Abstract
It is allegedly known that the thermal stability of the Mint Lift® (the Mint Lift® 17 and the Mint Lift® Fine; HansBiomed Co., Ltd., Seoul, Korea) over time might be lower as compared with thread-lifts processed through ultrasonic molding technology, such as the MEDI ROPE (WSM 19-03; CNG Co., Ltd., Gyeonggi, Korea), because the Mint Lift® undergoes a thermal treatment during the manufacturing process. We conducted this accelerated aging test to compare the thermal stability over time between the Mint Lift® and the MEDI ROPE. Thus, we measured the degrees of strength of the MEDI ROPE, the Mint Lift® 17, and the Mint Lift® Fine at 0, 2, 3, 4, 5, 7, 8, 9, 10, 11, 14, and 17 weeks. Between 0 and 14 weeks, the Mint Lift® 17 and the Mint Lift® Fine had significantly higher degrees of strength as compared with the MEDI ROPE (p < 0.05). At 0, 5, and 10 weeks, the Mint Lift® 17 and the Mint Lift® Fine showed no notable differences in microscopic findings as compared with the MEDI ROPE. At 20 weeks, however, the integrities of the Mint Lift® 17 and the Mint Lift® Fine were better preserved as compared with the MEDI ROPE. In conclusion, our results indicate that the Mint Lift® 17 and the Mint Lift® Fine might be less vulnerable to degradation over time as compared with the MEDI ROPE under thermal conditions.
1. Introduction
In the past decades, there has been a dramatic increase in the demand for non-invasive rejuvenation procedures. According to the American Society of Plastic Surgeons (ASPS) statistics, 15.9 million minimally-invasive aesthetic procedures were performed in 2018. This corresponded to a 2% and 228% increase from 2017 and 2000, respectively. However, 1.8 million surgical aesthetic procedures were performed in 2018. This corresponded to a 5% decrease from 2000. Thus, there has been a tremendous growth of the market of non-invasive aesthetic procedures as compared with surgical ones. This should be addressed using effective, safe non-surgical aesthetic procedures [1].
The increased number of patients receiving non-invasive aesthetic procedures may be attributable to technological advancements in the pharmaceuticals and medical devices that are used for patients. Thus, soft tissue fillers, laser therapies, and radiofrequency- or ultrasound-based skin tightening methods have been introduced. It would therefore be mandatory to perform the above treatment modalities for patients who are in need of aesthetic rejuvenation procedures, which is essential for optimizing outcomes and minimizing complications [1].
A thread-lifting is a minimally-invasive technique using a thread-lift; it is performed to correct the skin laxity by improving the contours of soft tissues [2,3]. The emergence of thread-lift has opened new era of non-surgical aesthetic procedures. To date, fillers or other procedures controlling hyperactive facial mimetic muscles have been used for the restoration of volume of the deflated facial areas. This is complemented by non-surgical facial rejuvenation using a barbed thread-lift [4].A barbed suture is a special type of suture that is used to anastomose the tissue without tying a knot [5]. It is characterized by the presence of barbs on its surface. Conventional types of sutures are dependent on surgeons’ technical skills in tying secure knots. However, barbed sutures serve as a knotless alternative in some surgical settings; they are commonly used in the field of cosmetic surgery [6].
With technological advancements in the manufacturing process and the popularity of a barbed thread-lift in the field of aesthetic procedures, diverse types of polydioxanone (PDO) thread-lifts have become commercially available [7,8,9,10]. Meanwhile, PDO thread-lifts have undergone scientific evolution, leading to improvements in their physicochemical and biomechanical properties [8,9].
Of various types of PDO thread-lifts, the Mint Lift® (HansBiomed Co., Ltd., Seoul, Korea) deserves special attention because it was approved by the U.S. Food and Drug Administration (US FDA) [11]. Moreover, its clinical efficacy and safety have been well described in the literature [4,12,13,14,15,16,17,18,19,20].
The accelerated aging test is commonly performed to assess the stability of a medical device, for which it accelerates the chemical or physical degradation of the device in such conditions as severe temperature for a short period of time relative to the actual shelf-life of the device [21]. The accelerated aging test for biomaterials has been traditionally performed using either solvent or thermal methods. Use of solvents or solutions for the accelerated aging test is a type of method for assessing the performance of the material once it has been implanted; it is advantageous in predicting toxicological concerns and changes in the properties of the material. Thermal methods have also been used for the accelerated aging test, although they are much less accurate as compared with those using solvents or solutions. Nevertheless, they have successfully predicted the long-term performance of the material in assessing the shelf-life and packaging of devices [22].
It is allegedly known that the thermal stability of the Mint Lift® over time might be lower as compared with thread-lifts processed through ultrasonic molding technology, such as the MEDI ROPE (WSM 19-03; CNG Co., Ltd., Gyeonggi, Korea), because the Mint Lift® undergoes a thermal treatment during the manufacturing process.
Given the above background, we conducted this accelerated aging test to compare the thermal stability over time between the Mint Lift® and the MEDI ROPE.
2. Materials and Methods
2.1. Experimental Rationale
For the current study, the accelerated aging test was performed based on the following rationale: first, the accelerated aging of materials refers to the accelerated variation of their properties, particularly including those related to safety and function of the material or package, over time. Second, the material or package were subjected to external stress to a greater extent or more frequently as compared to normal conditions for relatively short periods of time. Third, the accelerated aging test was performed based on the assumption that the chemical reactions involved in the deterioration of materials followed the Arrhenius reaction rate equation. This suggests that the rate of a chemical reaction (Q10) is increased by twice or decreased by half when there is a 10 °C change in the temperature of a homogeneous process. Fourth, the Q10 was determined by performing the accelerated aging test at varying temperatures and, thereby, assessing changes in the reaction rate when there was a 10 °C change in the temperature [23,24,25,26,27].
2.2. Definitions of Test Variables
To simulate real-time aging of the sample, the accelerated aging of it is induced at an elevated temperature (TAA) for short periods of time [28]. For the current experiment, the following test variables are defined, as previously described [28]:
- (1)
- Accelerated aging factor (AAF): the estimated time to real-time changes in the physical property of the sample
- (2)
- Accelerated aging temperature (TAA): the elevated temperature during the accelerated aging test
- (3)
- Accelerated aging time (AAT): the length of time elapsed during the accelerated aging test
- (4)
- Ambient temperature (TRT): storage temperature for the sample as a measure of storage conditions in real-time conditions.
2.3. The Accelerated Aging Conditions
The following equations define the relationships between the above variables, as previously described [28,29,30]:
AAF = Q10[(TAA − TRT)/10]
AAT = Desired (RT)/AAF
The estimated AAT might differ from its actual value because the Formula (1) did not consider the treatment of samples with the phosphate buffered saline (PBS) solution. Moreover, it is necessary to adjust temporal changes in the strength considering results of a real-time degradation test [29,30]. Based on the Formula (2), the AAT was estimated at 90 days/4 = 23 days at a TAA of 57.5 °C. Therefore, the accelerated aging conditions include a temperature of 57.5 °C and a duration of 23 days.
2.4. Experimental Materials
The MEDI ROPE served as the control material, whose dimensions included a 19-G cannula with an external diameter of 1.030–1.100 mm and a suture size of United States Pharmacopeia (USP) 3. It was equipped with a depressed barb, whose strength was closely associated with its engraved central part. It was therefore expected to be vulnerable to degradation.
The Mint Lift® 17 and the Mint Lift® Fine served as the trial materials, whose characteristics have been described in previous literature [12,13,19,20].
The MEDI ROPE, the Mint Lift® 17, and the Mint Lift® Fine were cut at a length of 4.5 cm and then weighed. Thus, the MEDI ROPE (0.014 g), the Mint Lift® 17 (0.009 g), and the Mint Lift® Fine (0.009 g) were prepared for the current experiment.
2.5. Experimental Procedure
For the current experiment, the accelerated aging test was performed in compliance with guidelines of the American Society for Testing and Materials (ASTM) F 1980-2 Standard Guide for Accelerated Aging of Sterile Medical Device Packages for the purposes of assessing the thermal stability over time of the Mint Lift® 17 and the Mint Lift® Fine as compared with the MEDI ROPE, as previously described [31,32,33].
Three samples each were placed in a 15-mL conical tube and then treated with PBS solution 10 mL. Following this, the experiment was performed in an oven at a temperature of 57.5 °C.
Following the thermal treatment of samples, we measured the strength in triplicate. Then, we compared microscopic findings between the Mint Lift® 17, the Mint Lift® Fine and the MEDI ROPE under a light microscope (Industrial Microscope Division, Nikon Instruments, Inc., Melville, NY, USA) at a magnification of 4×.
In the current study, we compared the strength between the Mint Lift® 17, the Mint Lift® Fine, and the MEDI ROPE at 0, 2, 3, 4, 5, 7, 8, 9, 10, 11, 14, and 17 weeks.
2.6. Statistical Analysis
All data were expressed as mean ± standard deviation. Differences in the degree of the initial strength between the experimental materials were analyzed using the two-way analysis of variance with Duncan’s post-hoc analysis. Statistical analysis was carried out using the SPSS ver. 18.0 for windows (SPSS Inc., Chicago, IL, USA). Statistical significance was set at p < 0.05.
3. Results
3.1. Time-Dependent Changes in the Thermal Stability
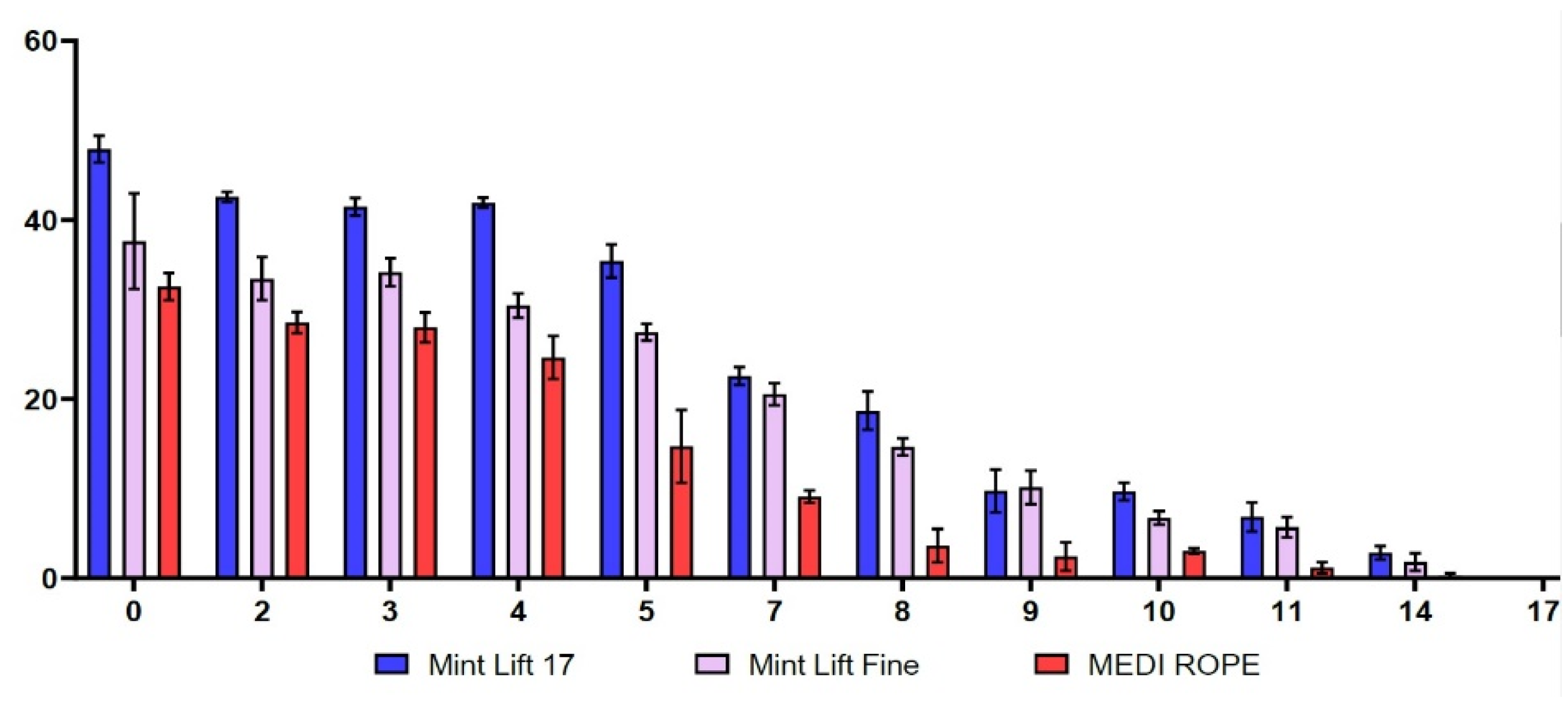
As shown in Table 1 and Figure 1, the initial strengths of the Mint Lift® 17 and the Mint Lift® Fine were higher as compared with the MEDI ROPE. More specifically, between 0 and 14 weeks, the Mint Lift® 17 and the Mint Lift® Fine had significantly higher degrees of strength as compared with the MEDI ROPE (p < 0.05).

Table 1.
Time-dependent changes in the thermal stability.
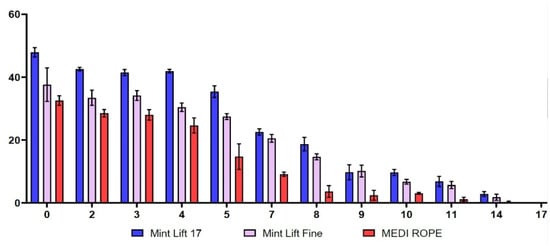
Figure 1.
Time-dependent changes in the thermal stability. Between 0 and 14 weeks, the Mint Lift® 17 and the Mint Lift® Fine had significantly higher degrees of strength as compared with the MEDI ROPE (p < 0.05). (X- and Y-axis indicate the time and the strength (N), respectively).
3.2. Microscopic Findings
At 0, 5, and 10 weeks, the Mint Lift® 17 and the Mint Lift® Fine showed no notable differences in microscopic findings as compared with the MEDI ROPE (Figure 2). At 20 weeks, however, the integrities of the Mint Lift® 17 and the Mint Lift® Fine were better preserved as compared with the MEDI ROPE (Figure 3).
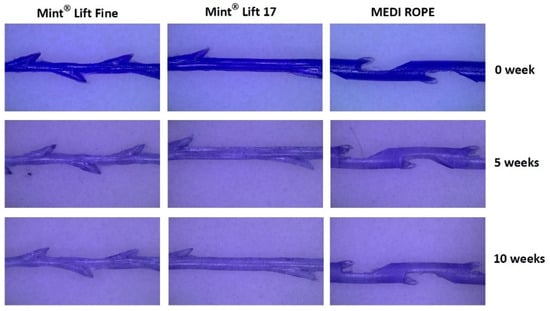
Figure 2.
Microscopic findings observed at 0, 5, and 10 weeks. At 0, 5, and 10 weeks, the Mint Lift® 17 and the Mint Lift® Fine showed no notable differences in microscopic findings as compared with the MEDI ROPE.
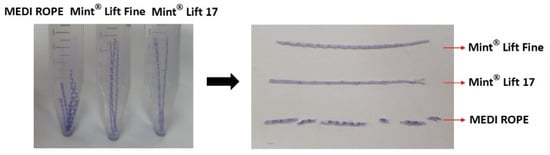
Figure 3.
Microscopic findings observed at 20 weeks. At 20 weeks, the integrities of the Mint Lift® 17 and the Mint Lift® Fine were better preserved as compared with the MEDI ROPE.
4. Discussion
Both synthetic and natural polymers are vulnerable to degradation at varying levels during their lives, which may eventually lead to alterations in their mechanical, thermal, optical, functional, or other properties. Such vulnerability to the degradation plays a role in impairing the performance of polymers and thereby shortening their life cycles [34,35,36,37,38,39]. The long-term behavior of polymers may be affected by diverse factors, such as their chemical properties, the manufacturing process, and the type and level of environmental exposure. Indeed, even the same polymers would perform differently if exposed to different conditions [40].
The accelerated aging test is commonly performed to obtain the experimental data from a new product and, thereby, to estimate its clinical performance and shelf-life claims [41]. To our knowledge, this is the first study to assess the thermal stability of the Mint Lift® 17 and the Mint Lift® Fine over time as compared with the MEDI ROPE.
Adequate tensile strength for tissue anchorage as well as durability is considered a requirement for thread-lifts, for which both larger cross-sectional diameters and irregular shapes serve as factors causing a tissue reaction, and barbs are involved in the even distribution of the tensile strength in the dermal and subcutaneous layers [42]. There are several literature studies advocating the effects of barbed thread-lifts; these effects are classified as mechanical and biochemical ones. The former arises from the helical and bidirectional arrangement of barbs, which is followed by the generation of the tensile force, applied to both sides, allowing the barbs to act as a hook without a wire slip [43,44]. The latter leads to neocollagenesis; Jang et al. [45] used a rat experimental model and thereby inserted cog threads in the skin. These authors observed that myofibroblasts were a key player in the contracture of the fibrous tissue at 4 weeks following the insertion of thread. In addition, histological examinations showed the formation of a homogeneous fibrous capsule around the thread, leading to the preservation of the traction and compactness of tissues. Moreover, there was an increase in the thickness of the dermal papillae on histopathology, which is suggestive of the interstitial growth of collagen components [7].
A limited durability, in vivo, of thread-lifts deserves special attention; it may commonly arise from changes in their thermal stability over time. This is closely associated with clinical failure of the facial rejuvenation using them. Therefore, their clinical lifetimes should be prolonged through improvements in their thermal stabilities over time.
To summarize, our results are as follows: First, between 0 and 14 weeks, the Mint Lift® 17 and the Mint Lift® Fine had significantly higher degrees of strength as compared with the MEDI ROPE (p < 0.05). Second, at 0, 5, and 10 weeks, the Mint Lift® 17 and the Mint Lift® Fine showed no notable differences in microscopic findings as compared with the MEDI ROPE. Third, at 20 weeks, the integrity of the Mint Lift® 17 and the Mint Lift® Fine was better preserved as compared with the MEDI ROPE.
However, our results cannot be generalized, because we failed to perform histologic and molecular analyses in vivo. It is mandatory to consider inherent differences in the elastic and thread-holding properties between in vitro and in vivo conditions. This deserves further studies.
5. Conclusions
In conclusion, our results indicate that the Mint Lift® 17 and the Mint Lift® Fine might be less vulnerable to degradation over time as compared with the MEDI ROPE under thermal conditions.
Author Contributions
Conceptualization, J.H.P., T.S.K. and J.E.S.; data curation, J.H.P., C.J. and S.S.K.; formal analysis, J.H.P. and J.E.S.; investigation, J.H.P. and J.E.S.; methodology, C.J.; supervision, J.E.S.; writing—original draft, J.H.W. and R.K.; writing—review and editing, R.K. and J.E.S. All authors have read and agreed to the published version of the manuscript.
Funding
The current study was sponsored by the HansBiomed Co., Ltd. (Seoul, Korea).
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
We greatly thank the Advanced Medical Device R&D Center of the HansBiomed Co., Ltd. (Seoul, Korea) for performing the experimental procedures.
Conflicts of Interest
Robert Kim was a paid consultant for key opinion leaders of the HansBiomed Co., Ltd. (Seoul, Korea) between November of 2018 and February of 2020; the other authors had no conflicts of interest in relation to the current work.
References
- Farber, S.E.; Epps, M.T.; Brown, E.; Krochonis, J.; McConville, R.; Codner, M.A. A review of nonsurgical facial rejuvenation. Plast. Aesthetic Res. 2020, 7, 72. [Google Scholar] [CrossRef]
- Lee, S.; Isse, N. Barbed polypropylene sutures for midface elevation: Early results. Arch. Facial Plast. Surg. 2005, 7, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Bisaccia, E.; Kadry, R.; Saap, L.; Rogachefsky, A.; Scarborough, D. A novel specialized suture and inserting device for the resuspension of ptotic facial tissues: Early results. Dermatol. Surg. 2009, 35, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Song, J.K.; Chang, J.; Cho, K.W.; Choi, C.Y. Favorable Crisscrossing Pattern With Polydioxanone: Barbed Thread Lifting in Constructing Fibrous Architecture. Aesthetic Surg. J. 2021, 41, NP875–NP886. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.A.; Goldman, R.H. Barbed suture: A review of the technology and clinical uses in obstetrics and gynecology. Rev. Obstet. Gynecol. 2013, 6, 107–115. [Google Scholar]
- Paul, M.D. Using barbed sutures in open/subperiosteal midface lifting. Aesthetic Surg. J. 2006, 26, 725–732. [Google Scholar] [CrossRef][Green Version]
- Savoia, A.; Accardo, C.; Vannini, F.; Di Pasquale, B.; Baldi, A. Outcomes in thread lift for facial rejuvenation: A study performed with happy lift™ revitalizing. Dermatol. Ther. 2014, 4, 103–114. [Google Scholar] [CrossRef]
- Wu, W.T. Barbed sutures in facial rejuvenation. Aesthetic Surg. J. 2004, 24, 582–587. [Google Scholar] [CrossRef]
- Sulamanidze, M.; Sulamanidze, G. Facial lifting with Aptos Methods. J. Cutan. Aesthetic Surg. 2008, 1, 7–11. [Google Scholar] [CrossRef]
- Park, T.H.; Seo, S.W.; Whang, K.W. Facial rejuvenation with fine-barbed threads: The simple Miz lift. Aesthetic Plast. Surg. 2014, 38, 69–74. [Google Scholar] [CrossRef]
- Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf19/K192423.pdf (accessed on 2 January 2021).
- Yarak, S.; de Carvalho, J.A.R. Facial Rejuvenation with Absorbable and Barbed Thread Lift: Case Series with Mint Lift™. J. Clin. Exp. Dermatol. Res. 2017, 8, 415. [Google Scholar] [CrossRef]
- Baek, W.I.; Kim, W.S.; Suh, J.H.; Kim, B.J. Lower Facial Rejuvenation Using Absorbable Casting Barbed Thread. Dermatol. Surg. 2017, 43, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.H.; Lee, Y.B.; Han, D.G. Early complications from absorbable anchoring suture following thread-lift for facial rejuvenation. Arch. Aesthetic Plast. Surg. 2017, 23, 11–16. [Google Scholar] [CrossRef]
- Bae, K.I.; Han, D.G.; Kim, S.E.; Lee, Y.B. Minimally invasive facial rejuvenation combining thread lifting with liposuction: A clinical comparison with thread lifting alone. Arch. Aesthetic Plast. Surg. 2019, 25, 52–58. [Google Scholar] [CrossRef]
- Moon, H.J.; Chang, D.; Lee, W. Short-term Treatment Outcomes of Facial Rejuvenation Using the Mint Lift Fine. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2775. [Google Scholar] [CrossRef] [PubMed]
- Myung, Y.; Jung, C. Mini-midface Lift Using Polydioxanone Cog Threads. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2920. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Byeon, H.S.; Moon, H.J. Short-term safety of facial rejuvenation using an absorbable polydioxanone monofilament thread in patients with mild-to-moderate facial skin sagging. Arch. Aesthetic Plast. Surg. 2020, 26, 53–56. [Google Scholar] [CrossRef]
- Chang, D.Y.; Kim, H.M.; Ahn, T.H.; Lee, S.B.; Moon, H.J. Proposed Treatment Protocols for Facial Rejuvenation Using a Novel Absorbable Polydioxanone Monofilament Threadlift in Koreans: Empirical Perspectives of Aesthetic Physicians and Surgeons. Aesthetic Surg. J. Open Forum 2020, 3, ojaa049. [Google Scholar] [CrossRef]
- Kim, T.S.; Kim, S.S.; Jeong, C.; Song, Y.K. Expert consensus on the facial rejuvenation using the Mint Lift® in Koreans: Perspectives of plastic surgeons. J. Cosmet. Dermatol. 2021, 20, 2224–2231. [Google Scholar] [CrossRef]
- Jang, B.S.; Cheon, J.Y.; Jung, M.Y.; Kwon, O.H.; Park, W.H. Effect of pH on the Accelerated Aging Test of Gastrointestinal Absorption Inhibiting Stent Materials. Polym. Korea 2019, 43, 261–267. [Google Scholar] [CrossRef]
- Weems, A.C.; Boyle, A.J.; Maitland, D.J. Two-year performance study of porous, thermoset, shape memory polyurethanes intended for vascular medical devices. Smart Mater. Struct. 2017, 26, 035054. [Google Scholar] [CrossRef] [PubMed]
- Rydz, J.; Sikorska, W.; Musioł, M.; Janeczek, H.; Włodarczyk, J.; Misiurska-Marczak, M.; Łęczycka, J.; Kowalczuk, M. 3D-Printed Polyester-Based Prototypes for Cosmetic Applications—Future Directions at the Forensic Engineering of Advanced Polymeric Materials. Materials 2019, 12, 994. [Google Scholar] [CrossRef] [PubMed]
- Frigione, M.; Rodríguez-Prieto, A. Can Accelerated Aging Procedures Predict the Long Term Behavior of Polymers Exposed to Different Environments? Polymers 2021, 13, 2688. [Google Scholar] [CrossRef]
- Rad, F.H.; Ghaffari, T.; Tamgaji, R. Evaluation of the Color Stability of Methyl Methacrylate and Nylon Base Polymer. J. Dent. 2017, 18, 136–142. [Google Scholar]
- Men, S.; Yan, L.; Liu, J.; Qian, H.; Luo, Q. A Classification Method for Seed Viability Assessment with Infrared Thermography. Sensors 2017, 17, 845. [Google Scholar] [CrossRef]
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Echegoyen Sanz, Y.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U.; et al. Review on the Processing and Properties of Polymer Nanocomposites and Nanocoatings and Their Applications in the Packaging, Automotive and Solar Energy Fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef]
- Hukins, D.W.; Mahomed, A.; Kukureka, S.N. Accelerated aging for testing polymeric biomaterials and medical devices. Med. Eng. Phys. 2008, 30, 1270–1274. [Google Scholar] [CrossRef]
- Mendoza García, M.A.; Izadifar, M.; Chen, X. Evaluation of PBS Treatment and PEI Coating Effects on Surface Morphology and Cellular Response of 3D-Printed Alginate Scaffolds. J. Funct. Biomater. 2017, 8, 48. [Google Scholar] [CrossRef]
- Uquillas, J.A.; Kishore, V.; Akkus, O. Effects of phosphate-buffered saline concentration and incubation time on the mechanical and structural properties of electrochemically aligned collagen threads. Biomed. Mater. 2011, 6, 035008. [Google Scholar] [CrossRef]
- Loynachan, C.N.; Thomas, M.R.; Gray, E.R.; Richards, D.A.; Kim, J.; Miller, B.S.; Brookes, J.C.; Agarwal, S.; Chudasama, V.; McKendry, R.A.; et al. Platinum Nanocatalyst Amplification: Redefining the Gold Standard for Lateral Flow Immunoassays with Ultrabroad Dynamic Range. ACS Nano 2018, 12, 279–288. [Google Scholar] [CrossRef]
- Jurkiewicz, E.; Husemann, U.; Greller, G.; Barbaroux, M.; Fenge, C. Verification of a new biocompatible single-use film formulation with optimized additive content for multiple bioprocess applications. Biotechnol. Prog. 2014, 30, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Lester, B.R.; Alexander, A.A.; Miller, K.; Boser, N.P.; Sullivan, B.F.; Brucker, G.G. Comparison of performance characteristics between new and reprocessed electrophysiology catheters. J. Interv. Card. Electrophysiol. 2006, 17, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Liu, S.; Fifield, L.S.; Bowler, N. Aging mechanisms of filled cross-linked polyethylene (XLPE) cable insulation material exposed to simultaneous thermal and gamma radiation. Radiat. Phys. Chem. 2021, 185, 109486. [Google Scholar] [CrossRef]
- Mohammadi, H.; Morovati, V.; Korayem, A.-E.; Poshtan, E.; Dargazany, R. Constitutive modeling of elastomers during photo and thermo-oxidative aging. Polym. Degrad. Stab. 2021, 191, 109663. [Google Scholar] [CrossRef]
- Deshoulles, Q.; Le Gall, M.; Dreanno, C.; Arhant, M.; Stoclet, G.; Priour, D.; Le Gac, P.Y. Origin of embrittlement in Polyamide induced by chemical degradations: Mechanisms and governing factors. Polym. Degrad. Stab. 2021, 191, 109657. [Google Scholar] [CrossRef]
- Kotanen, S.; Poikelispää, M.; Efimov, A.; Harjunalanen, T.; Mills, C.; Laaksonen, T.; Sarlin, E. Hydrolytic stability of polyurethane/polyhydroxyurethane hybrid adhesives. Int. J. Adhes. Adhes. 2021, 110, 102950. [Google Scholar] [CrossRef]
- Asadi, H.; Uhlemann, J.; Stranghoener, N.; Ulbricht, M. Artificial weathering mechanisms of uncoated structural Polyethylene Terephthalate fabrics with focus on tensile strength degradation. Materials 2021, 14, 618. [Google Scholar] [CrossRef]
- Lam, C.X.; Savalani, M.M.; Teoh, S.H.; Hutmacher, D.W. Dynamics of in vitro polymer degradation of polycaprolactone-based scaffolds: Accelerated versus simulated physiological conditions. Biomed. Mater. 2008, 3, 34108. [Google Scholar] [CrossRef]
- Chaves, F.O.; Farias, N.C.; Medeiros, L.M.; Alonso, R.C.; Di Hipólito, V.; D’Alpino, P.H. Mechanical properties of composites as functions of the syringe storage temperature and energy dose. J. Appl. Oral Sci. 2015, 23, 120–128. [Google Scholar] [CrossRef][Green Version]
- Kim, J.; Zheng, Z.; Kim, H.; Nam, K.A.; Chung, K.Y. Investigation on the Cutaneous Change Induced by Face-Lifting Monodirectional Barbed Polydioxanone Thread. Dermatol. Surg. 2017, 43, 74–80. [Google Scholar] [CrossRef]
- Villa, M.T.; White, L.E.; Alam, M.; Yoo, S.S.; Walton, R.L. Barbed sutures: A review of the literature. Plast. Reconstr. Surg. 2008, 121, 102e–108e. [Google Scholar] [CrossRef] [PubMed]
- Consiglio, F.; Pizzamiglio, R.; Parodi, P.C.; De Biasio, F.; Machin, P.N.; Di Loreto, C.; Gamboa, M. Suture With Resorbable Cones: Histology and Physico-Mechanical Features. Aesthetic Surg. J. 2016, 36, NP122–NP127. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Lee, W.S.; Hwang, K.; Park, J.H.; Kim, D.J. Effect of cog threads under rat skin. Dermatol. Surg. 2005, 31, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).