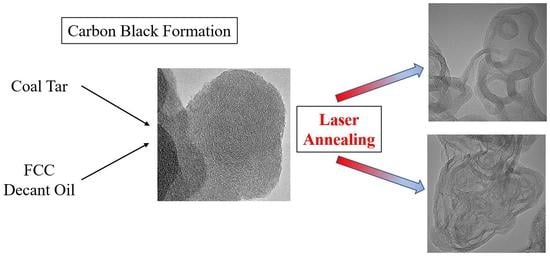
Effect of Fuel Composition on Carbon Black Formation Pathways
Abstract
:1. Introduction
2. Materials and Experimental Methods
2.1. Feedstocks
2.2. Transmission Electron Microscopy (TEM)
2.3. Electron Energy Loss Spectroscopy (EELS)
2.4. Fringe Analysis
2.5. Thermogravimetric Analysis
2.6. Laser Annealing
3. Results and Discussion
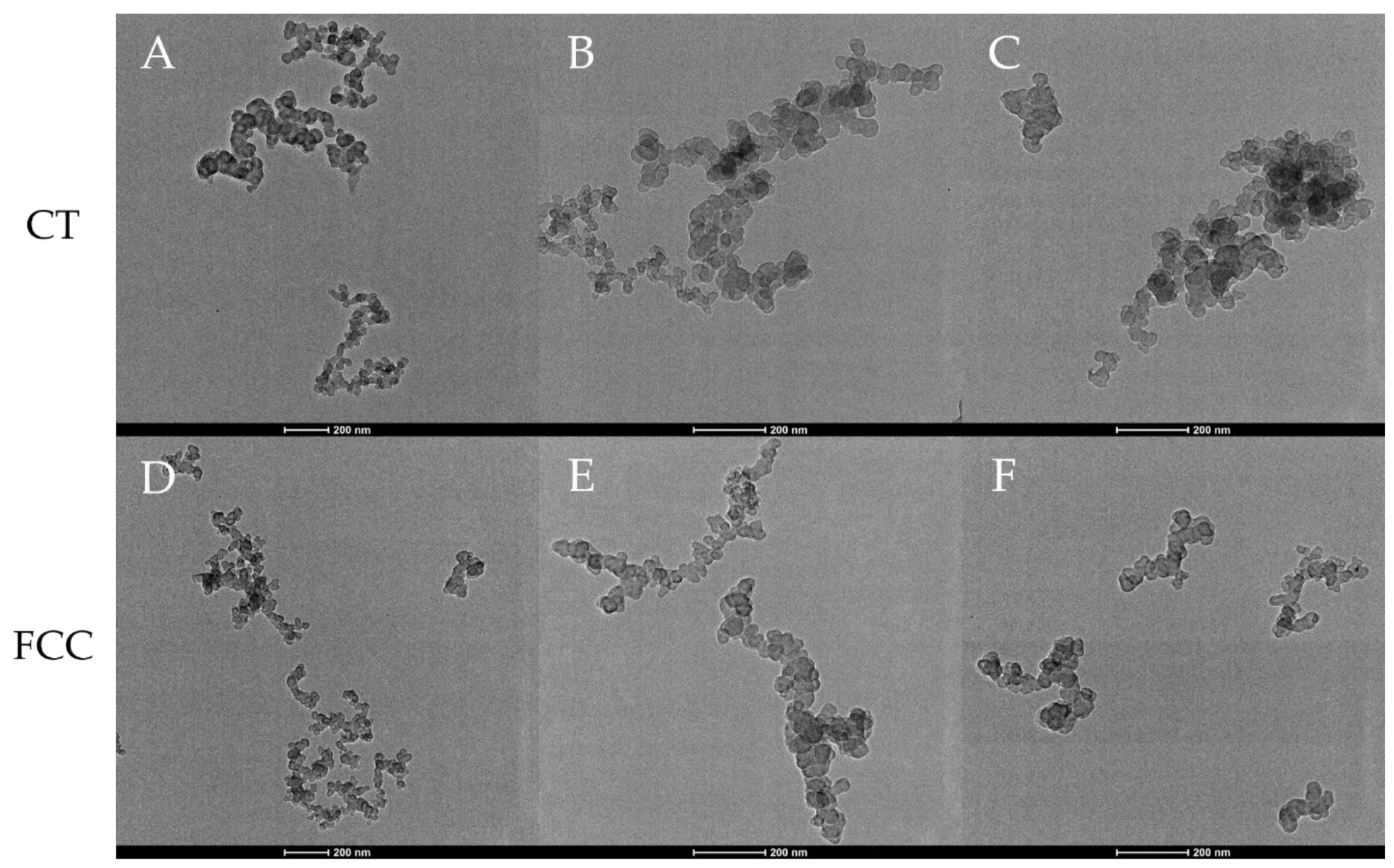
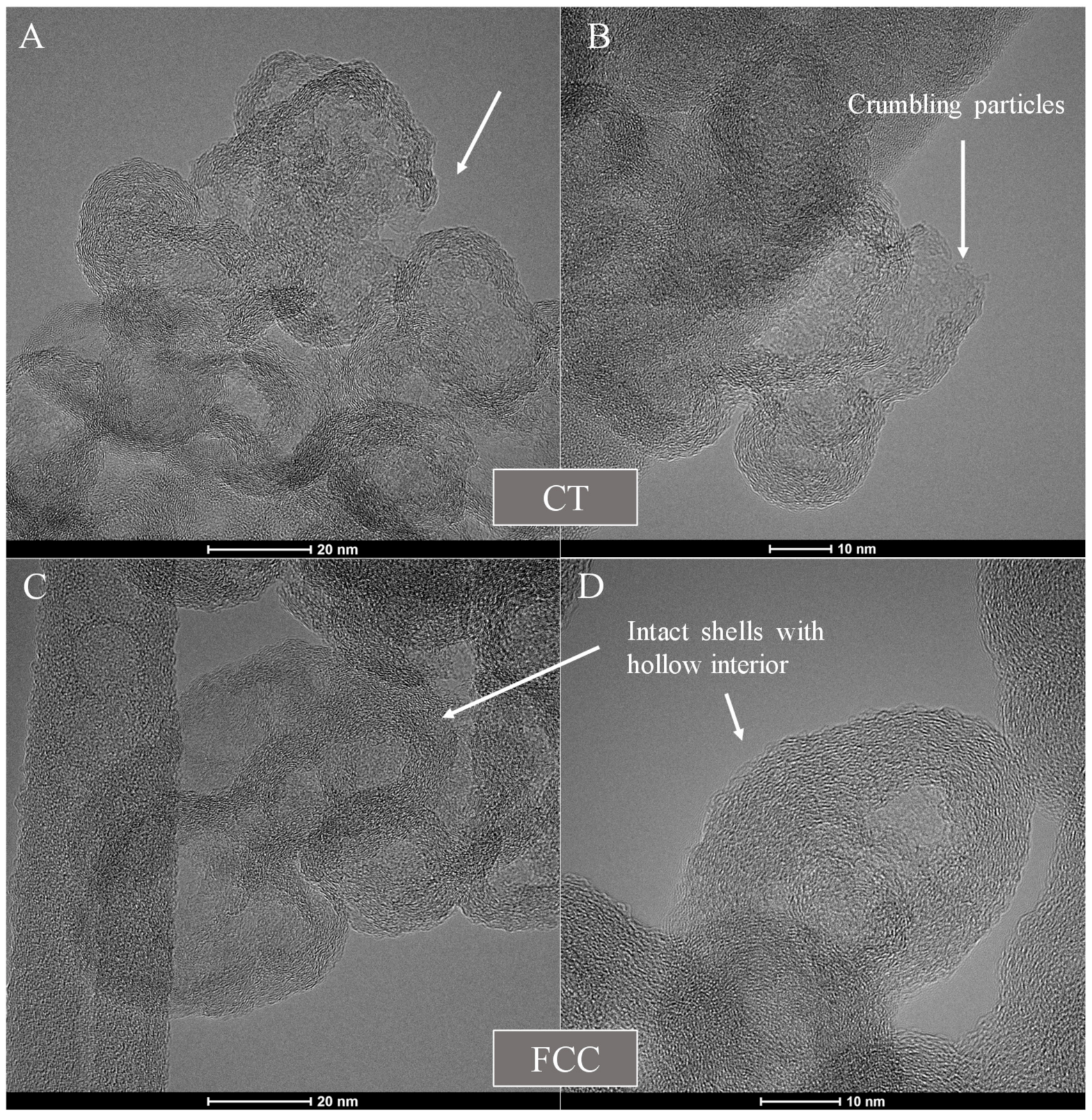
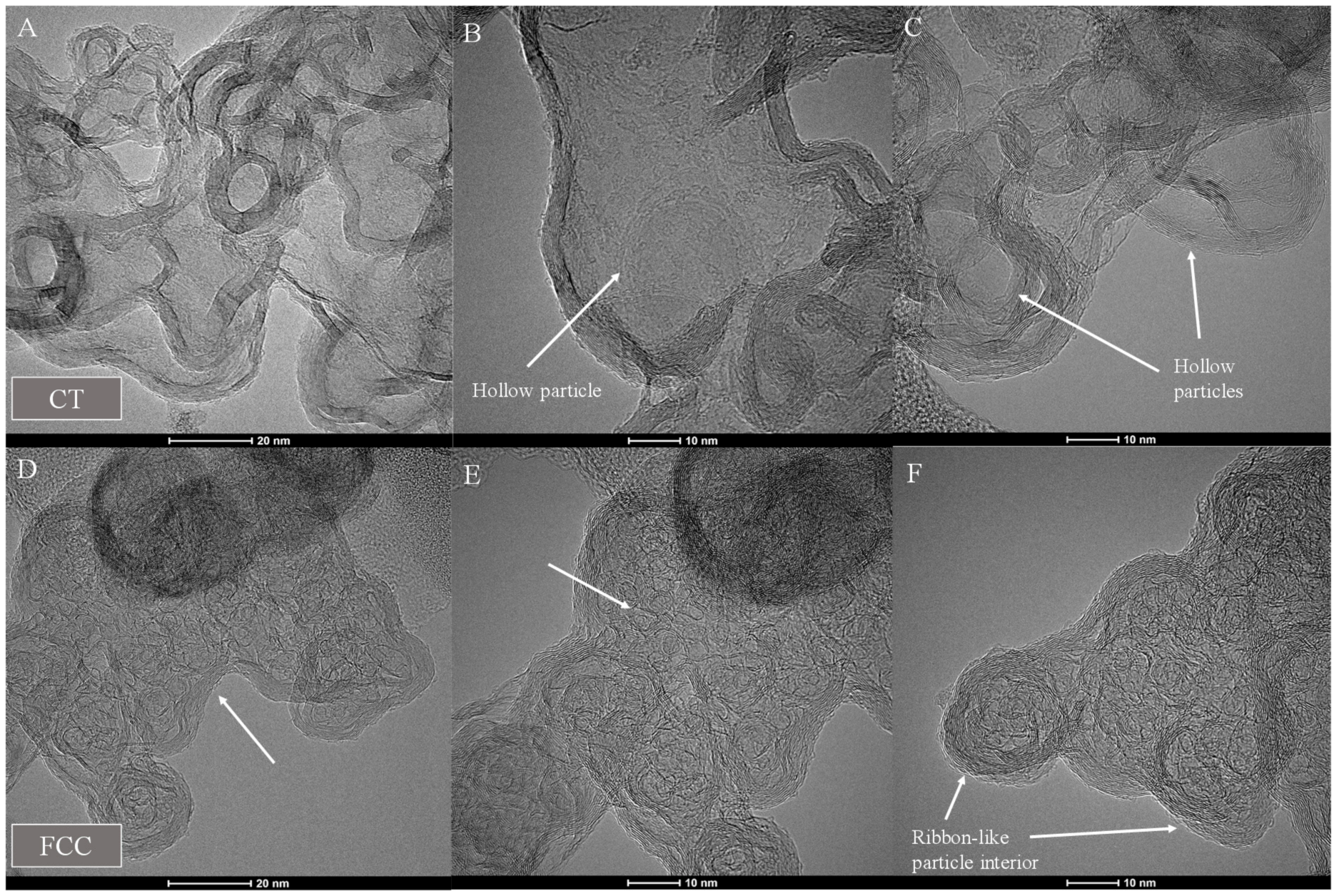
3.1. Carbon Black Aggregate Morphology and Particle Structure
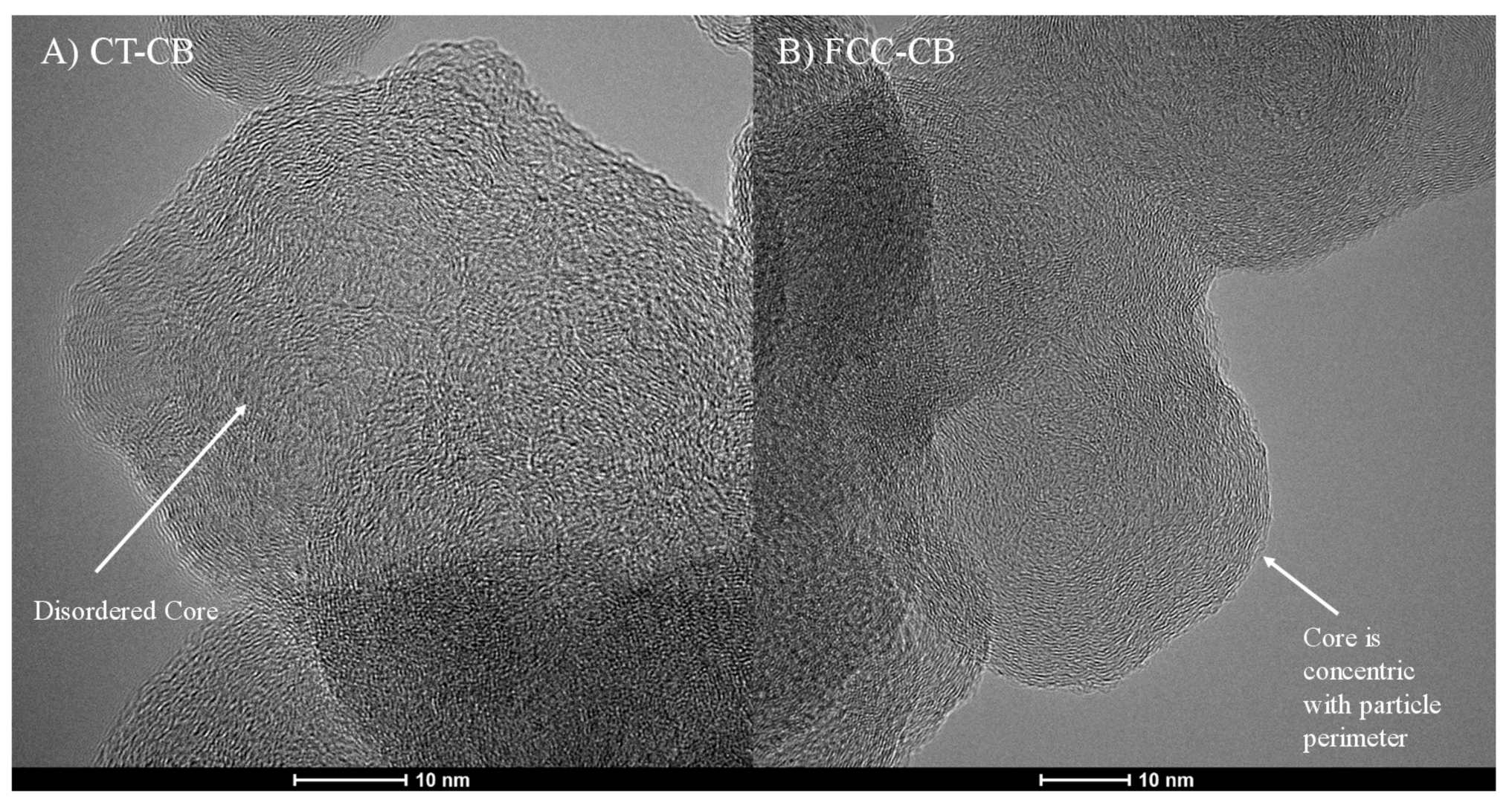
3.2. Carbon Black Nanostructure
3.3. Lattice Fringe Analysis
3.4. Electron Energy Loss Spectroscopy (EELS)
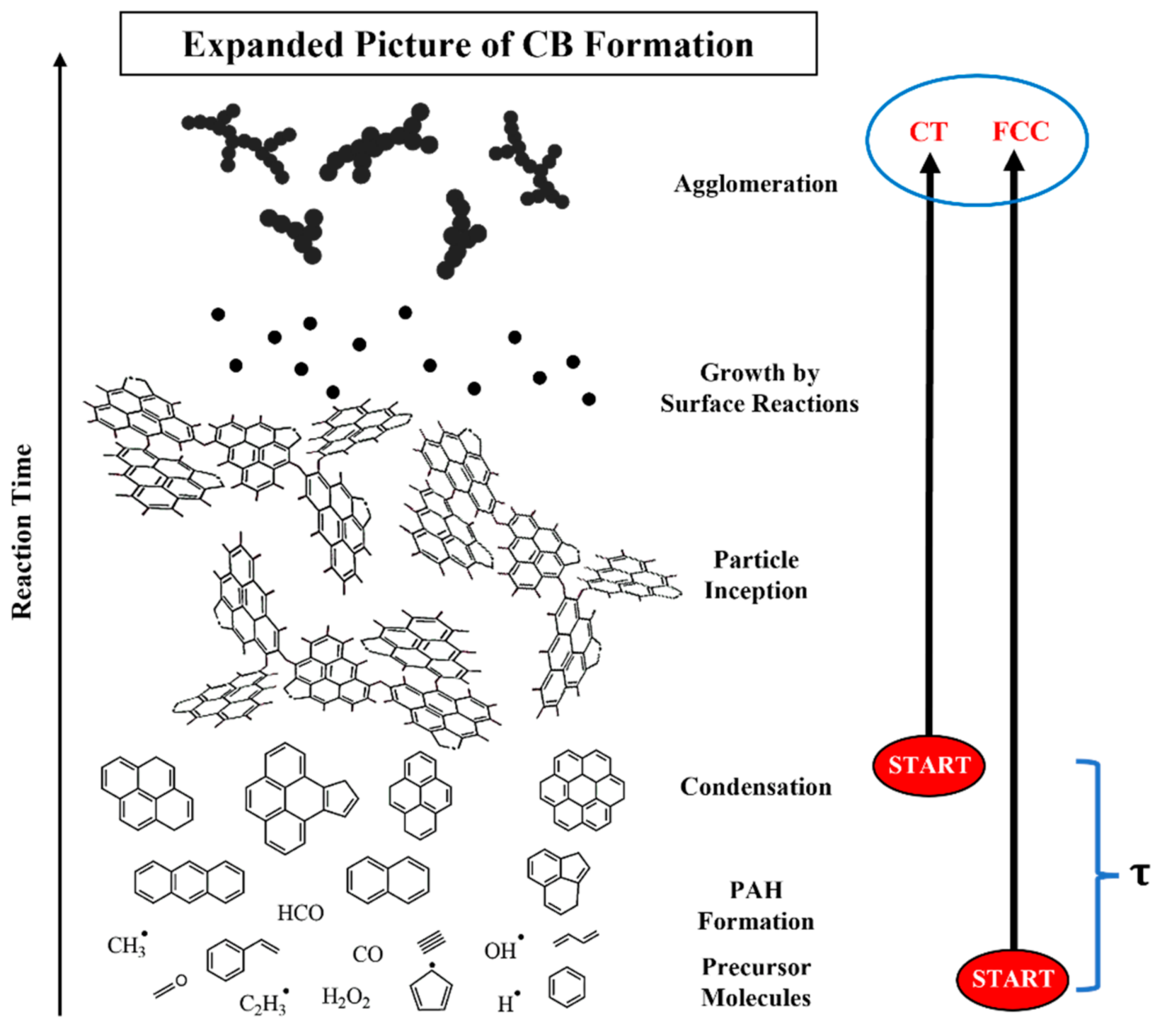
3.5. Carbon Black Formation—Dependence upon Fuel Compositional Differences
3.5.1. CT-CB
3.5.2. FCC-CB
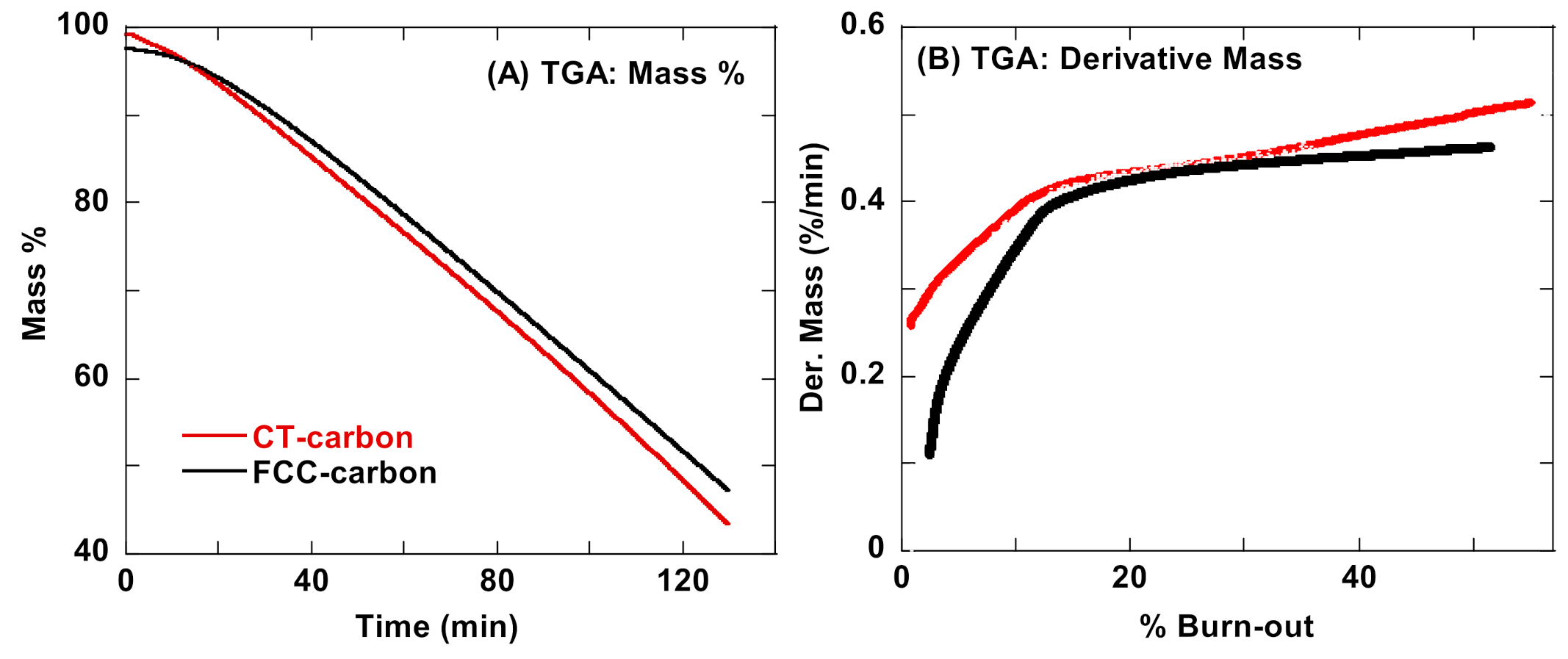
3.6. Nanostructure Inferences Reconciled Using Oxidative Thermal Treatment on CT- and FCC-Carbon Black Nanostructure
3.7. Laser Heat Treatment
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Anna, A.; Violi, A.; D’Alessio, A.; Sarofim, A.F. A reaction pathway for nanoparticle formation in rich premixed flames. Combust. Flame 2001, 127, 1995–2003. [Google Scholar] [CrossRef]
- Siegmann, K.; Sattler, K.; Siegmann, H.C. Clustering at high temperatures: Carbon formation in combustion. J. Electron Spectrosc. Relat. Phenom. 2002, 126, 191–202. [Google Scholar] [CrossRef]
- Lai, J.Y.W.; Elvati, P.; Violi, A. Stochastic atomistic simulation of polycyclic aromatic hydrocarbon growth in combustion. Phys. Chem. Chem. Phys. 2014, 16, 7969–7979. [Google Scholar] [CrossRef] [PubMed]
- Kholghy, M.R.; Afarin, Y.; Sediako, A.D.; Barba, J.; Lapuerta, M.; Chu, C.; Weingarten, J.; Borshanpour, B.; Chernov, V.; Thomson, M.J. Comparison of multiple diagnostic techniques to study soot formation and morphology in a diffusion flame. Combust. Flame 2017, 176, 567–583. [Google Scholar] [CrossRef]
- Vitiello, G.; De Falco, G.; Picca, F.; Commodo, M.; D’Errico, G.; Minutolo, P.; D’Anna, A. Role of radicals in carbon clustering and soot inception: A combined EPR and Raman spectroscopic study. Combust. Flame 2019, 205, 286–294. [Google Scholar] [CrossRef]
- Johansson, K.O.; Head-Gordon, M.P.; Schrader, P.E.; Wilson, K.R.; Michelsen, H.A. Resonance-stabilized hydrocarbon-radical chain reactions may explain soot inception and growth. Science 2018, 361, 997–1000. [Google Scholar] [CrossRef] [Green Version]
- Wang, H. Formation of nascent soot and other condensed-phase materials in flames. Proc. Combust. Inst. 2011, 33, 41–67. [Google Scholar] [CrossRef]
- Mercier, X.; Carrivain, O.; Irimiea, C.; Faccinetto, A.; Therssen, E. Dimers of polycyclic aromatic hydrocarbons: The missing pieces in the soot formation process. Phys. Chem. Chem. Phys. 2019, 21, 8282–8294. [Google Scholar] [CrossRef]
- Singh, M.; Gaddam, C.K.; Abrahamson, J.P.; Wal, R.L.V. Soot differentiation by laser derivatization. Aerosol Sci. Technol. 2018, 53, 207–229. [Google Scholar] [CrossRef]
- Betts, W.D. Tar and Pitch. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Mochida, I.; Korai, Y.; Hieida, T.; Azuma, A.; Kitajima, E. Detailed Analyses of FCC Decant Oil as a Starting Feedstock for Mesophase Pitch. Fuel Sci. Technol. Int. 1991, 9, 485–504. [Google Scholar] [CrossRef]
- Wal, R.L.V.; Tomasek, A.J.; Street, K.; Hull, D.R.; Thompson, W.K. Carbon Nanostructure Examined by Lattice Fringe Analysis of High-Resolution Transmission Electron Microscopy Images. Appl. Spectrosc. 2004, 58, 230–237. [Google Scholar]
- Wal, R.L.V.; Tomasek, A.J. Soot oxidation: Dependence upon initial nanostructure. Combust. Flame 2003, 134, 1–9. [Google Scholar]
- Wal, R.L.V.; Tomasek, A.J. Soot nanostructure: Dependence upon synthesis conditions. Combust. Flame 2004, 136, 129–140. [Google Scholar]
- Wal, R.L.V.; Bryg, V.M.; Hays, M.D. Fingerprinting soot (towards source identification): Physical structure and chemical composition. J. Aerosol Sci. 2010, 41, 108–117. [Google Scholar]
- Egerton, R.F. Electron Energy-Loss Spectroscopy in the Electron Microscope; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Abrahamson, J.P.; Singh, M.; Mathews, J.P.; Wal, R.L.V. Pulsed laser annealing of carbon black. Carbon N. Y. 2017, 124, 380–390. [Google Scholar] [CrossRef]
- Gong, X.; Wang, Z.; Deng, S.; Li, S.; Song, W.; Lin, W. Impact of the Temperature, Pressure, and Particle Size on Tar Composition from Pyrolysis of Three Ranks of Chinese Coals. Energy Fuels 2014, 28, 4942–4948. [Google Scholar] [CrossRef]
- Fardhyanti, D.; Damayanti, A. Analysis of Coal Tar Compositions Produced from Sub-Bituminous Kalimantan Coal Tar. World Acad. Sci. Eng. Technol. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2015, 9, 1022–1025. [Google Scholar]
- Richards, A.P.; Fletcher, T.H. A comparison of simple global kinetic models for coal devolatilization with the CPD model. Fuel 2016, 185, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Schobert, H. Chemistry of Fossil Fuels and Biofuels. Cambridge Series in Chemical Engineering; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar] [CrossRef]
- Chu, C.; Zhang, T.; Thomson, M.J. The chemical structure effects of alkylbenzenes on soot formation in a laminar co-flow flame. Combust. Flame 2019, 204, 237–249. [Google Scholar] [CrossRef]
- Genetti, D.B. The Chemical Structure Effects of Alkylbenzenes on Soot Formation in a Laminar Co-Flow Flame; Birmingham Young University: Provo, UT, USA, 1999. [Google Scholar]
- Filley, R.M.; Eser, S. Analysis of hydrocarbons and sulfur compounds in two FCC decant oils and their carbonization products. Energy Fuels 1997, 11, 623–630. [Google Scholar] [CrossRef]
- Tsang, W. The stability of alkyl radicals. J. Am. Chem. Soc. 1985, 107, 2872–2880. [Google Scholar] [CrossRef]
- Forrester, A.R.; Hay, J.M.; Thomson, R.H. Organic Chemistry of Stable Free Radicals; Academic Press: London, UK, 1968. [Google Scholar]
- Ono, K.; Matsukawa, Y.; Dewa, K.; Watanabe, A.; Takahashi, K.; Saito, Y.; Matsushita, Y.; Aoki, H.; Era, K.; Aoki, T.; et al. Formation mechanisms of soot from high-molecular-weight polycyclic aromatic hydrocarbons. Combust. Flame 2015, 162, 2670–2678. [Google Scholar] [CrossRef]
- Mitra, T.; Zhang, T.; Sediako, A.D.; Thomson, M.J. Understanding the formation and growth of polycyclic aromatic hydrocarbons (PAHs) and young soot from n-dodecane in a sooting laminar coflow diffusion flame. Combust. Flame 2019, 202, 33–42. [Google Scholar] [CrossRef]
- Pretorius, G.N.; Bunt, J.; Gräbner, M.; Neomagus, H.; Waanders, F.B.; Everson, R.C.; Strydom, C. Evaluation and prediction of slow pyrolysis products derived from coals of different rank. J. Anal. Appl. Pyrolysis 2017, 128, 156–167. [Google Scholar] [CrossRef]
- Thomson, M.; Mitra, T. A radical approach to soot formation. Science 2018, 361, 978–979. [Google Scholar] [CrossRef]
- Martin, J.W.; Salamanca, M.; Kraft, M. Soot inception: Carbonaceous nanoparticle formation in flames. Prog. Energy Combust. Sci. 2022, 88, 100956. [Google Scholar] [CrossRef]
- Frenklach, M.; Mebel, A.M. On the mechanism of soot nucleation. Phys. Chem. Chem. Phys. 2020, 22, 5314–5331. [Google Scholar] [CrossRef]
- Rodrigues, P.; Franzelli, B.; Vicquelin, R.; Gicquel, O.; Darabiha, N. Coupling an LES approach and a soot sectional model for the study of sooting turbulent non-premixed flames. Combust. Flame 2018, 190, 477–499. [Google Scholar] [CrossRef] [Green Version]
- Bockhorn, H. Soot Formation in Combustion; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Baum, T.; Löffler, S.; Löffler, P.; Weilmünster, P.; Homann, K.-H. Fullerene ions and their relation to PAH and soot in low-pressure hydrocarbon flames. Ber. Bunsenges. Phys. Chem. 1992, 96, 841–857. [Google Scholar] [CrossRef]
- Kholghy, M.R.; Kelesidis, G.A.; Pratsinis, S.E. Reactive polycyclic aromatic hydrocarbon dimerization drives soot nucleation. Phys. Chem. Chem. Phys. 2018, 20, 10926–10938. [Google Scholar] [CrossRef]
- Genetti, D.B. An Advanced Model of Coal Devolatilization Based on Chemical Structure; Birmingham Young University: Provo, UT, USA, 1999. [Google Scholar]
- Yuan, H.; Kong, W.; Liu, F.; Chen, D. Study on soot nucleation and growth from PAHs and some reactive species at flame temperatures by ReaxFF molecular dynamics. Chem. Eng. Sci. 2019, 195, 748–757. [Google Scholar] [CrossRef]
- Gaddam, C.K.; Wal, R.L.V.; Chen, X.; Yezerets, A.; Kamasamudram, K. Reconciliation of carbon oxidation rates and activation energies based on changing nanostructure. Carbon N. Y. 2016, 98, 545–556. [Google Scholar] [CrossRef]




| Feedstock | Saturates | Aromatics | Resins | Asphaltenes |
|---|---|---|---|---|
| Coal tar | 0.8 | 97.19 | 0.91 | 0.38 |
| FCC decant oil | 7.3 | 90.8 | 0.4 | 1.5 |
| CT | FCC | |
|---|---|---|
| No. Measurements | 7 | 15 |
| Ratio: Edge/Center | 0.86 | 0.83 |
| Normalized Mean sp2 (%) | 7.2 ± 1.8 | 24.4 ± 1.6 |
| sp2 referenced value | 46 | 78 |
| Rel. Ratio | 1 | 3.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, M.; Gharpure, A.; Vander Wal, R.L.; Kollar, J.; Herd, C.R. Effect of Fuel Composition on Carbon Black Formation Pathways. Appl. Sci. 2022, 12, 2569. https://doi.org/10.3390/app12052569
Singh M, Gharpure A, Vander Wal RL, Kollar J, Herd CR. Effect of Fuel Composition on Carbon Black Formation Pathways. Applied Sciences. 2022; 12(5):2569. https://doi.org/10.3390/app12052569
Chicago/Turabian StyleSingh, Madhu, Akshay Gharpure, Randy L. Vander Wal, James Kollar, and Charles R. Herd. 2022. "Effect of Fuel Composition on Carbon Black Formation Pathways" Applied Sciences 12, no. 5: 2569. https://doi.org/10.3390/app12052569
APA StyleSingh, M., Gharpure, A., Vander Wal, R. L., Kollar, J., & Herd, C. R. (2022). Effect of Fuel Composition on Carbon Black Formation Pathways. Applied Sciences, 12(5), 2569. https://doi.org/10.3390/app12052569