Multi-Directional Shape Change Analysis of Biotensegrity Model Mimicking Human Spine Curvature
Abstract
:Featured Application
Abstract
1. Introduction
2. Spine Biotensegrity Models
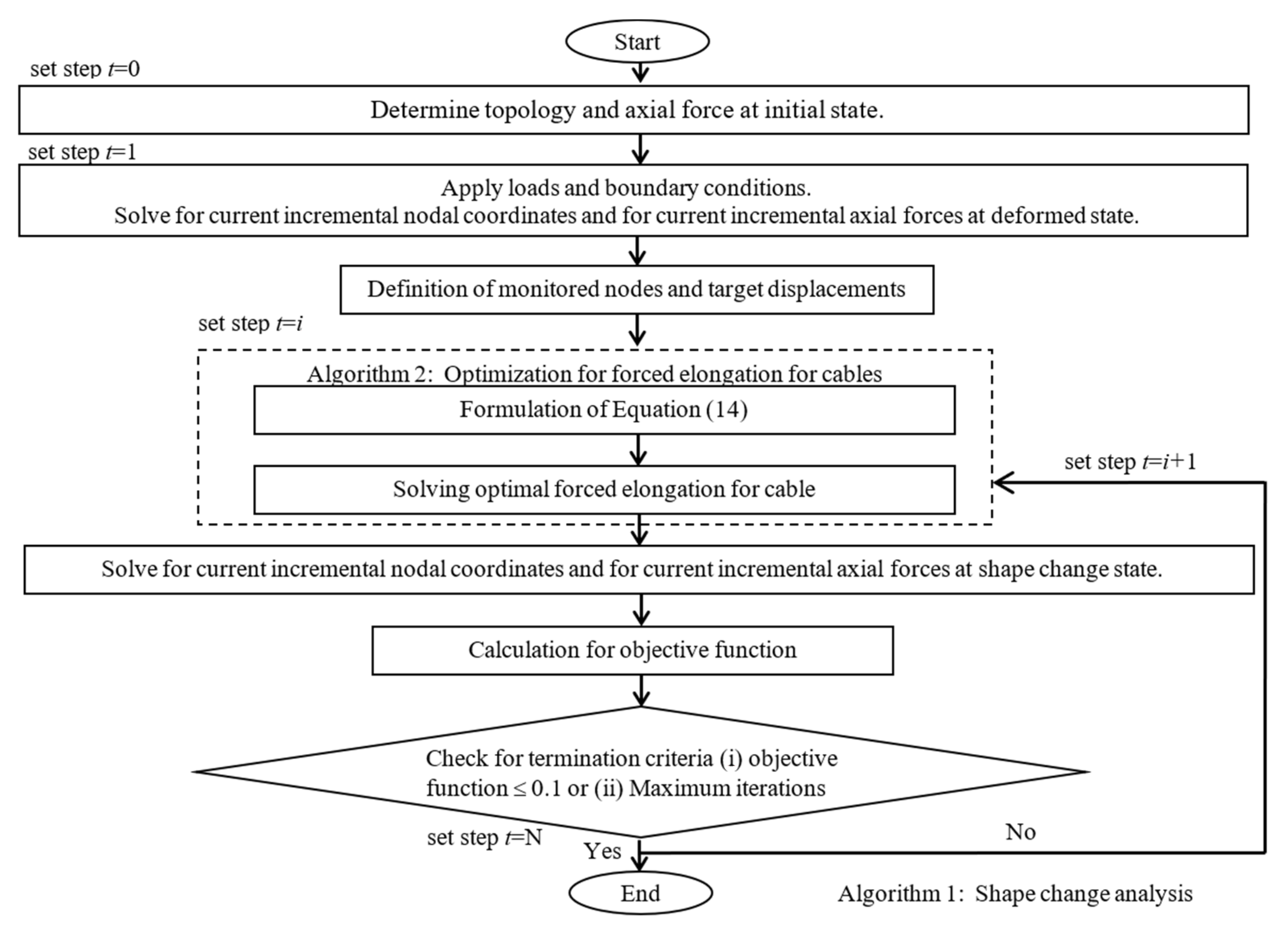
3. Shape Change Strategies for Spine Biotensegrity Models
3.1. Formulation of Incremental Equilibrium Equations
3.2. Optimization by Sequential Quadratic Programming
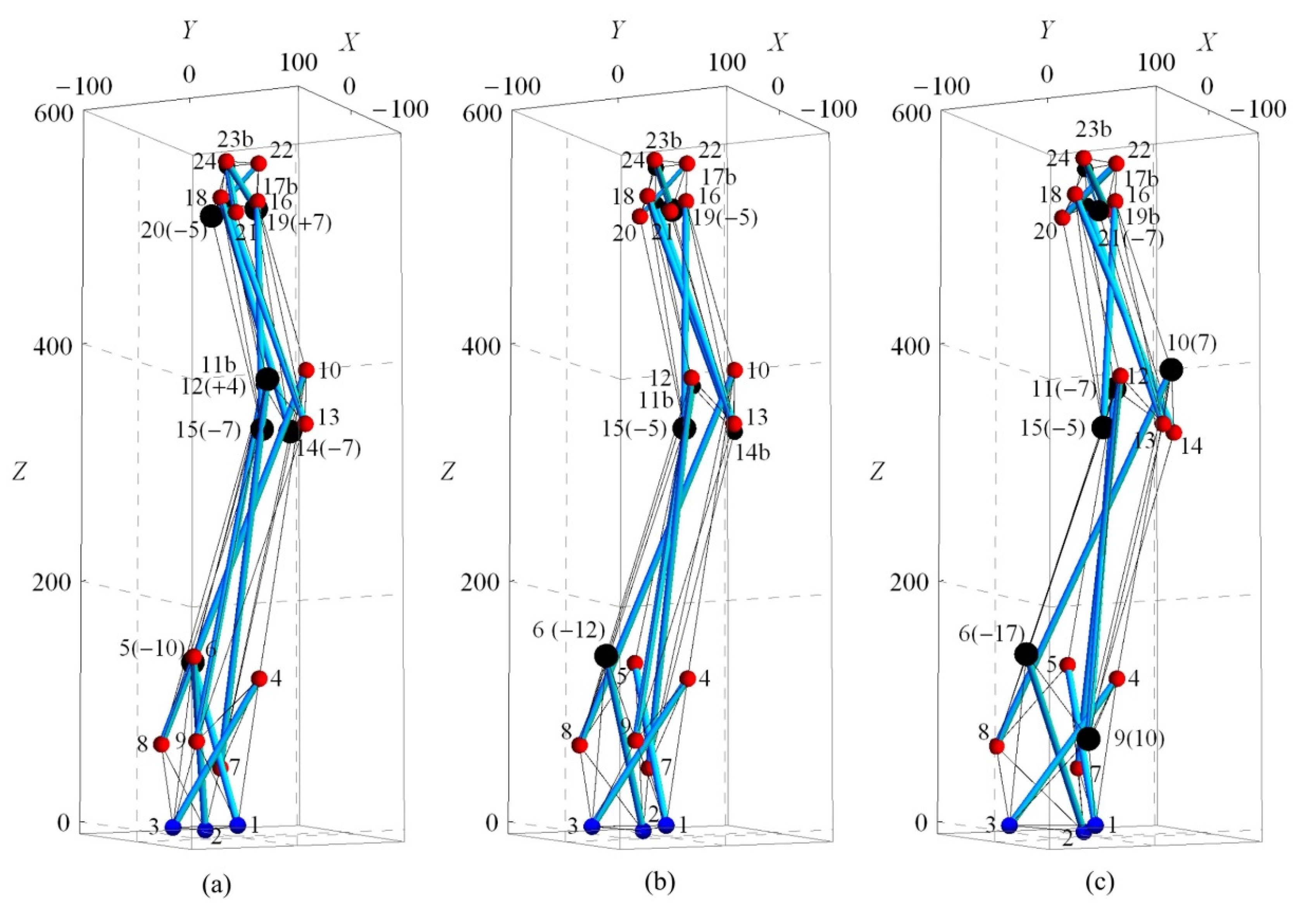
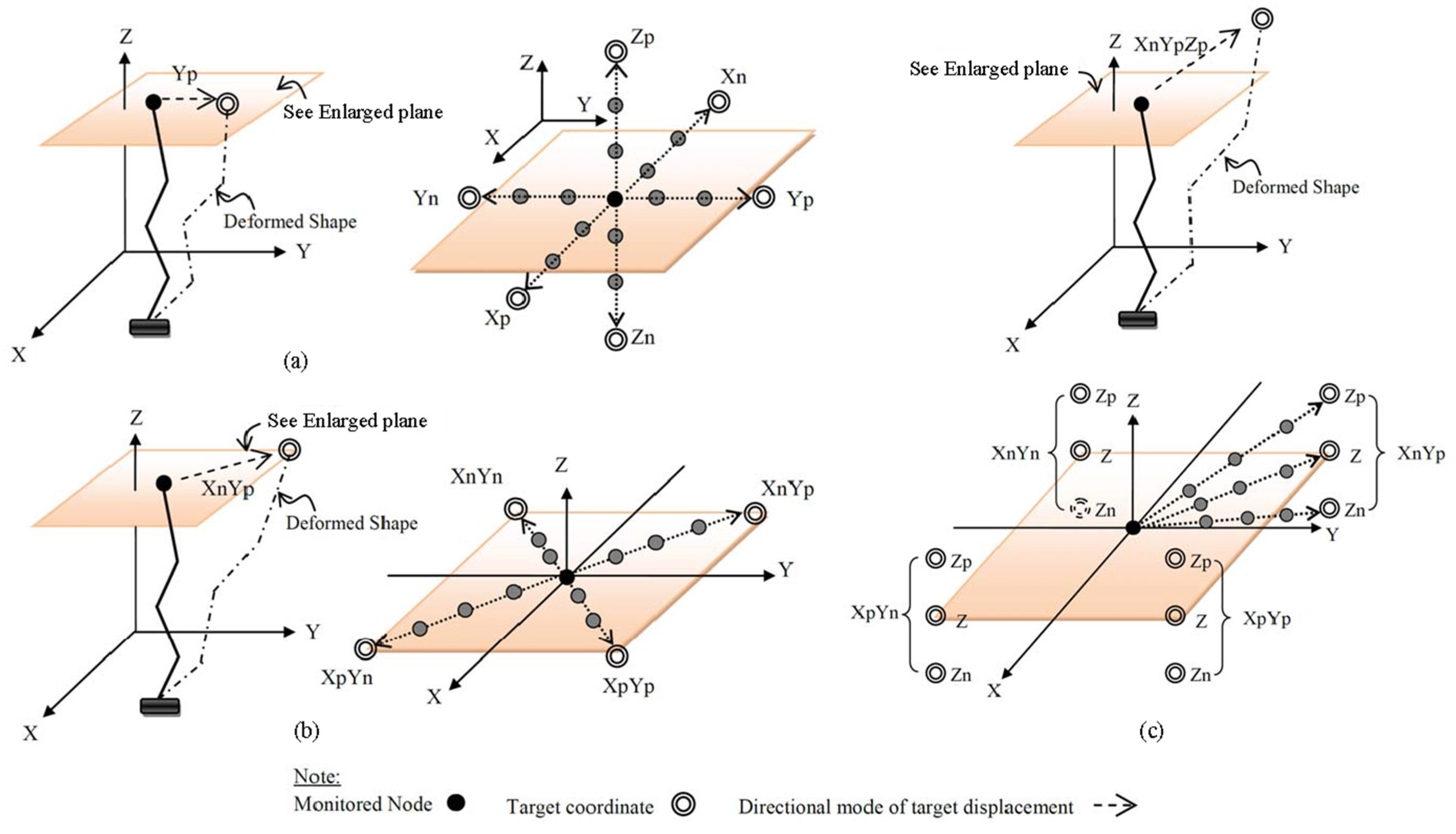
4. Numerical Examples
4.1. Total Computational Step
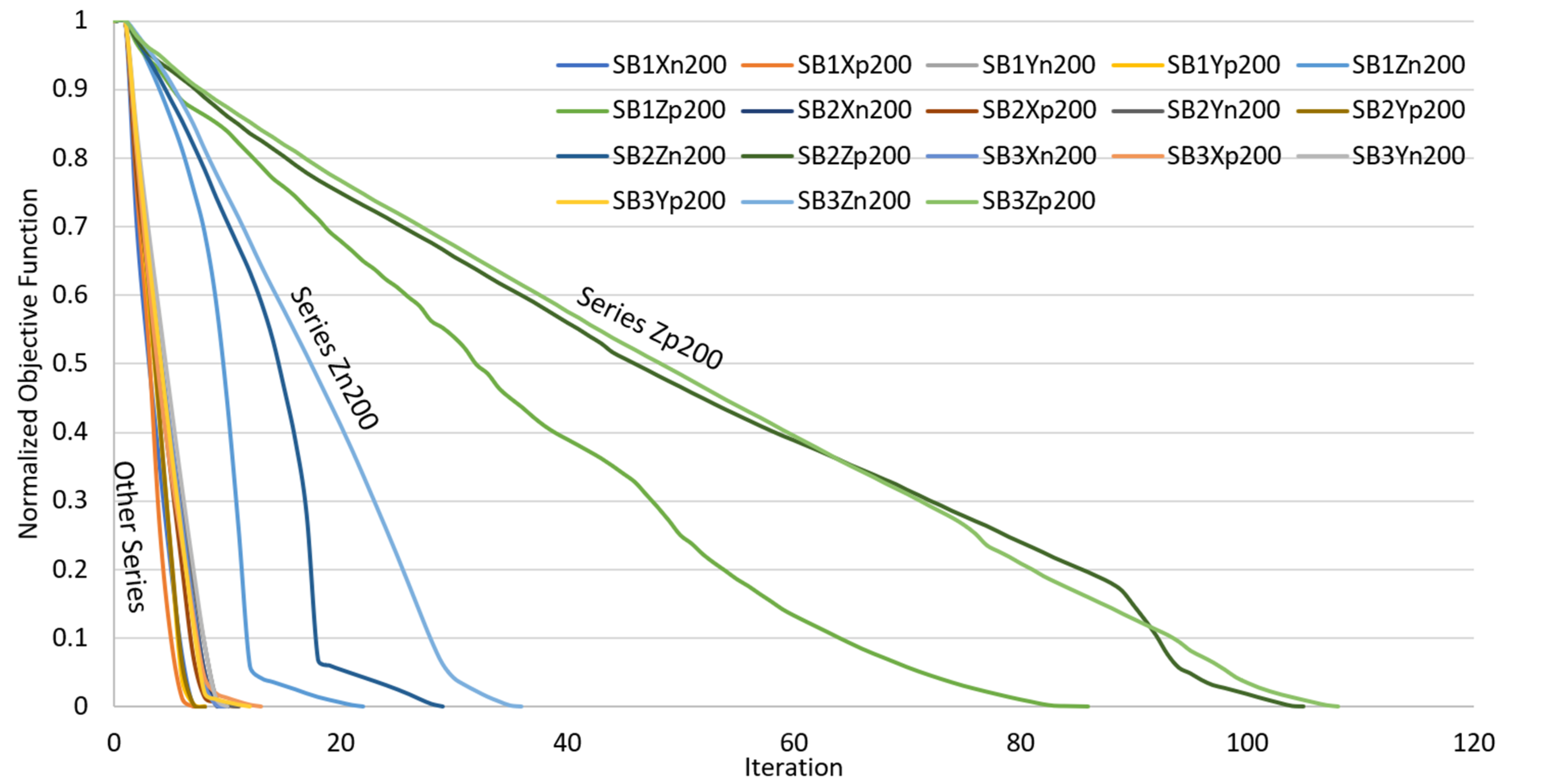
4.2. Convergence History
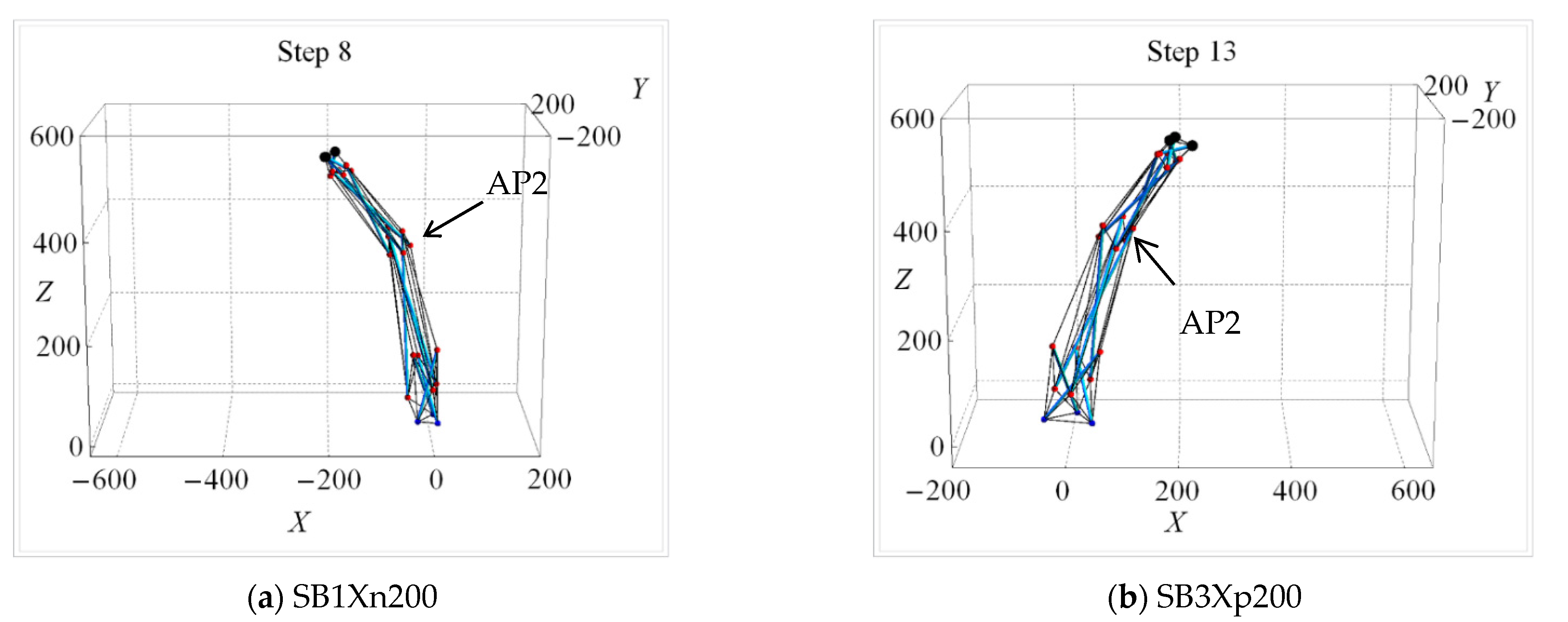
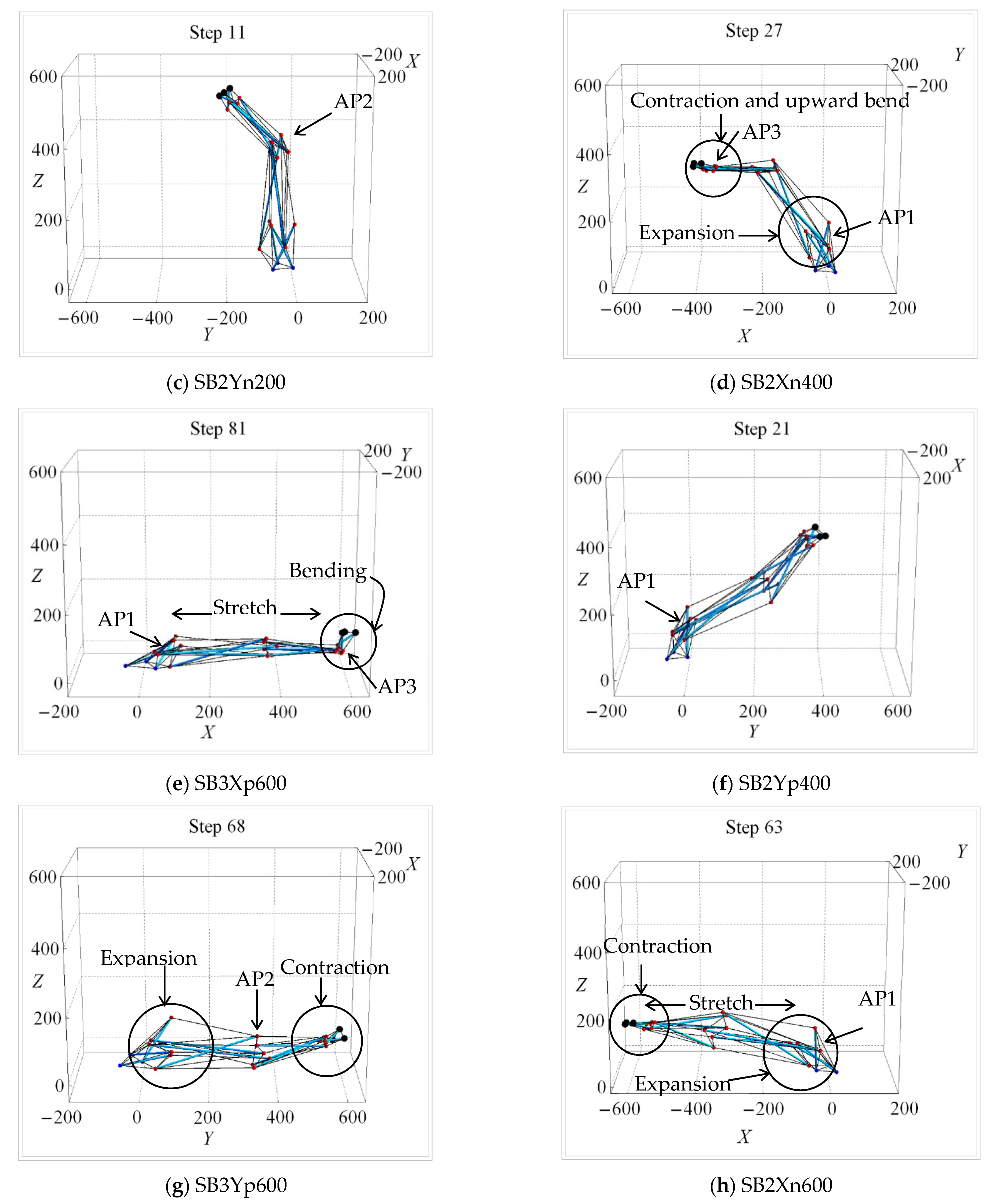
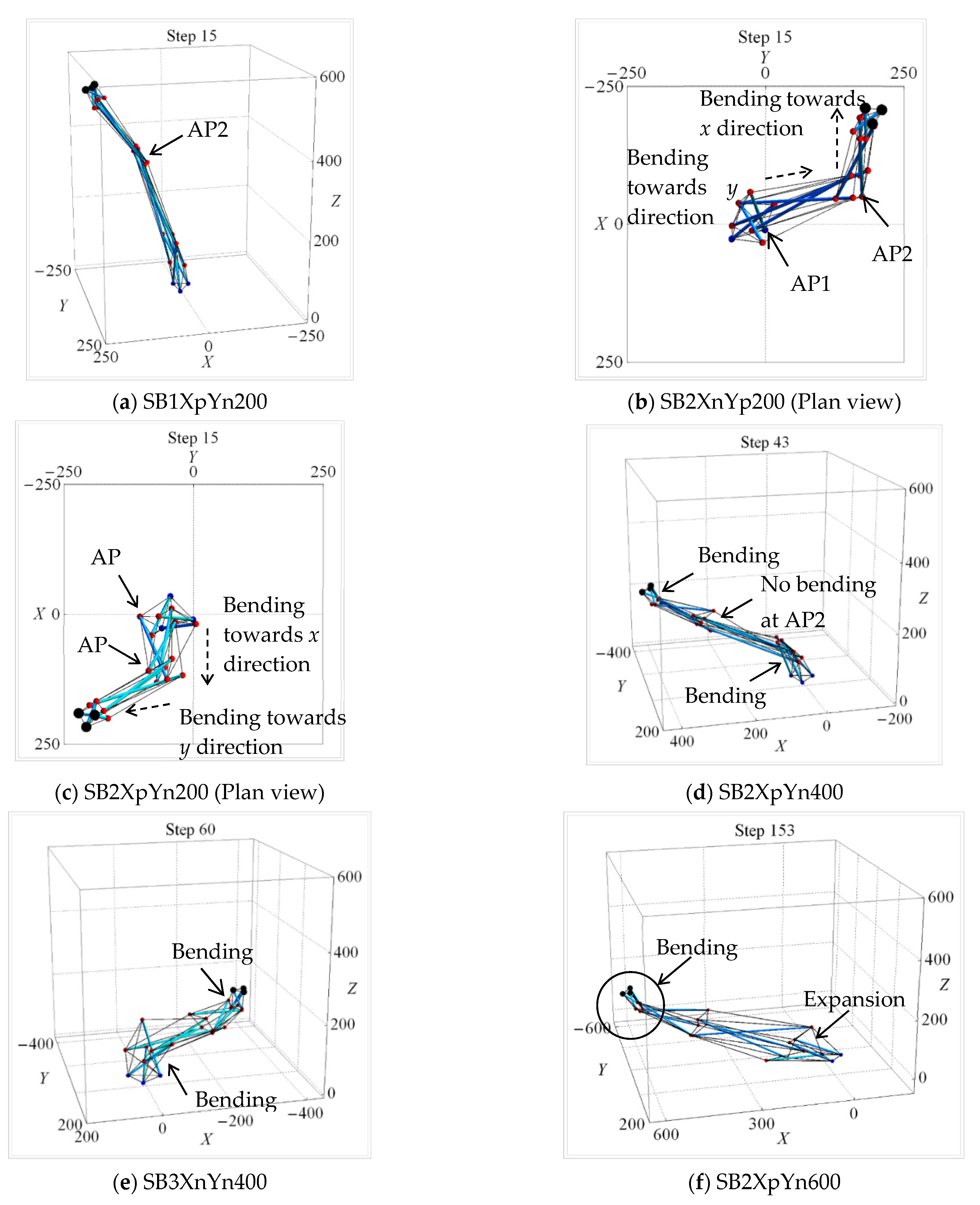
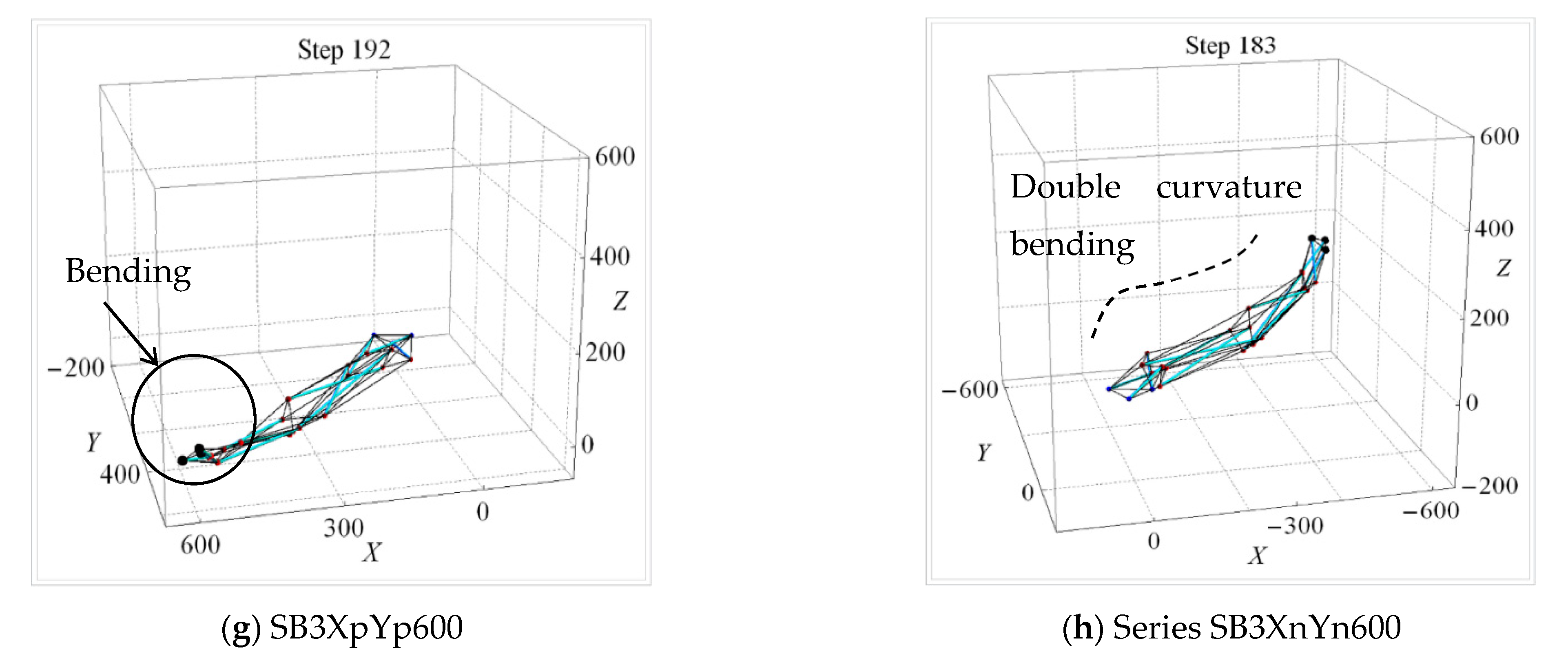
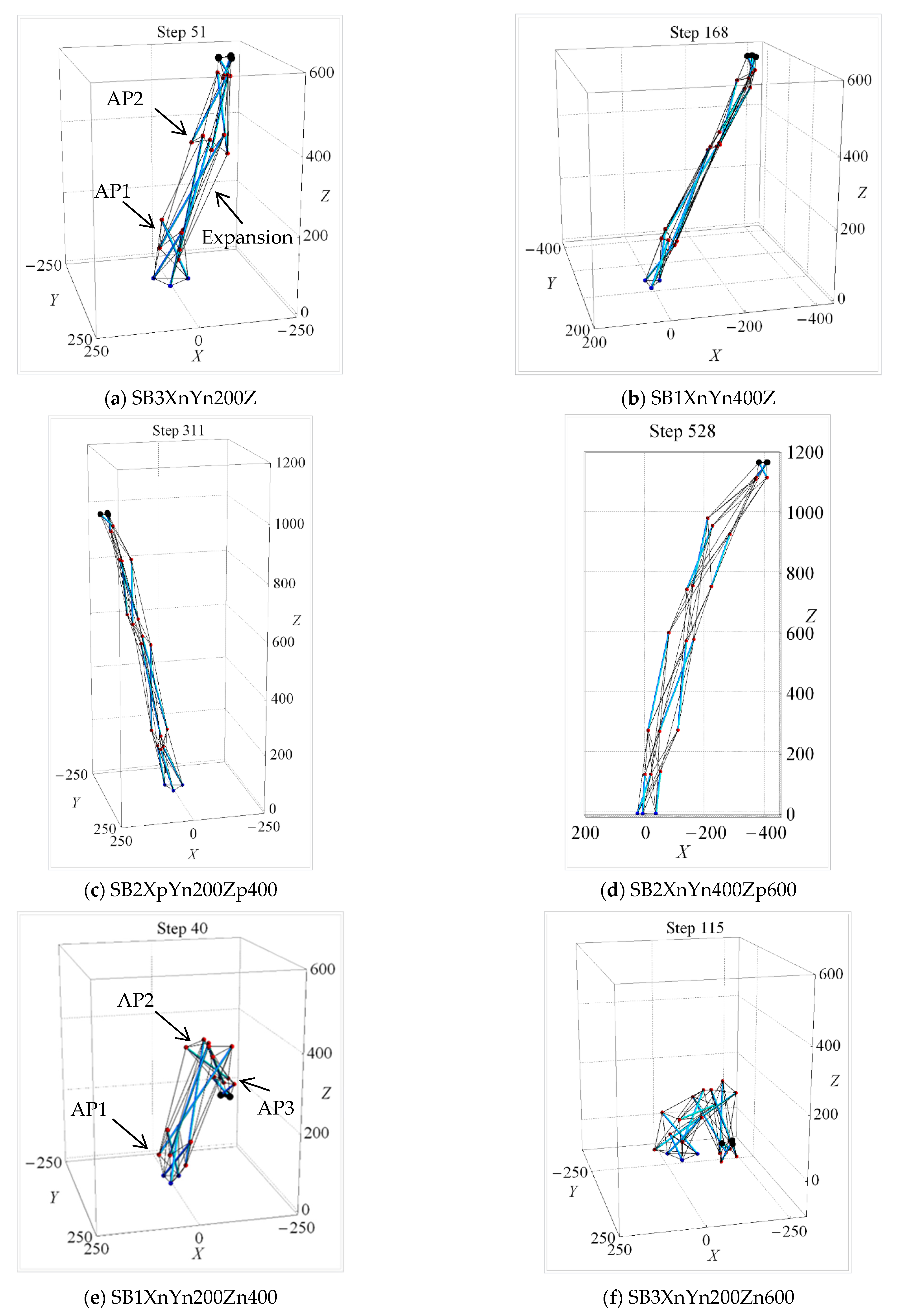
4.3. Deformed Modes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Ac | Cross sectional area of cable |
| As | Cross sectional area of strut |
| B | Matrix of directional cosines |
| Es | Young modulus of strut |
| F | Vector of nodal forces |
| H | Hessian matrix |
| Is | Moment inertia of section |
| kg | Geometrical stiffness matrix |
| ke | Linear stiffness matrix |
| K | structure tangent stiffness matrix |
| l | Length of member |
| Vector of incremental force elongation | |
| nc | Axial forces of cable |
| ns | Axial forces of strut |
| n | Vector of axial forces |
| Vector of incremental axial forces | |
| t | Current step |
| t − 1 | Previous step |
| u | Elastic elongation |
| x | Vector of nodal coordinates |
| Vector of incremental nodal coordinates | |
| Prescribed target coordinates | |
| Nodal coordinates for monitored node | |
| λ | Vector of directional cosines |
| σc | Yield stress of cable |
| σs | Yield stress of strut |
Appendix A
| Nodes | SB1 | SB2 | SB3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | x | y | z | |
| 1 | 9.33 | −1.23 | 0 | 9.33 | -1.23 | 0 | 9.33 | −1.23 | 0 |
| 2 | −24.01 | −48.3 | 0 | −35.34 | −45.27 | 0 | −49.24 | -41.54 | 0 |
| 3 | 14.67 | −58.67 | 0 | 26 | −61.7 | 0 | 39.91 | −65.43 | 0 |
| 4 | −8.9 | 9.49 | 123.78 | −8.9 | 9.49 | 123.78 | −8.9 | 9.49 | 123.78 |
| 5 | −14.71 | −55.48 | 141.36 | −25.95 | −48.49 | 141.36 | −39.72 | −52.18 | 141.36 |
| 6 | 23.61 | −35.21 | 141.36 | 34.85 | −44.2 | 141.36 | 48.62 | −45.51 | 141.36 |
| 7 | −33.22 | -39 | 52.83 | −33.22 | −39 | 52.83 | −33.22 | −39 | 52.83 |
| 8 | 11.48 | −71.52 | 70.41 | 8.47 | −82.75 | 70.41 | 4.77 | −96.53 | 70.41 |
| 9 | 21.75 | −33.19 | 70.41 | 24.76 | −21.96 | 70.41 | 28.45 | 1.82 | 70.41 |
| 10 | −10.04 | 52.87 | 382.82 | −10.04 | 52.87 | 382.82 | −10.04 | 59.87 | 382.82 |
| 11 | −7.19 | 14.89 | 372.81 | −14.34 | 11.56 | 372.81 | −23.12 | 0.46 | 372.81 |
| 12 | 17.23 | 30.28 | 372.81 | 24.38 | 29.61 | 372.81 | 33.16 | 33.7 | 372.81 |
| 13 | 14.15 | 63.71 | 332.85 | 14.15 | 63.71 | 332.85 | 14.15 | 63.71 | 332.85 |
| 14 | −18.11 | 34.13 | 332.85 | −24.57 | 45.65 | 332.85 | −32.51 | 51.21 | 332.85 |
| 15 | 3.96 | 18.67 | 332.85 | 10.42 | 16.15 | 332.85 | 18.36 | 10.49 | 332.85 |
| 16 | −5.08 | 10.05 | 531.04 | −5.08 | 10.05 | 531.04 | −5.08 | 10.05 | 531.04 |
| 17 | −7.84 | −21.15 | 533.31 | −13.91 | −22.77 | 533.31 | −21.37 | −24.77 | 533.31 |
| 18 | 12.91 | −15.59 | 533.31 | 18.99 | −13.96 | 533.31 | 26.44 | −11.96 | 533.31 |
| 19 | −18.29 | 2.21 | 527.57 | −18.29 | −9.79 | 527.57 | −18.29 | −4.79 | 527.57 |
| 20 | 6.37 | −27.52 | 519.59 | 4.74 | −28.59 | 519.59 | 2.74 | −36.05 | 519.59 |
| 21 | 11.93 | −1.77 | 519.59 | 13.56 | 4.31 | 519.59 | 15.55 | 4.77 | 519.59 |
| 22 | −7.58 | 9.77 | 564.29 | −7.58 | 9.77 | 564.29 | −7.58 | 9.77 | 564.29 |
| 23 | −5.12 | −18.78 | 564.29 | −10.33 | −21.21 | 564.29 | −16.74 | −24.21 | 564.29 |
| 24 | 12.7 | −10.47 | 564.29 | 17.91 | −8.04 | 564.29 | 24.33 | −5.05 | 564.29 |
| Elastic Modulus, E (MPa) | Strut and Cable Yield Stress, σs, σc (MPa) | Density, γ (kg/mm3) | Strut Cross Sectional Area, As (mm2) | Cable Cross Sectional Area, Ac (mm2) |
|---|---|---|---|---|
| 20,000 | 250 | 7.7 × 10-6 | 50.30 | 3.14 |
References
- Swanson, R.L. Biotensegrity: A Unifying Theory of Biological Architecture with Applications to Osteopathic Practice, Education, and Research—A Review and Analysis. J. Osteopat. Med. 2013, 113, 34–52. [Google Scholar] [CrossRef] [Green Version]
- Bordoni, B.; Myers, T. A Review of the Theoretical Fascial Models: Biotensegrity, Fascintegrity, and Myofascial Chains. Cureus 2020, 12, e7092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowle, A.; Harley, C. Development of a biotensegrity focused therapy for the treatment of pelvic organ prolapse: A retrospective case series. J. Bodyw. Mov. Ther. 2020, 24, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, J. Biotensegrity-Anatomy for the 21st Century Informing Yoga and Physiotherapy Concerning New Findings in Fascia Research. J. Yoga Physiother. 2018, 6, 1–4. [Google Scholar] [CrossRef]
- Oh, C.L.; Choong, K.K.; Nishimura, T.; Kim, J.-Y. Form-finding of spine inspired biotensegrity model. Appl. Sci. 2020, 10, 6344. [Google Scholar] [CrossRef]
- Kan, Z.; Peng, H.; Chen, B.; Zhong, W. Nonlinear dynamic and deployment analysis of clustered tensegrity structures using a positional formulation FEM. Compos. Struct. 2018, 187, 241–258. [Google Scholar] [CrossRef]
- Kan, Z.; Peng, H.; Chen, B.; Zhong, W. A sliding cable element of multibody dynamics with application to nonlinear dynamic deployment analysis of clustered tensegrity. Int. J. Solids Struct. 2018, 130–131, 61–79. [Google Scholar] [CrossRef]
- Zhu, D.; Deng, H. Deployment of tensegrities subjected to load-carrying stiffness constraints. Int. J. Solids Struct. 2020, 206, 224–235. [Google Scholar] [CrossRef]
- Cai, H.; Wang, M.; Xu, X.; Luo, Y. A General Model for Both Shape Control and Locomotion Control of Tensegrity Systems. Front. Built Environ. 2020, 6, 98. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Q.; Liu, Y.; Zhang, H. An efficient finite element formulation for nonlinear analysis of clustered tensegrity. Eng. Comput. 2016, 33, 252–273. [Google Scholar] [CrossRef]
- Veuve, N.; Safaei, S.D.; Smith, I.F. Active control for mid-span connection of a deployable tensegrity footbridge. Eng. Struct. 2016, 112, 245–255. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Sultan, C. Deployment of foldable tensegrity-membrane systems via transition between tensegrity configurations and tensegrity-membrane configurations. Int. J. Solids Struct. 2019, 160, 103–119. [Google Scholar] [CrossRef]
- Yıldız, K.; Lesieutre, G.A. Deployment of n-Strut Cylindrical Tensegrity Booms. J. Struct. Eng. 2020, 146, 04020247. [Google Scholar] [CrossRef]
- Shintake, J.; Zappetti, D.; Peter, T.; Ikemoto, Y.; Floreano, D. Bio-inspired Tensegrity Fish Robot. In Proceedings of the 2020 IEEE International Conference on Robotics and Automation (ICRA), Paris, France, 31 May–31 August 2020; pp. 2887–2892. [Google Scholar]
- Ramadoss, V.; Sagar, K.; Ikbal, M.S.; Calles, J.H.L.; Zoppi, M. HEDRA: A Bio-Inspired Modular Tensegrity Soft Robot with Polyhedral Parallel Modules. arXiv 2011, arXiv:142402020. [Google Scholar]
- Wei, D.; Gao, T.; Mo, X.; Xi, R.; Zhou, C. Flexible Bio-tensegrity Manipulator with Multi-degree of Freedom and Variable Structure. Chin. J. Mech. Eng. 2020, 33, 3. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Li, Q.; Wang, P.; Guo, F. Kinematic and static analysis of a novel tensegrity robot. Mech. Mach. Theory 2020, 149, 103788. [Google Scholar] [CrossRef]
- Oh, C.L.; Choong, K.K.; Nishimura, T.; Kim, J.-Y.; Hassanshahi, O. Shape change analysis of tensegrity models. Lat. Am. J. Solids Struct. 2019, 16, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Busscher, I.; Ploegmakers, J.J.W.; Verkerke, G.J.; Veldhuizen, A.G. Comparative anatomical dimensions of the complete human and porcine spine. Eur. Spine J. 2010, 19, 1104–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]





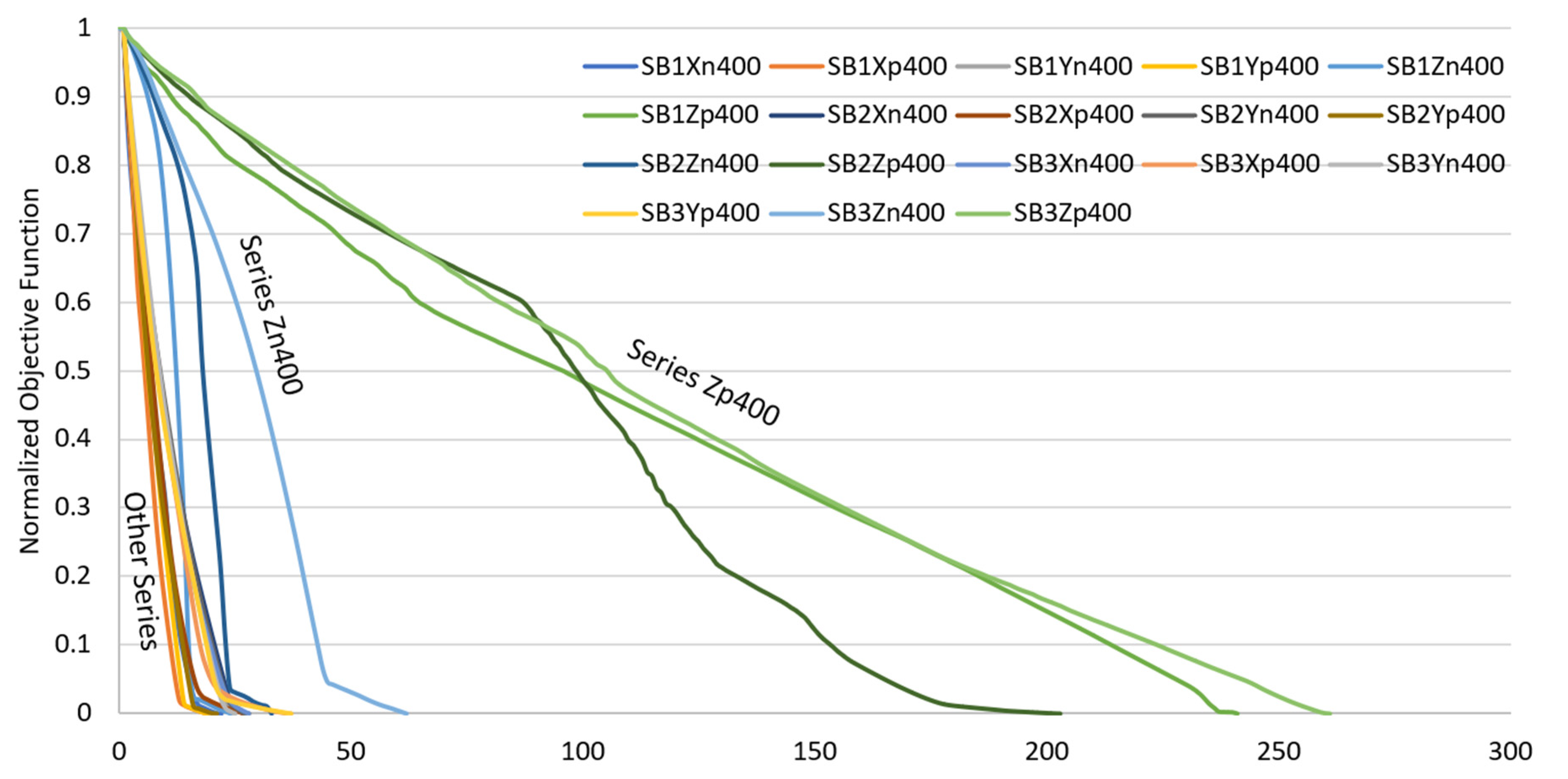
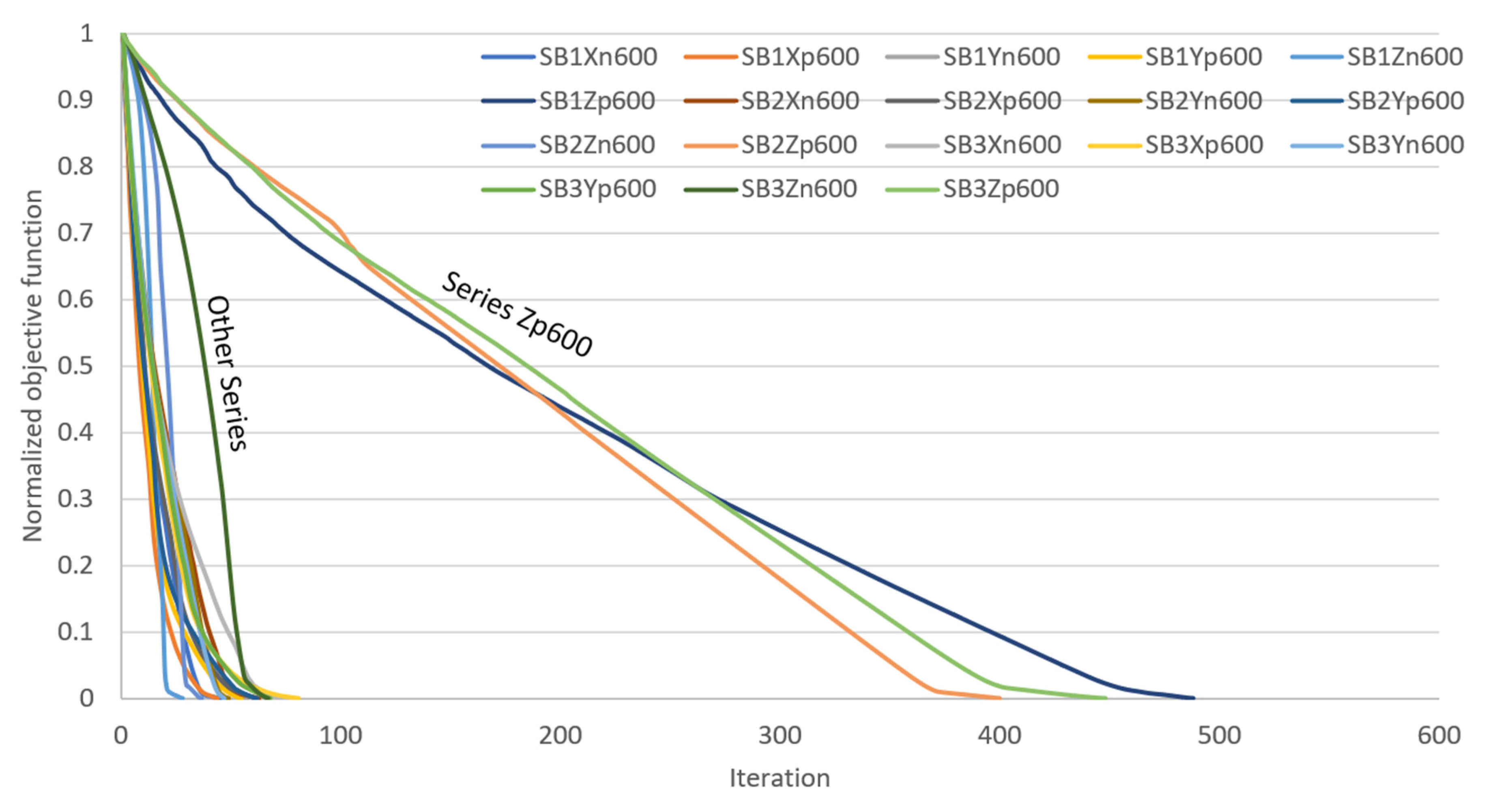
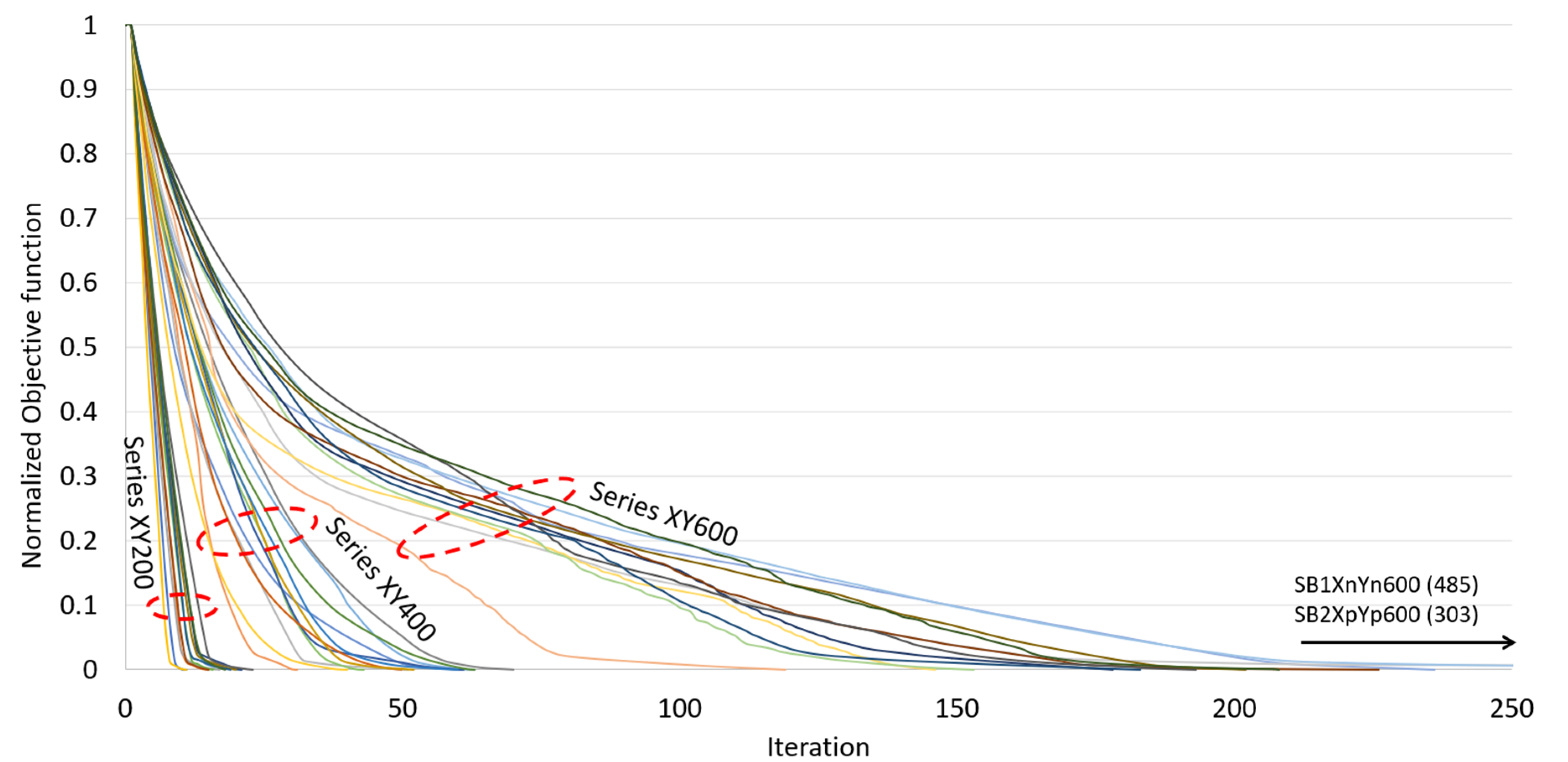

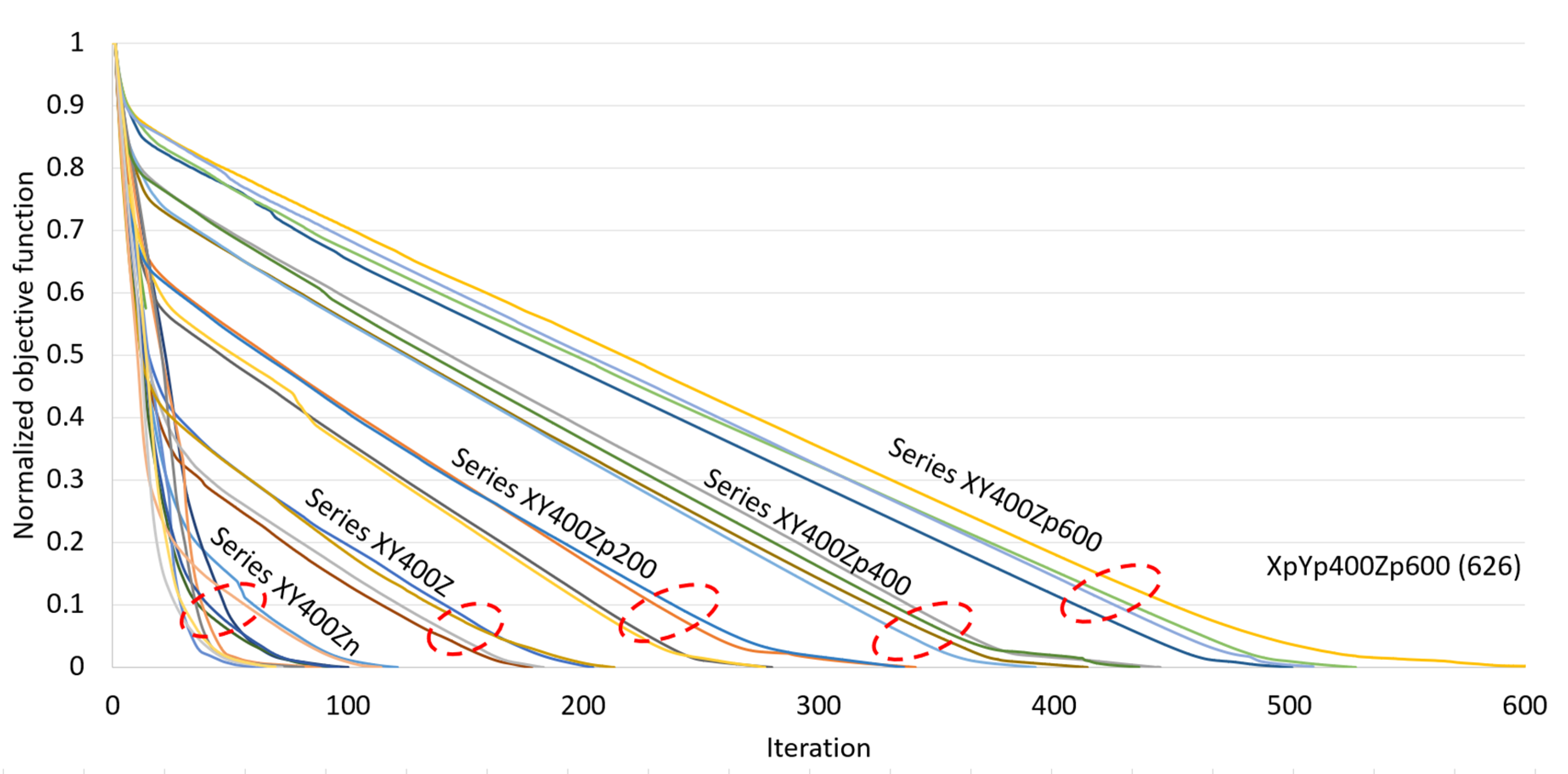


| Uni-Directional Mode | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Target Displacement (mm) | Case | Target Displacement (mm) | Case | Target Displacement (mm) | ||||||
| x | y | z | x | y | z | x | y | z | |||
| Xn200 | −200 | - | - | Xp200 | 200 | - | - | Zp200 | - | - | 200 |
| Xn400 | −400 | - | - | Xp400 | 400 | - | - | Zp400 | - | - | 400 |
| Xn600 | −600 | - | - | Xp600 | 600 | - | - | Zp600 | - | - | 600 |
| Bi-directional mode | |||||||||||
| XpYp200 | 200 | 200 | - | XnYn200 | −200 | −200 | - | XpYn200 | 200 | −200 | - |
| XpYp400 | 400 | 400 | - | XnYn400 | −400 | −400 | - | XpYn400 | 400 | −400 | - |
| XpYp600 | 600 | 600 | - | XnYn600 | −600 | −600 | - | XpYn600 | 600 | −600 | - |
| Tri-directional mode | |||||||||||
| XpYp200Z | 200 | 200 | - | XpYn200Z | 200 | −200 | - | XnYn200Z | −200 | −200 | - |
| XpYp200 Zp200 | 200 | 200 | 200 | XpYn200 Zn400 | 200 | −200 | −400 | XnYn200 Zp600 | −200 | −200 | 600 |
| XpYp400 Zp200 | 400 | 400 | 200 | XpYn400 Zn200 | 400 | −400 | −200 | XnYn400 Zn200 | −400 | −400 | −200 |
| XpYp400 Zn200 | 400 | 400 | −200 | XpYn400 Zp400 | 400 | −400 | 400 | XnYn400 Zn600 | −400 | −400 | −600 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, C.L.; Choong, K.K.; Nishimura, T.; Kim, J.-Y. Multi-Directional Shape Change Analysis of Biotensegrity Model Mimicking Human Spine Curvature. Appl. Sci. 2022, 12, 2377. https://doi.org/10.3390/app12052377
Oh CL, Choong KK, Nishimura T, Kim J-Y. Multi-Directional Shape Change Analysis of Biotensegrity Model Mimicking Human Spine Curvature. Applied Sciences. 2022; 12(5):2377. https://doi.org/10.3390/app12052377
Chicago/Turabian StyleOh, Chai Lian, Kok Keong Choong, Toku Nishimura, and Jae-Yeol Kim. 2022. "Multi-Directional Shape Change Analysis of Biotensegrity Model Mimicking Human Spine Curvature" Applied Sciences 12, no. 5: 2377. https://doi.org/10.3390/app12052377







