The Effects of Photobioreactor Type on Biomass and Lipid Production of the Green Microalga Monoraphidium pusillum in Laboratory Scale
Abstract
:1. Introduction
- Are there significant changes of the main physical and chemical parameters in the two different types of photobioreactors in the same (laboratory) scale;
- Does culturing in a hybrid-type reactor result in the production of more biomass than using a conventional culturing method at the same (laboratory) scale;
- What is the efficiency of nutrient uptake in different types of photobioreactors in the same (laboratory) scale;
- How is the lipid composition of biomass influenced by the reactor type;
- Which technological parameters are the most important to ensure more efficient biomass and lipid production?
2. Materials and Methods
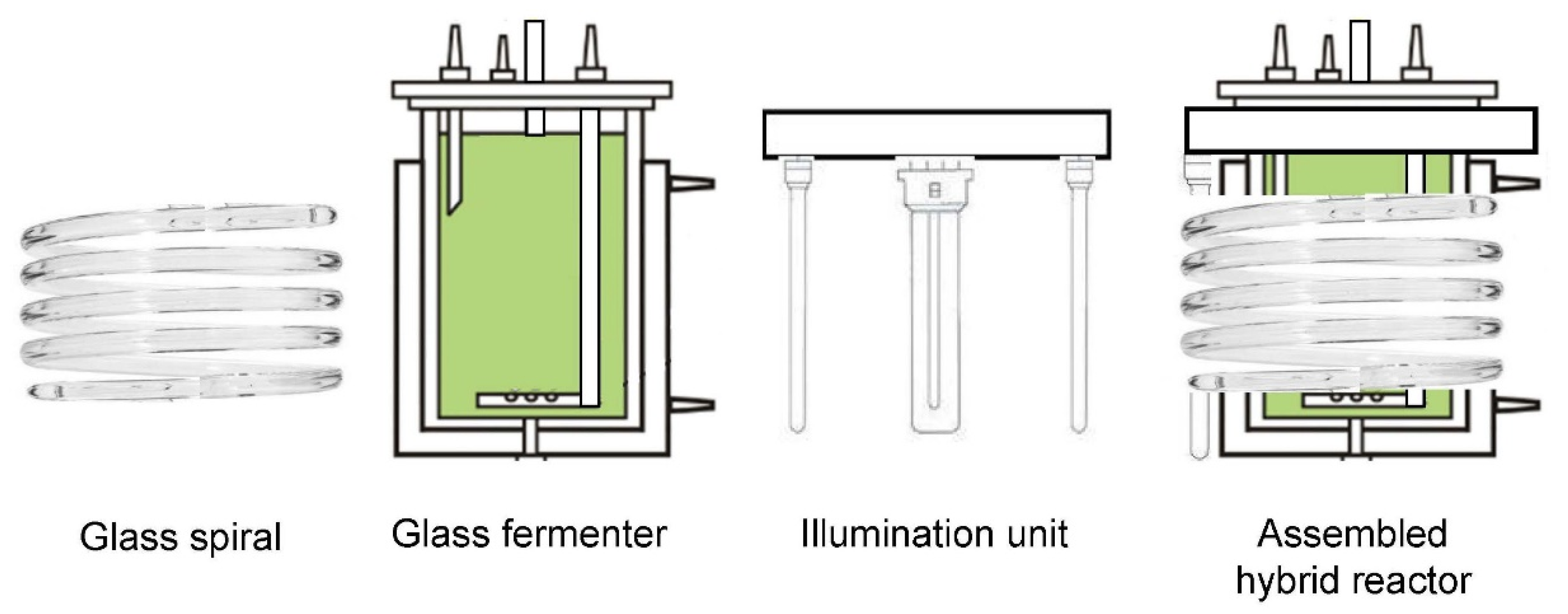
2.1. Algal Strain and Experimental Setup
2.2. Measurement of Culture Growth
2.3. Following of Nitrate and Phosphate Contents of the Cultures
2.4. Detection and Basic Analysis of Lipid-Content
2.5. Statistical Analysis
3. Results
3.1. Illumination Changes of the Hybrid Reactor
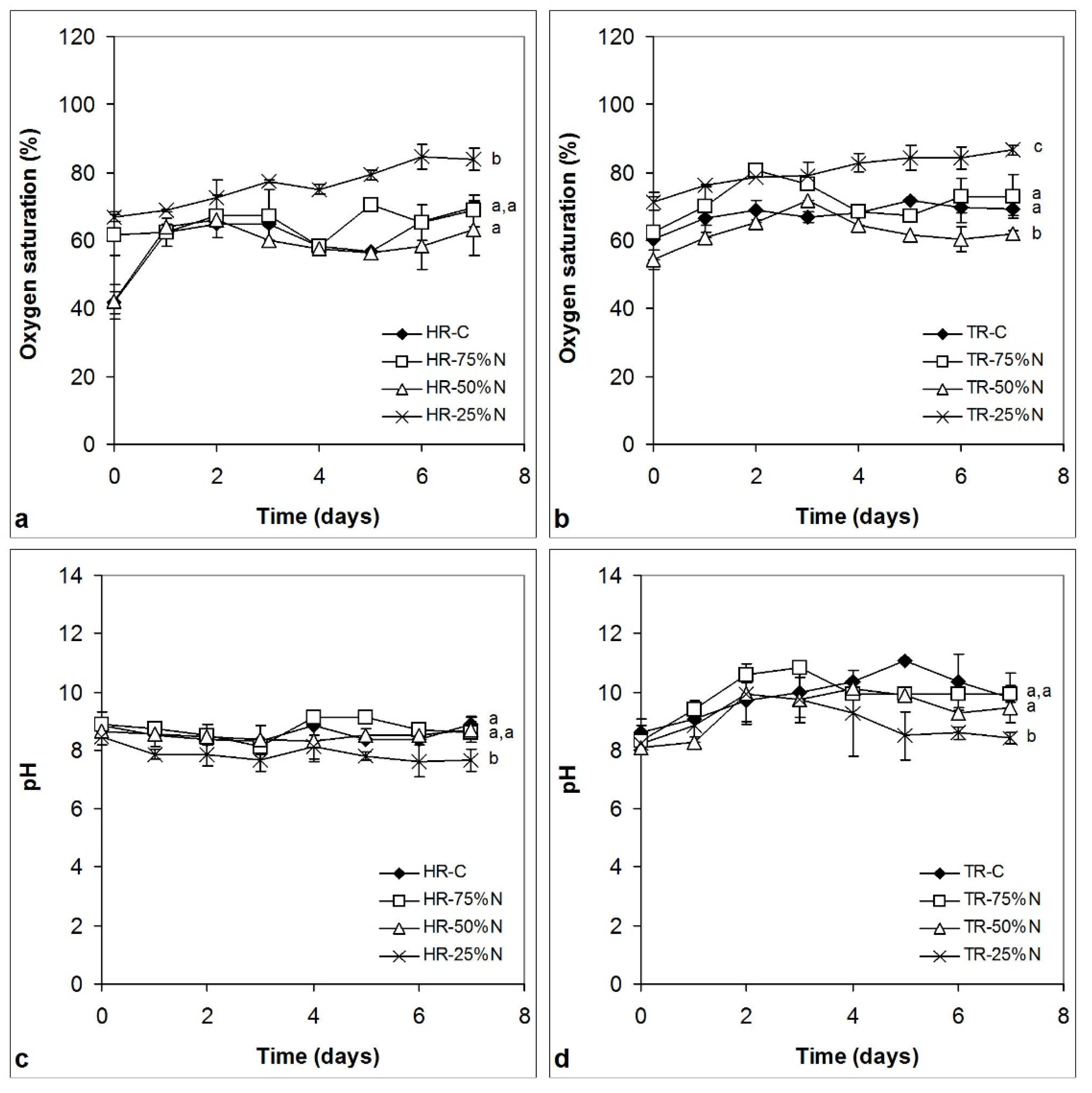
3.2. Changes of Oxygen Saturation and pH
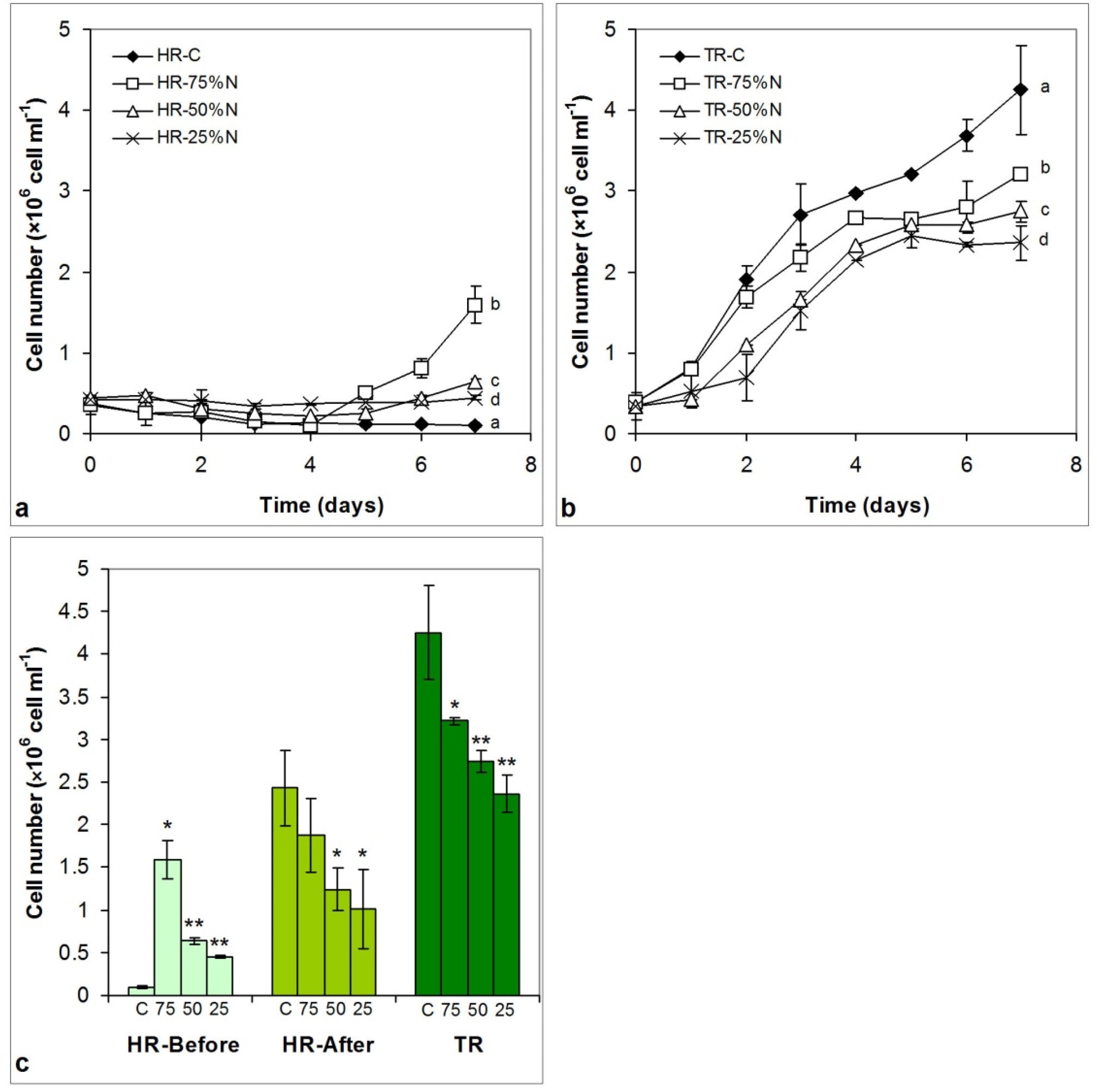
3.3. Growth of the Cultures
3.3.1. Cell Number Changes
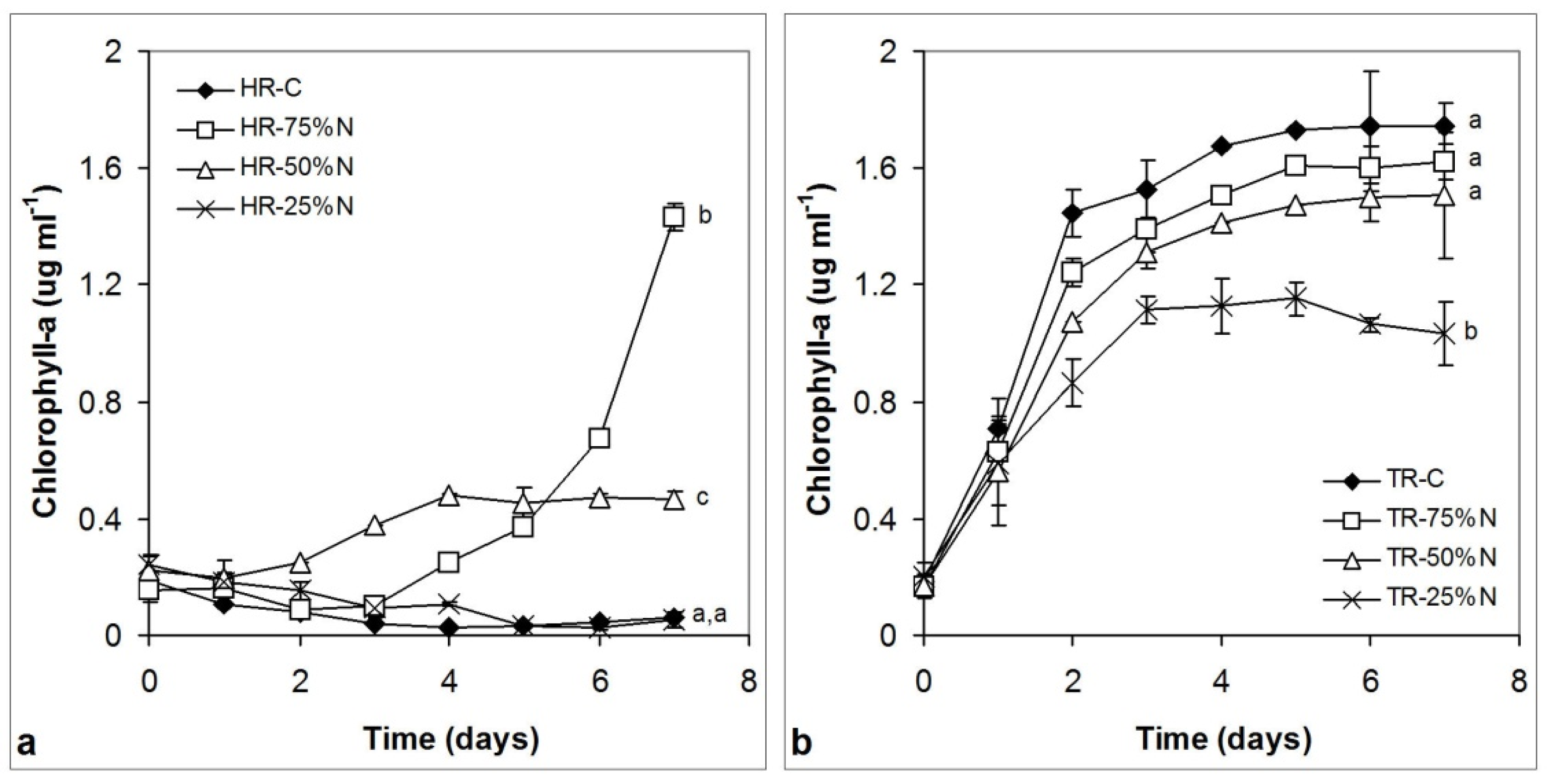
3.3.2. Chlorophyll Content Changes
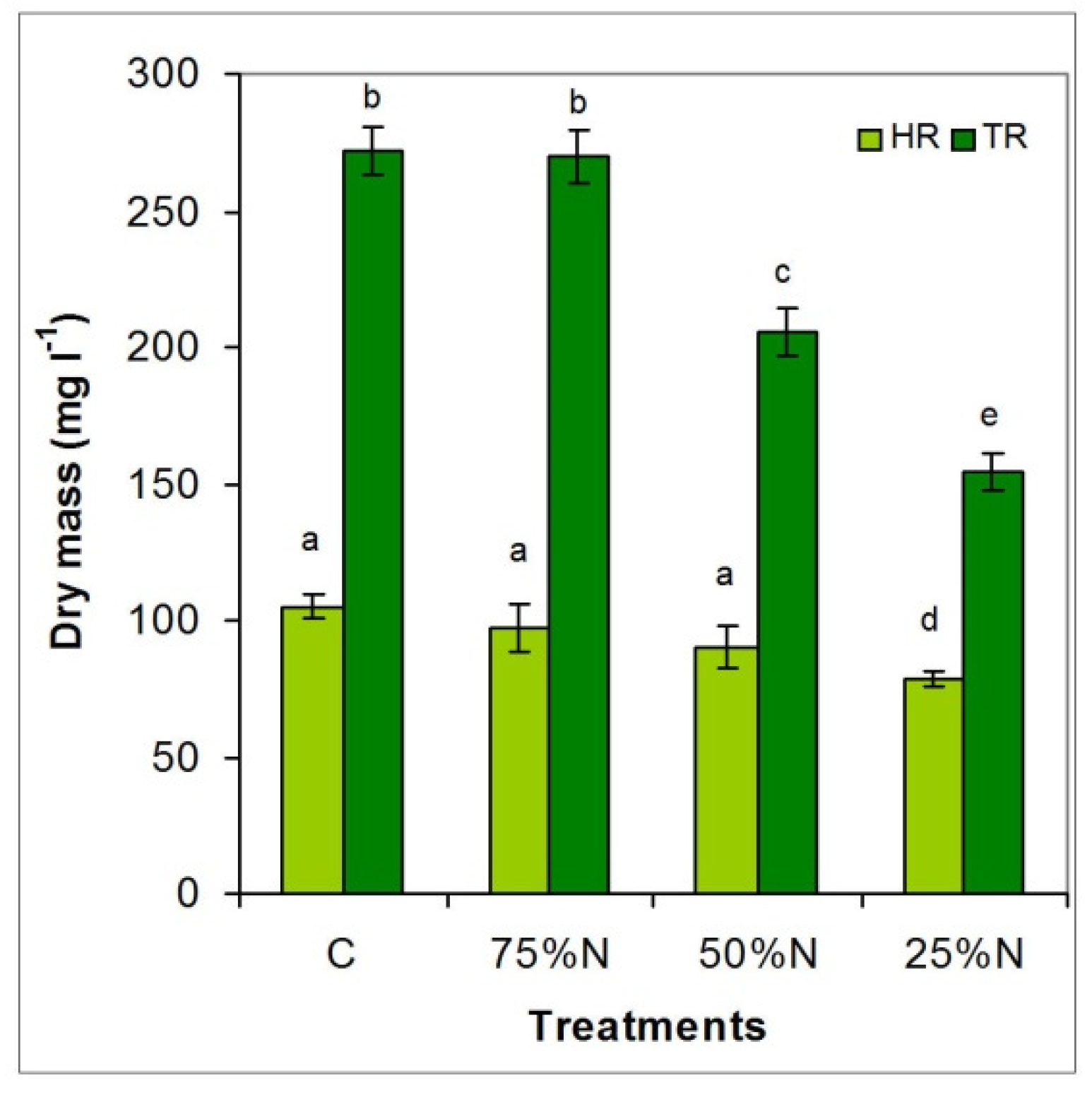
3.3.3. Differences in Net Dry Biomass Production
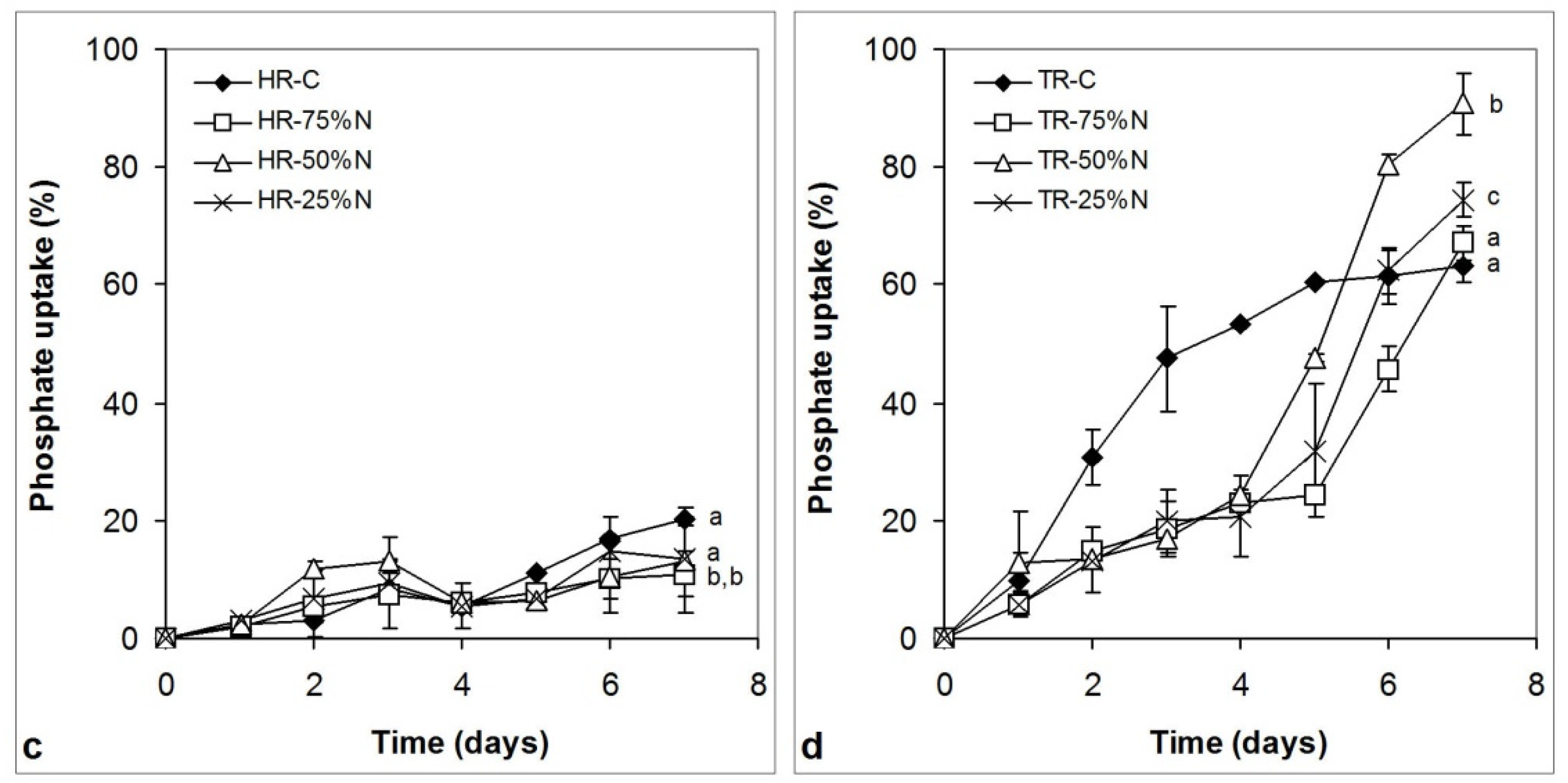
3.4. Nitrate and Phosphate Uptake
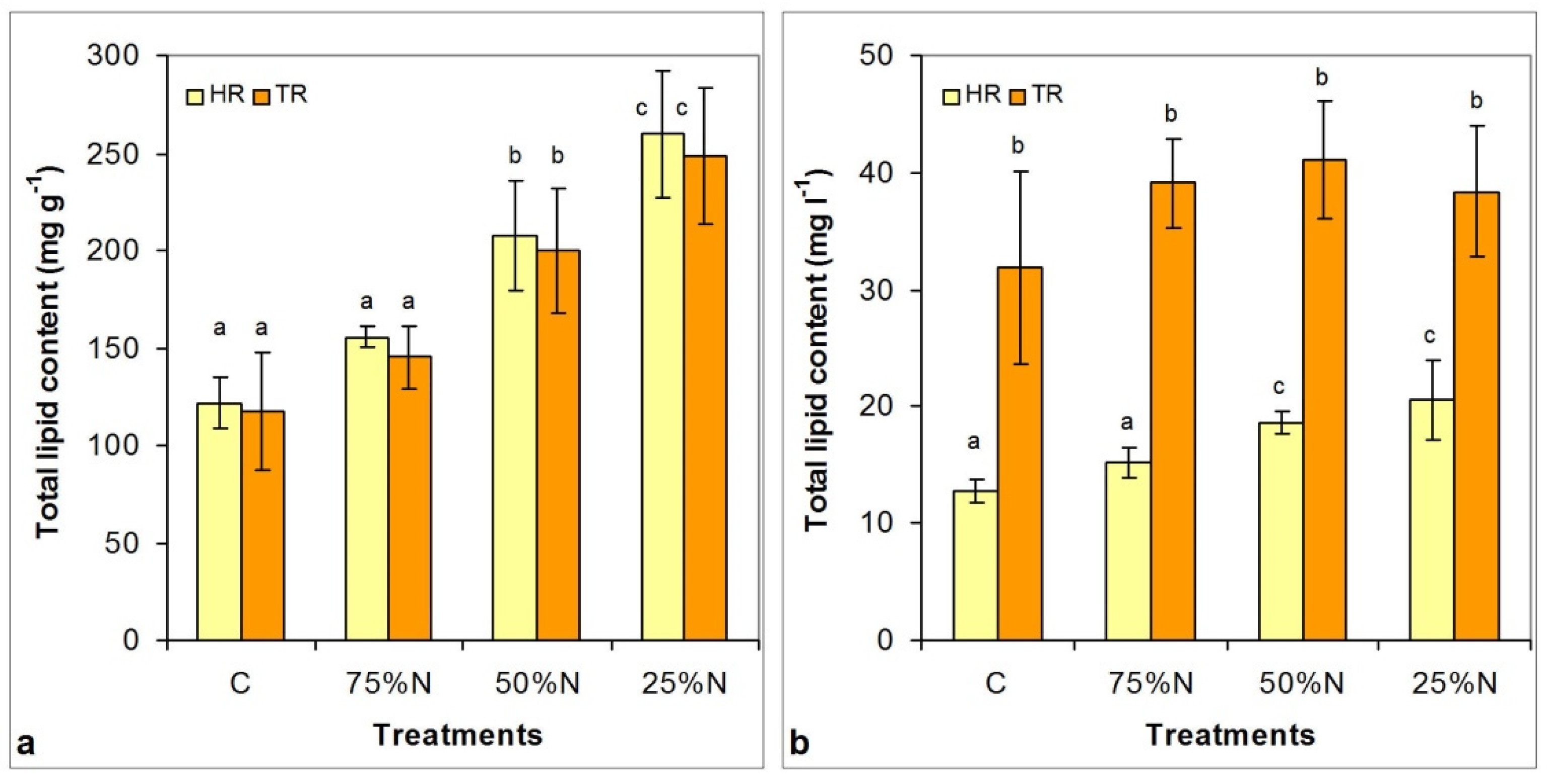
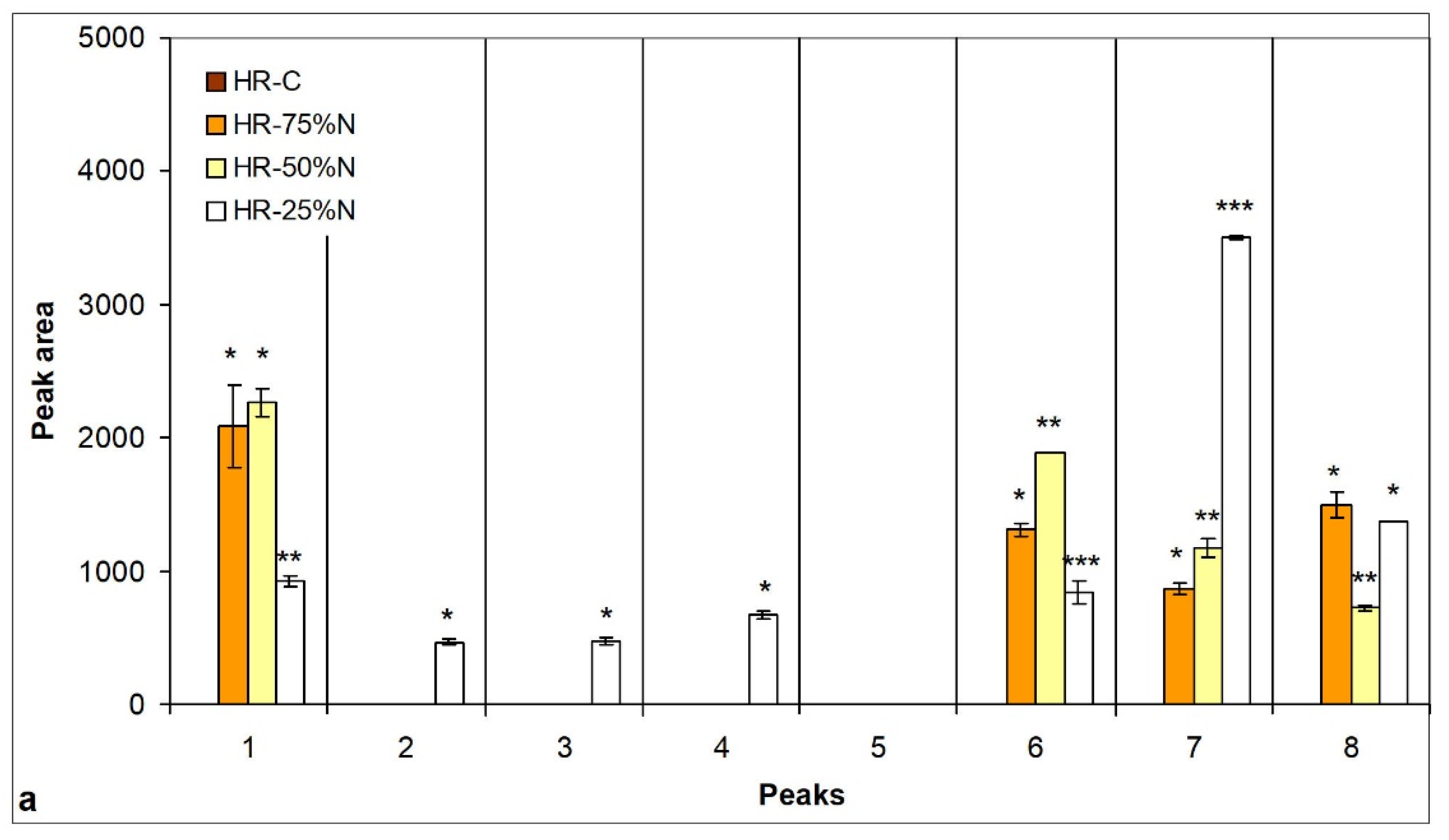
3.5. Changes in Lipid Content and Neutral Lipid Composition
4. Discussion
4.1. Oxygen Saturation and pH
4.2. Growth of the Cultures
4.3. Nutrient Uptake
4.4. Lipid Content
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walter, C.; Steinau, T.; Gerbsch, N.; Buchholz, R. Monoseptic cultivation of phototrophic microorganisms–development and scale-up of a photobioreactor system with thermal sterilization. Biomol. Eng. 2003, 20, 261–271. [Google Scholar] [CrossRef]
- Singh, R.N.; Sharma, S. Development of suitable photobioreactor for algae production–A review. Renew. Sust. Energ. Rev. 2012, 16, 2347–2353. [Google Scholar] [CrossRef]
- Gupta, P.L.; Lee, S.M.; Choi, H.J. A mini review: Photobioreactors for large scale algal cultivation. World J. Microbiol. Biotechnol. 2015, 31, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Assunção, J.; Malcata, F.X. Enclosed “non-conventional” photobioreactors for microalga production: A review. Algal Res. 2020, 52, 102107. [Google Scholar] [CrossRef]
- Lee, Y.K. Microalgal mass culture systems and methods: Their limitation and potential. J. Appl. Phycol. 2001, 13, 307–315. [Google Scholar] [CrossRef]
- Lee, Y.K. Enclosed bioreactors for the mass cultivation of photosynthetic microorganisms: The future trend. Trends. Biotechnol. 1986, 4, 186–189. [Google Scholar] [CrossRef]
- Tsoglin, L.N.; Gabel, B.V.; Fal’kovich, T.N.; Semenenko, V.E. Closed photobioreactors for microalgal production. Russ. J. Plant Physiol. 1996, 43, 131–136. [Google Scholar]
- Acién Fernández, F.G.; Fernández Sevilla, J.M.; Molina Grima, E. Photobioreactors for the production of microalgae. Rev. Environ. Sci. Bio. 2013, 12, 131–151. [Google Scholar] [CrossRef]
- Legrand, J.; Artu, A.; Pruvost, J. A review on photobioreactor design and modelling for microalgae production. React. Chem. Eng. 2021, 6, 1134–1151. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae–A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sust. Energ. Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Pulz, O. Photobioreactors: Production systems for phototrophic microorganisms. Appl. Microbiol. Biot. 2001, 57, 287–293. [Google Scholar]
- Watanabe, Y.; Saiki, H. Development of a photobioreactor incorporating Chlorella sp. for removal of CO2 in stack gas. Energ. Convers. Manag. 1997, 38, S499–S503. [Google Scholar] [CrossRef]
- Mirón, A.S.; Gómez, A.C.; Camacho, F.G.; Grima, E.M.; Chisti, Y. Comparative evaluation of compact photobioreactors for large-scale monoculture of microalgae. Prog. Ind. Microbiol. 1999, 35, 249–270. [Google Scholar]
- Molina, E.; Fernández, J.; Acién, F.G.; Chisti, Y. Tubular photobioreactor design for algal cultures. J. Biotechnol. 2001, 92, 113–131. [Google Scholar] [CrossRef]
- Ugwu, C.; Ogbonna, J.; Tanaka, H. Improvement of mass transfer characteristics and productivities of inclined tubular photobioreactors by installation of internal static mixers. Appl. Microbiol. Biotechnol. 2002, 58, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Harun, R.; Singh, M.; Forde, G.M.; Danquah, M.K. Bioprocess engineering of microalgae to produce a variety of consumer products. Renew. Sust. Energ. Rev. 2010, 14, 1037–1047. [Google Scholar] [CrossRef]
- Norsker, N.H.; Barbosa, M.J.; Vermuë, M.H.; Wijffels, R.H. Microalgal production—A close look at the economics. Biotechnol. Adv. 2011, 29, 24–27. [Google Scholar] [CrossRef]
- Dasgupta, C.N.; Gilbert, J.J.; Lindblad, P.; Heidorn, T.; Borgvang, S.A.; Skjanes, K.; Das, D. Recent trends on the development of photobiological processes and photobioreactors for the improvement of hydrogen production. Int. J. Hydrogen Energ. 2010, 35, 10218–10238. [Google Scholar] [CrossRef]
- Grima, E.M.; Camacho, F.G.; Perez, J.A.S.; Sevilla, J.M.F.; Fernandez, F.G.A.; Gomez, A.C. A mathematical model of microalgal growth in light-limited chemostat culture. J. Chem. Technol. Biotechnol. 1994, 61, 167–173. [Google Scholar] [CrossRef]
- Richmond, A.; Boussiba, S.; Vonshak, A.; Kopel, R. A new tubular reactor for mass production of microalgae outdoors. J. Appl. Phycol. 1993, 5, 327–332. [Google Scholar] [CrossRef]
- Fernandez, F.G.A.; Sevilla, J.M.F.; Perez, J.A.S.; Grima, E.M.; Chisti, Y. Airlift driven external loop tubular photobioreactors for outdoor production of microalgae: Assessment of design and performance. Chem. Eng. Sci. 2001, 56, 2721–2732. [Google Scholar] [CrossRef] [Green Version]
- Kirnev, P.C.S.; Carvalho, J.C.; Vandenberghe, L.P.S.; Karp, S.G.; Soccol, C.R. Technological mapping and trends in photobioreactors for the production of microalgae. World J. Microb. Biot. 2020, 36, 42. [Google Scholar] [CrossRef]
- Yee, W. Microalgae from the Selenastraceae as emerging candidates for biodiesel production: A mini review. World J. Microb. Biot. 2016, 32, 64. [Google Scholar] [CrossRef]
- Guiry, M.D. AlgaeBase; Guiry, M.D., Guiry, G.M., Eds.; World-wide Electronic Publication, National University of Ireland: Galway, Ireland, 2020; Available online: https://www.algaebase.org (accessed on 15 January 2022).
- Aparicio, P.J.; Witt, F.G.; Ramírez, J.M.; Quiñones, M.A.; Balandín, T. Blue-light-induced pH changes associated with NO3−, NO2− and Cl− uptake by the green alga Monoraphidium braunii. Plant. Cell Environ. 1994, 17, 1323–1330. [Google Scholar] [CrossRef]
- Alhama, J.; López-Ruiz, A.; Diez, J.; García-Fernández, J.M. Effect of carbon and nitrogen availability on intracellular amino acids and ammonium pools in the green alga Monoraphidium braunii. J. Plant. Physiol. 1998, 153, 529–533. [Google Scholar] [CrossRef]
- Giráldez, N.; Aparicio, P.J.; Quiñones, M.A. Limiting CO2 levels induce a blue light-dependent HCO3− uptake system in Monoraphidium braunii. J. Exp. Bot. 2000, 51, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Mora, C.; Witt, F.G.; Aparicio, P.J.; Quiñones, M.A. Independent induction of two blue light-dependent monovalent anion transport systems in the plasma membrane of Monoraphidium braunii. J. Exp. Bot. 2002, 53, 1909–1918. [Google Scholar] [CrossRef] [Green Version]
- Corzo, A.; Plasa, R.; Ullrich, W.R. Extracellular ferricyanide reduction and nitrate reductase activity in the green alga Monoraphidium braunii. Plant. Sci. 1991, 75, 221–228. [Google Scholar] [CrossRef]
- Navarro, J.A.; Roncel, M.; De la Rosa, M.A. On the reaction mechanism of flavin-sensitized photoregulation of Monoraphidium braunii nitrate reductase. J. Photochem. Photobiol. B Biol. 1991, 10, 211–220. [Google Scholar] [CrossRef]
- Vigara, A.J.; GarciaSanchez, M.I.; Gotor, C.; Vega, J.M. Interaction between glutamate synthase and ferredoxin from Monoraphidium braunii. Chemical modifications and cross-linking studies. Plant. Physiol. Biochem. 1996, 34, 707–711. [Google Scholar]
- García-Fernández, J.M.; López-Ruiz, A.; Humanes, L.; Dapena, J.D. Purification and characterization of glutamine synthetase from green alga Monoraphidium braunii. Plant. Sci. 1997, 123, 77–84. [Google Scholar] [CrossRef]
- González-Arroyo, J.G.; Vega, J.M.; Pérez-Castiñeira, J.R. Regulation of the O-acetyl-L-serine(thiol)lyase activity in Monoraphidium braunii. J. Physiol. Biochem. 1998, 54, 141–148. [Google Scholar]
- Hörnström, E.; Harbom, A.; Edberg, F.; Andrén, C. The influence of ph on aluminium toxicity in the phytoplakton species Monoraphidium dybowskii and M. griffithii. Water Air Soil Poll. 1995, 85, 817–822. [Google Scholar] [CrossRef]
- Takami, R.; Almeida, J.V.; Vardaris, C.V.; Colepicolo, P.; Barros, M.P. The interplay between thiol-compounds against chromium (VI) in the freshwater green alga Monoraphidium convolutum: Toxicology, photosynthesis, and oxidative stress at a glance. Aquat. Toxicol. 2012, 118, 80–87. [Google Scholar] [CrossRef]
- Bácsi, I.; Novák, Z.; Jánószky, M.; B-Béres, V.; Grigorszky, I.; Nagy, S.A. The sensitivity of two Monoraphidium species to zinc—Their possible future role in bioremediation. Int. J. Environ. Sci. Tech. 2015, 12, 2455–2466. [Google Scholar] [CrossRef] [Green Version]
- Novák, Z.; Harangi, S.; Baranyai, E.; Gonda, S.; B-Béres, V.; Bácsi, I. Effects of metal quantity and quality to the removal of zinc and copper by two common green microalgae (Chlorophyceae) species. Phycol. Res. 2020, 68, 227–235. [Google Scholar] [CrossRef]
- Levy, J.L.; Stauber, J.L.; Adams, M.S.; Maher, W.A.; Kirby, J.K.; Jolley, D.F. Toxicity, biotransformation, and mode of action of arsenic in two freshwater microalgae (Chlorella sp. and Monoraphidium arcuatum). Environ. Toxicol. Chem. 2005, 24, 2630–2639. [Google Scholar] [CrossRef]
- Fujii, K.; Nakashima, H.; Hashidzume, Y.; Uchiyama, T.; Mishiro, K.; Kadota, Y. Potential use of the astaxathin-producing microalga, Monoraphidium sp. GK12, as a functional aquafeed for prawns. J. Appl. Phycol. 2010, 22, 363–369. [Google Scholar] [CrossRef]
- Culture Collection of Algae and Protozoa. CCAP Media Recipes B. Available online: https://www.ccap.ac.uk/wp-content/uploads/MR_JM.pdf (accessed on 15 January 2022).
- Felföldy, L. Biological Water Qualification; Vízgazdálkodási Intézet (Institute of Water Management): Budapest, Hungary, 1987. [Google Scholar]
- Hungarian Standard MSZ 1484-13:2009; Water Quality. Part 13: Determination of Nitrate and Nitrite Content by Spectrophotometric Method. Hungarian Standards Institution: Budapest, Hungary, 2009. Available online: http://www.mszt.hu/web/guest/webaruhaz (accessed on 15 January 2022).
- ISO 6878:2004; Water Quality. Determination of Phosphorus. Ammonium Molybdate Spectrometric Method. International Organization for Standardization: Geneva, Switzerland, 2004. Available online: https://www.iso.org/standard/36917.html (accessed on 15 January 2022).
- Chen, W.; Zhang, C.; Song, L.; Sommerfeld, M.; Hu, Q. A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J. Microbiol. Methods 2009, 77, 41–47. [Google Scholar] [CrossRef]
- Tarandjiiska, R.B.; Marekov, I.N. Precise classification of virgin olive oils with various linoleic acid contents based on triacylglycerol analysis. Anal. Chim. Acta 1998, 364, 83–91. [Google Scholar] [CrossRef]
- Thimijan, R.W.; Heins, R.D. Photometric, radiometric, and quantum light units of measure: A review of procedures for interconversion. HortScience 1983, 18, 818–822. [Google Scholar]
- Tamburic, B.; Evenhuis, C.R.; Sugget, D.J.; Larkum, A.W.D.; Raven, J.A.; Ralph, P.J. Gas transfer controls carbon limitation during biomass production by marine microalgae. ChemSusChem 2015, 8, 2727–2736. [Google Scholar] [CrossRef]
- Tamburic, B.; Evenhuis, C.R.; Crosswell, J.R.; Ralph, P.J. An empirical process model to predict microalgal carbon fixation rates in photobioreactors. Algal Res. 2018, 31, 334–346. [Google Scholar] [CrossRef]
- Zavřel, T.; Szabó, M.; Tamburic, B.; Evenhuis, C.R.; Kuzhiumparambil, U.; Literáková, P.; Larkum, A.W.D.; Raven, J.A.; Červený, J.; Ralph, P.J. Effect of carbon limitation on photosynthetic electron transport in Nannochloropsis oculata. J. Photochem. Photobiol. B Biol. 2018, 181, 31–43. [Google Scholar] [CrossRef] [Green Version]
- Gluszcz, P.; Klepacz-Smółka, A.; Ledakowicz, S. Experimental evaluation of a helical laboratory photobioreactor for cultivation of thermophilic cyanobacteria—Hydrodynamics and mass transfer studies. Chem. Process. Eng. 2018, 39, 457–473. [Google Scholar]
- Eriksen, N.T. The technology of microalgal culturing. Biotechnol. Lett. 2008, 30, 1525–1536. [Google Scholar] [CrossRef]
- Posten, C. Design principles of photo-bioreactors for cultivation of microalgae. Eng. Life Sci. 2009, 9, 165–177. [Google Scholar] [CrossRef]
- Wang, B.; Lan, C.Q.; Horsman, M. Closed photobioreactors for production of microalgal biomasses. Biotechnol. Adv. 2012, 30, 904–912. [Google Scholar] [CrossRef]
- Brewer, P.G.; Goldman, J.C. Alkalinity changes generated by phytoplankton growth. Limnol. Oceanogr. 1976, 21, 108–117. [Google Scholar] [CrossRef]
- Jacobi, A.; Ivanova, D.; Posten, C. Photobioreactors: Hydrodynamics and mass transfer. In Proceedings of the 11th International Symposium on Computer Applications in Biotechnology, Leuven, Belgium, 7–9 July 2010. [Google Scholar] [CrossRef]
- Pruvost, J.; Cornet, J.F. Knowledge models for the engineering and optimization of photobioreactors. In Microalgal Biotechnology: Potential and Production; Posten, C., Walter, C., Eds.; Walter de Gruyter: Berlin, Germany, 2012; pp. 181–224. [Google Scholar]
- Richmond, A.; Hu, Q. Handbook of Microalgal Culture Applied Phycology and Biotechnology, 2nd ed.; Wiley Blackwell: Chichester, UK, 2013. [Google Scholar]
- Shriwastav, A.; Gupta, S.K.; Ansari, F.A.; Rawat, I.; Bux, F. Adaptability of growth and nutrient uptake potential of Chlorella sorokiniana with variable nutrient loading. Bioresour. Technol. 2014, 174, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Lee, S.M. Effect of the N/P ratio on biomass productivity and nutrient removal from municipal wastewater. Bioproc. Biosyst. Eng. 2015, 38, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Vyverman, W. Differences in nutrient uptake capacity of the benthic filamentous algae Cladophora sp., Klebsormidium sp. and Pseudanabaena sp. under varying N/P conditions. Bioresour. Technol. 2015, 179, 234–242. [Google Scholar] [CrossRef]
- Alketife, A.M.; Judd, S.; Znad, H. Synergistic effects and optimization of nitrogen and phosphorus concentrations on the growth and nutrient uptake of a freshwater Chlorella vulgaris. Environ Technol. 2017, 38, 94–102. [Google Scholar] [CrossRef]
- Arora, N.; Laurens, L.M.L.; Sweeney, N.; Pruthi, V.; Poluri, K.M.; Pienkos, P.T. Elucidating the unique physiological responses of halotolerant Scenedesmus sp. cultivated in sea water for biofuel production. Algal Res. 2019, 37, 260–268. [Google Scholar] [CrossRef]
- Figler, A.; Márton, K.; B-Béres, V.; Bácsi, I. Effects of nutrient content and nitrogen to phosphorus ratio on the growth, nutrient removal and desalination properties of the green alga Coelastrum morus on a laboratory scale. Energies 2021, 14, 2112. [Google Scholar] [CrossRef]
- Bogen, C.; Al-Dilaimi, A.; Albersmeier, A.; Wichmann, J.; Grundmann, M.; Rupp, O.; Lauersen, K.J.; Bilfernez-Klassen, O.; Kalinowski, J.; Goesmann, A.; et al. Reconstruction of the lipid metabolism for the microalga Monoraphidium neglectum from its genome sequence reveals characteristics suitable for biofuel production. BMC Genom. 2013, 14, 926. [Google Scholar] [CrossRef] [Green Version]
- Dhup, S.; Dhawan, V. Effect of nitrogen contentration on lipid productivity and fatty acid composition of Monoraphidium sp. Bioresour. Technol. 2014, 152, 572–575. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Yang, H.; Wu, L.; Hu, C. Effect of light intensity on physiological changes, carbon allocation and neutral lipid accumulation in oleaginous microalgae. Bioresour. Technol. 2015, 191, 219–228. [Google Scholar] [CrossRef] [PubMed]
- George, B.; Pancha, I.; Desai, C.; Chokshi, K.; Paliwal, C.; Ghosh, T.; Mishra, S. Effect of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus—A pontential strain for bio-fuel production. Bioresour. Technol. 2014, 171, 367–374. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bácsi, I.; Tóthfalusi, F.; Márton, K.; B-Béres, V.; Gonda, S. The Effects of Photobioreactor Type on Biomass and Lipid Production of the Green Microalga Monoraphidium pusillum in Laboratory Scale. Appl. Sci. 2022, 12, 2196. https://doi.org/10.3390/app12042196
Bácsi I, Tóthfalusi F, Márton K, B-Béres V, Gonda S. The Effects of Photobioreactor Type on Biomass and Lipid Production of the Green Microalga Monoraphidium pusillum in Laboratory Scale. Applied Sciences. 2022; 12(4):2196. https://doi.org/10.3390/app12042196
Chicago/Turabian StyleBácsi, István, Fruzsina Tóthfalusi, Kamilla Márton, Viktória B-Béres, and Sándor Gonda. 2022. "The Effects of Photobioreactor Type on Biomass and Lipid Production of the Green Microalga Monoraphidium pusillum in Laboratory Scale" Applied Sciences 12, no. 4: 2196. https://doi.org/10.3390/app12042196
APA StyleBácsi, I., Tóthfalusi, F., Márton, K., B-Béres, V., & Gonda, S. (2022). The Effects of Photobioreactor Type on Biomass and Lipid Production of the Green Microalga Monoraphidium pusillum in Laboratory Scale. Applied Sciences, 12(4), 2196. https://doi.org/10.3390/app12042196





