Y(III) Ion Migration in AlF3–(Li,Na)F–Y2O3 Molten Salt
Abstract
:1. Introduction
2. Materials and Methods
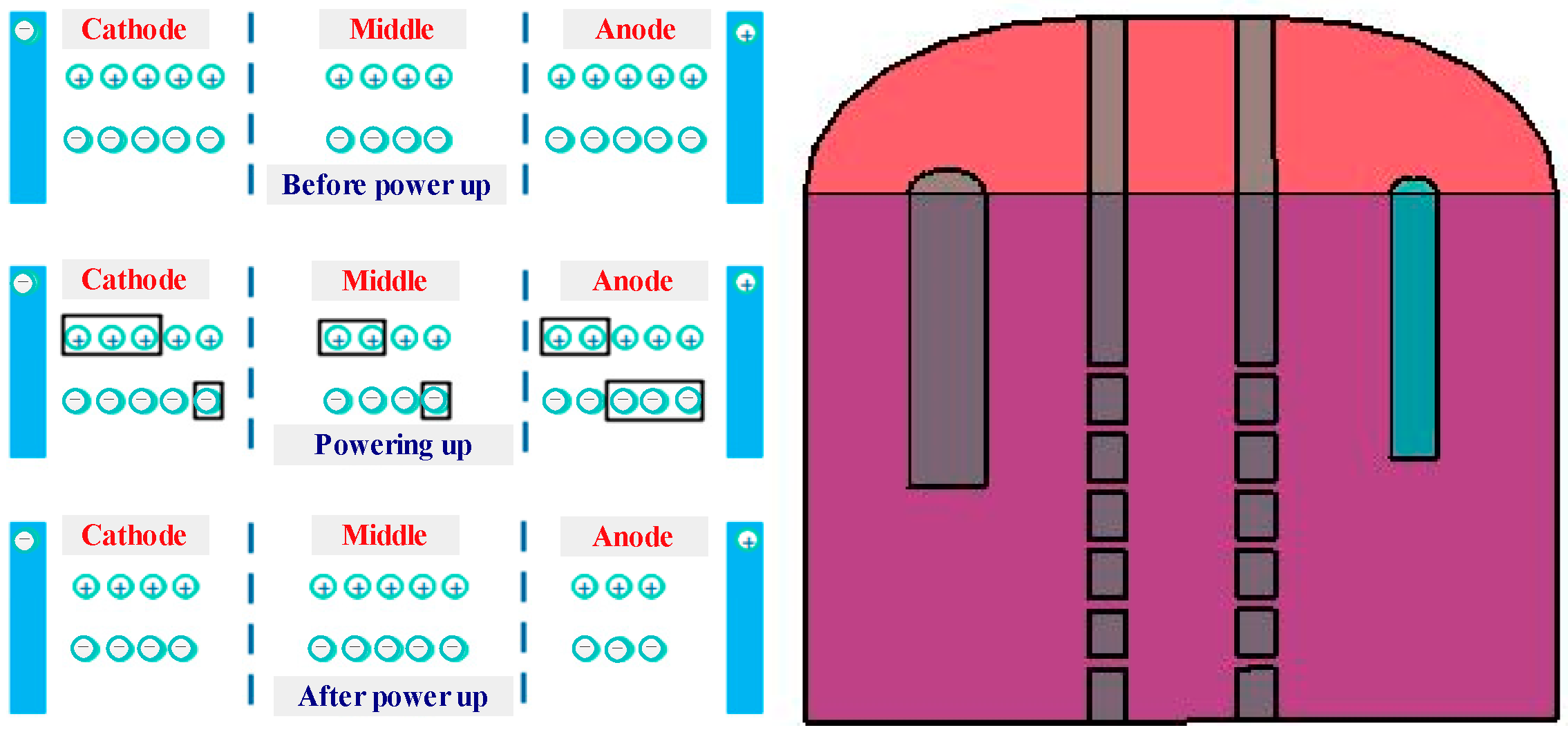
2.1. Measuring Principle
2.2. Experimental Method
3. Results and Discussion
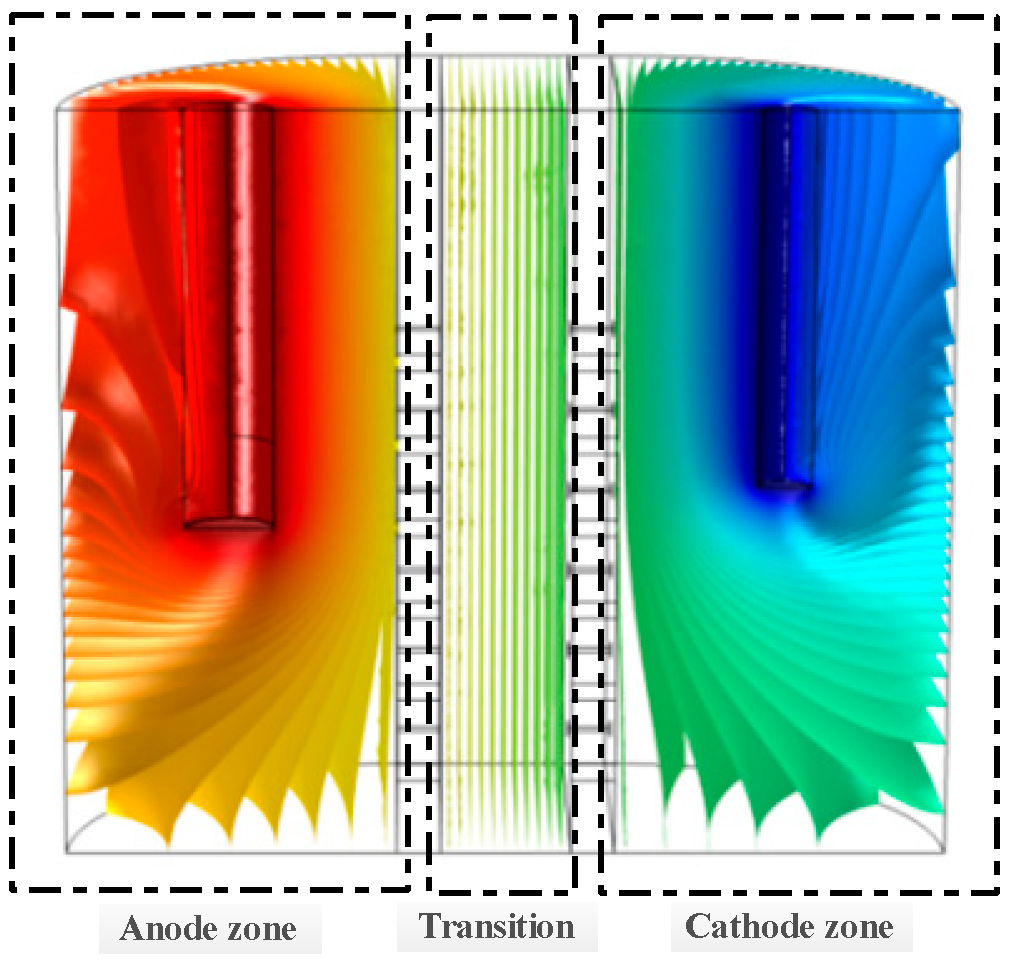
3.1. Simulation Analysis of the Migration Tank
3.1.1. Model Construction
- (1)
- (2)
- Preset Conditions [20]
- (3)
3.1.2. Simulation Results and Their Analysis
3.2. Influence of Temperature on the Migration Number of Y(III) Ions
3.3. Influence of the (Li,Na)F/AlF3 Molar Ratio on the Migration Number of Y(III) Ions
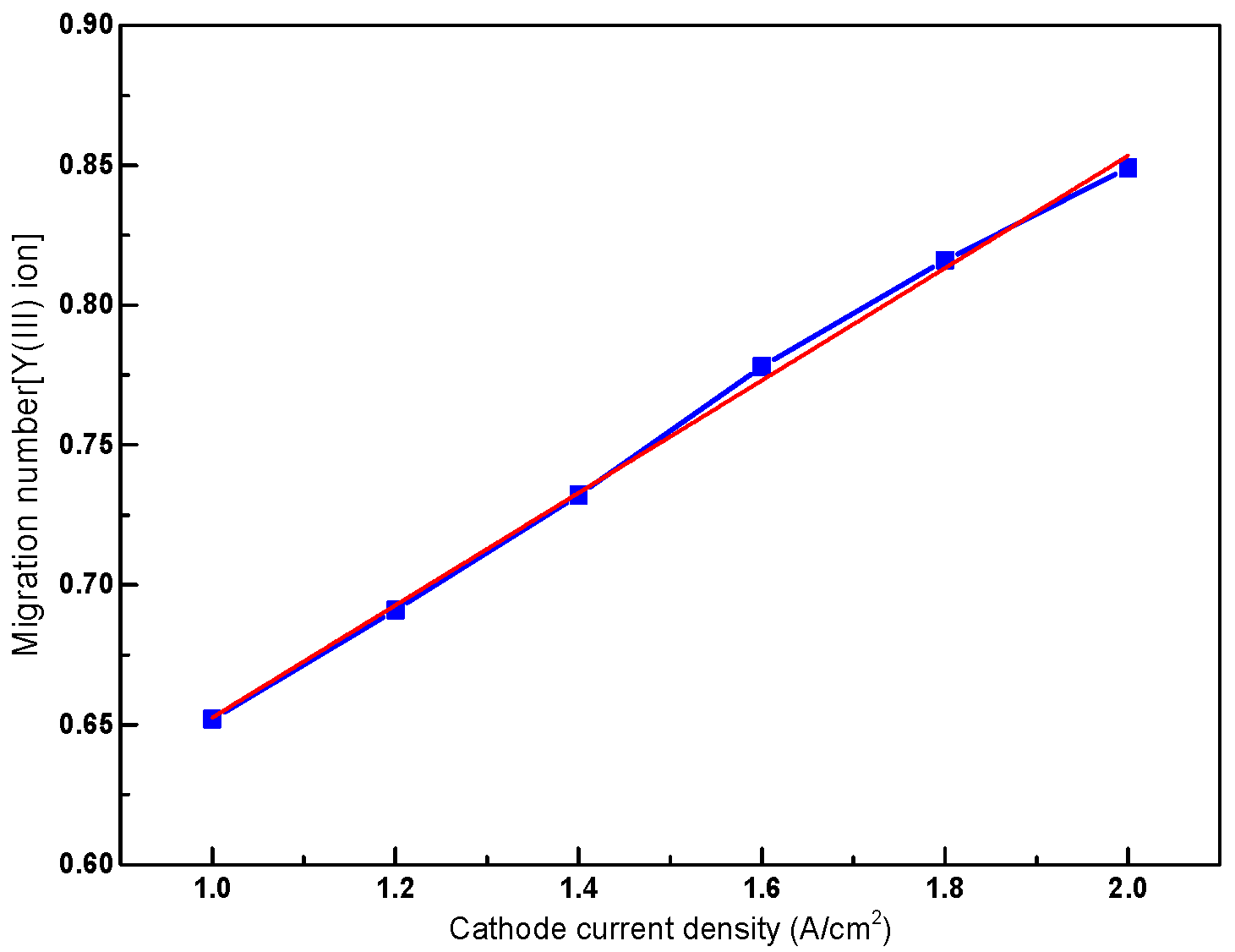
3.4. Influence of Current Density on the Migration Number of Y(III) Ions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pang, S.; Yan, S.; Li, Z.; Chen, D.; Xu, L.; Zhao, B. Development on Molten Salt Electrolytic Methods and Technology for Preparing Rare Earth Metals and Alloys in China. Rare Met. 2011, 35, 440–450. [Google Scholar]
- Guo, T.; Wang, S.; Ye, X.; Li, Q.; Liu, H.; Guo, M.; Wu, Z. Research Progress in Preparation of Rare Earth Alloys by Molten Salt Electrolysis. Sci. Sin. Chim. 2012, 42, 1328–1336. [Google Scholar]
- Wang, X.-S.; Wang, Z.-Q.; Chen, D.-H.; Pang, S.-M.; Xu, L.-H.; Li, Z.-A.; Yan, S.-H. Development and Status quo of Rare Earth Metals Preparation Technologies. Chin. Rare Earths 2015, 36, 123–132. [Google Scholar]
- Lu, Y.; Yuan, L.; Li, W.; Jin, Y. Corrosion resistance of Cu14%Al-X aibronze alloy in 3.5% NaCl solution. J. Mech. Eng. 2005, 41, 42–45. [Google Scholar] [CrossRef]
- Cao, D.; Shi, Z.; Yang, S. Effects of Rare Earth on Aluminium and Its Alloys. Chin. Rare Earths 2006, 27, 88–93. [Google Scholar]
- Wang, H.; An, Y.; Li, C.; Chao, B. The Research Progress of Rare Earth Application in Aluminum and Aluminum Alloys. Chin. Rare Earths 2012, 33, 74–78. [Google Scholar]
- Xu, W.; Chunfa, L.; Jiao, Y.; Tang, H. Cathodic reduction process of Al-Cu-Y alloy in fluoride-oxide eutectic system via molten salt electrolysis. J. Rare Earths 2018, 36, 324–330. [Google Scholar]
- Gheribi, A.E.; Machado, K.; Zanghi, D.; Bessada, C.; Salanne, M.; Chartrand, P. On the determination of ion transport numbers in molten salts using molecular dynamics. Electrochim. Acta 2018, 274, 266–273. [Google Scholar] [CrossRef]
- Lv, X.; Xu, Z.; Li, J.; Chen, J.; Liu, Q. Molecular dynamics investigation on structural and transport properties of Na3AlF6–Al2O3 molten salt. J. Mol. Liq. 2016, 221, 26–32. [Google Scholar] [CrossRef]
- Feng, N.; Kvande, H. Calculation of molar volume, equivalent conductance and migration number of sodium fluoride-aluminum fluoride melt. Non-Ferr. Met. 1988, 40, 60–64. [Google Scholar]
- Vink, H. A new modified Hittorf method for the determination of transport numbers in polyelectrolyte solutions. J. Chem. Soc. Faraday Trans. 1984, 80, 1297–1304. [Google Scholar] [CrossRef]
- Li, X.; Ding, H.H.; Li, G.H.; Wang, Y.; Fang, Z.M.; Yang, S.F.; Ju, H.X.; Zhu, J.F. In situ investigations of interfacial degradation and ion migration at CH3-NH3-PbI3 perovskite/Ag interface. Chin. J. Chem. Phys. 2019, 32, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Ottoy, M.; Forland, F.; Ratkje, S.K.; Moller-Holst, S. Membrane transference numbers from a new emf method. J. Membr. Sci. 1992, 74, 1–8. [Google Scholar] [CrossRef]
- Kontturi, K.; Mafé, S.; Manzanares, J.A.; Pellicer, J.; Vuoristo, M. A new method for determining transport numbers of charged membranes from convective diffusion experiments. J. Electroanal. Chem. 1994, 378, 111–116. [Google Scholar] [CrossRef]
- Zugmann, S.; Fleischmann, M.; Amereller, M.; Gschwind, R.M.; Wiemhofer, H.D.; Gores, H.J. Measurement of transference numbers for lithium ion electrolytes via four different methods, a comparative study. Electrochim. Acta 2011, 56, 3926–3933. [Google Scholar] [CrossRef]
- Macinnes, D.A.; Dole, M. The transference numbers of potassium chloride. New determinations by the hittorf method and comparison with results obtained by the moving boundary method. J. Am. Chem. Soc. 2002, 53, 1357–1364. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, J.; Gao, W.; Chun, Y. Influence of the Shape of Transfer Tube on Determining Ion Transference Number by Hittorf Method. Univ. Chem. 2017, 32, 45–50. [Google Scholar] [CrossRef]
- Nie, J.; Chen, Y. Numerical modeling of three-dimensional two-phase gas–liquid flow in the flow field plate of a PEM electrolysis cell. Int. J. Hydrog. Energy 2010, 35, 3183–3197. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Yin, C.; Yang, S.; Zhang, H.; Lu, X. Method of strongly coupled modeling and computing for thermal-electrical field in aluminium reduction cells. Chin. J. Nonferrous Met. 2014, 24, 239–245. [Google Scholar]
- Liu, Z.; Li, Z.; Zhang, X.; Pang, S.; Chen, D.; Wang, Z. Numerical simulation of flow field in rare earth electrolysis cell. Chin. J. Nonferrous Met. 2015, 11, 3209–3215. [Google Scholar]
- Zhou, N.J.; Mei, C.; Jiang, C.; Zhou, P. Coupled computation method of physics fields in aluminium reduction cells. Trans. Nonferrous Met. Soc. China 2013, 13, 431–437. [Google Scholar]
- Peipei, S.; Qianqian, S.; Yang, Z.; Zeng, G.; Xu, H.; Li, X.; Xiong, W. Numerical simulation and exploration of electrocoagulation process for arsenic and antimony removal: Electric field, flow field, and mass transfer studies. J. Environ. Manage. 2018, 228, 336–345. [Google Scholar]
- Wu, Y.; Ju, Y.; Liu, Z.; Hu, Y.; Han, W. Numerical Simulation of Flow Field-electric Field Coupling in Rare Earth Electrolytic Cell. Chin. Rare Earths 2015, 36, 76–80. [Google Scholar]
- Liu, Q.; Tang, W.; Wang, L. Numerical Simulation of Anode Bubble Behavior in Rare Earth Electrolysis Cell. J. Chin. Soc. Rare Earths 2015, 33, 737–746. [Google Scholar]
- Zoric, J.; Solheim, A. On gas bubbles in industrial aluminium cells with prebaked anodes and their influence on the current distribution. J. Appl. Electro-Chem. 2000, 30, 787–794. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, T.; Wang, X.; Zhai, Y.; Chang, Y. Y(III) Ion Migration in AlF3–(Li,Na)F–Y2O3 Molten Salt. Appl. Sci. 2022, 12, 2200. https://doi.org/10.3390/app12042200
Hao T, Wang X, Zhai Y, Chang Y. Y(III) Ion Migration in AlF3–(Li,Na)F–Y2O3 Molten Salt. Applied Sciences. 2022; 12(4):2200. https://doi.org/10.3390/app12042200
Chicago/Turabian StyleHao, Tingting, Xu Wang, Yuchun Zhai, and Yunlong Chang. 2022. "Y(III) Ion Migration in AlF3–(Li,Na)F–Y2O3 Molten Salt" Applied Sciences 12, no. 4: 2200. https://doi.org/10.3390/app12042200




