilva, M.-J. A new hydroxytyrosol metabolite identified in human plasma: Hydroxytyrosol acetate sulphate. Food Chem. 2012, 134, 1132–1136. [Google Scholar] [CrossRef]
- Khymenets, O.; Crespo, M.C.; Dangles, O.; Rakotomanomana, N.; Andres-Lacueva, C.; Visioli, F. Human hydroxytyrosol’s absorption and excretion from a nutraceutical. J. Funct. Foods 2016, 23, 278–282. [Google Scholar] [CrossRef]
- Chen, C.; Wei, Y.-h. Potential role of hydroxytyrosol in neuroprotection. J. Funct. Foods 2021, 82, 104506. [Google Scholar] [CrossRef]
- Serra, A.; Rubió, L.; Borràs, X.; Macià, A.; Romero, M.P.; Motilva, M.J. Distribution of olive oil phenolic compounds in rat tissues after administration of a phenolic extract from olive cake. Mol. Nutr. Food Res. 2012, 56, 486–496. [Google Scholar] [CrossRef]
- Caruso, D.; Visioli, F.; Patelli, R.; Galli, C.; Galli, G. Urinary excretion of olive oil phenols and their metabolites in humans. Metab.-Clin. Exp. 2001, 50, 1426–1428. [Google Scholar] [CrossRef] [PubMed]
- Miro-Casas, E.; Covas, M.-I.; Farre, M.; Fito, M.; Ortuno, J.; Weinbrenner, T.; Roset, P.; De La Torre, R. Hydroxytyrosol disposition in humans. Clin. Chem. 2003, 49, 945–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alemán-Jiménez, C.; Domínguez-Perles, R.; Medina, S.; Prgomet, I.; López-González, I.; Simonelli-Muñoz, A.; Campillo-Cano, M.; Auñón, D.; Ferreres, F.; Gil-Izquierdo, Á. Pharmacokinetics and bioavailability of hydroxytyrosol are dependent on the food matrix in humans. Eur. J. Nutr. 2021, 60, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.L.; Sanchez-Rovira, P.; Ramirez-Tortosa, M.C. Hydroxytyrosol: From laboratory investigations to future clinical trials. Nutr. Rev. 2010, 68, 191–206. [Google Scholar] [CrossRef] [Green Version]
- Vilaplana-Pérez, C.; Auñón, D.; García-Flores, L.A.; Gil-Izquierdo, A. Hydroxytyrosol and Potential Uses in Cardiovascular Diseases, Cancer, and AIDS. Front. Nutr. 2014, 1, 18. [Google Scholar] [CrossRef] [Green Version]
- López de las Hazas, M.-C.; Piñol, C.; Macià, A.; Romero, M.-P.; Pedret, A.; Solà, R.; Rubió, L.; Motilva, M.-J. Differential absorption and metabolism of hydroxytyrosol and its precursors oleuropein and secoiridoids. J. Funct. Foods 2016, 22, 52–63. [Google Scholar] [CrossRef] [Green Version]
- Bernini, R.; Merendino, N.; Romani, A.; Velotti, F. Naturally occurring hydroxytyrosol: Synthesis and anticancer potential. Curr. Med. Chem. 2013, 20, 655–670. [Google Scholar] [CrossRef]
- Rodríguez-Morató, J.; Xicota, L.; Fitó, M.; Farré, M.; Dierssen, M.; De la Torre, R. Potential role of olive oil phenolic compounds in the prevention of neurodegenerative diseases. Molecules 2015, 20, 4655–4680. [Google Scholar] [CrossRef] [Green Version]
- Merra, E.; Calzaretti, G.; Bobba, A.; Storelli, M.M.; Casalino, E. Antioxidant role of hydroxytyrosol on oxidative stress in cadmium-intoxicated rats: Different effect in spleen and testes. Drug Chem. Toxicol. 2014, 37, 420–426. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).