Hybrid In Situ Reinforcement of EPDM Rubber Compounds Based on Phenolic Novolac Resin and Ionic Coagent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Processing of EPDM Compounds
2.3. Characterization of Nonvulcanized Compounds
2.4. Material Characterization of EPDM Vulcanizates
2.5. Dynamic Mechanical Analysis
2.6. Morphology
2.7. Equilibrium Swelling and Rubber Gel Content
3. Results and Discussion
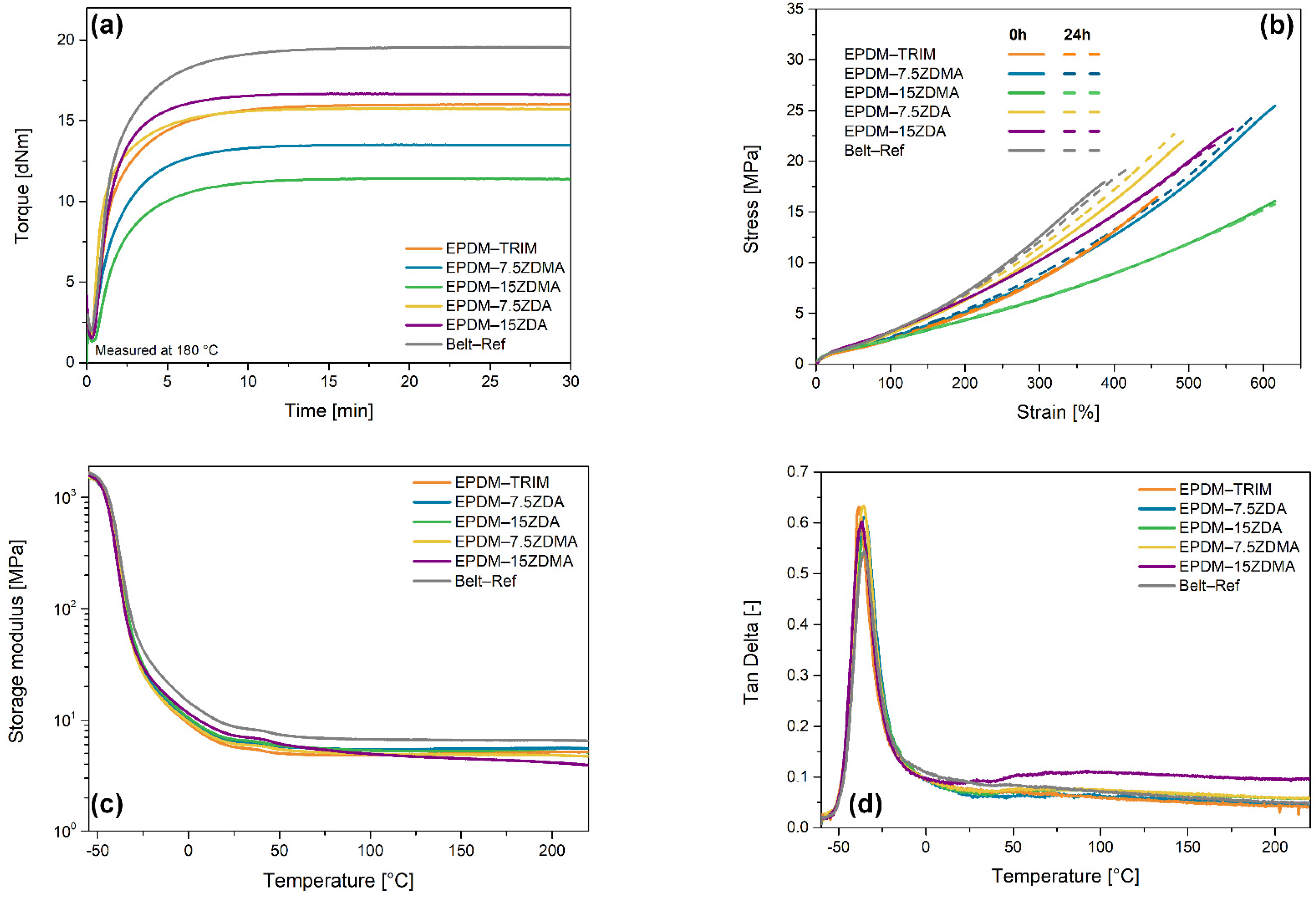
3.1. Influence of Ionic Coagents on Reinforcement
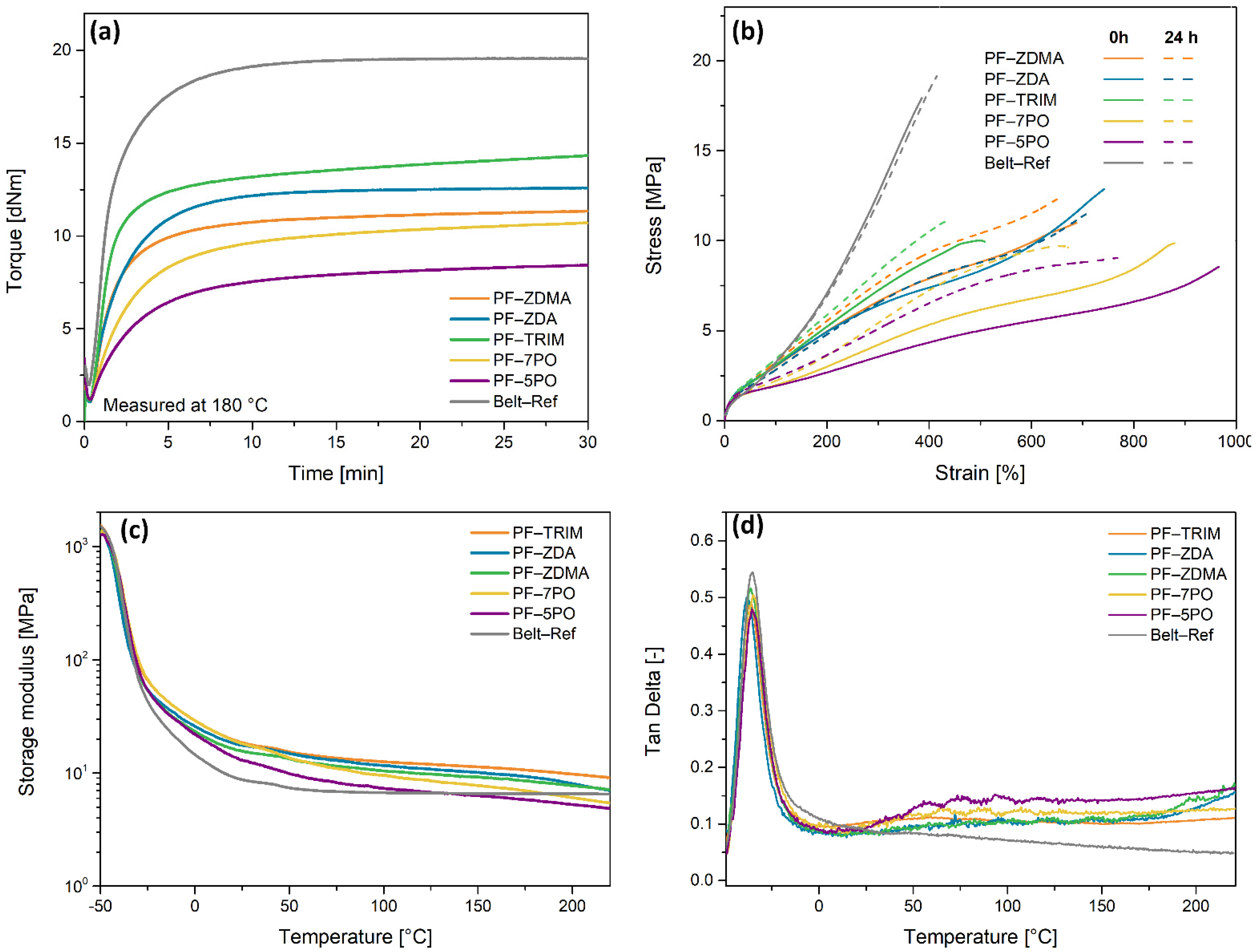
3.2. Rheological Characterization and Curing Behavior of Hybrid Reinforced EPDM Compounds
3.3. Mechanical Properties of EPDM–PF Compounds
3.4. Microscopic Analysis of Tear Fractures
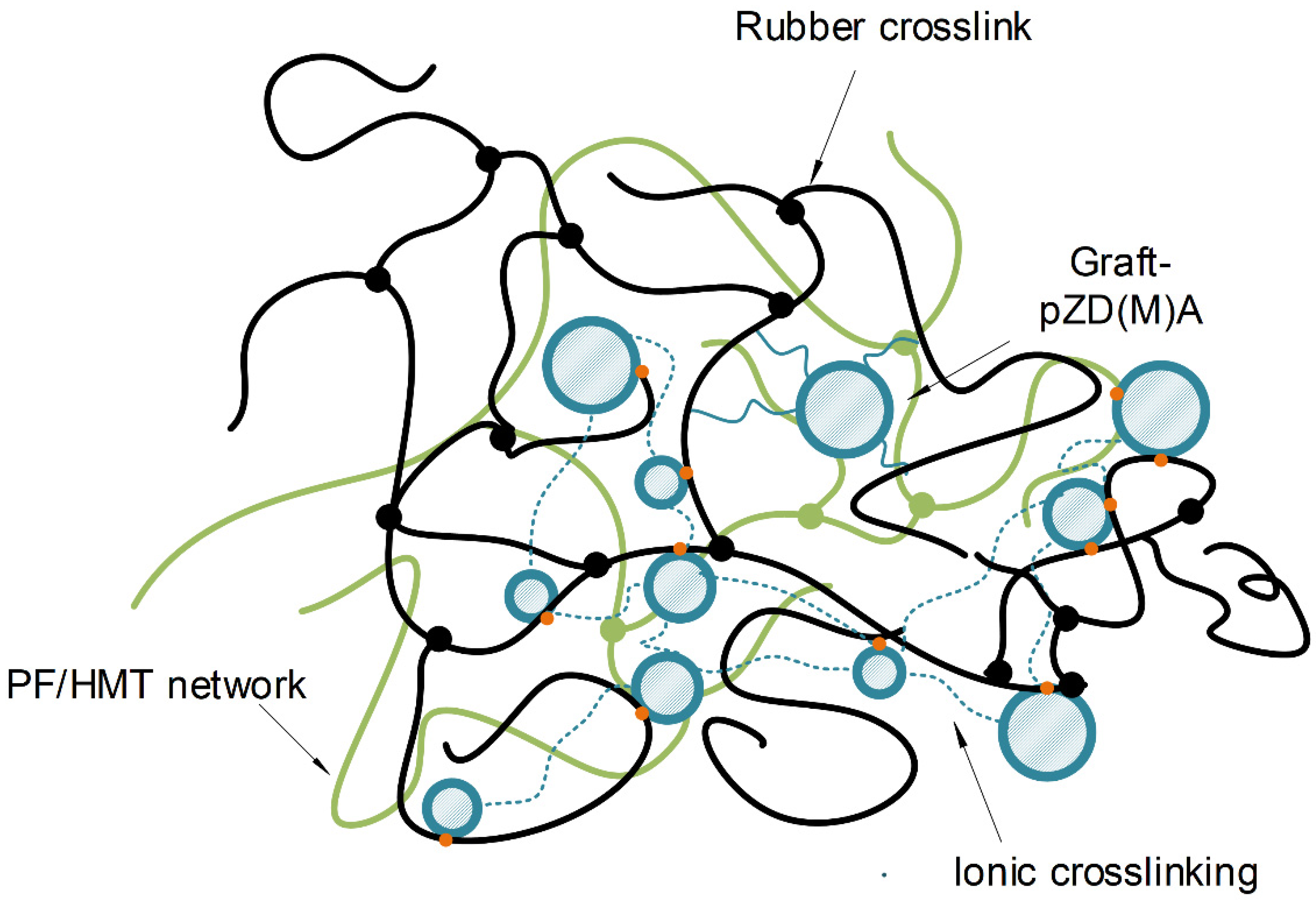
3.5. Proposed Structure of Ionic Hybrid Systems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arlanxeo, D.G. (Ed.) Handbook of Synthetic Rubber, 1st ed.; Arlanxeo Deutschland GmbH: Dormagen, Germany, 2020; ISBN 978-3-00-067354-2. [Google Scholar]
- Ling, X.; Hu, Y.L.; Lai, W.W.; Wang, Y. Formulation of EPDM heat-resistant V-ribbed belt. IOP Conf. Ser. Mater. Sci. Eng. 2020, 770, 12029. [Google Scholar] [CrossRef] [Green Version]
- Strohmeier, L.; Schrittesser, B.; Schlögl, S. Approaches Toward In Situ Reinforcement of Organic Rubbers: Strategy and Recent Progress. Polym. Rev. 2021, 62, 142–174. [Google Scholar] [CrossRef]
- Koga, T.; Takenaka, M.; Aizawa, K.; Nakamura, M.; Hashimoto, T. Structure factors of dispersible units of carbon black filler in rubbers. Langmuir 2005, 21, 11409–11413. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, J.L. Rubber-filler interactions and rheological properties in filled compounds. Prog. Polym. Sci. 2002, 27, 627–687. [Google Scholar] [CrossRef]
- Yuan, W.Q.; Mark, J.E. Reinforcement of poly(dimethylsiloxane) networks by blended and in-situ generated silica fillers having various sizes, size distributions, and modified surfaces. Macromol. Chem. Phys. 1999, 200, 206–220. [Google Scholar] [CrossRef]
- Balasooriya, W.; Schrittesser, B.; Pinter, G.; Schwarz, T.; Conzatti, L. The Effect of the Surface Area of Carbon Black Grades on HNBR in Harsh Environments. Polymers 2019, 11, 61. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, Y.; Kohjiya, S. In situ formed silica particles in rubber vulcanizate by the sol-gel method. Polymer 1997, 38, 4417–4423. [Google Scholar] [CrossRef]
- Pal, P.K.; De, S.K. Effect of reinforcing silica on vulcanization, network structure, and technical properties of natural rubber. Rubber Chem. Technol. 1982, 55, 1370–1388. [Google Scholar] [CrossRef]
- Wang, G.; Yuan, J. Interpenetrating Polymer Networks; Klempner, D., Sperling, L.H., Utracki, L.A., Eds.; American Chemical Society: Washington, DC, USA, 1994; ISBN 0-8412-2528-1. [Google Scholar]
- Sperling, L.H.; Arnts, R.R. Simultaneous interpenetrating networks. J. Appl. Polym. Sci. 1971, 15, 2317–2319. [Google Scholar] [CrossRef]
- Frisch, K.C.; Klempner, D.; Mukherjee, S.K.; Frisch, H.L. Stress-strain properties and thermal resistance of polyurethane-polyepoxide interpenetrating polymer networks. J. Appl. Polym. Sci. 1974, 18, 689–698. [Google Scholar] [CrossRef]
- Blum, A.W. Phenolic resins in rubber compounds: Applications and new developments. Rubber World 2011, 243, 28. [Google Scholar]
- Akrochem. Reinforcing Phenolic Resins. Available online: https://www.akrochem.com/solutions_technical_papers.php (accessed on 11 November 2021).
- Nieberle, J.; Paulus, G.; Queins, H.; Schöppl, H. Über die Wirkung von Phenol-Formaldehyd-Novokalen im Kaustschuk. Kautsch. Und Gummi Kunstst. 1986, 39, 108–114. [Google Scholar]
- Syed, I.H.; Klat, D.; Braer, A.; Fleck, F.; Lacayo-Pineda, J. Characterizing the influence of reinforcing resin on the structure and the mechanical response of filled isoprene rubber. Soft Mater. 2018, 16, 275–288. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, Y. In-situ polymerized phenolic resin for reinforcement of chloroprene and ethylene-propylene rubber vulcanizates. J. Appl. Polym. Sci. 2009, 111, 1177–1184. [Google Scholar] [CrossRef]
- Preedy, V.R. Coffee in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2014; ISBN 0124167160. [Google Scholar]
- Strohmeier, L.; Frommwald, H.; Schlögl, S. Digital light processing 3D printing of modified liquid isoprene rubber using thiol-click chemistry. RSC Adv. 2020, 10, 23607–23614. [Google Scholar] [CrossRef]
- Henning, S.K.; Costin, R. Fundamentals of curing elastomers with peroxides and coagents I: Coagent structure-property relationships. In 167th Technical Meeting of the Rubber Division; American Chemical Society: Washington, DC, USA, 2005; pp. 16–18. [Google Scholar]
- Costin, R.; Nagel, W.; Ekwall, R. New Metallic Coagents for Curing Elastomers. Rubber Chem. Technol. 1991, 64, 152–161. [Google Scholar] [CrossRef]
- Testing of Rubber—Determination of Tensile Strength at Break, Tensile Stress at Yield, Elongation at Break and Stress Values in a Tensile Test; Beuth Verlag GmbH: Berlin, Germany, 2017; p. 53504.
- Rubber, Vulcanized or Thermoplastic—Determination of Tear Strength—Part 1: Trouser, Angle and Crescent Test Pieces; Beuth Verlag GmbH: Berlin, Germany, 2016; 34p.
- Rubber, Vulcanized or Thermoplastic—Determination of Indentation Hardness. Part 1: Durometer Method (Shore Hardness); Beuth Verlag GmbH: Berlin, Germany, 2010; 7619p.
- Rubber, Vulcanized or Thermoplastic—Determination of Compression Se—Part 1: At Ambient or Elevated Temperatures; Beuth Verlag GmbH: Berlin, Germany, 2016; 815p.
- Liu, G.; Hoch, M.; Wrana, C.; Kulbaba, K.; Liu, S.; Bi, W.; Zhao, S. Investigation of the swelling response and quantitative prediction for hydrogenated nitrile rubber. Polym. Test. 2014, 34, 72–77. [Google Scholar] [CrossRef]
- Chassé, W.; López Valentín, J.; Genesky, G.D.; Cohen, C.; Saalwächter, K. Precise dipolar coupling constant distribution analysis in proton multiple-quantum NMR of elastomers. J. Chem. Phys. 2011, 134, 44907. [Google Scholar] [CrossRef]
- Manin, L.; Liang, X.; Lorenzon, C. Power losses prediction in poly-v belt transmissions: Application to front engine accessory drives. In Proceedings of the International Gear Conference 2014, Lyon, France, 26–28 August 2014; pp. 1162–1171. [Google Scholar] [CrossRef]
- Starmer, P.H. Swelling of Nitrile Rubber Vulcanizates-Part 3: Factors Affecting Maximum Swelling. J. Elastomers Plast. 1993, 25, 188–215. [Google Scholar] [CrossRef]
- Gao, G.; Zhang, Z.; Zheng, Y.; Jin, Z. Effect of magnesium methacrylate and zinc methacrylate on bond properties of thermal insulation material based on NBR/EPDM blends. J. Appl. Polym. Sci. 2009, 113, 3901–3909. [Google Scholar] [CrossRef]
- de Risi, F.R.; Noordermeer, J.W.M. Effect of Methacrylate Co-Agents on Peroxide Cured PP/EPDM Thermoplastic Vulcanizates. Rubber Chem. Technol. 2007, 80, 83–99. [Google Scholar] [CrossRef]
- Yu, P.; He, H.; Jiang, C.; Wang, D.; Jia, Y.; Zhou, L.; Jia, D.M. Reinforcing styrene butadiene rubber with lignin-novolac epoxy resin networks. Express Polym. Lett. 2015, 9, 36–48. [Google Scholar] [CrossRef] [Green Version]
- Kader, M.; Bhowmick, A.K. Thermal ageing, degradation and swelling of acrylate rubber, fluororubber and their blends containing polyfunctional acrylates. Polym. Degrad. Stab. 2003, 79, 283–295. [Google Scholar] [CrossRef]
- Rajasekar, R.; Pal, K.; Heinrich, G.; Das, A.; Das, C.K. Development of nitrile butadiene rubber–nanoclay composites with epoxidized natural rubber as compatibilizer. Mater. Des. 2009, 30, 3839–3845. [Google Scholar] [CrossRef]
- Howse, S.; Porter, C.; Mengistu, T.; Pazur, R.J. Experimental determination of the quantity and distribution of chemical crosslinks in unaged and aged natural rubber, part 1: Peroxide vulcanization. Polym. Test. 2018, 70, 263–274. [Google Scholar] [CrossRef]
- Shelton, J.R. Review of Basic Oxidation Processes in Elastomers. Rubber Chem. Technol. 1972, 45, 359–380. [Google Scholar] [CrossRef]
- Kruželák, J.; Sýkora, R.; Hudec, I. Vulcanization Of Rubber Compounds With Peroxide Curing Systems. Rubber Chem. Technol. 2017, 90, 60–88. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, L.; Tian, M.; Geng, H.; Zhang, L. Study on mechanical properties of elastomers reinforced by zinc dimethacrylate. Eur. Polym. J. 2005, 41, 589–598. [Google Scholar] [CrossRef]
- Dreyfuss, P.; Gent, A.N.; Williams, J.R. Tear strength and tensile strength of model filled elastomers. J. Polym. Sci. Polym. Phys. Ed. 1980, 18, 2135–2142. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, L.; Yang, C.; Tian, M.; Zhang, L. The morphology of zinc dimethacrylate reinforced elastomers investigated by SEM and TEM. Eur. Polym. J. 2005, 41, 577–588. [Google Scholar] [CrossRef]
- Ikeda, T.; Yamada, B.; Tsuji, M.; Sakurai, S. In situ copolymerization behaviour of zinc dimethacrylate and 2-(N-ethylperfluoro-octanesulphonamido)ethyl acrylate in hydrogenated nitrile-butadiene rubber during peroxide crosslinking. Polym. Int. 1999, 48, 446–454. [Google Scholar] [CrossRef]
- Saito, Y.; Nishimura, K.; Asada, M.; Toyoda, A. Polymerization Behavior of Zinc Methacrylate Study of Zinc Methacrylat/Rubber/Peroxide Compounds; Part 2. Nihon Gomu Kyoukaishi 1994, 67, 867–872. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Liang, X.; Zhang, Y.; Zhang, Y. Reinforcement of EPDM byin situ prepared zinc dimethacrylate. J. Appl. Polym. Sci. 2002, 84, 1339–1345. [Google Scholar] [CrossRef]
- Balasooriya, W.; Clute, C.; Schrittesser, B.; Pinter, G. A Review on Applicability, Limitations, and Improvements of Polymeric Materials in High-Pressure Hydrogen Gas Atmospheres. Polym. Rev. 2021, 62, 175–209. [Google Scholar] [CrossRef]


| Time (min) | Steps |
|---|---|
| 0 | Addition of rubber |
| 0–0.5 | Crumbling of rubber |
| 0.5–1.0 | Addition of filler, oil, reinforcing agent, and antioxidants |
| 1.0–3.0 | Mixing (ram down) |
| 3.0–3.2 | Sweeping (ram up) |
| 3.2–5.0 | Mixing (ram down) |
| 5.0 | Dumping of compound |
| Compound | EPDM | CB N660 | Process Oil | PO | Vulkanox HS | Vulkanox ZMB2 | ZDMA | ZDA | TRIM | PF | HMT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Belt–Ref | 100 | 50 | 15 | 7 | 1.5 | 1.5 | - | - | 4 | - | - |
| EPDM–7.5ZDMA | 100 | 30 | 15 | 5 | 1.5 | 1.5 | 7.5 | - | - | - | - |
| EPDM–15ZDMA | 100 | 30 | 15 | 2.5 | 1.5 | 1.5 | 15 | - | - | - | - |
| EPDM–7.5ZDA | 100 | 30 | 15 | 5 | 1.5 | 1.5 | - | 7.5 | - | - | - |
| EPDM–15ZDA | 100 | 30 | 15 | 2.5 | 1.5 | 1.5 | - | 15 | - | - | - |
| EPDM–TRIM | 100 | 30 | 15 | 7 | 1.5 | 1.5 | - | - | 4 | - | - |
| PF–ZDMA | 100 | 30 | 15 | 5 | 1.5 | 1.5 | 7.5 | - | - | 30 | 3 |
| PF–ZDA | 100 | 30 | 15 | 5 | 1.5 | 1.5 | - | 7.5 | - | 30 | 3 |
| PF–TRIM | 100 | 30 | 15 | 7 | 1.5 | 1.5 | - | - | 4 | 30 | 3 |
| PF–5PO | 100 | 30 | 15 | 5 | 1.5 | 1.5 | - | - | - | 30 | 3 |
| PF–7PO | 100 | 30 | 15 | 7 | 1.5 | 1.5 | - | - | - | 30 | 3 |
| Sample | t90 (min) | t2 (min) | MH (dNm) | ML (dNm) | ΔM (dNm) | ML1+4 (MU) |
|---|---|---|---|---|---|---|
| EPDM–7.5ZDMA | 5.3 | 0.66 | 13.5 | 1.55 | 12.0 | 81 |
| EPDM–15ZDMA | 5.9 | 0.92 | 11.4 | 1.30 | 10.1 | 83 |
| EPDM–7.5ZDA | 4.2 | 0.50 | 15.8 | 1.59 | 14.2 | 76 |
| EPDM–15ZDA | 4.1 | 0.67 | 16.7 | 1.51 | 15.2 | 76 |
| EPDM–TRIM | 5.4 | 0.68 | 16.0 | 1.62 | 14.4 | 75 |
| PF–ZDA | 6.5 | 0.72 | 12.6 | 1.11 | 11.6 | 67 |
| PF–ZDMA | 6.2 | 0.77 | 11.4 | 1.11 | 10.3 | 84 |
| PF–TRIM | 8.4 | 0.72 | 14.3 | 1.14 | 13.2 | 59 |
| PF–7PO | 11.0 | 1.04 | 10.7 | 1.19 | 9.5 | 73 |
| PF–5PO | 11.6 | 1.33 | 8.4 | 1.19 | 7.2 | 74 |
| Belt–Ref | 5.4 | 0.61 | 19.6 | 1.95 | 17.6 | 89 |
| Sample | Swelling Degree (%) | Gel Content (%) | Mc (mol/g) | |||
|---|---|---|---|---|---|---|
| 0 h | 24 h | 0 h | 24 h | 0 h | 24 h | |
| EPDM–7.5ZDMA | 158 ± 0.6 | 161 ± 0.4 | 100 ± 0.1 | 100 ± 0.1 | 740 ± 3 | 730 ± 3 |
| EPDM–15ZDMA | 196 ± 1.0 | 187 ± 0.4 | 96 ± 0.9 | 98 ± 0.1 | 1089 ± 300 | 926 ± 7 |
| EPDM–7.5ZDA | 142 ± 0.1 | 142 ± 0.6 | 100 ± 0.1 | 100 ± 0.1 | 734 ± 225 | 603 ± 2 |
| EPDM–15ZDA | 138 ± 0.1 | 137 ± 0.1 | 99 ± 0.1 | 99 ± 0.2 | 786 ± 176 | 564 ± 2 |
| EPDM–TRIM | 162 ± 0.6 | 164 ± 0.3 | 100 ± 0.1 | 100 ± 0.1 | 766 ± 4 | 763 ± 4 |
| Belt–Ref | 137 ± 0.4 | 135 ± 0.2 | 100 ± 0.1 | 100 ± 0.1 | 703 ± 15 | 674 ± 3 |
| Sample | TS (MPa) | εb (%) | σ100 (MPa) | σ300 (MPa) | Tear (N/mm) |
|---|---|---|---|---|---|
| EPDM_7.5ZDMA-0 h | 25.4 ± 1.1 | 617 ± 16.7 | 2.6 ± 0.1 | 8.5 ± 0.2 | 16 ± 1.0 |
| EPDM–7.5ZDMA-24 h | 24.5 ± 0.3 | 587 ± 19 | 2.7 ± 0.1 | 8.8 ± 0.1 | 17 ± 0.4 |
| EPDM–15ZDMA-0 h | 16.0 ± 0.3 | 617 ± 16 | 2.4 ± 0.1 | 6.4 ± 0.1 | 26 ± 0.2 |
| EPDM–15ZDMA-24 h | 15.8 ± 0.4 | 619 ± 11 | 2.5 ± 0.1 | 6.5 ± 0.2 | 28 ± 0.1 |
| EPDM–7.5ZDA-0 h | 22.0 ± 0.8 | 492 ± 8 | 3.0 ± 0.1 | 10.7 ± 0.2 | 14 ± 0.8 |
| EPDM–7.5ZDA-24 h | 22.4 ± 1.2 | 476 ± 9 | 3.2 ± 0.1 | 11.5 ± 0.2 | 15 ± 1.2 |
| EPDM–15ZDA-0 h | 23.2 ± 1.2 | 560 ± 15 | 3.2 ± 0.1 | 10.2 ± 0.3 | 19 ± 1.5 |
| EPDM–15ZDA-24 h | 21.6 ± 1.2 | 535 ± 13 | 3.2 ± 0.1 | 10.2 ± 0.3 | 18 ± 0.3 |
| EPDM–TRIM-0 h | 16.3 ± 0.7 | 452 ± 9 | 2.4 ± 0.1 | 8.3 ± 0.1 | 12 ± 0.3 |
| EPDM–TRIM-24 h | 16.6 ± 0.5 | 459 ± 9 | 2.5 ± 0.1 | 8.5 ± 0.2 | 10 ± 0.7 |
| PF–ZDA-0 h | 12.9 ± 0.7 | 742 ± 14 | 3.1 ± 0.1 | 6.4 ± 0.1 | 22 ± 0.4 |
| PF–ZDA-24 h | 11.7 ± 0.9 | 715 ± 24 | 2.8 ± 0.1 | 6.5 ± 0.3 | 22 ± 0.2 |
| PF–ZDMA-0 h | 11.4 ± 1.1 | 688 ± 34 | 3.0 ± 0.1 | 6.6 ± 0.3 | 29 ± 1.4 |
| PF–ZDMA-24 h | 12.4 ± 0.7 | 658 ± 34 | 3.3 ± 0.1 | 7.7 ± 0.3 | 28 ± 0.5 |
| PF–TRIM-0 h | 10.0 ± 0.3 | 509 ± 33 | 3.1 ± 0.2 | 7.2 ± 0.3 | 32 ± 0.3 |
| PF–TRIM-24 h | 11.3 ± 0.3 | 442 ± 17 | 3.4 ± 0.1 | 8.3 ± 0.2 | 20 ± 1.0 |
| PF–7PO-0 h | 10.0 ± 0.7 | 880 ± 16 | 2.0 ± 0.1 | 4.2 ± 0.2 | 28 ± 1.2 |
| PF–7PO-24 h | 9.8 ± 0.5 | 672 ± 23 | 2.2 ± 0.2 | 5.4 ± 0.5 | 26 ± 0.8 |
| PF–5PO-0 h | 8.4 ± 0.3 | 966 ± 40 | 1.9 ± 0.2 | 3.5 ± 0.2 | 32 ± 0.6 |
| PF–5PO-24 h | 9.3 ± 0.3 | 768 ± 95 | 2.3 ± 0.1 | 5.1 ± 0.2 | 30 ± 0.5 |
| Belt–Ref-0 h | 18.0 ± 1.1 | 386 ± 33 | 3.2 ± 0.1 | 12.6 ± 0.2 | 15 ± 0.3 |
| Belt–Ref-24 h | 17.4 ± 0.7 | 386 ± 16 | 3.2 ± 0.1 | 12.2 ± 0.2 | 14 ± 0.5 |
| Sample | Tan δ (-) | Hardness (Shore A) | CS@RT (%) | CS@100 °C (%) |
|---|---|---|---|---|
| EPDM–7.5ZDMA-0 h | - | 58 ± 1.2 | 17 ± 0.5 | 32 ± 1.0 |
| EPDM–7.5ZDMA-24 h | 0.63 | 60 ± 0.6 | - | - |
| EPDM–15ZDMA-0 h | - | 62 ± 1.5 | 24 ± 0.7 | 47 ± 0.6 |
| EPDM–15ZDMA-24 h | 0.60 | 62 ± 0.6 | - | - |
| EPDM–7.5ZDA-0 h | - | 60 ± 1.2 | 26 ± 0.8 | 37 ± 1.2 |
| EPDM–7.5ZDA-24 h | 0.61 | 58 ± 0.6 | - | - |
| EPDM–15ZDA-0 h | - | 60 ± 0.6 | 25 ± 0.9 | 52 ± 1.9 |
| EPDM–15ZDA-24 h | 0.57 | 59 ± 1.2 | - | - |
| EPDM–TRIM-0 h | - | 56 ± 1.0 | 24 ± 0.6 | 26 ± 0.9 |
| EPDM–TRIM-24 h | 0.63 | 57 ± 0.6 | - | - |
| PF–ZDA-0 h | - | 72 ± 0.6 | 34 ± 0.6 | 58 ± 1.8 |
| PF–ZDA-24 h | 0.50 | 72 ± 0.6 | - | - |
| PF–ZDMA-0 h | - | 71 ± 0.6 | 33 ± 0.6 | 54 ± 1.4 |
| PF–ZDMA-24 h | 0.52 | 71 ± 1.2 | - | - |
| PF–TRIM-0 h | - | 65 ± 0.6 | 19 ± 0.9 | 32 ± 2.1 |
| PF–TRIM-24 h | 0.49 | 66 ± 0.5 | - | - |
| PF–7PO-0 h | - | 63 ± 0.6 | 25 ± 0.4 | 45 ± 1.2 |
| PF–7PO-24 h | 0.48 | 68 ± 1.2 | - | - |
| PF–5PO-0 h | - | 63 ± 1.0 | 22 ± 0.6 | 37 ± 1.7 |
| PF–5PO-24 h | 0.50 | 68 ± 1.2 | - | - |
| Belt–Ref-0 h | - | 63 ± 0.6 | 14 ± 0.3 | 16 ± 0.4 |
| Belt–Ref-24 h | 0.54 | 62 ± 0.6 | - | - |
| Sample | Swelling Degree (%) | Gel Content (%) | Mc (g/mol) | |||
|---|---|---|---|---|---|---|
| 0 h | 24 h | 0 h | 24 h | 0 h | 24 h | |
| PF–ZDA | 154 ± 0.8 | 142 ± 2.6 | 97 ± 0.1 | 99 ± 0.1 | 953 ± 4 | 821 ± 22 |
| PF–ZDMA | 166 ± 1.5 | 148 ± 0.4 | 97 ± 0.1 | 99 ± 0.1 | 1075 ± 16 | 872 ± 2 |
| PF–TRIM | 147 ± 5.2 | 141 ± 0.6 | 97 ± 0.2 | 99 ± 0.1 | 896 ± 15 | 823 ± 4 |
| PF–7PO | 192 ± 3.4 | 180 ± 1.1 | 96 ± 0.3 | 99 ± 0.2 | 1433 ± 48 | 1230 ± 11 |
| PF–5PO | 226 ± 0.8 | 206 ± 0.2 | 93 ± 0.3 | 97 ± 0.2 | 1944 ± 15 | 1571 ± 7 |
| Belt–Ref | 137 ± 0.4 | 135 ± 0.2 | 100 ± 0.1 | 100 ± 0.1 | 703 ± 15 | 674 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strohmeier, L.; Balasooriya, W.; Schrittesser, B.; van Duin, M.; Schlögl, S. Hybrid In Situ Reinforcement of EPDM Rubber Compounds Based on Phenolic Novolac Resin and Ionic Coagent. Appl. Sci. 2022, 12, 2432. https://doi.org/10.3390/app12052432
Strohmeier L, Balasooriya W, Schrittesser B, van Duin M, Schlögl S. Hybrid In Situ Reinforcement of EPDM Rubber Compounds Based on Phenolic Novolac Resin and Ionic Coagent. Applied Sciences. 2022; 12(5):2432. https://doi.org/10.3390/app12052432
Chicago/Turabian StyleStrohmeier, Lara, Winoj Balasooriya, Bernd Schrittesser, Martin van Duin, and Sandra Schlögl. 2022. "Hybrid In Situ Reinforcement of EPDM Rubber Compounds Based on Phenolic Novolac Resin and Ionic Coagent" Applied Sciences 12, no. 5: 2432. https://doi.org/10.3390/app12052432
APA StyleStrohmeier, L., Balasooriya, W., Schrittesser, B., van Duin, M., & Schlögl, S. (2022). Hybrid In Situ Reinforcement of EPDM Rubber Compounds Based on Phenolic Novolac Resin and Ionic Coagent. Applied Sciences, 12(5), 2432. https://doi.org/10.3390/app12052432







