Similarities and Differences in Quorum Sensing-Controlled Bioluminescence between Photobacterium phosphoreum T3 and Vibrio qinghaiensis sp.-Q67
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria and Culture
2.2. Growth and Bioluminescence Measurements
2.3. Quantification of Relative Expression Levels of the Selected Luminant Gene
2.4. Preparation of Crude Extract from Cell-Free Supernatants
2.5. Influence of Crude Extract on Bacterial Luminescence
2.6. Statistical Analysis
3. Results and Discussion
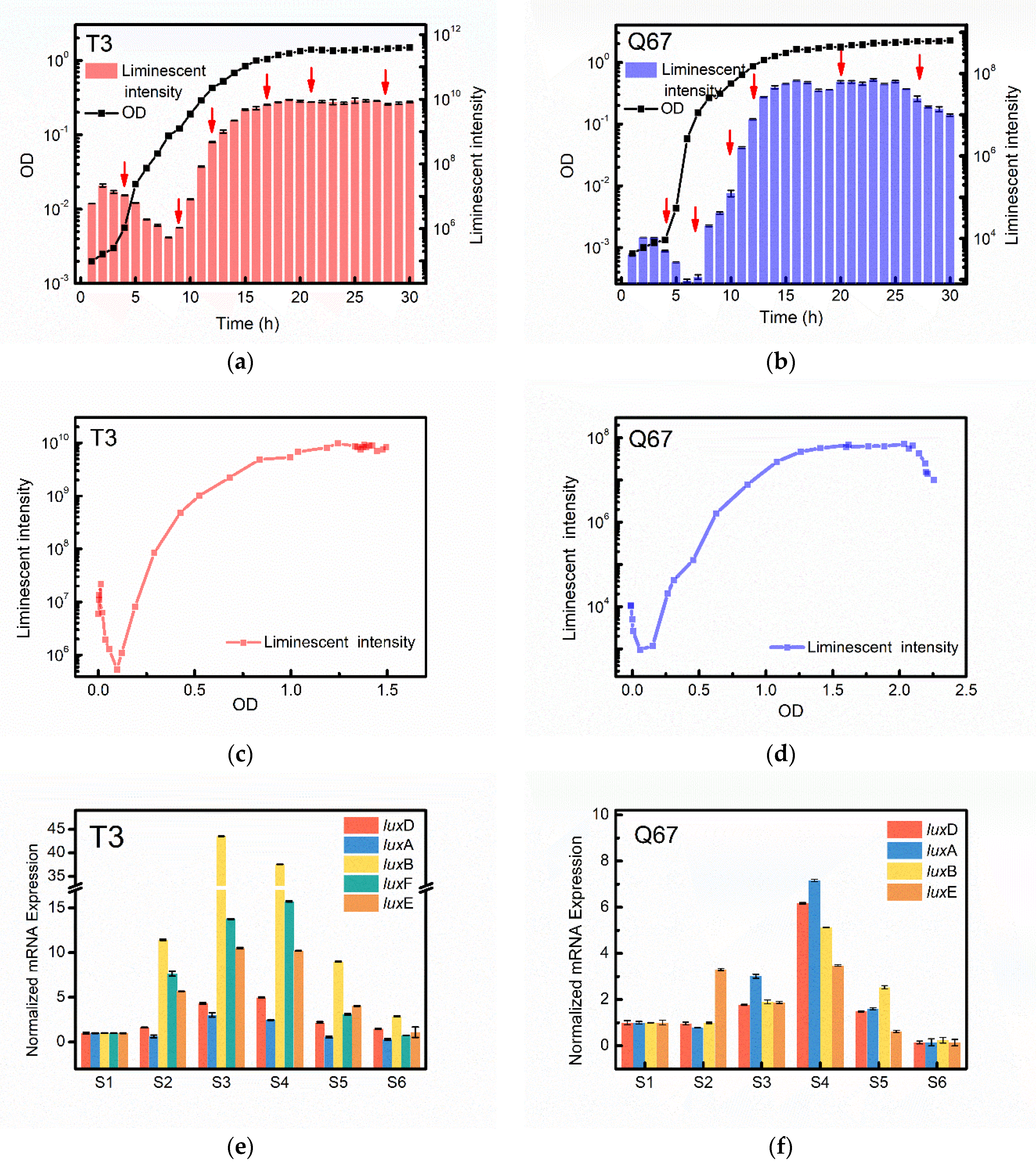
3.1. The Genes Associated with Luminescence in P. phosphoreum and V. qinghaiensis
3.2. Similarities in Cell-Density-Dependent Control of Bioluminescence between T3 and Q67
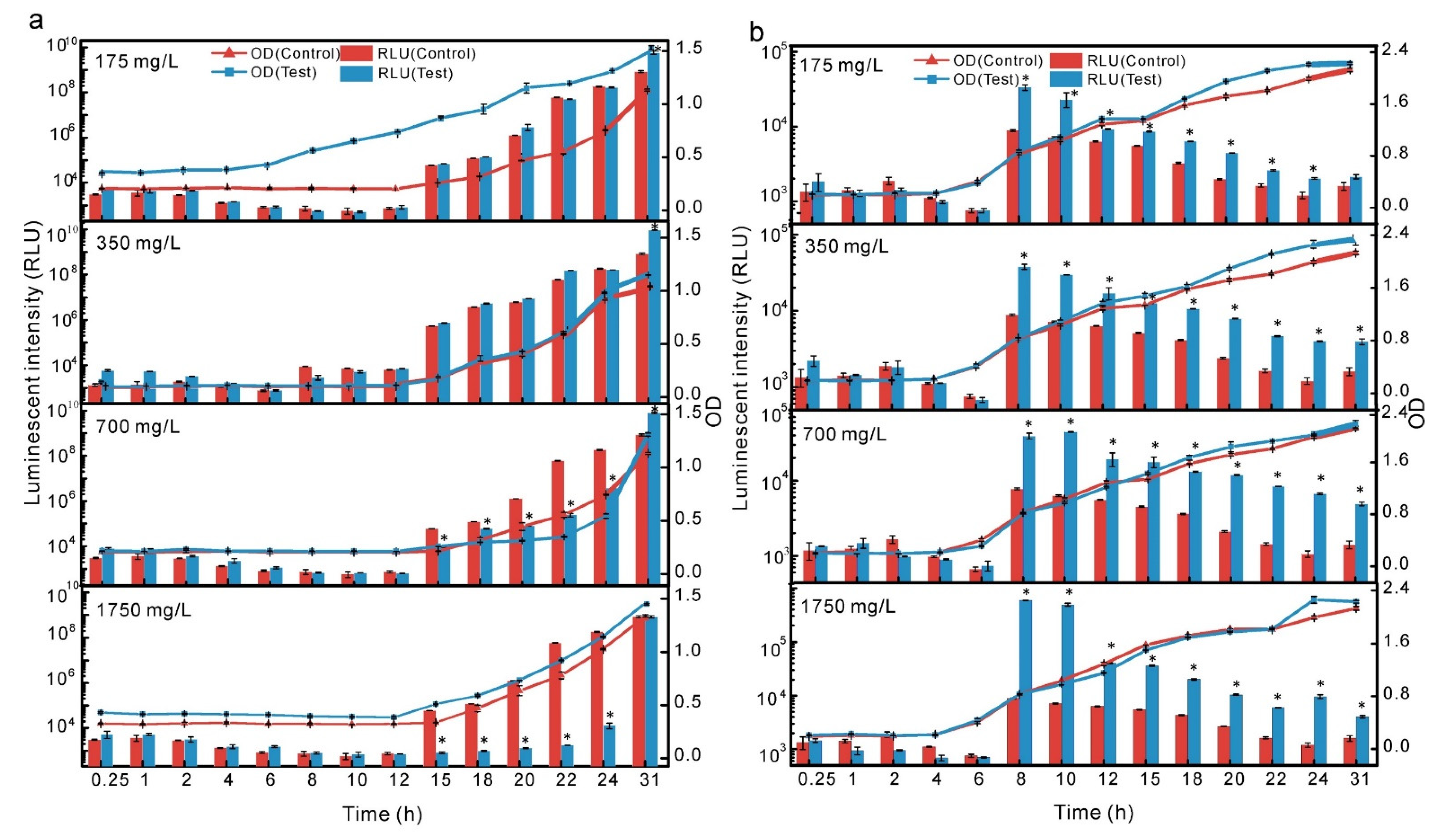
3.3. Differences in the Stimulations of Crude Culture Extract between T3 and Q67
3.4. Toxicological Significance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mukherjee, S.; Bassler, B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Chen, J.; Yang, C.; Yin, Y.; Yao, K. Quorum Sensing: A Prospective Therapeutic Target for Bacterial Diseases. Biomed Res. Int. 2019, 2019, 2015978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiteley, M.; Diggle, S.P.; Greenberg, E.P. Progress in and promise of bacterial quorum sensing research. Nature 2017, 551, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [Green Version]
- Kimbrough, J.H.; Stabb, E.V. Comparative analysis reveals regulatory motifs at the ainS/ainR pheromone-signaling locus of Vibrio fischeri. Sci. Rep. 2017, 7, 11734. [Google Scholar] [CrossRef]
- Watve, S.; Barrasso, K.; Jung, S.A.; Davis, K.J.; Hawver, L.A.; Khataokar, A.; Palaganas, R.G.; Neiditch, M.B.; Perez, L.J.; Ng, W.L. Parallel quorum-sensing system in Vibrio cholerae prevents signal interference inside the host. PLoS Pathog. 2020, 16, e1008313. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [Green Version]
- Abisado, R.G.; Benomar, S.; Klaus, J.R.; Dandekar, A.A.; Chandler, J.R. Bacterial Quorum Sensing and Microbial Community Interactions. mBio 2018, 9, e02331-17. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Wang, S.; Bai, L.; Nasir, M.S.; Li, S.; Yan, W. Mathematical Modeling Approaches for Assessing the Joint Toxicity of Chemical Mixtures Based on Luminescent Bacteria: A Systematic Review. Front. Microbiol. 2020, 11, 1651. [Google Scholar] [CrossRef]
- Zhu, W.J.; Wang, J.; Chen, X.Y.; Zhaxi, C.R.; Yang, Y.; Song, Y. A new species of luminous bacteria Vibrio qinghaiensis sp. Oceanol. Limnol. Sin. 1994, 25, 273–279. (In Chinese) [Google Scholar]
- Wang, Z.J.; Chen, F.; Xu, Y.Q.; Huang, P.; Liu, S.S. Protein Model and Function Analysis in Quorum-Sensing Pathway of Vibrio qinghaiensis sp.-Q67. Biology 2021, 10, 638. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, C.; Shi, J.; Chen, L. Evaluation on joint toxicity of chlorinated anilines and cadmium to Photobacterium phosphoreum and QSAR analysis. J. Hazard. Mater. 2014, 279, 156–162. [Google Scholar] [CrossRef]
- Ma, X.Y.; Wang, X.C.; Liu, Y.J. Study of the variation of ecotoxicity at different stages of domestic wastewater treatment using Vibrio-qinghaiensis sp.-Q67. J. Hazard. Mater. 2011, 190, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Sharifian, S.; Homaei, A.; Hemmati, R.; Khajeh, K. Light emission miracle in the sea and preeminent applications of bioluminescence in recent new biotechnology. J. Photochem. Photobiol. B 2017, 172, 115–128. [Google Scholar] [CrossRef]
- Deeva, A.A.; Zykova, E.A.; Nemtseva, E.V.; Kratasyuk, V.A. Functional divergence between evolutionary-related LuxG and Fre oxidoreductases of luminous bacteria. Proteins 2019, 87, 723–729. [Google Scholar] [CrossRef] [Green Version]
- Hoepker, A.C.; Wang, A.; Le Marois, A.; Suhling, K.; Yan, Y.; Marriott, G. Genetically encoded sensors of protein hydrodynamics and molecular proximity. Proc. Natl. Acad. Sci. USA 2015, 112, E2569–E2574. [Google Scholar] [CrossRef] [Green Version]
- Gong, L.; Wu, Y.; Jian, Q.J.; Yin, C.X.; Li, T.T.; Gupta, V.K.; Duan, X.W.; Jiang, Y.M. Complete genome sequencing of the luminescent bacterium, Vibrio qinghaiensis sp. Q67 using PacBio technology. Sci. Data 2018, 5, 170205. [Google Scholar] [CrossRef] [Green Version]
- Tabib, C.R.; Brodl, E.; Macheroux, P. Evidence for the generation of myristylated FMN by bacterial luciferase. Mol. Microbiol. 2017, 104, 1027–1036. [Google Scholar] [CrossRef]
- Tanet, L.; Tamburini, C.; Baumas, C.; Garel, M.; Simon, G.; Casalot, L. Bacterial Bioluminescence: Light Emission in Photobacterium phosphoreum Is Not under Quorum-Sensing Control. Front. Microbiol. 2019, 10, 365. [Google Scholar] [CrossRef]
- Nealson, K.H.; Hastings, J.W. Bacterial bioluminescence: Its control and ecological significance. Microbiol. Rev. 1979, 43, 496–518. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, P. Biochemistry and genetics of bacterial bioluminescence. Adv. Biochem. Eng. Biotechnol. 2014, 144, 37–64. [Google Scholar] [CrossRef] [PubMed]
- Bergner, T.; Tabib, C.R.; Winkler, A.; Stipsits, S.; Kayer, H.; Lee, J.; Malthouse, J.P.; Mayhew, S.; Muller, F.; Gruber, K.; et al. Structural and biochemical properties of LuxF from Photobacterium leiognathi. Biochim. Biophys. Acta 2015, 1854, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Visick, K.G.; Ruby, E.G. Construction and symbiotic competence of a luxA-deletion mutant of Vibrio fischeri. Gene 1996, 175, 89–94. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Hou, J.; Wang, P.; You, G.; Miao, L. Mechanistic understanding of cerium oxide nanoparticle-mediated biofilm formation in Pseudomonas aeruginosa. Environ. Sci. Pollut. Res. Int. 2018, 25, 34765–34776. [Google Scholar] [CrossRef]
- Lv, L.; Feng, C.; Li, W.; Zhang, G.; Wang, P.; Ren, Z. Exogenous N-acyl-homoserine lactones promote the degradation of refractory organics in oligotrophic anaerobic granular sludge. Sci. Total Environ. 2021, 761, 143289. [Google Scholar] [CrossRef]
- Ren, T.T.; Yu, H.Q.; Li, X.Y. The quorum-sensing effect of aerobic granules on bacterial adhesion, biofilm formation, and sludge granulation. Appl. Microbiol. Biotechnol. 2010, 88, 789–797. [Google Scholar] [CrossRef] [Green Version]
- Perez, P.D.; Hagen, S.J. Heterogeneous response to a quorum-sensing signal in the luminescence of individual Vibrio fischeri. PLoS ONE 2010, 5, e15473. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.P.; Salisbury, V.C. Investigation of the mechanism by which sublethal erythromycin inhibits bacterial cell-cell communication. In Abstracts of the Interscience Conference on Antimicrobial Agents and Chemotherapy, 1st ed.; American Society for Microbiology: Chicago, IL, USA, 2003; p. 92. [Google Scholar]
- Schaefer, A.L.; Hanzelka, B.L.; Eberhard, A.; Greenberg, E.P. Quorum sensing in Vibrio fischeri: Probing autoinducer-LuxR interactions with autoinducer analogs. J. Bacteriol. 1996, 178, 2897–2901. [Google Scholar] [CrossRef] [Green Version]
- Hawver, L.A.; Jung, S.A.; Ng, W.L. Specificity and complexity in bacterial quorum-sensing systems. FEMS Microbiol. Rev. 2016, 40, 738–752. [Google Scholar] [CrossRef] [Green Version]
- Alav, I.; Sutton, J.M.; Rahman, K.M. Role of bacterial efflux pumps in biofilm formation. J. Antimicrob. Chemother. 2018, 73, 2003–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, R.; Unsal, T.; Xu, D.K.; Lekbach, Y.; Gu, T.Y. Microbiologically influenced corrosion and current mitigation strategies: A state of the art review. Int. Biodeterior. Biodegrad. 2019, 137, 42–58. [Google Scholar] [CrossRef]
- Shastry, R.P.; Rekha, P.D.; Rai, V.R. Biofilm inhibitory activity of metallo-protein AHL-lactonase from cell-free lysate of endophytic Enterobacter species isolated from Coscinium fenestratum Gaertn. Biocatal. Agric. Biotechnol. 2019, 18, 101009. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Dong, D.; Hu, H.; Wu, B.; Ren, H. AHLs-mediated quorum sensing threshold and its response towards initial adhesion of wastewater biofilms. Water Res. 2021, 194, 116925. [Google Scholar] [CrossRef]
- Hashmi, M.Z.; Naveedullah; Shen, H.; Zhu, S.; Yu, C.; Shen, C. Growth, bioluminescence and shoal behavior hormetic responses to inorganic and/or organic chemicals: A review. Environ. Int. 2014, 64, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Shen, C.; Lu, Y.; Tang, X.; Zhang, C.; Chen, X.; Shi, J.; Lin, Q.; Chen, Y. Hormesis response of marine and freshwater luminescent bacteria to metal exposure. Biol. Res. 2009, 42, 183–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Yao, Z.; Wang, D.; Wu, X.; Lin, Z.; Liu, Y. A deep insight into the toxic mechanism for sulfonamides based on bacterial cell-cell communication. Environ. Int. 2019, 129, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.Y.; Liu, Y.A.; Zhu, J.; Qin, L.T.; Liang, Y.P.; Zeng, H.H. Benefits from hazards, benefits from nothing, and benefits from benefits: The combined effects of five quaternary ammonium compounds to Vibrio qinghaiensis Q67. Environ. Sci. Eur. 2020, 32, 35. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.J.; Liu, S.S.; Yuan, J.; Liu, H.L. Remarkable hormesis induced by 1-ethyl-3-methyl imidazolium tetrafluoroborate on Vibrio qinghaiensis sp.-Q67. Chemosphere 2011, 84, 1440–1445. [Google Scholar] [CrossRef]
- Deng, Z.; Lin, Z.; Zou, X.; Yao, Z.; Tian, D.; Wang, D.; Yin, D. Model of hormesis and its toxicity mechanism based on quorum sensing: A case study on the toxicity of sulfonamides to Photobacterium phosphoreum. Environ. Sci. Technol. 2012, 46, 7746–7754. [Google Scholar] [CrossRef]

| Bacterium | Gene Upstream the luxCDAB(F)EG Operon | Location in Genomes | NCBI Accession Number | Fragment Size (nt) | EncoProtein Length (aa) |
|---|---|---|---|---|---|
| V. fischeri | luxI | 1048783-1049355 | HQ436485 | 573 | 190 |
| P. phosphoreum | luxR | 1049571-1050323 | L21989 | 753 | 250 |
| V. qinghaiensis | luxL | 1127275-1127847 | CP022742 REGION: 900787.901089 | 573 | 478 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Bai, L.; Li, S.; Yan, W. Similarities and Differences in Quorum Sensing-Controlled Bioluminescence between Photobacterium phosphoreum T3 and Vibrio qinghaiensis sp.-Q67. Appl. Sci. 2022, 12, 2066. https://doi.org/10.3390/app12042066
Wang D, Bai L, Li S, Yan W. Similarities and Differences in Quorum Sensing-Controlled Bioluminescence between Photobacterium phosphoreum T3 and Vibrio qinghaiensis sp.-Q67. Applied Sciences. 2022; 12(4):2066. https://doi.org/10.3390/app12042066
Chicago/Turabian StyleWang, Dan, Linming Bai, Shanshan Li, and Wei Yan. 2022. "Similarities and Differences in Quorum Sensing-Controlled Bioluminescence between Photobacterium phosphoreum T3 and Vibrio qinghaiensis sp.-Q67" Applied Sciences 12, no. 4: 2066. https://doi.org/10.3390/app12042066
APA StyleWang, D., Bai, L., Li, S., & Yan, W. (2022). Similarities and Differences in Quorum Sensing-Controlled Bioluminescence between Photobacterium phosphoreum T3 and Vibrio qinghaiensis sp.-Q67. Applied Sciences, 12(4), 2066. https://doi.org/10.3390/app12042066






