Effect of Photo-Mediated Ultrasound Therapy on Nitric Oxide and Prostacyclin from Endothelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Circular Cross-Section Microchannel
2.2. Flow System
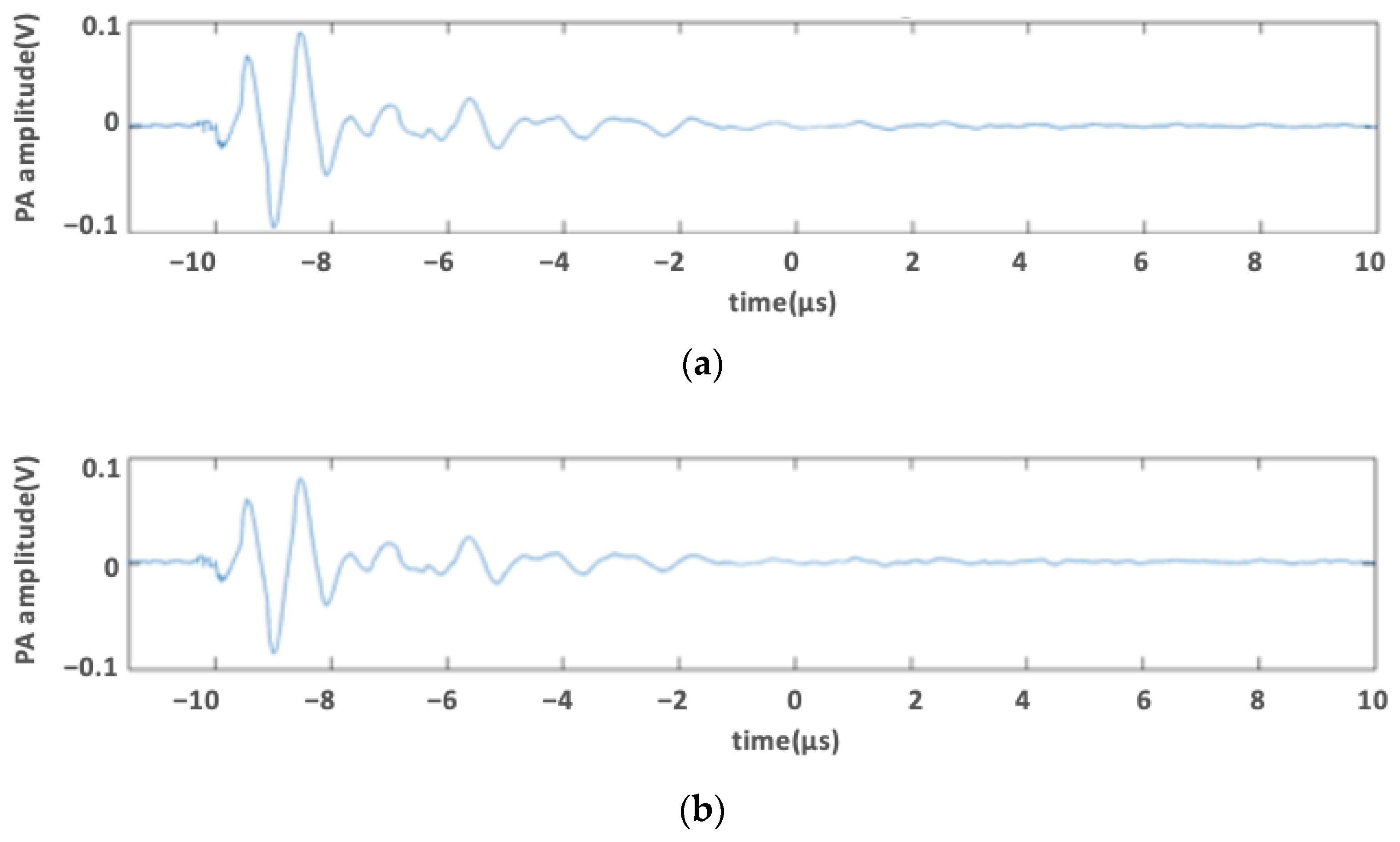
2.3. Photoacoustic Signal of Medium Containing Phenol Red
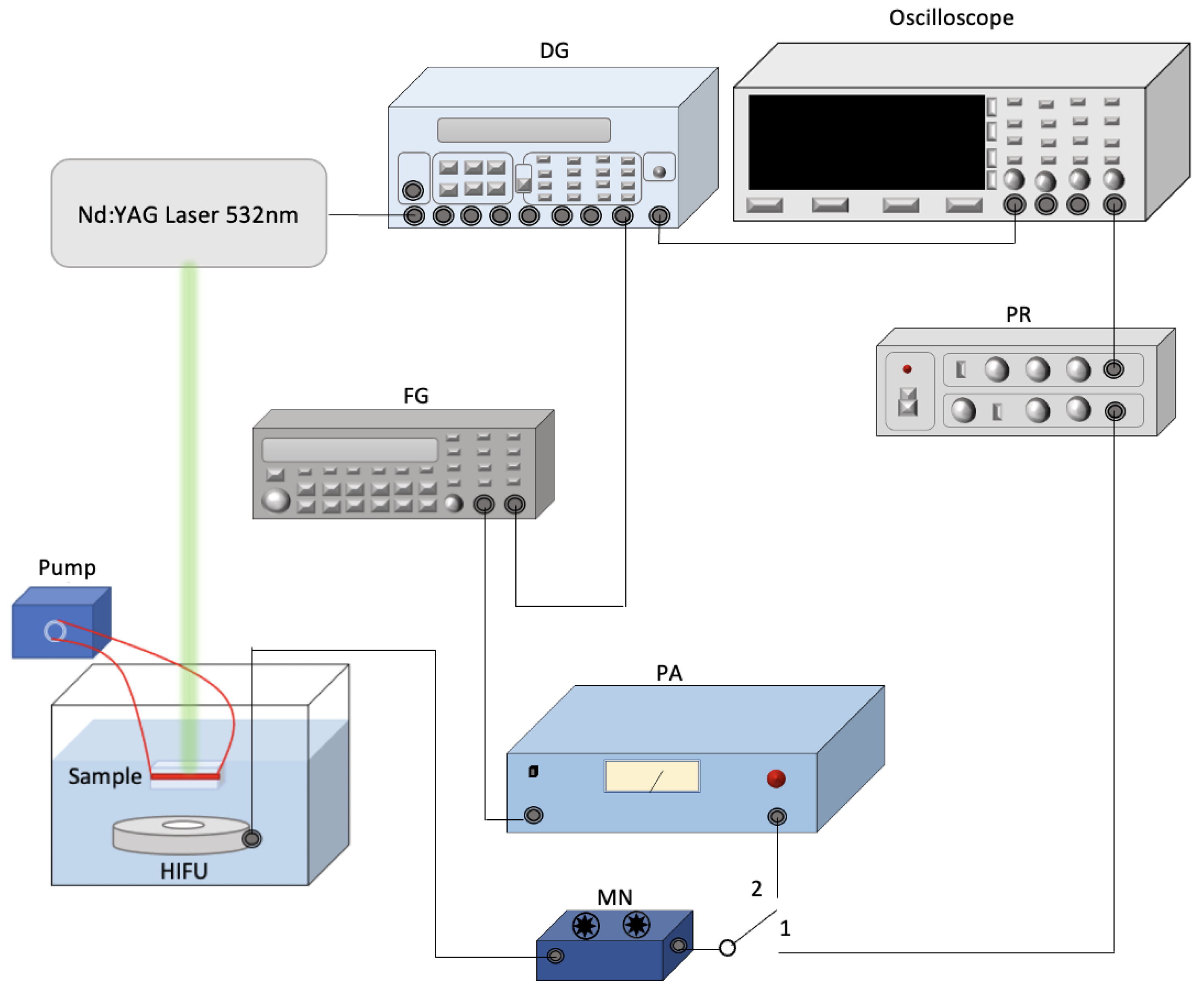
2.4. Ultrasound and Laser System Setup for Assessing NO and PGI2 Release
2.5. Nitrate Reduction Assay
2.6. PGI2 Assay
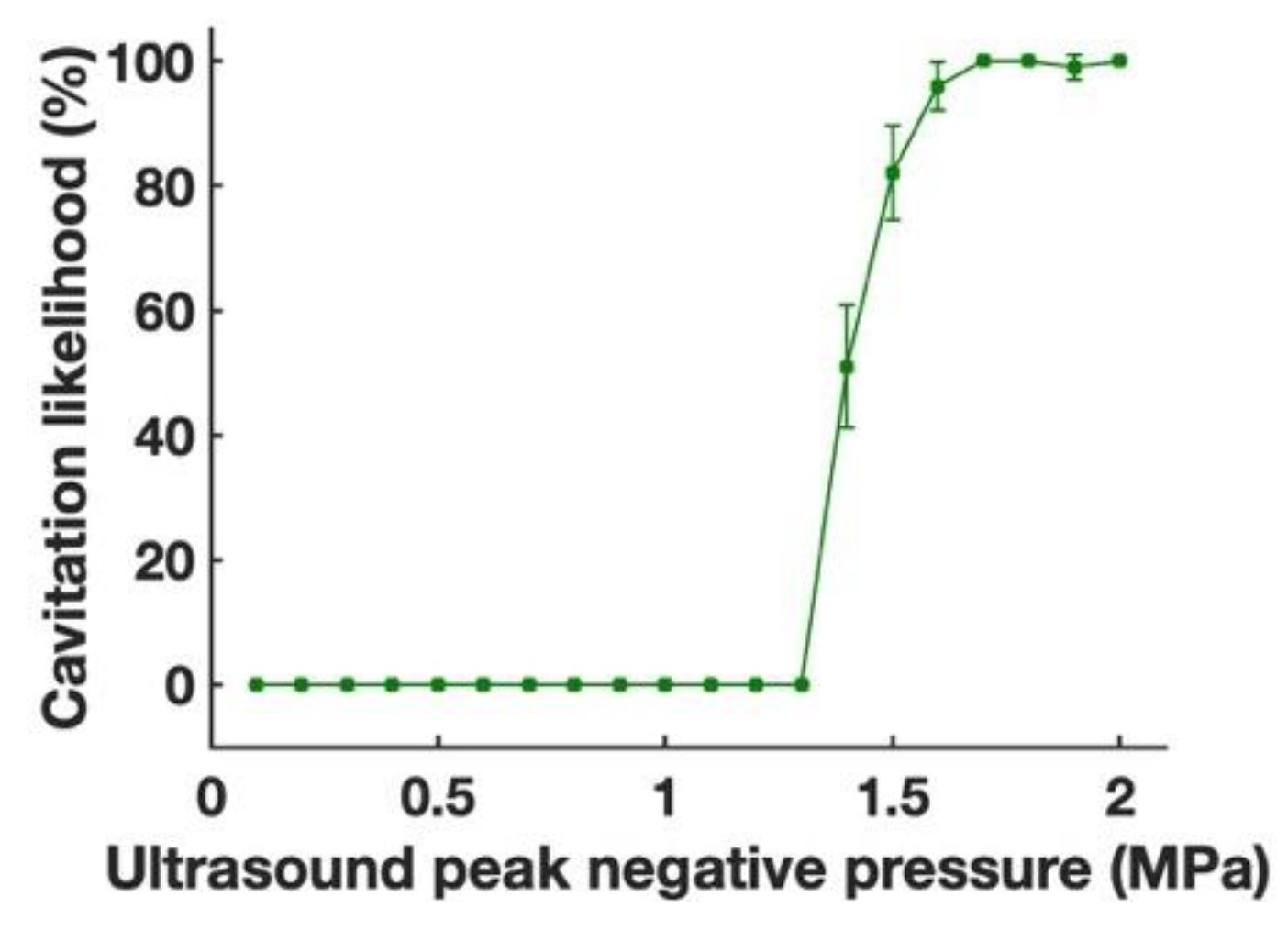
2.7. Cavitation Detection
2.8. Apoptosis Detection
2.9. Statistical Analysis
3. Results
3.1. Flow System
3.2. Photoacoustic Signal of Medium Containing Phenol Red
3.3. Effect of Ultrasound-Only Treatment on NO Level
3.4. Effect of Laser-Only Treatment on NO Level
3.5. Effect of Photo-Mediated Ultrasound Therapy on NO Level
3.5.1. Constant Ultrasound with Varying Laser Fluence
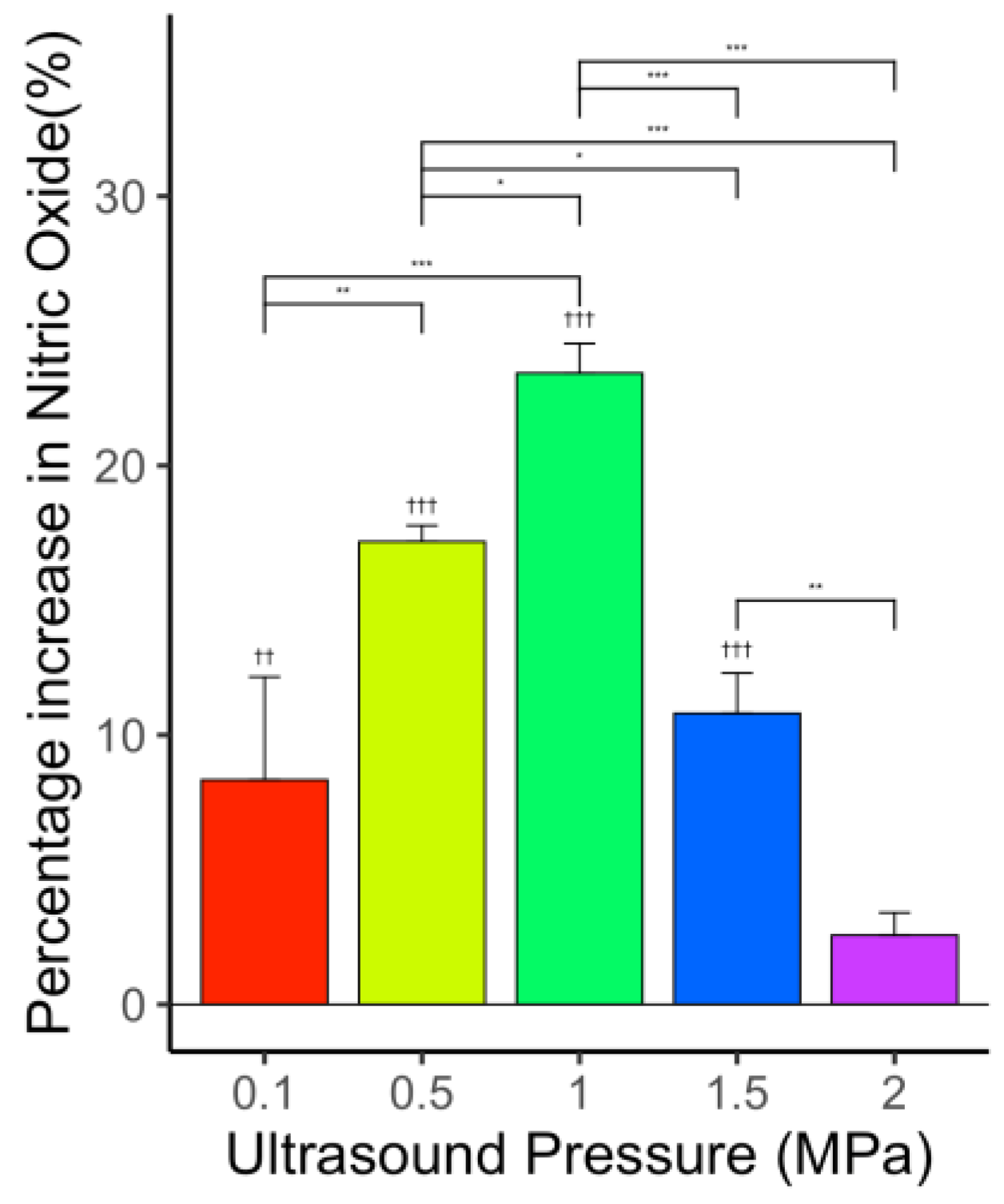
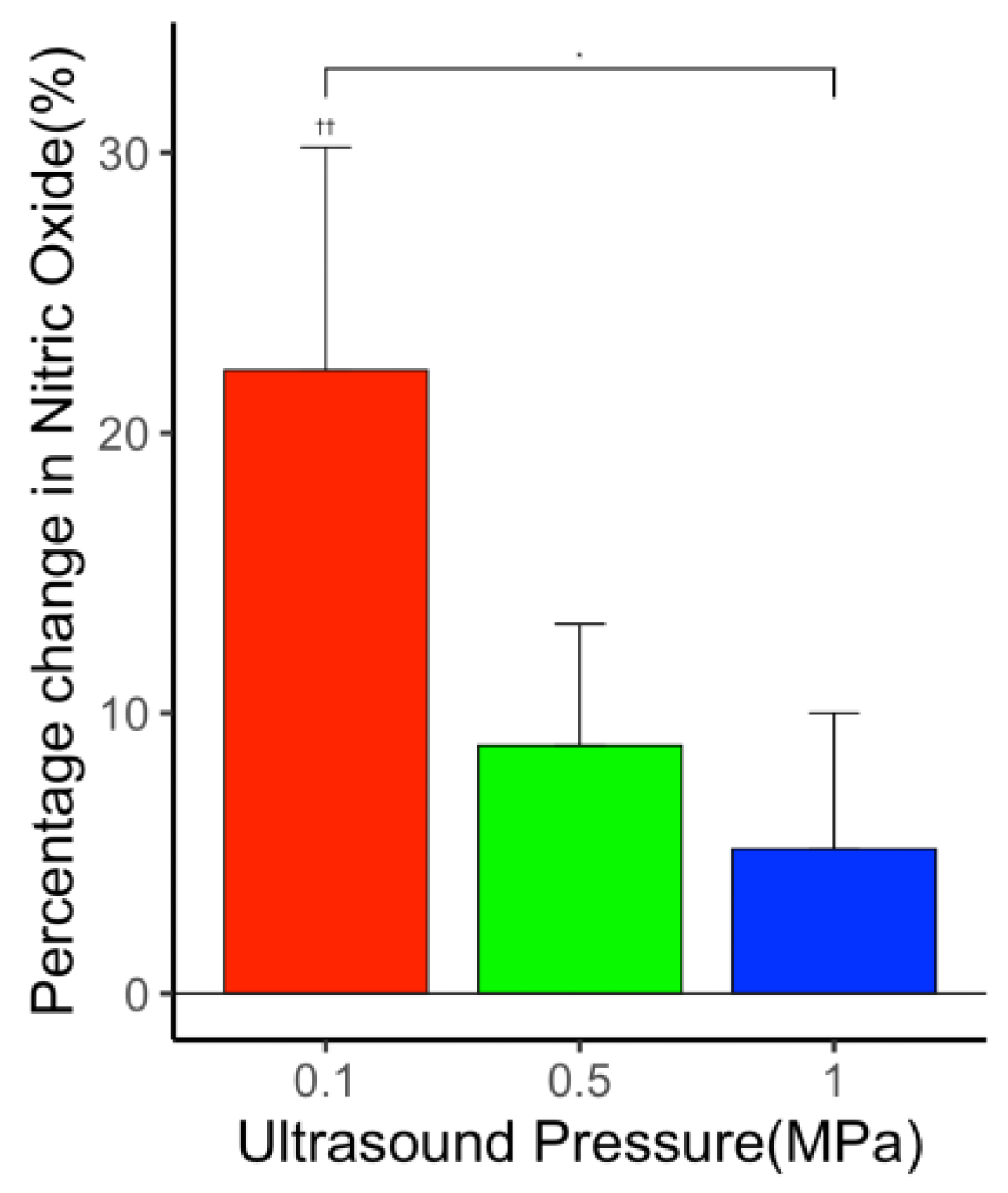
3.5.2. Constant Laser Fluence with Varying Ultrasound Pressure
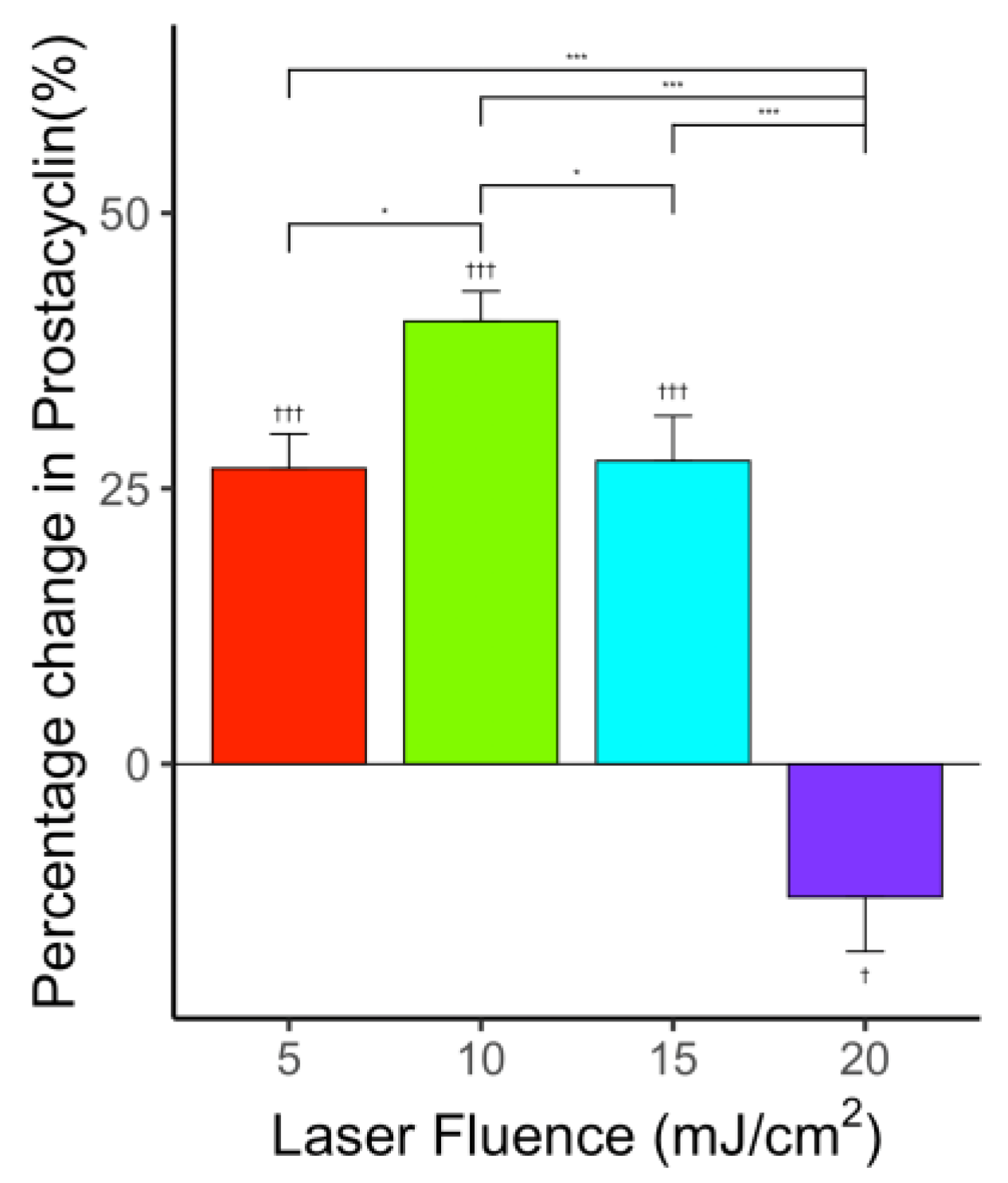
3.6. Effect of Ultrasound-Only Treatment on PGI2 Level
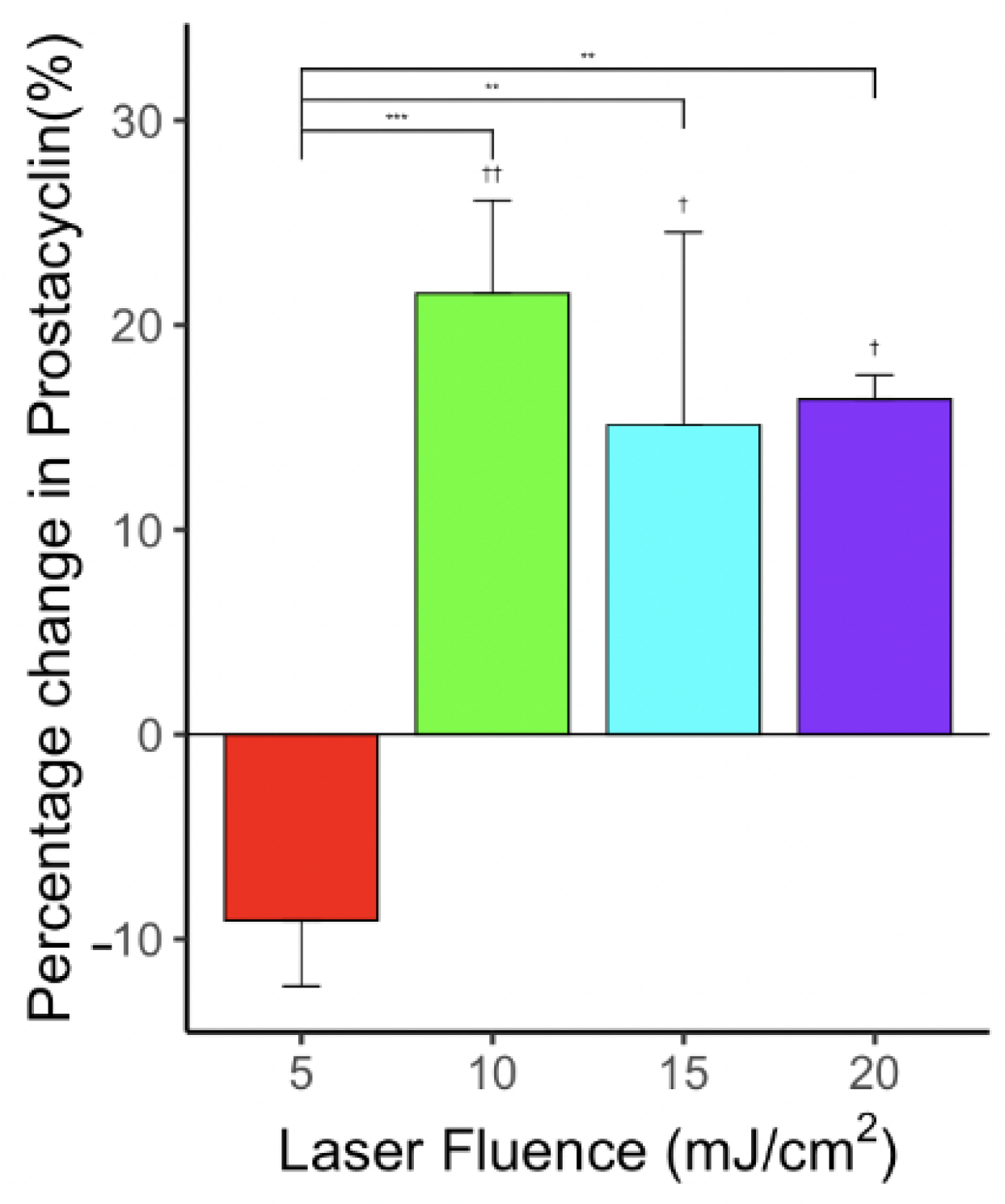
3.7. Effect of Laser-Only Treatment on PGI2 Level
3.8. Effect of Photo-Mediated Ultrasound Therapy on PGI2 Level
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, H.; Yang, X. Enhanced-heating effect during photoacoustic imaging-guided high-intensity focused ultrasound. Appl. Phys. Lett. 2011, 99, 231113. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Zhang, T.; Yang, X. Laser-enhanced cavitation during high intensity focused ultrasound: An in vivo study. Appl. Phys. Lett. 2013, 102, 133702. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, H.; Mordovanakis, A.; Paulus, Y.; Liu, Q.; Wang, X.; Yang, X. High-precision, non-invasive anti-microvascular approach via concurrent ultrasound and laser irradiation. Sci. Rep. 2017, 7, 40243. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, X.; Li, J.; Qin, Y.; Zhang, W.; Cheng, Q.; Yuan, S.; Liu, Q.; Paulus, Y.M.; Wang, X.; et al. Removal of choroidal vasculature using concurrently applied ultrasound bursts and nanosecond laser pulses. Sci. Rep. 2018, 8, 12848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Zhang, W.; Qin, Y.; Xie, X.; Yang, X.; Wang, X.; Paulus, Y.M. Photoacoustic signal-guided photo-mediated ultrasound therapy as a novel method to remove microvasculature. Investig. Ophthalmol. Vis. Sci. 2019, 60, PB0172. [Google Scholar]
- Qin, Y.; Yu, Y.; Xie, X.; Zhang, W.; Fu, J.; Paulus, Y.M.; Yang, X.; Wang, X. The Effect of Laser and Ultrasound Synchronization in Photo-Mediated Ultrasound Therapy. IEEE Trans. Biomed. Eng. 2020, 67, 3363–3370. [Google Scholar] [CrossRef] [PubMed]
- Paulus, Y.M.; Qin, Y.; Yu, Y.; Fu, J.; Wang, X.; Yang, X. Photo-mediated Ultrasound Therapy to Treat Retinal Neovascularization. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 5244–5247. [Google Scholar]
- Paulus, Y.M.; Hu, Z.; Yang, X.; Mordovanakis, A.; Wang, X. Photo-Mediated Ultrasound Therapy as a Novel Method to Selectively Treat Small Blood Vessels. Investig. Ophthalmol. Vis. Sci. 2016, 57. Available online: https://iovs.arvojournals.org/article.aspx?articleid=2558449 (accessed on 29 January 2022).
- Mukundakrishnan, K.; Ayyaswamy, P.S.; Eckmann, D.M. Bubble Motion in a Blood Vessel: Shear Stress Induced Endothelial Cell Injury. J. Biomech. Eng. 2009, 131, 074516. [Google Scholar] [CrossRef]
- Hwang, J.H.; Brayman, A.A.; Reidy, M.A.; Matula, T.J.; Kimmey, M.B.; Crum, L.A. Vascular effects induced by combined 1-MHz ultrasound and microbubble contrast agent treatments in vivo. Ultrasound Med. Biol. 2005, 31, 553–564. [Google Scholar] [CrossRef]
- Wood, A.K.; Ansaloni, S.; Ziemer, L.S.; Lee, W.M.-F.; Feldman, M.D.; Sehgal, C.M. The antivascular action of physiotherapy ultrasound on murine tumors. Ultrasound Med. Biol. 2005, 31, 1403–1410. [Google Scholar] [CrossRef] [Green Version]
- Wood, A.K.; Bunte, R.M.; Cohen, J.D.; Tsai, J.H.; Lee, W.M.-F.; Sehgal, C.M. The Antivascular Action of Physiotherapy Ultrasound on a Murine Tumor: Role of a Microbubble Contrast Agent. Ultrasound Med. Biol. 2007, 33, 1901–1910. [Google Scholar] [CrossRef] [Green Version]
- Feng, G.; Liu, J.; Zhao, X.; Wei, J.; Ou, W.; Xiao, S.; Hu, Z.; Wei, H.; Liu, Z. Hemostatic Effects of Microbubble-Enhanced Low-Intensity Ultrasound in a Liver Avulsion Injury Model. PLoS ONE 2014, 9, e95589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Kheirolomoom, A.; Mahakian, L.M.; Beegle, J.R.; Kruse, D.E.; Lam, K.S.; Ferrara, K.W. Insonation of Targeted Microbubbles Produces Regions of Reduced Blood Flow Within Tumor Vasculature. Investig. Radiol. 2012, 47, 398–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballermann, B.J.; Dardik, A.; Eng, E.; Liu, A. Shear stress and the endothelium. Kidney Int. 1998, 54, S100–S108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.-S.J.; Haga, J.H.; Chien, S. Molecular basis of the effects of shear stress on vascular endothelial cells. J. Biomech. 2005, 38, 1949–1971. [Google Scholar] [CrossRef]
- Vine, A.K.; Stader, J.; Branham, K.; Musch, D.C.; Swaroop, A. Biomarkers of Cardiovascular Disease as Risk Factors for Age-Related Macular Degeneration. Ophthalmology 2005, 112, 2076–2080. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Blood vessels and endothelial cells. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Michiels, C. Endothelial cell functions. J. Cell. Physiol. 2003, 196, 430–443. [Google Scholar] [CrossRef]
- Deng, J.; Huang, Q.; Wang, F.; Liu, Y.; Wang, Z.; Wang, Z.; Zhang, Q.; Lei, B.; Cheng, Y. The Role of Caveolin-1 in Blood–Brain Barrier Disruption Induced by Focused Ultrasound Combined with Microbubbles. J. Mol. Neurosci. 2011, 46, 677–687. [Google Scholar] [CrossRef]
- Lelu, S.; Afadzi, M.; Berg, S.; Åslund, A.; Torp, S.H.; Sattler, W.; Davies, C.d.L. Primary Porcine Brain Endothelial Cells as In Vitro Model to Study Effects of Ultrasound and Microbubbles on Blood–Brain Barrier Function. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 64, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Hallow, D.M.; Mahajan, A.D.; Prausnitz, M.R. Ultrasonically targeted delivery into endothelial and smooth muscle cells in ex vivo arteries. J. Control. Release 2007, 118, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Fan, Z.; Kumon, R.E.; El-Sayed, M.E.; Deng, C.X. Modulation of Intracellular Ca2+ Concentration in Brain Microvascular Endothelial Cells in vitro by Acoustic Cavitation. Ultrasound Med. Biol. 2010, 36, 1176–1187. [Google Scholar] [CrossRef] [Green Version]
- Juffermans, L.J.; van Dijk, A.; Jongenelen, C.A.; Drukarch, B.; Reijerkerk, A.; de Vries, H.E.; Kamp, O.; Musters, R.J. Ultrasound and Microbubble-Induced Intra- and Intercellular Bioeffects in Primary Endothelial Cells. Ultrasound Med. Biol. 2009, 35, 1917–1927. [Google Scholar] [CrossRef]
- Karthikesh, M.S.; Yang, X. The effect of ultrasound cavitation on endothelial cells. Exp. Biol. Med. 2021, 246, 758–770. [Google Scholar] [CrossRef]
- Zhigang, W.; Zhiyu, L.; Haitao, R.; Hong, R.; Qunxia, Z.; Ailong, H.; Qi, L.; Chunjing, Z.; Hailin, T.; Lin, G.; et al. Ultrasound-mediated microbubble destruction enhances VEGF gene delivery to the infarcted myocardium in rats. Clin. Imaging 2004, 28, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, N.; Seliktar, D.; Kimmel, E. Ultrasound-Induced Angiogenic Response in Endothelial Cells. Ultrasound Med. Biol. 2007, 33, 1818–1829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, X.; Liu, C.; Wang, J.; Liu, X.; Li, H.; Wang, J.; Wu, C. Expression of endostatin mediated by a novel non-viral delivery system inhibits human umbilical vein endothelial cells in vitro. Mol. Biol. Rep. 2009, 37, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Raz, D.; Zaretsky, U.; Einav, S.; Elad, D. Cellular Alterations in Cultured Endothelial Cells Exposed to Therapeutic Ultrasound Irradiation. Endothelium 2005, 12, 201–213. [Google Scholar] [CrossRef]
- Liu, X.; Wang, B.; Ding, H.; Shi, H.; Liu, J.; Sun, H. Low-intensity pulsed ultrasound in combination with SonoVue induces cytotoxicity of human renal glomerular endothelial cells via repression of the ERK1/2 signaling pathway. Ren. Fail. 2018, 40, 458–465. [Google Scholar] [CrossRef]
- Karsch, U.; Henn, W.; Seyfert, U.T.; Steudel, W.-I.; Reif, J. Effect of high power ultrasound on endothelial cells—an in vitro study of the endothelium—hemostasis interaction. Clin. Hemorheol. Microcirc. 2002, 27, 123–135. [Google Scholar]
- Eisenbrey, J.R.; Shraim, R.; Liu, J.-B.; Li, J.; Stanczak, M.; Oeffinger, B.; Leeper, D.B.; Keith, S.W.; Jablonowski, L.J.; Forsberg, F.; et al. Sensitization of Hypoxic Tumors to Radiation Therapy Using Ultrasound-Sensitive Oxygen Microbubbles. Int. J. Radiat. Oncol. 2018, 101, 88–96. [Google Scholar] [CrossRef]
- Al-Mahrouki, A.A.; Karshafian, R.; Giles, A.; Czarnota, G.J. Bioeffects of Ultrasound-Stimulated Microbubbles on Endothelial Cells: Gene Expression Changes Associated with Radiation Enhancement In Vitro. Ultrasound Med. Biol. 2012, 38, 1958–1969. [Google Scholar] [CrossRef]
- Kim, H.C.; Al-Mahrouki, A.; Gorjizadeh, A.; Karshafian, R.; Czarnota, G.J. Effects of Biophysical Parameters in Enhancing Radiation Responses of Prostate Tumors with Ultrasound-Stimulated Microbubbles. Ultrasound Med. Biol. 2013, 39, 1376–1387. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Lim, W.; Kim, J.; Jeon, S.; Kim, S.; Karna, S.; Cha, H.; Kim, O.; Choi, H. Effect of 635 nm irradiation on high glucose-boosted inflammatory responses in LPS-induced MC3T3-E1 cells. Lasers Med. Sci. 2012, 28, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Esmaeelinejad, M.; Bayat, M. Effect of low-level laser therapy on the release of interleukin-6 and basic fibroblast growth factor from cultured human skin fibroblasts in normal and high glucose mediums. J. Cosmet. Laser Ther. 2013, 15, 310–317. [Google Scholar] [CrossRef]
- Moskvin, S.; Askhadulin, E.; Kochetkov, A. Low-Level Laser Therapy in Prevention of the Development of Endothelial Dysfunction and Clinical Experience of Treatment and Rehabilitation of COVID-19 Patients. Rehabil. Res. Pract. 2021, 2021, 6626932. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-J.; Teng, Y.-H.; Cheng, Y.-J. Low Level LASER Therapy Induces Therapeutic Angiogenesis in Diabetic Mice with Hindlimb Ischemia. bioRxiv 2018, 496208. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef]
- Palmer, R.M.J.; Ferrige, A.G.; Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, M.; Ohara, Y.; Navas, J.P.; Nishida, K.; Murphy, T.J.; Alexander, R.W.; Nerem, R.M.; Harrison, D.G. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am. J. Physiol. Physiol. 1995, 269, C1371–C1378. [Google Scholar] [CrossRef] [PubMed]
- Ayajiki, K.; Kindermann, M.; Hecker, M.; Fleming, I.; Busse, R. Intracellular pH and Tyrosine Phosphorylation but Not Calcium Determine Shear Stress–Induced Nitric Oxide Production in Native Endothelial Cells. Circ. Res. 1996, 78, 750–758. [Google Scholar] [CrossRef]
- Corson, M.A.; James, N.L.; Latta, S.E.; Nerem, R.M.; Berk, B.C.; Harrison, D.G. Phosphorylation of Endothelial Nitric Oxide Synthase in Response to Fluid Shear Stress. Circ. Res. 1996, 79, 984–991. [Google Scholar] [CrossRef]
- Fleming, I.; Bauersachs, J.; Fisslthaler, B.; Busse, R. Ca2+-Independent Activation of the Endothelial Nitric Oxide Synthase in Response to Tyrosine Phosphatase Inhibitors and Fluid Shear Stress. Circ. Res. 1998, 82, 686–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Nucci, G.; Gryglewski, R.J.; Warner, T.; Vane, J.R. Receptor-mediated release of endothelium-derived relaxing factor and prostacyclin from bovine aortic endothelial cells is coupled. Proc. Natl. Acad. Sci. USA 1988, 85, 2334–2338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanada, T.; Hashimoto, M.; Nosaka, S.; Sasaki, T.; Nakayama, K.; Masumura, S.; Yamauchi, M.; Tamura, K. Shear stress enhances prostacyclin release from endocardial endothelial cells. Life Sci. 1999, 66, 215–220. [Google Scholar] [CrossRef]
- Qiu, Y.; Tarbell, J.M. Interaction between Wall Shear Stress and Circumferential Strain Affects Endothelial Cell Biochemical Production. J. Vasc. Res. 2000, 37, 147–157. [Google Scholar] [CrossRef]
- Wong, A.D.; Searson, P.C. Live-Cell Imaging of Invasion and Intravasation in an Artificial Microvessel Platform. Cancer Res. 2014, 74, 4937–4945. [Google Scholar] [CrossRef] [Green Version]
- Sfriso, R.; Zhang, S.; Bichsel, C.; Steck, O.; Despont, A.; Guenat, O.T.; Rieben, R. 3D artificial round section micro-vessels to investigate endothelial cells under physiological flow conditions. Sci. Rep. 2018, 8, 5898. [Google Scholar] [CrossRef] [PubMed]
- Bogorad, M.I.; Searson, P.C. Real-time imaging and quantitative analysis of doxorubicin transport in a perfusable microvessel platform. Integr. Biol. 2016, 8, 976–984. [Google Scholar] [CrossRef]
- Linville, R.M.; DeStefano, J.G.; Sklar, M.B.; Xu, Z.; Farrell, A.M.; Bogorad, M.I.; Chu, C.; Walczak, P.; Cheng, L.; Mahairaki, V. Human iPSC-derived blood-brain barrier microvessels: Validation of barrier function and endothelial cell behavior. Biomaterials 2019, 190, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Wipff, P.-J.; Majd, H.; Acharya, C.; Buscemi, L.; Meister, J.-J.; Hinz, B. The covalent attachment of adhesion molecules to silicone membranes for cell stretching applications. Biomaterials 2009, 30, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Visser, S.; Hergenrother, R.; Cooper, S. Polymers Biomaterials Science: An Introduction to Materials in Medicine; Academic Press: Cambridge, MA, USA, 1996. [Google Scholar]
- Yim, E.K.; Reano, R.M.; Pang, S.W.; Yee, A.F.; Chen, C.S.; Leong, K.W. Nanopattern-induced changes in morphology and motility of smooth muscle cells. Biomaterials 2005, 26, 5405–5413. [Google Scholar] [CrossRef] [Green Version]
- Bélanger, M.C.; Marois, Y. Hemocompatibility, biocompatibility, inflammatory and in vivo studies of primary reference materials low-density polyethylene and polydimethylsiloxane: A review. J. Biomed. Mater. Res. 2001, 58, 467–477. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Schueller, O.J.; Whitesides, G.M. Fabrication of microfluidic systems in poly (dimethylsiloxane). Electrophor. Int. J. 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Kuncova-Kallio, J.; Kallio, P.J. PDMS and its suitability for analytical microfluidic devices. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 2486–2489. [Google Scholar]
- Lamberti, A.; Marasso, S.L.; Cocuzza, M. PDMS membranes with tunable gas permeability for microfluidic applications. RSC Adv. 2014, 4, 61415–61419. [Google Scholar] [CrossRef]
- Hao-Ran, L.; Xiao, L.; Xin-Ye, N.; Hui-Lin, Y.; Lei, Y. A novel hydrogel-polydimethylsiloxane elastomer for wound dressing application. Ferroelectrics 2018, 523, 104–111. [Google Scholar] [CrossRef]
- Horn, G. Silicone Polymer Contact Lens Compositions and Methods of Use. 2005. Available online: https://patents.google.com/patent/US20050288196A1/en (accessed on 14 January 2020).
- Raub, C.B.; Suresh, V.; Krasieva, T.; Lyubovitsky, J.; Mih, J.D.; Putnam, A.J.; Tromberg, B.J.; George, S.C. Noninvasive Assessment of Collagen Gel Microstructure and Mechanics Using Multiphoton Microscopy. Biophys. J. 2007, 92, 2212–2222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrobak, K.M.; Potter, D.R.; Tien, J. Formation of perfused, functional microvascular tubes in vitro. Microvasc. Res. 2006, 71, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Lagacé, R.; Grimaud, J.-A.; Schürch, W.; Seemayer, T.A. Myofibroblastic stromal reaction in carcinoma of the breast: Variations of collagenous matrix and structural glycoproteins. Virchows. Archiv. A Pathol. Anat. Histopathol. 1985, 408, 49–59. [Google Scholar] [CrossRef]
- Wilson, M.E.; Kota, N.; Kim, Y.; Wang, Y.; Stolz, D.B.; LeDuc, P.R.; Ozdoganlar, O.B. Fabrication of circular microfluidic channels by combining mechanical micromilling and soft lithography. Lab Chip 2011, 11, 1550–1555. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, M.; Wu, C.; Chien, W.-Y.; Geddie, W.R.; Jewett, M.A.S.; Sun, Y. A fast and simple method to fabricate circular microchannels in polydimethylsiloxane (PDMS). Lab Chip 2010, 11, 545–551. [Google Scholar] [CrossRef]
- Singh, R.; Wang, X.; Yang, X. Cavitation induced shear and circumferential stresses on blood vessel walls during photo-mediated ultrasound therapy. AIP Adv. 2020, 10, 125227. [Google Scholar] [CrossRef]
- Hazlewood, D.; Yang, X. Enhanced cavitation activity in a slab-shaped optical absorber during photo-mediated ultrasound therapy. Phys. Med. Biol. 2019, 65, 055006. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qin, Y.; Wang, X.; Yang, X. Bubble growth in cylindrically-shaped optical absorbers during photo-mediated ultrasound therapy. Phys. Med. Biol. 2018, 63, 125017. [Google Scholar] [CrossRef] [PubMed]
- Tzima, E.; Irani-Tehrani, M.; Kiosses, W.B.; Dejana, E.; Schultz, D.A.; Engelhardt, B.; Cao, G.; Delisser, H.; Schwartz, M.A. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 2005, 437, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, S.; Yokoyama, M. Dysfunction of Endothelial Nitric Oxide Synthase and Atherosclerosis. Arter. Thromb. Vasc. Biol. 2004, 24, 998–1005. [Google Scholar] [CrossRef]
- Altland, O.D.; Dalecki, D.; Suchkova, V.N.; Francis, C.W. Low-intensity ultrasound increases endothelial cell nitric oxide synthase activity and nitric oxide synthesis. J. Thromb. Haemost. 2004, 2, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Sugita, Y.; Mizuno, S.; Nakayama, N.; Iwaki, T.; Murakami, E.; Wang, Z.; Endoh, R.; Furuhata, H. Nitric Oxide Generation Directly Responds to Ultrasound Exposure. Ultrasound Med. Biol. 2008, 34, 487–493. [Google Scholar] [CrossRef]
- Tam, G. Low power laser therapy and analgesic action. J. Clin. Laser Med. Surg. 1999, 17, 29–33. [Google Scholar] [CrossRef]
- Francis, P.J.; Appukuttan, B.; Simmons, E.; Landauer, N.; Stoddard, J.; Hamon, S.; Ott, J.; Ferguson, B.; Klein, M.; Stout, J.T.; et al. Rhesus monkeys and humans share common susceptibility genes for age-related macular disease. Hum. Mol. Genet. 2008, 17, 2673–2680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsikas, D. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: Appraisal of the Griess reaction in the l-arginine/nitric oxide area of research. J. Chromatogr. B 2007, 851, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Marletta, M.A.; Yoon, P.S.; Iyengar, R.; Leaf, C.D.; Wishnok, J.S. Macrophage oxidation of L-arginine to nitrite and nitrate: Nitric oxide is an intermediate. Biochemistry 1988, 27, 8706–8711. [Google Scholar] [CrossRef] [PubMed]
- Wennmalm, A.; Benthin, G.; Edlund, A.; Jungersten, L.; Kieler-Jensen, N.; Lundin, S.; Westfelt, U.N.; Petersson, A.S.; Waagstein, F. Metabolism and excretion of nitric oxide in humans. An experimental and clinical study. Circ. Res. 1993, 73, 1121–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wennmalm, Å.; Benthin, G.; Petersson, A.-S. Dependence of the metabolism of nitric oxide (NO) in healthy human whole blood on the oxygenation of its red cell haemoglobin. J. Cereb. Blood Flow Metab. 1992, 106, 507–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsikas, D. ReviewMethods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free Radic. Res. 2005, 39, 797–815. [Google Scholar] [CrossRef]
- Miles, A.M.; Wink, D.A.; Cook, J.C.; Grisham, M.B. Determination of nitric oxide using fluorescence spectroscopy. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1996; Volume 268, pp. 105–120. [Google Scholar]
- Alnahdi, A.; John, A.; Raza, H. N-acetyl cysteine attenuates oxidative stress and glutathione-dependent redox imbalance caused by high glucose/high palmitic acid treatment in pancreatic Rin-5F cells. PLoS ONE 2019, 14, e0226696. [Google Scholar] [CrossRef] [PubMed]
- Tijssen, P. Practice and Theory of Enzyme Immunoassays; Elsevier: Amsterdam, The Netherlands, 1985. [Google Scholar]
- Vallance, P.; Collier, J.; Moncada, S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet 1989, 334, 997–1000. [Google Scholar] [CrossRef]
- Kunisaki, M.; Umeda, F.; Inoguchi, T.; Nawata, H. Vitamin E restores reduced prostacyclin synthesis in aortic endothelial cells cultured with a high concentration of glucose. Metabolism 1992, 41, 613–621. [Google Scholar] [CrossRef]
- Ono, H.; Umeda, F.; Inoguchi, T.; Ibayashi, H. Glucose Inhibits Prostacyclin Production by Cultured Aortic Endothelial Cells. Thromb. Haemost. 1988, 60, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Yang, X. Laser-enhanced high-intensity focused ultrasound heating in an in vivo small animal model. Appl. Phys. Lett. 2016, 109, 213702. [Google Scholar] [CrossRef] [Green Version]
- Lachaux, J.; Hwang, G.; Arouche, N.; Naserian, S.; Harouri, A.; Lotito, V.; Casari, C.; Lok, T.; Menager, J.B.; Issard, J.; et al. A compact integrated microfluidic oxygenator with high gas exchange efficiency and compatibility for long-lasting endothelialization. Lab Chip 2021, 21, 4791–4804. [Google Scholar] [CrossRef]
- Naserian, S.; Abdelgawad, M.E.; Bakshloo, M.A.; Ha, G.; Arouche, N.; Cohen, J.L.; Salomon, B.; Uzan, G. The TNF/TNFR2 signaling pathway is a key regulatory factor in endothelial progenitor cell immunosuppressive effect. Cell Commun. Signal. 2020, 18, 94. [Google Scholar] [CrossRef]
- Barkestani, M.N.; Shamdani, S.; Bakshloo, M.A.; Arouche, N.; Bambai, B.; Uzan, G.; Naserian, S. TNFα priming through its interaction with TNFR2 enhances endothelial progenitor cell immunosuppressive effect: New hope for their widespread clinical application. Cell Commun. Signal. 2021, 19, 1–16. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subramanian Karthikesh, M.; Wu, S.; Singh, R.; Paulus, Y.; Wang, X.; Yang, X. Effect of Photo-Mediated Ultrasound Therapy on Nitric Oxide and Prostacyclin from Endothelial Cells. Appl. Sci. 2022, 12, 2617. https://doi.org/10.3390/app12052617
Subramanian Karthikesh M, Wu S, Singh R, Paulus Y, Wang X, Yang X. Effect of Photo-Mediated Ultrasound Therapy on Nitric Oxide and Prostacyclin from Endothelial Cells. Applied Sciences. 2022; 12(5):2617. https://doi.org/10.3390/app12052617
Chicago/Turabian StyleSubramanian Karthikesh, Madhumithra, Sa Wu, Rohit Singh, Yannis Paulus, Xueding Wang, and Xinmai Yang. 2022. "Effect of Photo-Mediated Ultrasound Therapy on Nitric Oxide and Prostacyclin from Endothelial Cells" Applied Sciences 12, no. 5: 2617. https://doi.org/10.3390/app12052617







