Baseline Concentrations of Various Immune Biomarkers Determine Their Increase after Consumption of a Postbiotic Based on Cow’s Milk Fermented with Lactobacillus paracasei CBA L74 in Both Newborns and Young Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Studies Involved
- -
- age below 12 months or above 48 months.
- -
- concomitant chronic infections, chronic systemic diseases, chronic inflammatory bowel diseases, autoimmune diseases, immunodeficiency, malignancy, metabolic diseases, chronic respiratory tract diseases including respiratory allergies and cystic fibrosis, malformations of gastrointestinal or urinary or respiratory tract, history of respiratory or gastrointestinal or urinary tract surgery, congenital cardiac defects, functional bowel disorders, suspected or challenge-proved food allergy, food intolerances, severe malnutrition (z-score for weight-for-height) and use of antibiotics or pre/pro/synbiotics or immune stimulating products in the 2 weeks before the enrolment.
- -
- the presence of congenital diseases, chromosomal abnormalities, and/or conditions that could interfere with growth, such as brain, metabolic, cardiac and gastrointestinal diseases, perinatal infections, being born to a mother affected by endocrine and/or metabolic diseases or having a family history of allergic disease.
2.3. Ethics
2.4. Data
2.5. Analysis of the Outcome Using the Data of the Three Studies
2.6. Association between Parameters
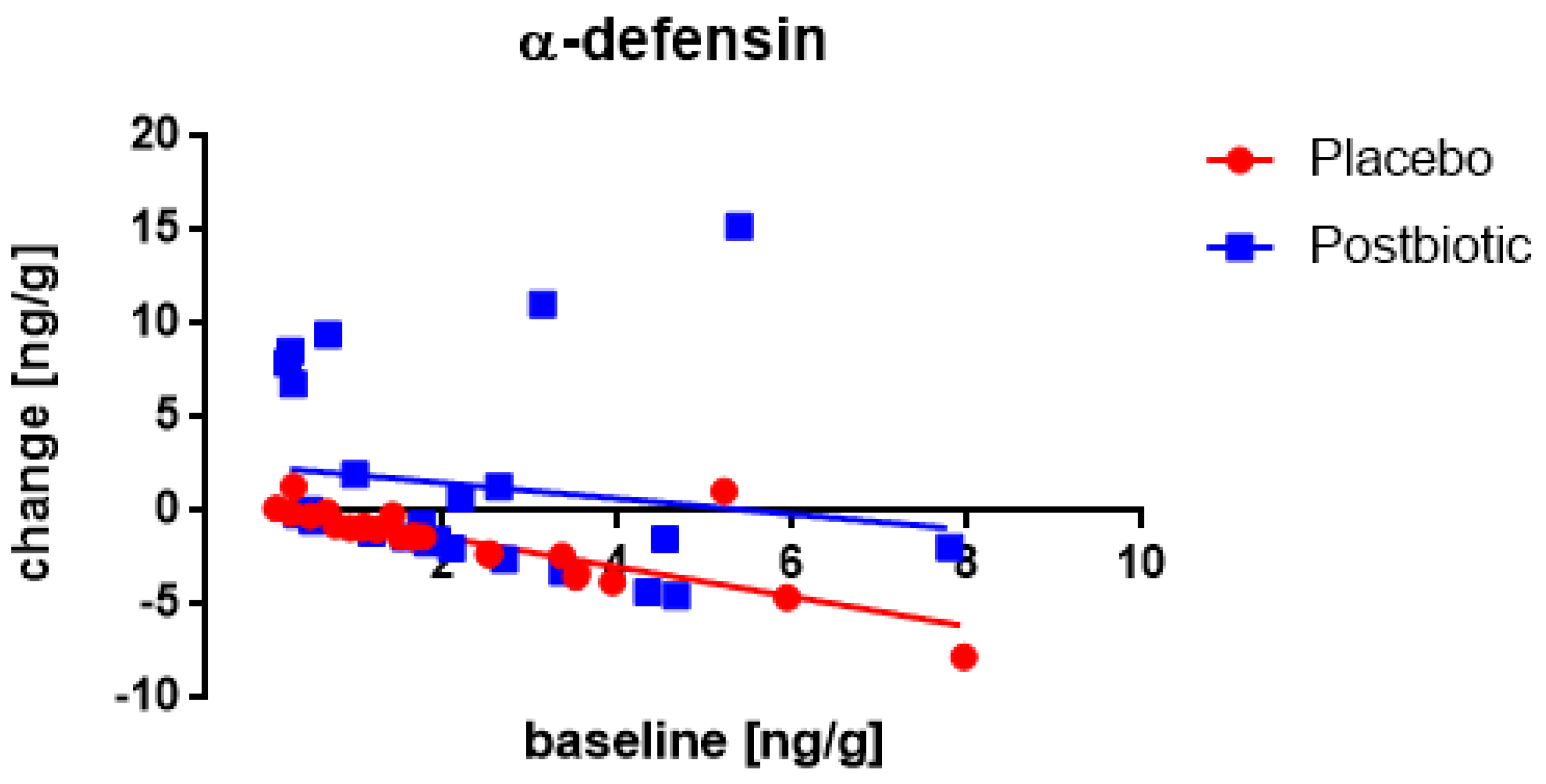
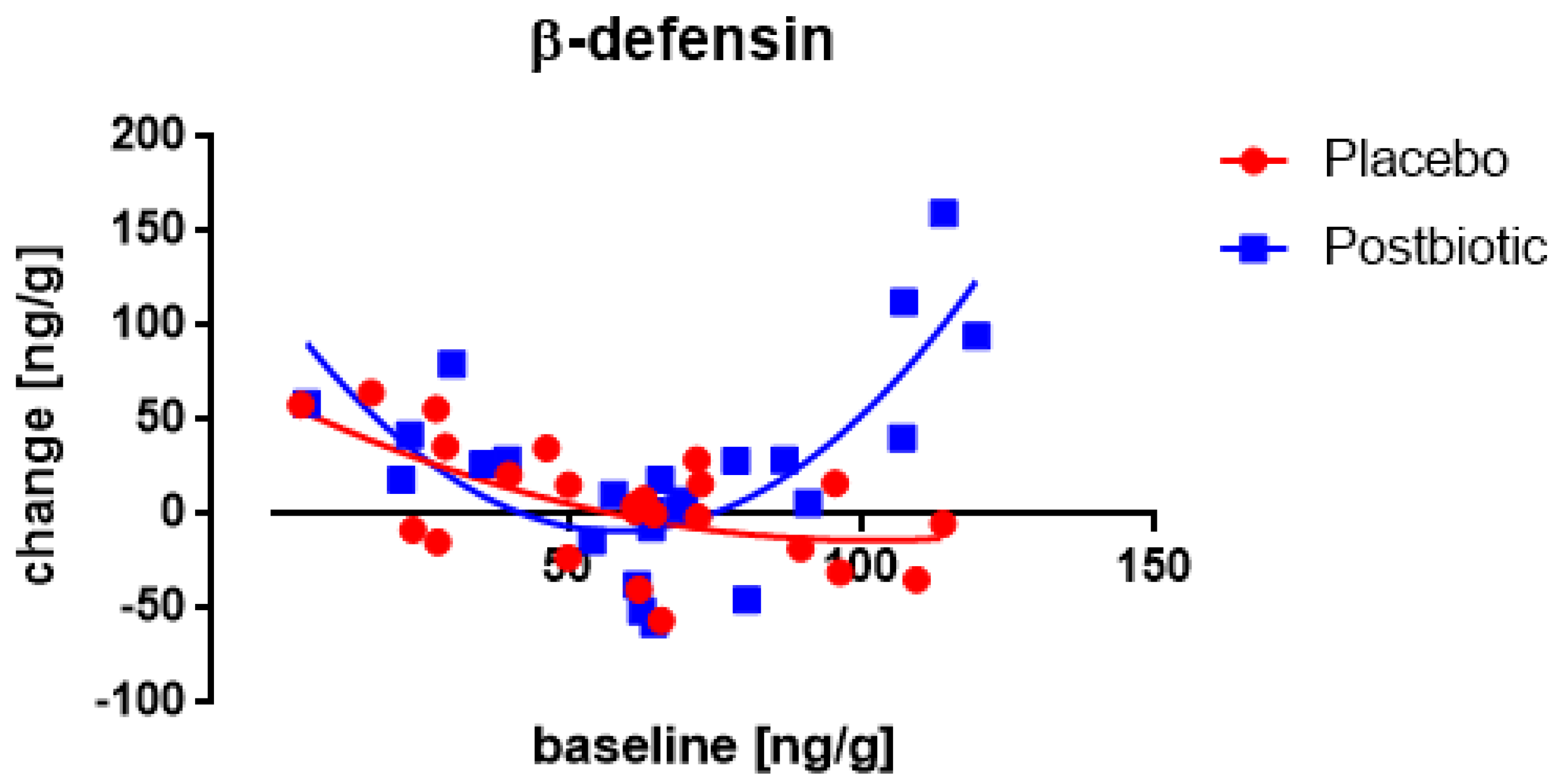
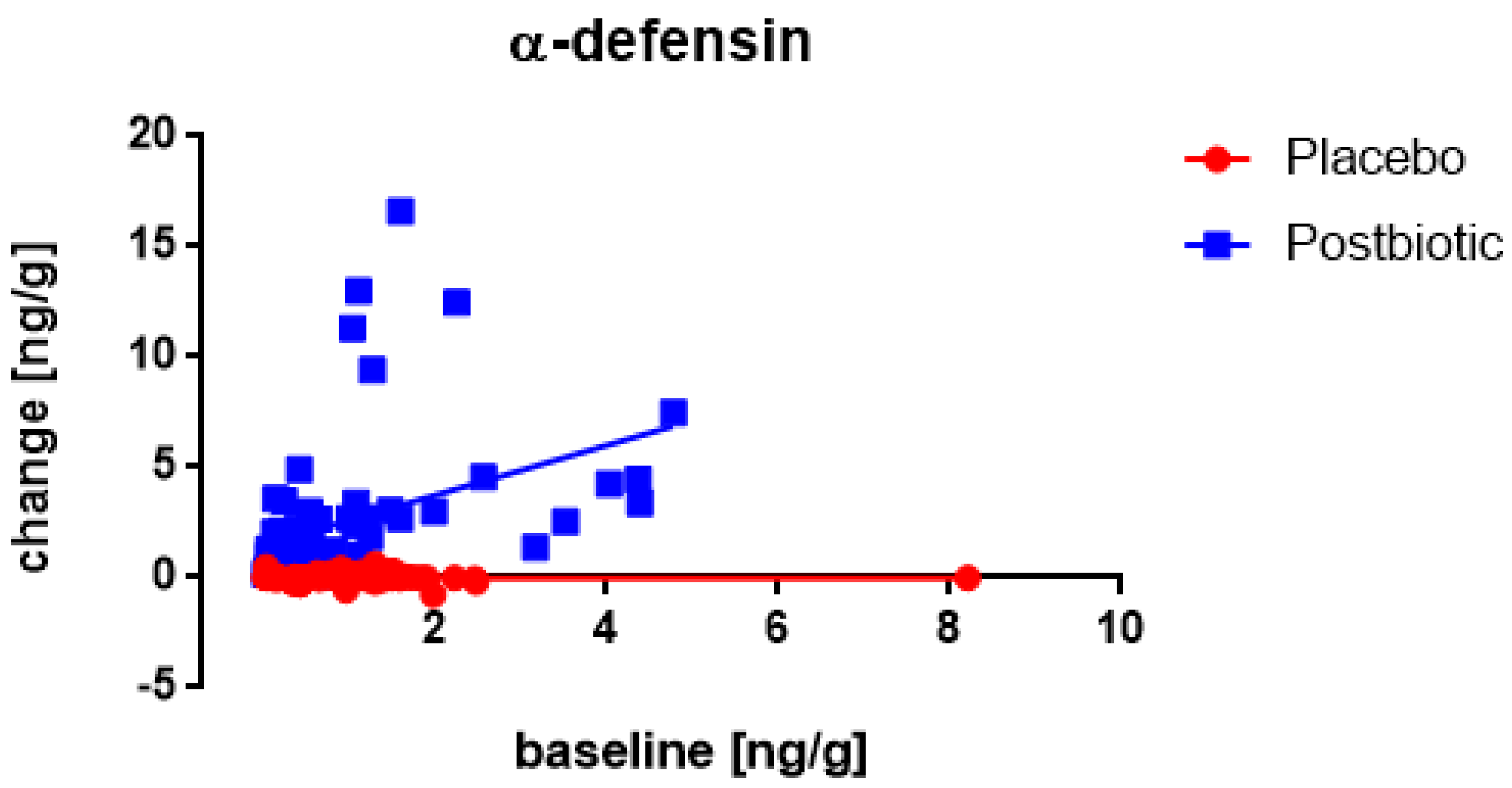
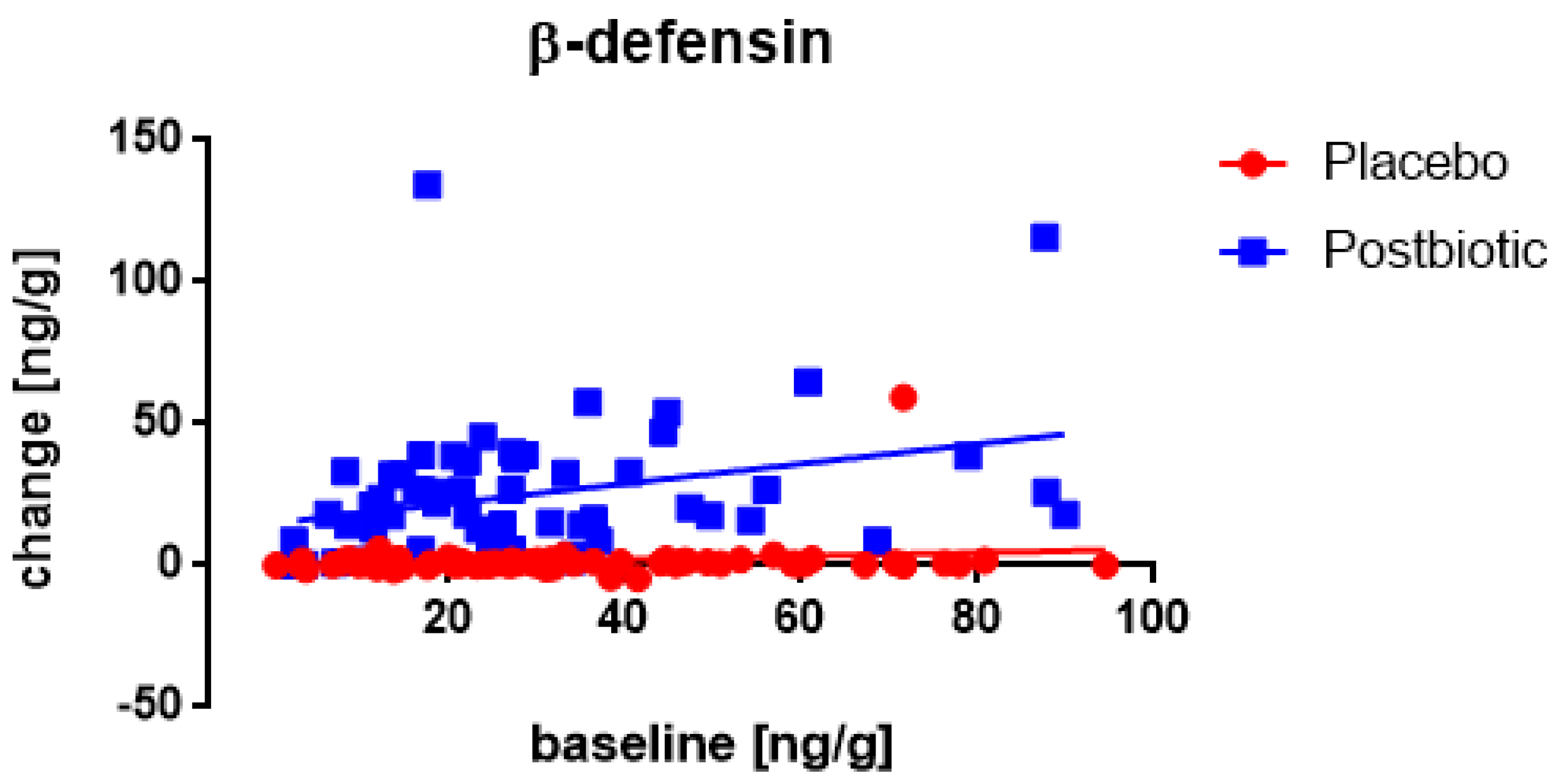
3. Results
3.1. Start-Values
3.2. The Roggero Study: Newborns
3.3. Nocerino and Corsello Studies: Children Aged 1–4 Years
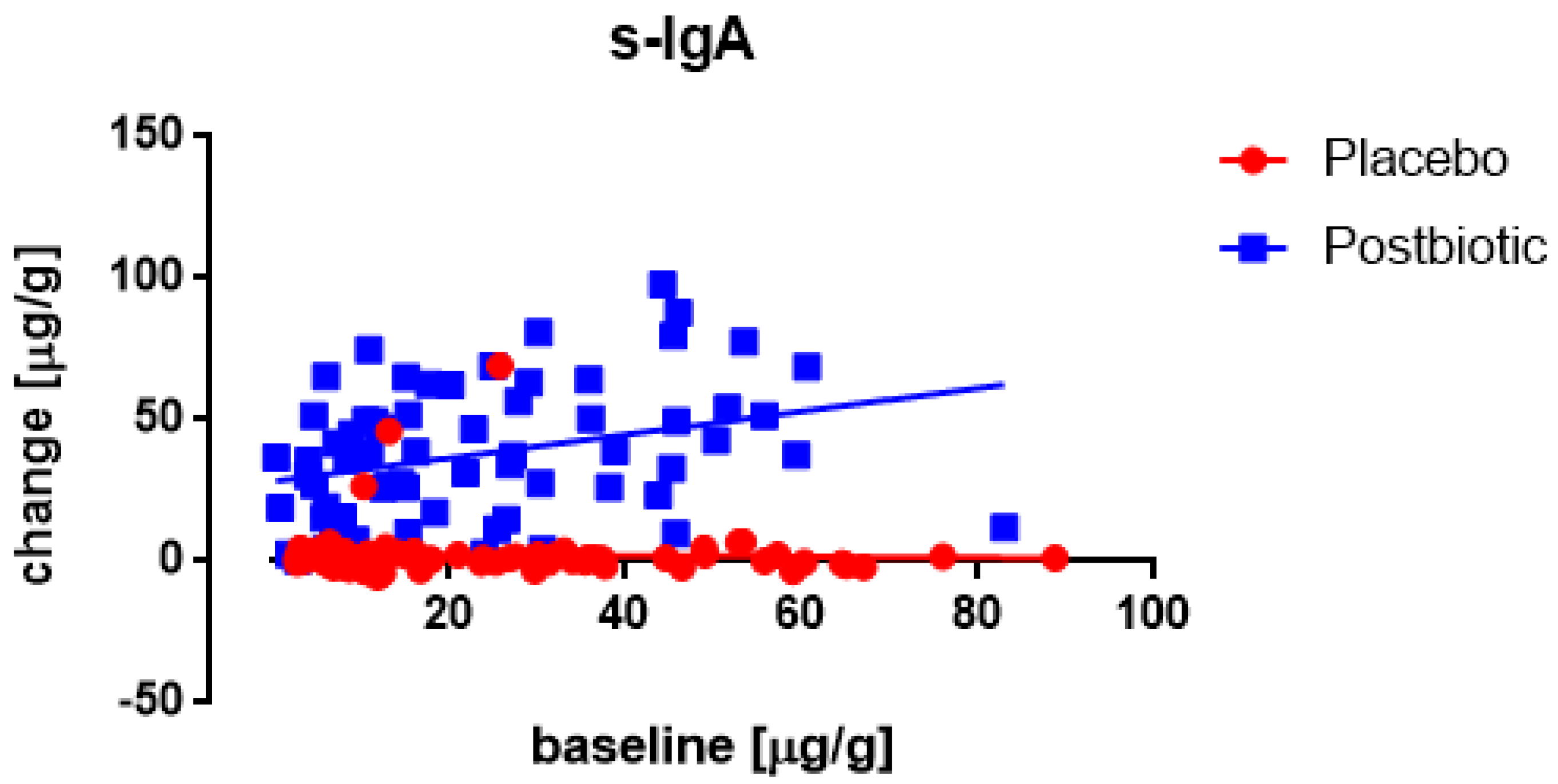
3.4. Associations between the Changes in the Various Immunological Factors
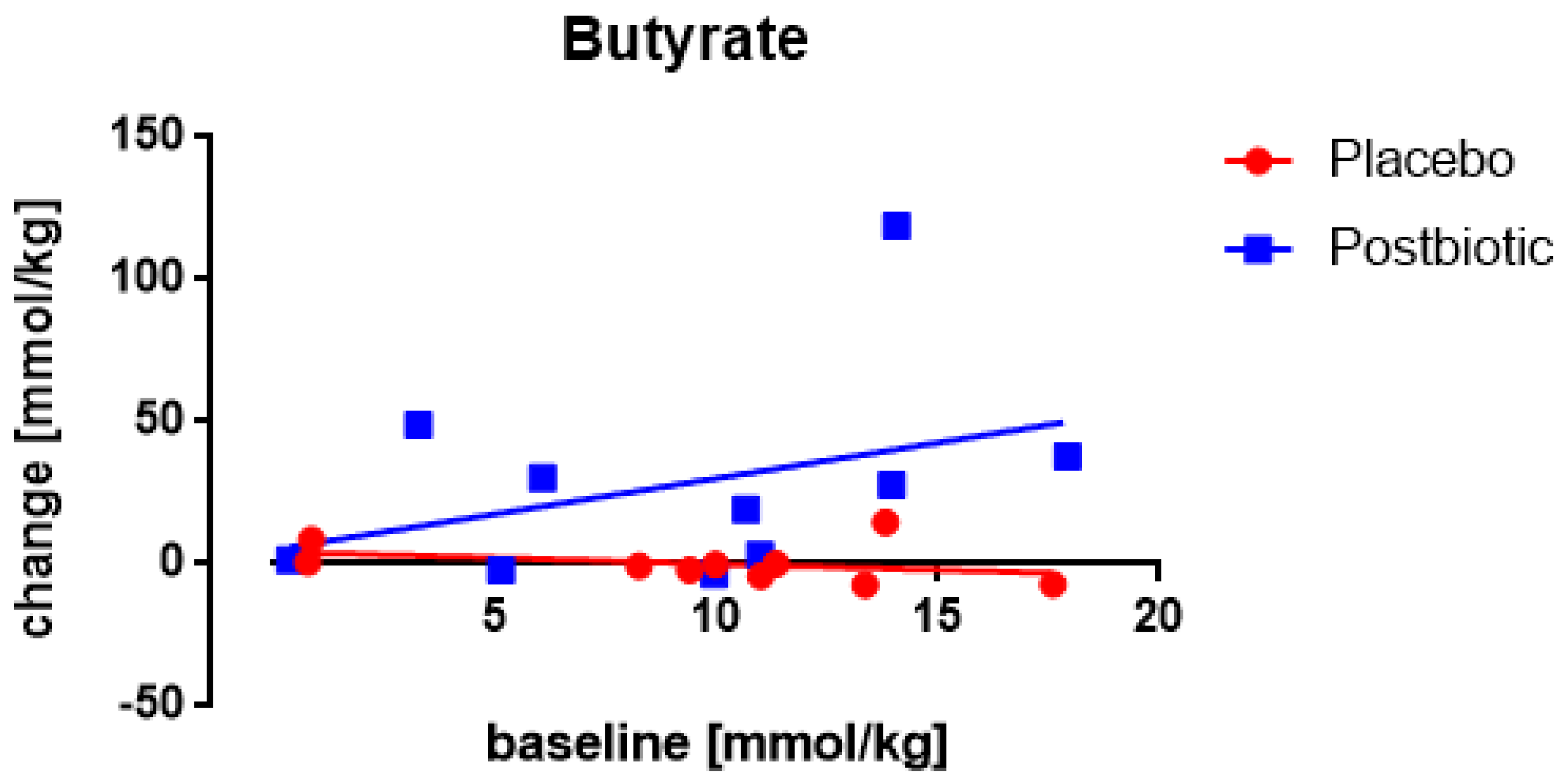
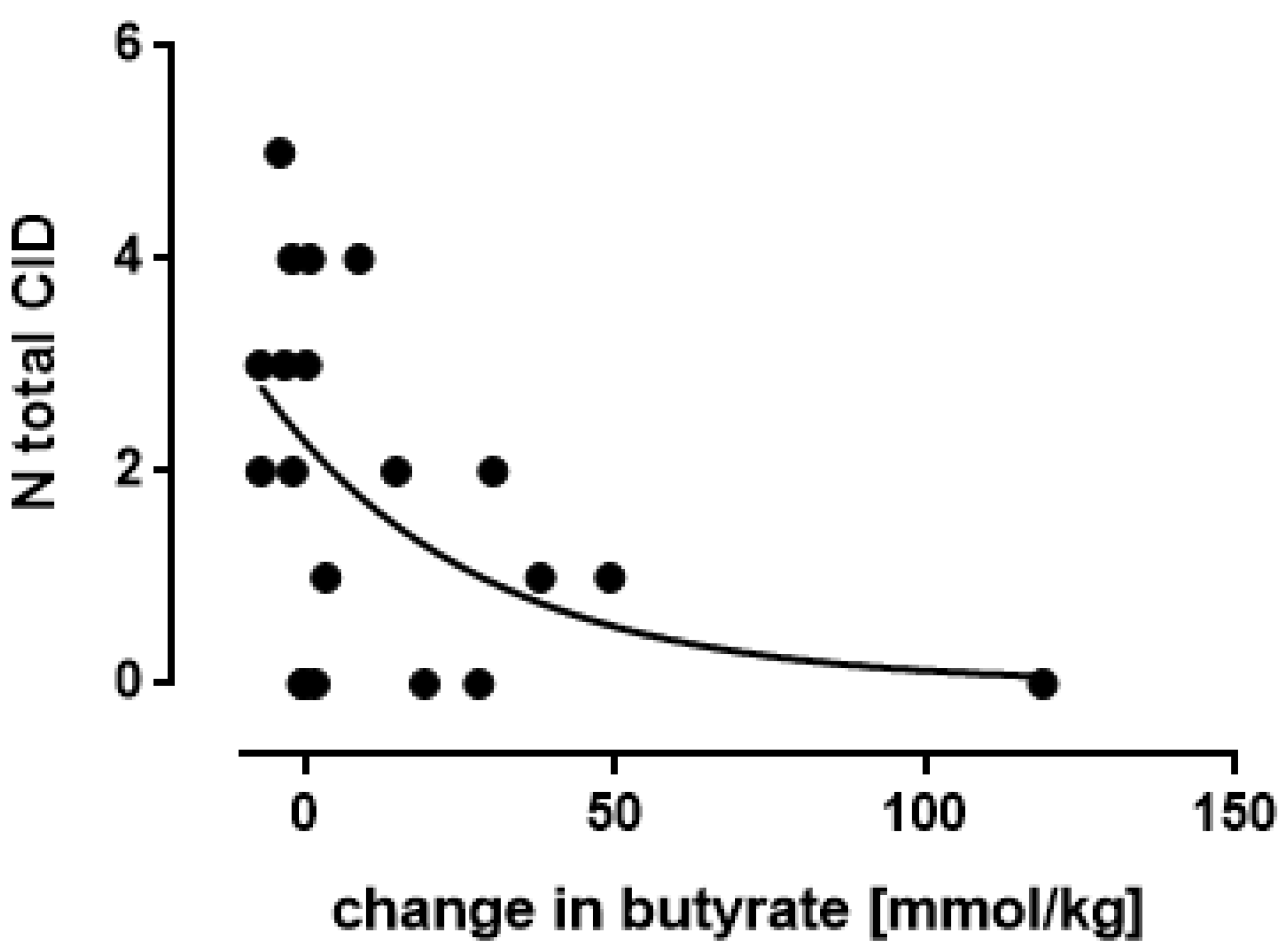
3.5. Butyrate: Only One Study Nocerino et al., 2017: Young Children Aged 1–4 Years
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Berni Canani, R.; Flint, H.J.; Salminen, S.; et al. Expert consensus statement: The international scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document. The international scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar-Toalá, J.E.; Arioli, S.; Behare, P.; Belzer, C.; Berni Canani, R.; Chatel, J.M.; D’Auria, E.; de Freitas, M.Q.; Elinav, E.; Esmerino, E.A.; et al. Postbiotics—When simplification fails to clarify. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 825–826. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics-A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef] [PubMed]
- Doron, S.; Snydman, D.R. Risk and safety of probiotics. Clin. Infect. Dis. 2015, 60 (Suppl 2), S129–S134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corsello, G.; Carta, M.; Marinello, R.; Picca, M.; de Marco, G.; Micillo, M.; Ferrara, D.; Vigneri, P.; Cecere, G.; Ferri, P. Preventive effect of cow’s milk fermented with Lactobacillus paracasei CBA L74 on common infectious diseases in children: A multicenter randomized controlled trial. Nutrients 2017, 9, 669. [Google Scholar] [CrossRef]
- Nocerino, R.; Paparo, L.; Terrin, G.; Pezzella, V.; Amoroso, A.; Cosenza, L.; Cecere, G.; De Marco, G.; Micillo, M.; Albano., F.; et al. Cow’s milk and rice fermented with Lactobacillus paracasei CBA L74 prevent infectious diseases in children: A randomized controlled trial. Clin. Nutr. 2017, 36, 118–125. [Google Scholar] [CrossRef]
- Zagato, E.; Mileti, E.; Massimiliano, L.; Fasano, F.; Budelli, A.; Penna, G.; Rescigno, M. Lactobacillus paracasei CBA L74 metabolic products and fermented milk for infant formula have anti-inflammatory activity on dendritic cells in vitro and protective effects against colitis and an enteric pathogen in vivo. PLoS ONE 2014, 9, e87615. [Google Scholar] [CrossRef]
- Paparo, L.; Aitoro, R.; Nocerino, R.; Fierro, C.; Bruno, C.; Berni Canani, R. Direct effects of fermented cow’s milk product with Lactobacillus paracasei CBA L74 on human enterocytes. Benef. Microbes 2018, 9, 165–172. [Google Scholar] [CrossRef]
- Berni Canani, R.; De Filippis, F.; Nocerino, R.; Laiola, M.; Paparo, L.; Calignano, A.; De Caro, C.; Coretti, L.; Chiariotti, L.; Gilbert, J.A.; et al. Specific Signatures of the Gut Microbiota and Increased Levels of Butyrate in Children Treated with Fermented Cow’s Milk Containing Heat-Killed Lactobacillus paracasei CBA L74. Appl. Environ. Microbiol. 2017, 83, e01206-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roggero, P.; Liotto, N.; Pozzi, C.; Braga, D.; Troisi, J.; Menis, C.; Giannì, M.L.; Berni Canani, R.; Paparo, L.; Nocerino, R.; et al. Analysis of immune, microbiota and metabolome maturation in infants in a clinical trial of Lactobacillus paracasei CBA L74-fermented formula. Nat. Commun. 2020, 11, 2703. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Selsted, M.E.; Lehrer, R.I. Defensins. Eur. J. Haematol. 1990, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hase, K.; Eckmann, L.; Leopard, J.D.; Varki, N.; Kagnoff, M.F. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Inf. Immun. 2002, 70, 953–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ren, C.; Han, X.; Huang, W.; You, Y.; Zhan, J. Role of IgA in the early-life establishment of the gut microbiota and immunity: Implications for constructing a healthy start. Gut Microbes 2021, 13, 1–21. [Google Scholar] [CrossRef]
- Bridgman, S.L.; Konya, T.; Azad, M.B.; Sears, M.R.; Becker, A.B.; Turvey, S.E.; Mandhane, P.J.; Subbarao, P.; CHILD Study Investigators; Scott, J.A.; et al. Infant gut immunity: A preliminary study of IgA associations with breastfeeding. J. Dev. Orig. Health Dis. 2016, 7, 68–72. [Google Scholar] [CrossRef]
- Raj, P.A.; Dentino, A.R. Current status of defensins and their role in innate and adaptive immunity. FEMS Microbiol. Lett. 2002, 206, 9–18. [Google Scholar] [CrossRef]
- Van der Does, A.M.; Beekhuizen, H.; Ravensbergen, B.; Vos, T.; Ottenhoff, T.H.M.; Van Dissel, J.T.; Drijfhout, J.W.; Hiemstra, P.S.; Nibbering, P.H. LL-37 direct macrophage differentiation towards macrophages with a proinflammatory signature. J. Immunol. 2010, 185, 1442–1449. [Google Scholar] [CrossRef] [Green Version]
- Campeotto, F.; Baldasarre, M.; Laforgia, N.; Viallon, V.; Kalach, N.; Amati, L.; Butel, M.J.; Dupont, C.; Kapel, N. Fecal expression of human β-defensin-2 following birth. Neonatology 2010, 98, 365–369. [Google Scholar] [CrossRef]
- Molès, J.P.; Tuaillon, E.; Kankasa, C.h.; Bedin, A.S.; Nagot, N.; Marchant, A.; McDermid, J.M.; Van de Perre, P. Breastmilk cell trafficking induces microchimerism-mediated immune system maturation in the infant. Pediatr. Allergy Immunol. 2018, 29, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Kolyva, S.; Triga, M.; Kritikou, D.; Chrysis, D. The effect of feeding patterns on serum zonulin levels in infants at 3-4 months of age. Eur. J. Pediatr. 2021, 180, 3273–3278. [Google Scholar] [CrossRef] [PubMed]
- Weström, B.; Sureda, E.A.; Pierzynowska, K.; Pierzynowski, S.; Pérez-Cano, F.J. The immature gut barrier and its importance in establishing immunity in newborn mammals. Front. Immunol. 2020, 11, 1153. [Google Scholar] [CrossRef] [PubMed]
- Kalbermatter, C.; Trigo, N.F.; Christensen, S.; Ganal-Vonarburg, S.S. Maternal microbiota, early life colonization and breast milk drive immune development in the newborn. Front. Immunol. 2021, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Goenka, A.; Kollmann, T.R. Development of immunity in early life. J. Infect. 2015, 71, S112–S120. [Google Scholar] [CrossRef]
- Fabiano, V.; Indrio, F.; Verducci, E.; Calacaterra, V.; Pop, T.L.; Mari, A.; Zuccotti, G.V.; Cokugras, F.C.; Pettoello-Mantovani, M.; Goulet, O. Term infant formulas influencing gut microbiota, an overview. Nutrients 2021, 13, 4200. [Google Scholar] [CrossRef]
- Selma-Royo, M.; Garcia-Mantrana, I.; Calatayud, M.; Parra-Llorca, A.; Martinez-Costa, C.; Collado, M.C. Maternal diet during pregnancy and intestinal markers are associated with early gut microbiota. Eur. J. Nutr. 2021, 60, 1429–1442. [Google Scholar] [CrossRef]
- Bolte, E.E.; Moorshead, D.; Aagaard, K.M. Maternal and early life exposures and their potential to influence development of the microbiome. Genome Med. 2022, 14, 4. [Google Scholar] [CrossRef]
- Verducci, E.; DÁuria, E.; Bosetti, A.; DI Profio, E.; Vizzuso, S.; Milanta, C.; Pendezza, E.; Borsani, B.; Zuccotti, G.V. Immunomodulatory diet in pedriatic age. Minerva Pediatr. 2021, 73, 128–149. [Google Scholar]
- Kapel, N.; Benahmed, N.; Morali, A.; Svahn, J.; Canioni, D.; Goulet, O.; Ruemmele, F.M. Fecal beta-defensin-2 in children with inflammatory bowel diseases. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 117–120. [Google Scholar] [CrossRef]
- Corebima, B.I.R.V.; Rohsiswatmo, R.; Gavatri, P.; Patole, S. Fecal human β-defensin-2 (hBD-2) levels and gut microbiota patterns in preterm neonates with different feeding patterns. Iran J. Microbiol. 2019, 11, 151–159. [Google Scholar] [PubMed]
- Scholtens, P.A.M.J.; Alliet, P.; Raes, M.; Alles, M.S.; Kroes, H.; Boehm, G.; Knippels, L.M.J.; Knol, J.; Vandenplas, Y. Fecal secretory immunoglobulin A is increased in healthy infants who receive a formula with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides. J. Nutr. 2008, 138, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.; Pires, E.; et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity 2019, 50, 432–445.e7. [Google Scholar] [CrossRef] [Green Version]
- Venegas, D.P.; De la Fuente, M.K.; Landskron, G.; Gonzalez, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SFCAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Studies | |||
|---|---|---|---|
| Characteristic | Corsello et al., 2017 | Nocerino et al., 2017 | Roggero et al., 2020 |
| Target group | healthy children | healthy children | healthy newborns |
| Age | 12–48 months | 12–48 months | 0–7 days |
| Number of participants | postbiotic: 66; control 60 | postbiotic: 137; control 122 | postbiotic: 13; control 13 |
| Male percentage | postbiotic: 53; control 62 | postbiotic: 51; control 51 | postbiotic: 46; control 24 |
| Postbiotic | fermented cow’s milk | fermented cow’s milk | fermented cow’s milk |
| Placebo | carbohydrate controlled | carbohydrate controlled | carbohydrate controlled |
| Energy (kcal) | postbiotic: 367; control: 388 | postbiotic: 367; control: 388 | postbiotic: 367; control: 388 |
| -proteins | |||
| (g/100 g product) | postbiotic: 24; control: 0 | postbiotic: 24; control: 0 | postbiotic: 24; control: 0 |
| -carbohydrates | |||
| (g/100 g product) | postbiotic: 66; control: 97 | postbiotic: 66; control: 97 | postbiotic: 66; control: 97 |
| -fat | |||
| (g/100 g product) | postbiotic: 0.6; control: 0.0 | postbiotic: 0.6; control: 0.0 | postbiotic: 0.6; control: 0.0 |
| Duration (months) | 3 | 3 | 3 |
| Number of lefts | 3 | 1 | 1 |
| Roggero et al. | Placebo | Postbiotic | |||||||
| Parameter | Mean | s.d. | N | s.e.m. | Mean | s.d. | N | s.e.m. | p-Value |
| α-defensin (ng/g) | 2.23 | 1.98 | 24 | 0.41 | 2.21 | 1.94 | 25 | 0.39 | 0.97 |
| β-defensin (ng/g) | 58.92 | 29.09 | 24 | 5.94 | 65.44 | 29.32 | 25 | 5.86 | 0.44 |
| Cathelicidin (ng/g) | 2.66 | 2.03 | 24 | 0.41 | 2.47 | 1.48 | 25 | 0.3 | 0.71 |
| s-IgA (μg/g) | 31.1 | 39.54 | 24 | 8.07 | 25.32 | 19.4 | 25 | 3.88 | 0.52 |
| Corsello/Nocerino | Placebo | Postbiotic | |||||||
| Parameter | Mean | s.d. | N | s.e.m. | Mean | s.d. | N | s.e.m. | p-Value |
| α-defensin (ng/g) | 1.01 | 1.04 | 73 | 0.12 | 1.01 | 1.18 | 60 | 0.15 | 0.98 |
| β-defensin (ng/g) | 31.18 | 22.03 | 80 | 2.46 | 29.36 | 21.25 | 60 | 2.74 | 0.62 |
| Cathelicidin (ng/g) | 12.97 | 10.29 | 80 | 1.15 | 16.93 | 19.61 | 64 | 2.45 | 0.15 |
| s-IgA (μg/g) | 23.12 | 19.99 | 86 | 2.16 | 23.01 | 18.1 | 69 | 2.18 | 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calame, W.; van Olderen, D.; Calabretta, V.; Bottari, L.; Paparo, L.; Bruno, C.; Carucci, L.; Voto, L.; Coppola, S.; Budelli, A. Baseline Concentrations of Various Immune Biomarkers Determine Their Increase after Consumption of a Postbiotic Based on Cow’s Milk Fermented with Lactobacillus paracasei CBA L74 in Both Newborns and Young Children. Appl. Sci. 2022, 12, 2009. https://doi.org/10.3390/app12042009
Calame W, van Olderen D, Calabretta V, Bottari L, Paparo L, Bruno C, Carucci L, Voto L, Coppola S, Budelli A. Baseline Concentrations of Various Immune Biomarkers Determine Their Increase after Consumption of a Postbiotic Based on Cow’s Milk Fermented with Lactobacillus paracasei CBA L74 in Both Newborns and Young Children. Applied Sciences. 2022; 12(4):2009. https://doi.org/10.3390/app12042009
Chicago/Turabian StyleCalame, Wim, Dick van Olderen, Veruska Calabretta, Luca Bottari, Lorella Paparo, Cristina Bruno, Laura Carucci, Luana Voto, Serena Coppola, and Andrea Budelli. 2022. "Baseline Concentrations of Various Immune Biomarkers Determine Their Increase after Consumption of a Postbiotic Based on Cow’s Milk Fermented with Lactobacillus paracasei CBA L74 in Both Newborns and Young Children" Applied Sciences 12, no. 4: 2009. https://doi.org/10.3390/app12042009
APA StyleCalame, W., van Olderen, D., Calabretta, V., Bottari, L., Paparo, L., Bruno, C., Carucci, L., Voto, L., Coppola, S., & Budelli, A. (2022). Baseline Concentrations of Various Immune Biomarkers Determine Their Increase after Consumption of a Postbiotic Based on Cow’s Milk Fermented with Lactobacillus paracasei CBA L74 in Both Newborns and Young Children. Applied Sciences, 12(4), 2009. https://doi.org/10.3390/app12042009






