Over and beyond the Primate baubellum Surface: A “Jewel Bone” Shielded in Museums
Abstract
:1. Introduction
2. Materials and Methods
2.1. Female Genital Bone Sampling
2.1.1. Fresh Sample
2.1.2. Museum Samples
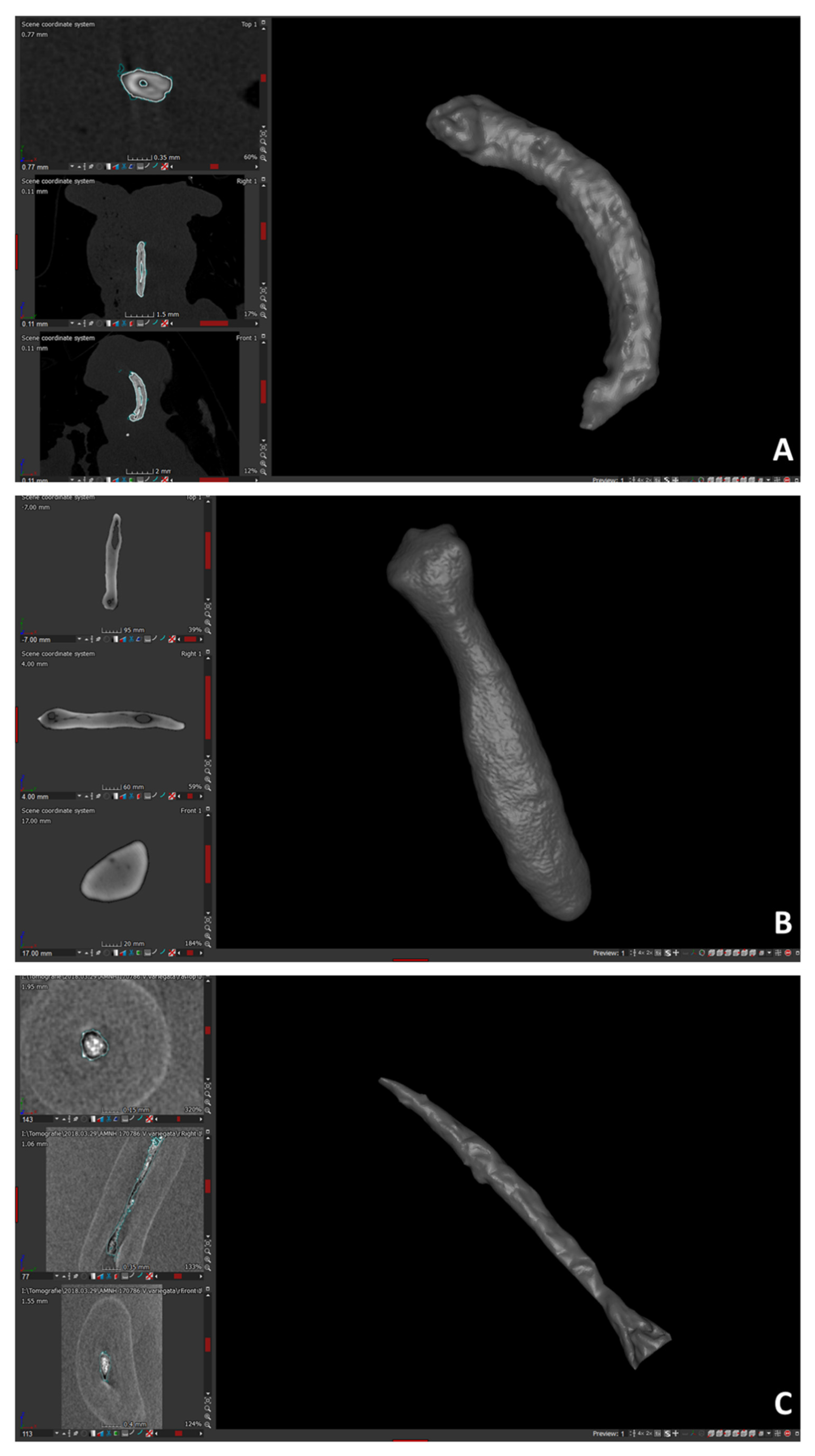
2.2. Anatomical Data Collection and 3D Morphological Data Acquisition
2.3. Morphological Analysis and Form Variation
2.3.1. Qualitative Analysis
2.3.2. Quantitative Analysis
- The product of segmentation (see ‘Anatomical data collection and 3D morphological data acquisition’ section) was extracted either as a 3D polygonal volumetric model (file format: ply) or mesh. The polygonal models were post-processed with Amira and Geomagic Studio 2014 software.
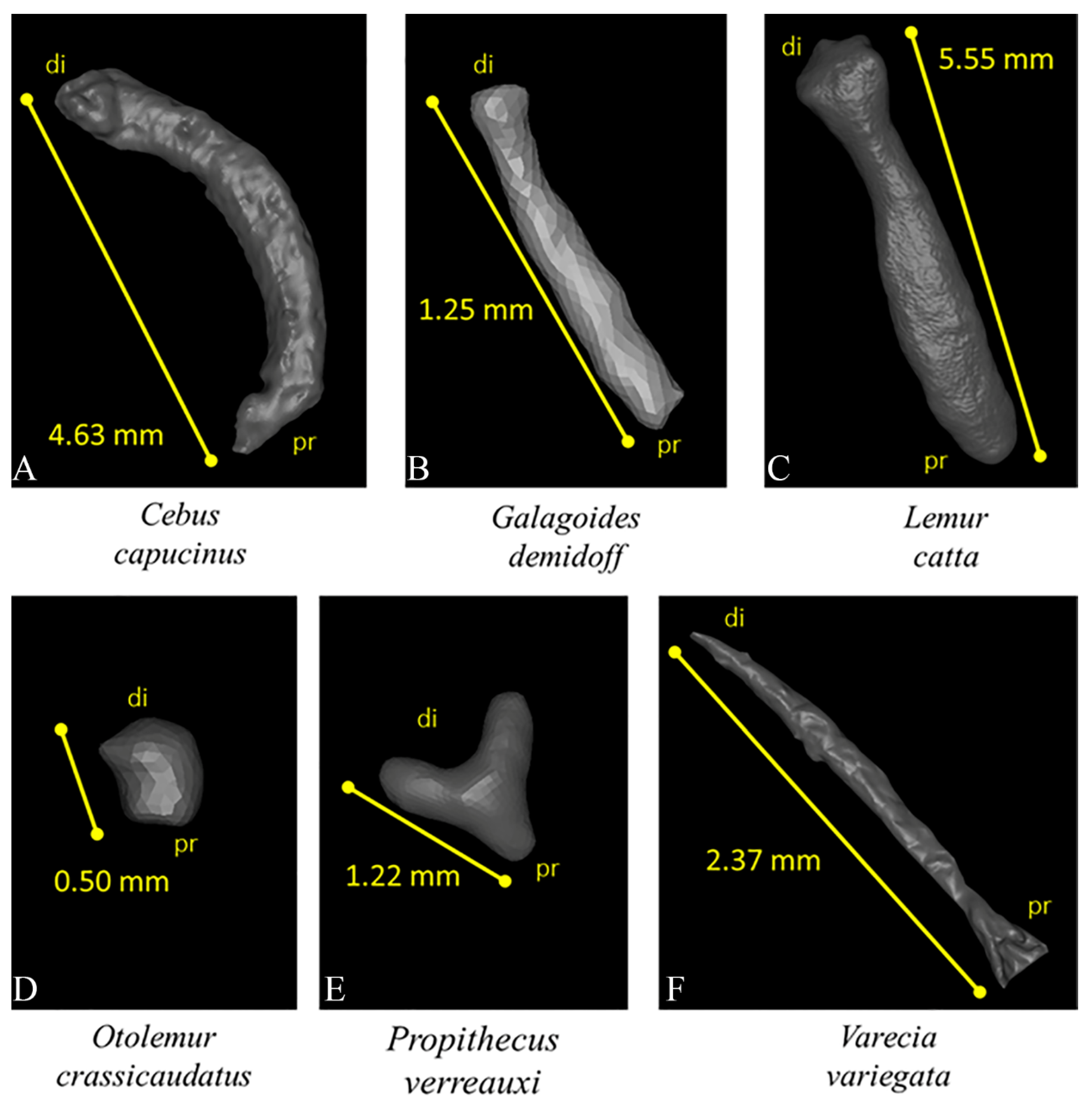
- The total number of polygons was taken down to 250,000 in Amira, and the extracted surface, loaded into Geomagic Studio 2014, was corrected by deleting (automatically and manually) computational errors in the mesh, such as non-manifold edges, self-intersections, highly creased edges, spikes, small components, small tunnels, and small holes. Only the external surface of bones was kept, by emptied 3D models, to avoid anatomical non-homologies in the bone’s internal structure. In addition, each baubellum length was measured by using the distal and proximal ends of the bones as reference points. The length was recorded 3 times by the same operator (FS), and the mean was calculated.
- The mesh was converted to a point cloud with Meshlab software, and the total number of points making up the cloud was set to 100,000.
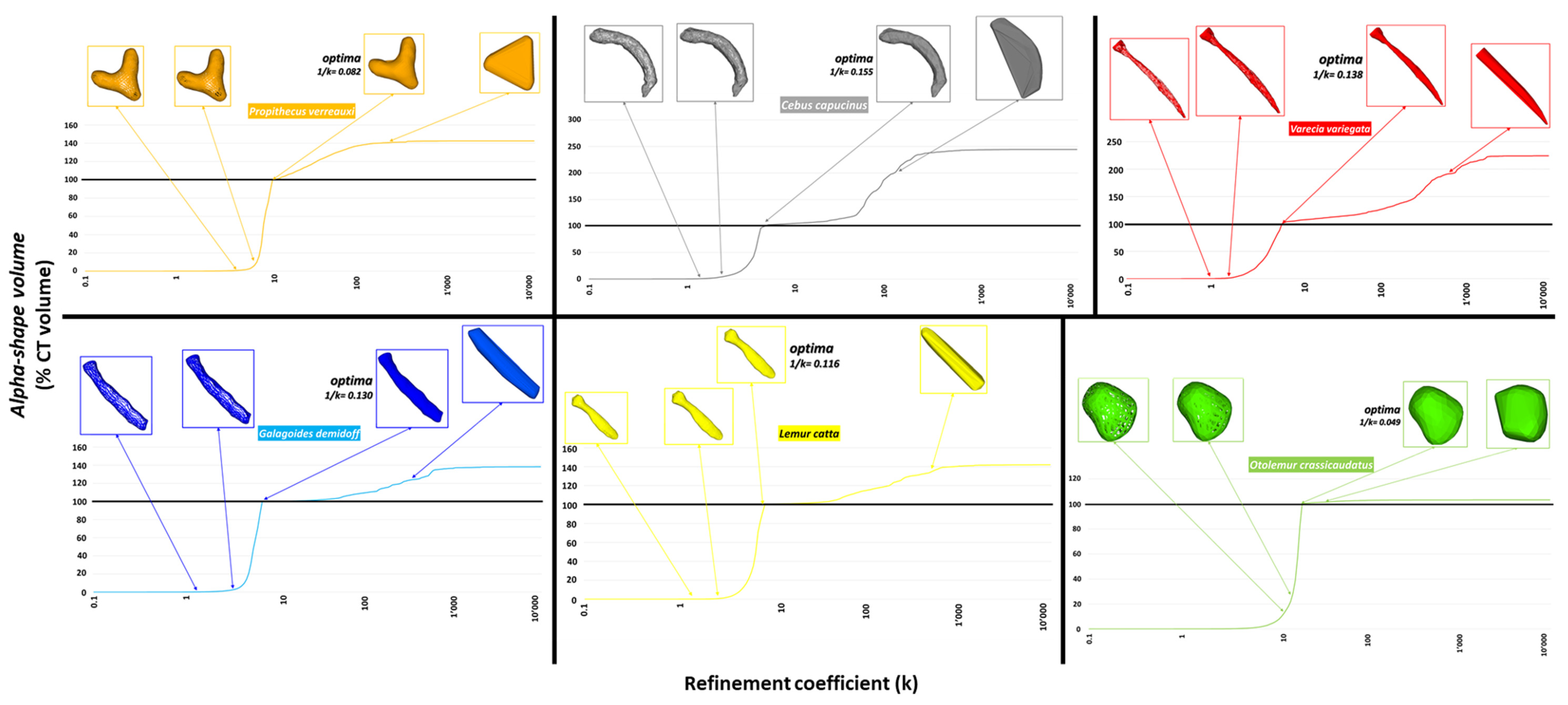
- An ‘alpha-shape’ is formed by the boundary of an alpha complex, which, in turn, is a sub-complex of the Delaunay triangulation for a given set of points [71]. For a given set of points in space, a family of ‘alpha-shapes’ can be defined, ranging from a conformation that fits the set of points very coarsely (forming a convex ‘shell’ around the points) to a conformation that fits very finely around the points. The following equation was used to calculate the typical radius α for each individual:where α is the radius, k is the refinement coefficient, and lref is the reference length (for more details see [62,63]).
- Since the present study is only interested in quantifying shape complexity regardless of size, all α-rays were scaled to the overall mesh size summarized by the lref factor. The size factor was then calculated as the average of the average distances of each point of the cloud from the 100 closest ones.
- The complexity of the baubella shapes analyzed in 3D was visualized by graphing the trend of a curve (representative of this morphological complexity) given by the ratio of the variables k to the percentage of the CT volume as described by the volume of the alpha-shapes; furthermore, morphological complexity was also quantified by the 1/k ratio, as suggested by Brassey and collaborators [63].
3. Results
3.1. 3-Step Protocol and baubellum Occurrence Data
3.2. Anatomical and 3D Morphological Data
3.3. Analysis of α-Shapes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Withers, P.J.; Bouman, C.; Carmignato, S.; Cnudde, V.; Grimaldi, D.; Hagen, C.K.; Maire, E.; Manley, M.; Du Plessis, A.; Stock, S.R. X-Ray Computed Tomography. Nat. Rev. Methods Primers 2021, 1, 18. [Google Scholar] [CrossRef]
- Mazansky, C. CT in the study of antiquities: Analysis of a basket-hilted sword relic from a 400-year-old shipwreck. Radiology 1993, 186, 55A–61A. [Google Scholar] [PubMed]
- Rossi, M.; Casali, F.; Chirco, P.; Morigi, M.P.; Nava, E.; Querzola, E.; Zanarini, M. X-ray 3D computed tomography of bronze archeological samples. IEEE Trans. Nucl. Sci. 1999, 46, 897–903. [Google Scholar] [CrossRef]
- Rossi, M.; Casali, F.; Bettuzzi, M.; Morigi, M.P.; Romani, D.; Golovkin, S.V.; Govorun, V.N. Experimental micro-CT system for X-ray NDT. Proc. SPIE 2001, 4503, 338–348. [Google Scholar]
- Applbaum, N.; Applbaum, Y.H. The use of medical computed tomography (CT) imaging in the study of ceramic and clay archaeological artifacts from the ancient near east. In X-rays for Archaeology; Uda, M., Demortier, G., Nakai, I., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 231–245. [Google Scholar]
- Casali, F. X-ray and neutron digital radiography and computed tomography for cultural heritage. In Physical Techniques in the Study of Art, Archaeology and Cultural Heritage; Bradley, D., Creagh, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 41–123. [Google Scholar]
- Freeth, T.; Bitsakis, Y.; Moussas, X.; Seiradakis, J.H.; Tselikas, A.; Mangou, H.; Zafeiropoulou, M.; Hadland, R.; Bate, D.; Ramsey, A. Decoding the ancient Greek astronomical calculator known as the Antikythera Mechanism. Nature 2006, 444, 587–591. [Google Scholar] [CrossRef]
- Morigi, M.P.; Casali, F.; Bettuzzi, M.; Brancaccio, R.; d’Errico, V. Application of X-ray computed tomography to cultural heritage diagnostics. Appl. Phys. A 2010, 100, 653–661. [Google Scholar] [CrossRef]
- Hughes, S. CT scanning in archeology. In Computed Tomography—Special Application; Saba, L., Ed.; InTech: Rijeka, Croatia, 2011; pp. 57–70. [Google Scholar]
- Re, A.; Albertin, F.; Bortolin, C.; Brancaccio, R.; Buscaglia, P.; Corsi, J.; Cotto, G.; Dughera, G.; Durisi, E.; Ferrarese, W. Results of the Italian neu_ART project. IOP Conf. Ser. Mater. Sci. Eng. 2012, 37, 012007. [Google Scholar] [CrossRef] [Green Version]
- Re, A.; Corsi, J.; Demmelbauer, M.; Martini, M.; Mila, G.; Ricci, C. X-ray tomography of a soil block: A useful tool for the restoration of archaeological finds. Herit. Sci. 2015, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pintus, R.; Pal, K.; Yang, Y.; Weyrich, T.; Gobbetti, E.; Rushmeier, H. A survey of geometric analysis in cultural heritage. CGF 2016, 35, 4–31. [Google Scholar] [CrossRef] [Green Version]
- Johnston, R.; Thomas, R.; Jones, R.; Graves-Brown, C.; Goodridge, W.; North, L. Evidence of diet, deification, and death within ancient Egyptian mummified animals. Sci. Rep. 2020, 10, 14113. [Google Scholar] [CrossRef]
- Albertin, F.; Bettuzzi, M.; Brancaccio, R.; Toth, M.B.; Baldan, M.; Morigi, M.P.; Casali, F. Inside the Construction Techniques of the Master Globe-Maker Vincenzo Coronelli. Microchem. J. 2020, 158, 105203. [Google Scholar] [CrossRef]
- Albertin, F.; Baumer, L.E.; Bettuzzi, M.; Brancaccio, R.; Caruso, E.; Casali, F.; Cifarelli, L.; Festa, G.; Griffo, M.G.; Mistretta, A.; et al. X-Ray Computed Tomography to Study Archaeological Clay and Wood Artefacts at Lilybaeum. Eur. Phys. J. Plus 2021, 136, 513. [Google Scholar] [CrossRef]
- Morigi, M.P.; Casali, F. Radiography and Computed Tomography for Works of Art. In Handbook of X-ray Imaging, Physics and Technology; Russo, P., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 1185–1210. [Google Scholar]
- Laycock, S.D.; Bell, G.D.; Mortimore, D.B.; Greco, M.K.; Corps, N.; Finkle, I. Combining x-ray micro-CT technology and 3D printing for the digital preservation and study of a 19th Century Cantonese chess piece with intricate internal structure. J. Comput. Cult. Herit. 2012, 5, 1–7. [Google Scholar] [CrossRef]
- Doney, E.; Krumdick, L.A.; Diener, J.M.; Wathen, C.A.; Chapman, S.E.; Stamile, B.; Scott, J.E.; Ravosa, M.J.; Van Avermaete, T.; Leevy, W.M. 3D printing of preclinical X-ray computed tomographic data sets. JoVE 2013, 73, 50250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawson, W.R.; Gray, P.H.K. Catalogue of Egyptian Antiquities in the British Museum 1: Mummies and Human Remains; The British Museum Press: London, UK, 1968. [Google Scholar]
- Marx, M.; D’Auria, S.H. CT examination of eleven Egyptian mummies. RadioGraph 1986, 6, 321–330. [Google Scholar] [CrossRef]
- Baldock, C.; Hughes, S.W.; Whittaker, D.K.; Taylor, J.; Davis, R.; Spencer, A.J.; Tonge, K.; Sofat, A. 3-D reconstruction of an ancient Egyptian mummy using X-ray computer tomography. J. R. Soc. Med. 1994, 87, 806–808. [Google Scholar]
- Hughes, S. Three-dimensional reconstruction of an ancient Egyptian mummy. In Imaging the Past; Higgins, T., Main, P., Lang, J., Eds.; British Museum Press: London, UK, 1996; Volume 114, pp. 211–225. [Google Scholar]
- Previgliano, C.H.; Ceruti, C.; Reinhard, J.; Araoz, F.A.; Diez, J.G. Radiologic evaluation of the Llullaillaco mummies. Am. J. Roentgenol. 2003, 181, 1473–1479. [Google Scholar] [CrossRef]
- Taylor, J. Mummy: The Inside Story; The British Museum Press: London, UK, 2004. [Google Scholar]
- Davis, R. Radiography: Archaeo-human and animal remains. Part I: Clinical radiography and archaeo-human remains. In Radiography of Cultural Material; Lang, J., Middleton, A., Eds.; Elsevier: Amsterdam, The Netherlands; Butterworth-Heinemann: Oxford, UK, 2005; pp. 130–149. [Google Scholar]
- Lynnerup, N. Medical imaging of mummies and bog bodies—A mini-review. Gerontology 2010, 56, 441–448. [Google Scholar] [CrossRef]
- Lynnerup, N.; Hjalgrim, H.; Nielsen, L.R.; Gregersen, H.; Thuesen, I. Non-invasive Archaeology of Skeletal Material by CT Scanning and Three-dimensional Reconstruction. Int. J. Osteoarch. 1997, 7, 91–94. [Google Scholar] [CrossRef]
- Wu, X.; Schepartz, L.A. Application of computed tomography in paleoanthropological research. Prog. Nat. Sci. 2009, 19, 913–921. [Google Scholar] [CrossRef]
- Anderson, T.; Fell, C. Analysis of Roman cremation vessels by computerized tomography. J. Arch. Sci. 1995, 22, 609–617. [Google Scholar] [CrossRef]
- Minozzi, S.; Giuffra, V.; Bagnoli, J.; Paribeni, E.; Giustini, D.; Caramella, D.; Fornaciari, G. An investigation of Etruscan cremations by Computed Tomography (CT). Antiquity 2010, 84, 195–201. [Google Scholar] [CrossRef]
- Harvig, L.; Lynnerup, N.; Ebsen, J.A. Computed tomography and computed radiography of late Bronze Age cremation urns from Denmark: An interdisciplinary attempt to develop methods applied in bioarchaeological cremation research. Archaeometry 2012, 54, 369–387. [Google Scholar] [CrossRef]
- Logan, H.; Wolfaardt, J.; Boulanger, P.; Hodgetts, B.; Seikaly, H. Evaluation of the accuracy of cone beam computerized tomography (CBCT): Medical imaging technology in head and neck reconstruction. J. Otolaryngol.—Head Neck Surg. 2013, 42, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanger, M.; Thostenson, J.; Hill, M.; Cain, H. Fibrous twists and turns: Early ceramic technology revealed through computed tomography. Appl. Phys. A 2013, 111, 829839. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Y.; Wang, C. A new 3D information acquisition method of micro-drilling marks on ancient perforated stone bead through micro-CT. J. X-ray Sci. Technol. 2011, 19, 333–343. [Google Scholar] [CrossRef]
- Haneca, K.; Deforce, K.; Boone, M.N.; Van Loo, D.; Dierick, M.; Van Acker, J.; Van den Bulcke, J. X-ray sub-micron tomography as a tool for the study of archaeological wood preserved through the corrosion of metal objects. Archaeometry 2012, 54, 893–905. [Google Scholar] [CrossRef]
- Bird, M.I.; Ascough, P.L.; Young, I.M.; Wood, C.V.; Scott, A.C. X-ray microtomographic imaging of charcoal. J. Archaeol. Sci. 2008, 35, 2698–2706. [Google Scholar] [CrossRef]
- Coubray, S.; Zech-Matterne, V.; Mazurier, A. The earliest remains of a Citrus fruit from a western Mediterranean archaeological context? A microtomographic-based re-assessment. Comptes. Rendus. Palevol. 2010, 9, 277–282. [Google Scholar] [CrossRef]
- Mensa, F.S.; Muzzi, M.; Spani, F.; Tromba, G.; Dullin, C.; Di Giulio, A. When the Utility of Micro-Computed Tomography Collides with Insect Sample Preparation: An Entomologist User Guide to Solve Post-Processing Issues and Achieve Optimal 3D Models. Appl. Sci. 2022, 12, 769. [Google Scholar] [CrossRef]
- Macchiarelli, R.; Bondioli, L.; Debénath, A.; Mazurier, A.; Tournepiche, J.F.; Birch, W.; Dean, M.C. How Neanderthal molar teeth grew. Nature 2006, 444, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Burrows, A.M.; Nash, L.T.; Hartstone-Rose, A.; Silcox, M.T.; López-Torres, S.; Selig, K.R. Dental signatures for exudativory in living primates, with comparisons to other gouging mammals. Anat. Rec. 2020, 303, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Andjelković, M.; Tomović, L.; Ivanović, A. Morphological integration of the kinetic skull in Natrix snakes. J. Zool. 2017, 303, 188–198. [Google Scholar] [CrossRef]
- Spani, F.; Morigi, M.P.; Bettuzzi, M.; Scalici, M.; Carosi, M. A 3D journey on virtual surfaces and inner structure of ossa genitalia in Primates by means of a non-invasive imaging tool. PLoS ONE 2020, 15, e0228131. [Google Scholar]
- Spani, F.; Morigi, M.P.; Bettuzzi, M.; Scalici, M.; Gentile, G.; Carosi, M. The ultimate database to (re) set the evolutionary history of primate genital bones. Sci. Rep. 2021, 11, 1–15. [Google Scholar]
- Kendall, D.G. Shape manifolds, procrustean metrics, and complex projective spaces. Bull. Lond. Math. 1984, 16, 81–121. [Google Scholar] [CrossRef] [Green Version]
- Bock, W.J. The definition and recognition of biological adaptation. Am. Zool. 1980, 20, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Gotthard, K.; Nylin, S. Adaptive plasticity and plasticity as an adaptation: A selective review of plasticity in animal morphology and life history. Oikos 1995, 74, 3–17. [Google Scholar] [CrossRef] [Green Version]
- O’Higgins, P.; Cobb, S.N.; Fitton, L.C.; Gröning, F.; Phillips, R.; Liu, J.; Fagan, M.J. Combining geometric morphometrics and functional simulation: An emerging toolkit for virtual functional analyses. J. Anat. 2011, 218, 3–15. [Google Scholar] [CrossRef]
- Dumont, M.; Wall, C.E.; Botton-Divet, L.; Goswami, A.; Peigné, S.; Fabre, A.-C. Do functional demands associated with locomotor habitat, diet, and activity pattern drive skull shape evolution in Musteloid carnivorans? Biol. J. Lin. Soc. 2016, 117, 858–878. [Google Scholar] [CrossRef] [Green Version]
- Scalici, M.; Spani, F.; Traversetti, L.; Carpaneto, G.M.; Piras, P. Cranial shape parallelism in soft-furred sengis: Moving on a geographic gradient. J. Mammal. 2018, 99, 1375–1386. [Google Scholar] [CrossRef]
- Spani, F.; Scalici, M.; Crandall, K.A.; Piras, P. Claw asymmetry in crabs: Approaching an old issue from a new point of view. Biol. J. Linn. Soc. 2020, 129, 162–176. [Google Scholar] [CrossRef]
- Spani, F.; Scalici, M. Carapace asymmetries in crabs. Crustaceana 2018, 91, 1281–1290. [Google Scholar] [CrossRef]
- Scalici, M.; Colamartino, M.; Spani, F.; Traversetti, L.; Persichini, T.; Maisano, M.; Fasulo, S.; Colasanti, M. Integrated early warning systems in marine bivalves reveal detrimental alterations of coastal habitats. Hydrobiologia 2020, 847, 2573–2585. [Google Scholar] [CrossRef]
- Scalici, M.; Traversetti, L.; Spani, F.; Bravi, R.; Malafoglia, V.; Persichini, T.; Colasanti, M. Using 3D virtual surfaces to investigate molluscan shell shape. Aquat. Living Resour. 2016, 29, 207. [Google Scholar] [CrossRef]
- Scalici, M.; Traversetti, L.; Spani, F.; Malafoglia, V.; Colamartino, M.; Persichini, T.; Cappello, S.; Mancini, G.; Guerriero, G.; Colasanti, M. Shell fluctuating asymmetry in the sea-dwelling benthic bivalve Mytilus galloprovincialis (Lamarck, 1819) as morphological markers to detect environmental chemical contamination. Ecotoxicology 2017, 26, 396–404. [Google Scholar] [CrossRef]
- Ray, R.P.; Nakata, T.; Henningsson, P.; Bomphrey, R.J. Enhanced flight performance by genetic manipulation of wing shape in drosophila. Nature Comm. 2016, 7, 10851. [Google Scholar] [CrossRef] [Green Version]
- Klein, L.L.; Caito, M.; Chapnick, C.; Kitchen, C.; O‘Hanlon, R.; Chitwood, D.H.; Miller, A.J. Digital Morphometrics of two north American grapevines (Vitis: Vitaceae) quantifies leaf variation between species, within species, and among Individuals. Front. Plant Sci. 2017, 8, 373. [Google Scholar] [CrossRef] [Green Version]
- Buchanan, B.; O’Brien, M.J.; Collard, M. Continent-wide or region-specific? A geometric morphometrics-based assessment of variation in Clovis point shape. Archaeol. Anthropol. Sci. 2014, 6, 145–162. [Google Scholar] [CrossRef]
- Ros, J.; Evin, A.; Bouby, L.; Ruas, M.-P. Geometric morphometric analysis of grain shape and the identification of two-rowed barley (Hordeum vulgare subsp. distichum L.) in southern France. J. Archaeol. Sci. 2014, 41, 568–575. [Google Scholar] [CrossRef] [Green Version]
- Ponton, D. Is geometric morphometrics efficient for comparing otolith shape of different fish species? J. Morphol. 2006, 267, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Weaver, A.A.; Schoell, S.L.; Stitzel, J.D. Morphometric analysis of variation in the ribs with age and sex. J. Anat. 2014, 225, 246–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frelat, M.A.; Katina, S.; Weber, G.W.; Bookstein, F.L. Technical note: A novel geometric morphometric approach to the study of long bone shape variation. Am. J. Phys. Anthrop. 2012, 149, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, J.D.; Behnsen, J.; Brassey, C.A. Alpha shapes: Determining 3D shape complexity across morphologically diverse structures. BMC Evol. Biol. 2018, 18, 184. [Google Scholar] [CrossRef] [PubMed]
- Brassey, C.A.; Behnsen, J.; Gardiner, J.D. Postcopulatory sexual selection and the evolution of shape complexity in the carnivoran baculum. Proc. Royal Soc. B 2020, 287, 20201883. [Google Scholar] [CrossRef] [PubMed]
- Arnqvist, G.; Thornhill, R.; Rowe, L. Evolution of animal genitalia: Morphological correlates of fitness components in a water strider. J. Evol. Biol. 1997, 10, 613–640. [Google Scholar] [CrossRef]
- Holwell, G.I.; Winnick, C.; Tregenza, T.; Herberstein, M.E. Genital shape correlates with sperm transfer success in the praying mantis Ciulfina klassi (Insecta: Mantodea). Behav. Ecol. Sociobiol. 2010, 64, 617–625. [Google Scholar] [CrossRef]
- Macagno, A.L.M.; Pizzo, A.; Parzer, H.F.; Palestrini, C.; Rolando, A.; Moczek, A.P. Shape—but not size—Codivergence between male and female copulatory structures in Onthophagus beetles. PLoS ONE 2011, 6, e28893. [Google Scholar] [CrossRef] [Green Version]
- Simmons, L.W.; Firman, R.C. Experimental evidence for the evolution of the mammalian baculum by sexual selection. Evolution 2013, 68, 276–283. [Google Scholar] [CrossRef] [Green Version]
- Hopwood, P.E.; Head, M.L.; Jordan, E.J.; Carter, M.J.; Davey, E.; Moore, A.J.; Royle, N.J. Selection on an antagonistic behavioral trait can drive rapid genital coevolution in the burying beetle, Nicrophorus vespilloides. Evolution 2016, 70, 1180–1188. [Google Scholar] [CrossRef] [Green Version]
- Pizzo, A.; Mercurio, D.; Palestrini, C.; Roggero, A.; Rolando, A. Male differentiation patterns in two polyphenic sister species of the genus Onthophagus Latreille, 1802 (Coleoptera: Scarabaeidae): A geometric morphometric approach. J. Zool. Syst. Evol. Res. 2006, 44, 54–62. [Google Scholar] [CrossRef]
- Dinca, V.; Dapporto, L.; Vila, R. A combined genetic-morphometric analysis unravels the complex biogeographical history of Polyommatus icarus and Polyommatus celina common blue butterflies. Mol. Ecol. 2011, 20, 3921–3935. [Google Scholar] [CrossRef] [PubMed]
- Edelsbrunner, H.; Mücke, E.P. Three-dimensional alpha shapes. ACM Trans. Graph. 1994, 13, 43–72. [Google Scholar] [CrossRef]
- Rohlf, F.J.; Slice, D. Extensions of the Procrustes method for the optimal superimposition of landmarks. Sys. Biol. 1990, 39, 40–59. [Google Scholar] [CrossRef] [Green Version]
- Hill, O.W.C. I-Strepsirrhini. In Primates-Comparative Anatomy and Taxonomy; University Press: Edinburgh, UK, 1953; p. 798. [Google Scholar]
- Harms, J.W. Primatologia—Handbook of Primatology; Hofer, H., Schultz, A.H., Starck, D., Eds.; Karger: Basel, Switzerland, 1956; Volume I, p. 1063. [Google Scholar]
- von Pehrson, T. Beiträge zur Kenntnis der äusseren weiblichen Genitalien bei Affen, Halbaffen, und Insectivoren. Anat. Anz. 1914, 46, 161–179. [Google Scholar]
- von Pohl, L. Beitrage zur Kenntnis des Os penis der Prosimier. Anat. Anz. 1910, 37, 225–231. [Google Scholar]
- Brindle, M.; Opie, C. Postcopulatory sexual selection influences baculum evolution in primates and carnivores. Proc. Royal Soc. B 2016, 283, 20161736. [Google Scholar] [CrossRef]
- Dixson, A.F. Observations on the evolution of the genitalia and copulatory behaviour in male primates. J. Zool. 1987, 213, 423–443. [Google Scholar] [CrossRef]
- Dixson, A.; Nyholt, J.; Anderson, M. A positive relationship between baculum length and prolonged intromission patterns in mammals. Acta Zool. Sin. 2004, 50, 490–503. [Google Scholar]
- Verrell, P.A. Primate penile morphologies and social systems: Further evidence for an association. Folia Primatol. 1992, 59, 114–120. [Google Scholar] [CrossRef]
- Ramm, S.A. Sexual selection and genital evolution in mammals: A phylogenetic analysis of baculum length. Am. Nat. 2007, 169, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Pomidor, B.J.; Makedonska, J.; Slice, D.E. A Landmark-Free Method for Three-Dimensional Shape Analysis. PLoS ONE 2016, 11, e0150368. [Google Scholar] [CrossRef] [PubMed]
- Besl, P.J.; McKay, N.D. Method for registration of 3-D shapes. Proc. SPIE 1992, 1611, 586–607. [Google Scholar] [CrossRef]
- Chen, Y.; Medioni, G. Object modelling by registration of multiple range images. Image Vis. Comput. 1992, 10, 145–155. [Google Scholar] [CrossRef]
- Dixson, A.F. Baculum length and copulatory behavior in primates. Am. J. Primatol. 1987, 13, 51–60. [Google Scholar] [CrossRef]
- Dixson, A.F. Primate Sexuality: Comparative Studies of the Prosimians, Monkeys, Apes, and Humans, 2nd ed; Oxford University Press: Oxford, UK, 2012; p. 808. [Google Scholar]
- Romer, S.A. The Vertebrate Body; Saunders: Philadelphia, PA, USA, 1962; p. 627. [Google Scholar]
- Hill, O.W.C. Evolutionary Biology of Primates; Academic Press: London, UK, 1972; p. 233. [Google Scholar]
- Hershkovitz, P. 14. External genitalia and accessory structures. In Living New World Monkeys (Platyrrhini) with an Introduction to Primates; Hershkovitz, P., Ed.; The University Chicago Press: Chicago, IL, USA, 1977; Volume 1, pp. 112–119. [Google Scholar]
- Hershkovitz, P. Male external genitalia of non-prehensile tailed South-American monkeys. Part, I. Subfamily Pitheciinae, Family Cebidae. Fieldiana Zool. 1993, 73, 1–17. [Google Scholar]
- Stockley, P. The baculum. Curr. Biol. 2012, 22, R1032–R1033. [Google Scholar] [CrossRef] [Green Version]
- Lough-Stevens, M.; Schultz, N.G.; Dean, M.D. The baubellum is more developmentally and evolutionarily labile than the baculum. Ecol. Evol. 2018, 8, 1073–1083. [Google Scholar] [CrossRef]
- Ah-King, M.; Barron, A.B.; Herberstein, M.E. Genital evolution: Why are females still understudied? PLoS Biol. 2014, 12, e1001851. [Google Scholar] [CrossRef] [Green Version]
- Estrada, A.; Garber, P.A.; Rylands, A.B.; Roos, C.; Fernandez-Duque, E.; Di Fiore, A.; Nekaris, K.A.I.; Nijman, V.; Heymann, E.W.; Lambert, J.E.; et al. Impending extinction crisis of the world’s primates: Why primates matter. Sci. Adv. 2017, 3, e1600946. [Google Scholar] [CrossRef] [Green Version]
- Carosi, M.; Spani, F.; Ulland, A.E.; Scalici, M.; Suomi, S.J. Clitoral length in immature and mature captive tufted capuchin (Sapajus spp.) females: A cross-sectional study. Am. J. Primatol. 2020, 82, e23135. [Google Scholar] [CrossRef] [PubMed]

| Samples | Manual Palpation | X-rays | Bone Extraction | Micro-CT | |
|---|---|---|---|---|---|
| Sample Preparation | Scan | ||||
| (1st step [42]) | (2nd step in [42]) | (3rd step in [42]) | (3rd step in [42]) | ||
| Fresh sample (Italy) | Double palpation along both mediolateral and anteroposterior axes performed by two operators (FS, MC) | 2D projectional radiography (X-ray plates) shot with Arcom Simply system for veterinary radiodiagnostics (20 ma—40 kV setting) (available at “Enrico Fermi” veterinary clinic, Rome, IT) | Sample positive to the presence of a genital bone was selected for manual dissection. For bone cleaning (tissues leftovers) and whitening, extracted bones were boiled for 30 min into a solution made of 50% oxidane (H2O) and 50% sodium hydrogen carbonate (NHCO3) | The fresh sample was immobilized in small pieces of polystyrene and placed on the high-precision rotary table to be scanned. The previous bone extraction guaranteed optimal 3D results (bones scanned directly without any surrounding soft tissues), and the 3D post-processing phase was subsequently simplified (see below) | Tomographic system * assembled at the Department of Physics and Astronomy of Bologna University (Italy). The settings used for micro-CT setup are reported in Table 2 |
| Museum wet samples (USA) | Same (performed only by FS) | X-rays performed directly by using the micro-CT scanner | Extraction not allowed | Each sample was packed into plastic bags and immobilized by using various kinds of supports for avoiding sample movements during scanning time due to alcohol evaporation | PHOENIX V|TOME|X S; PHOENIX V|TOME|X M. Settings used for micro-CT setup are reported in Table 2) |
| Settings | ||
|---|---|---|
| Parameters | (Italy) | (USA) |
| Voltage (kV) | 60–100 | auto |
| Beam current (µA) | 80–200 | auto |
| Al filter (mm) | no/1 | no/2 |
| N° projection | 900 | 1500–1800 |
| Total rotation angle | 360° | 360° |
| Exposure time (s) | 0.7–1 | 1 |
| Voxel size (µm) | 9.16–14 | 18 |
| Fresh Samples | Museum Samples | ||
|---|---|---|---|
| Step | T (min) | T (min) | |
| Manual Palpation | 5 | 2 | |
| X-rays | 10 | 3 | |
| Dissection | 60 | n/a | |
| Micro-CT | 215 | 65–95 | |
| a | alignment | 60 | 5 |
| b | scan | 75 | 30–60 |
| c | reconstruction | 60 | 20 |
| d | 3D rendering | 10 | 10 |
| T total | 290 | 70–100 | |
| Present Study Data | ||||||||
|---|---|---|---|---|---|---|---|---|
| Source | ID | Taxonomy | Age-Class | Specimen | Lit. | Palp. (1st Step) | X-ray (2nd Step) | Micro-CT (3rd Step) |
| NMNH | 257679 | Cebus capucinus | Adult | Clitoris | 1 | 1 | 1 | 1 |
| AMNH | 50984 | Galago demidoff | Adult | Whole-body | 1 | 1 | 1 | 1 |
| IZSLT | 22899 | Lemur catta | Adult | Clitoris | 1 | 0 | 1 | 1 |
| AMNH | 202613 | Otolemur crassicaudatus | Adult | Whole-body | 1 | 1 | 1 | 1 |
| AMNH | 31256 | Propithecus verreauxi | Adult | Whole-body | DD | 1 | 1 | 1 |
| AMNH | 170786 | Varecia variegata | Adult | Clitoris | 1 | 1 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spani, F.; Morigi, M.P.; Bettuzzi, M.; Scalici, M.; Carosi, M. Over and beyond the Primate baubellum Surface: A “Jewel Bone” Shielded in Museums. Appl. Sci. 2022, 12, 2096. https://doi.org/10.3390/app12042096
Spani F, Morigi MP, Bettuzzi M, Scalici M, Carosi M. Over and beyond the Primate baubellum Surface: A “Jewel Bone” Shielded in Museums. Applied Sciences. 2022; 12(4):2096. https://doi.org/10.3390/app12042096
Chicago/Turabian StyleSpani, Federica, Maria Pia Morigi, Matteo Bettuzzi, Massimiliano Scalici, and Monica Carosi. 2022. "Over and beyond the Primate baubellum Surface: A “Jewel Bone” Shielded in Museums" Applied Sciences 12, no. 4: 2096. https://doi.org/10.3390/app12042096
APA StyleSpani, F., Morigi, M. P., Bettuzzi, M., Scalici, M., & Carosi, M. (2022). Over and beyond the Primate baubellum Surface: A “Jewel Bone” Shielded in Museums. Applied Sciences, 12(4), 2096. https://doi.org/10.3390/app12042096








