Influence of Super Absorbent Polymer on Root Characteristics and Anchorage of Amorpha fruticosa on Rocky Slope
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
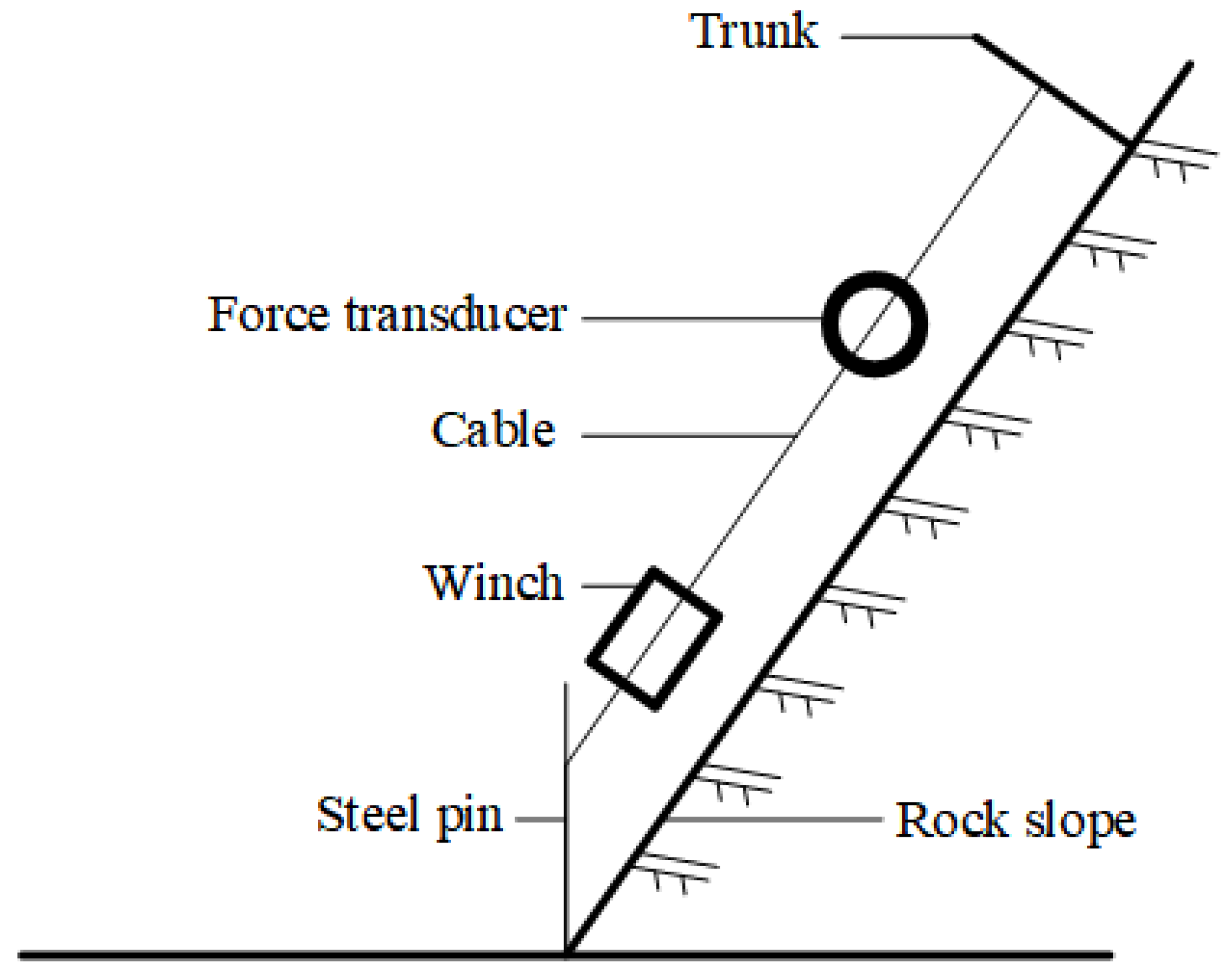
2.2. Pull-Out Test
2.3. Tensile Resistance Test
2.4. Measurement of Root System Anchorage Resistance
2.5. Soil Measurement
2.6. Data Analysis
3. Results
3.1. Soil Basic Properties
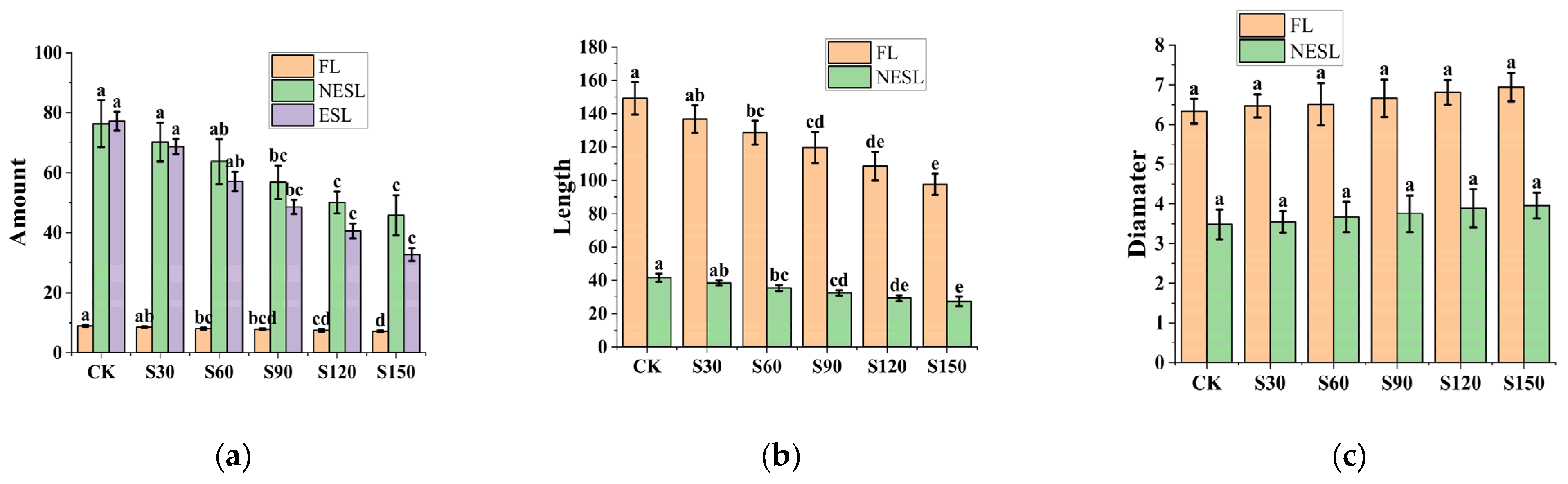
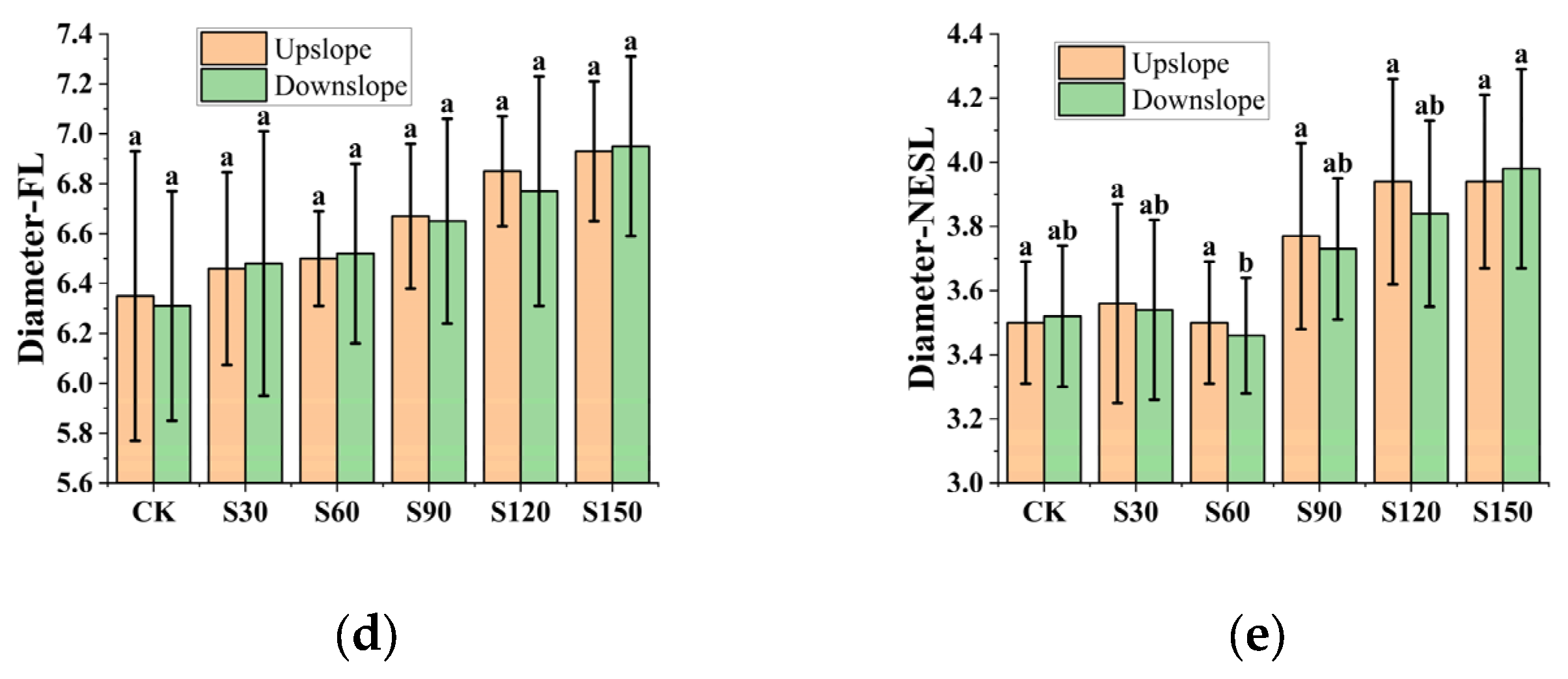
3.2. Distribution and Basic Data of Root System
3.3. Tensile Resistance of Root System
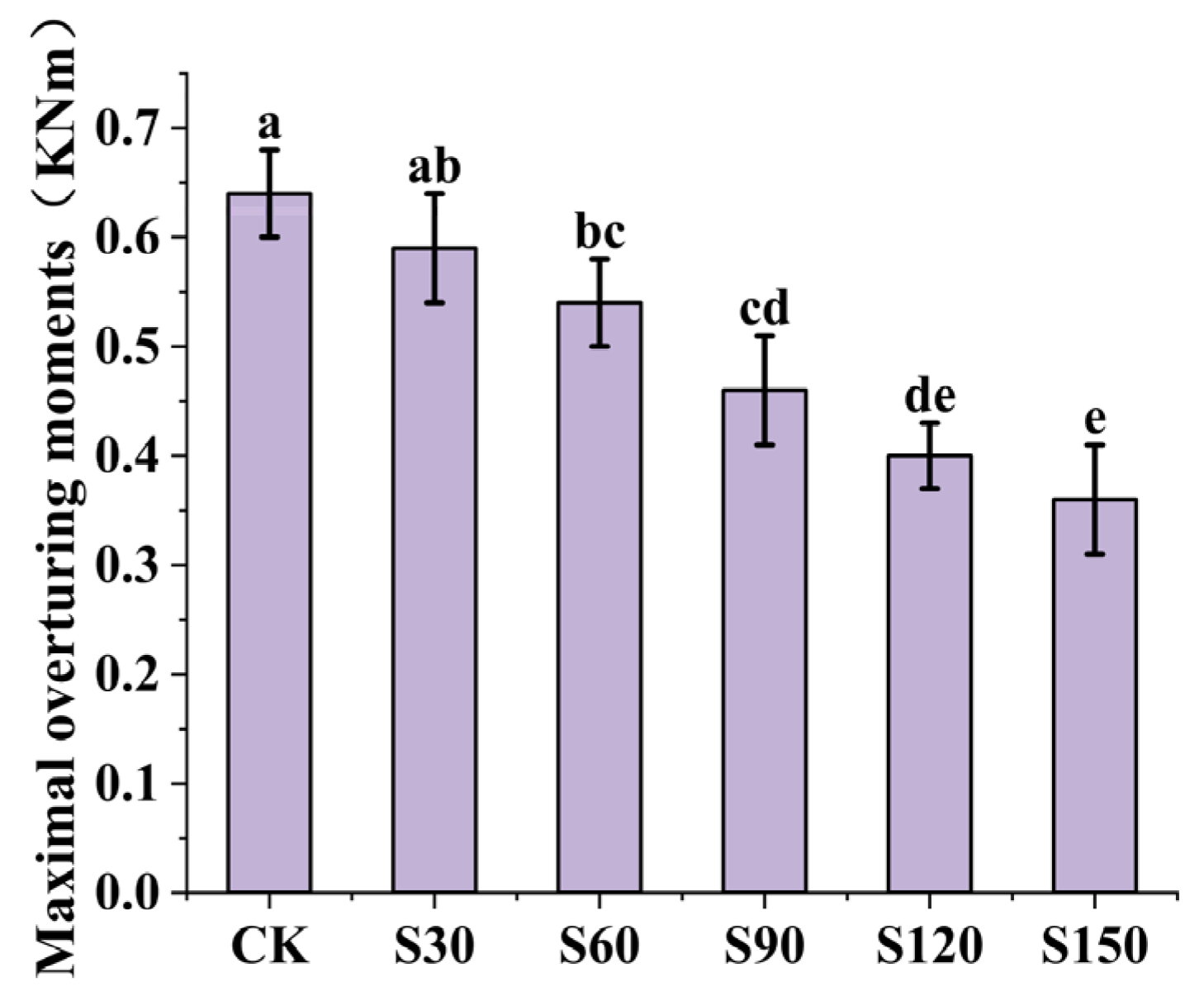
3.4. The Anchoring Force of Root System
4. Discussion
4.1. Soil Physicochemical Properties
4.2. Root Distribution
4.3. Mechanical Properties of the Root System
4.4. Anchorage and Breakage of Root System
5. Conclusions
- (1)
- The water retention agent effectively reduces soil bulk and increases soil water content. SAP can improve the environmental conditions at the root-soil interface, and enhances soil water storage capacity.
- (2)
- The soil nutrient content was found to be related to three factors: plant growth consumption, water retention agent adsorption, and active groups in the water retention agent. The first two reasons work against soil nutrient increase, while the third reason works in favor of nutrient growth. Three of these factors interact, and because the soil fertility in this experiment may be insufficient, the soil nutrients decline as the water-retention agent content increases.
- (3)
- The number and length of lateral roots decreases as the amount of SAP increases. When the soil moisture is sufficient, the water retention agent changes the soil structure due to its water absorption characteristics, limiting soil moisture transportation to the root system to a certain extent, thereby inhibiting the growth of the root system. Besides, we suppose that since Amorpha fruticosa is a woody shrub with tolerance to water stress and low or moderate water requirements, SAP does not provide additional benefits.
- (4)
- The tensile strength of the upslope root is greater than that of downslope root. The primary contributors to root anchoring are the upslope lateral roots. Mechanical stimulation enhances the tensile strength of the root system by increasing the quantity and diameter of up-slope lateral roots that resist the external load pressure, hence strengthening the slope stability. Simultaneously, water retention agents alter the water content of the root system, which results in changes to the root system’s internal structure in terms of lignin, cellulose, and xylem fiber area, which influences the anchorage of the root system.
- (5)
- Plant anchorage decreases as SAP increases. SAP increases the water content below the root-soil plate, causing the soil pore water pressure to increase, so the soil is hydraulically fractured, the friction between the root-soil decreases, and the plant anchorage is weakened.
- (6)
- The significant addition of SAP could enhance the tensile strength of upslope embedded secondary lateral roots but would adversely affect soil nutrients, root distribution, and root anchorage. The addition of SAP in this test had no significant effect on improving slope stability. From the perspective of reinforcement capacity, we cannot blindly pursue the survival rate and other high dosage use of water retention agents to increase the risk of slope destabilization.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Li, Y.; Qi, S.; Liang, B.; Ma, J.; Cheng, B.; Ma, C.; Qiu, Y.; Chen, Q. Dangerous degree forecast of soil loss on highway slopes in mountainous areas of the Yunnan–Guizhou Plateau (China) using the Revised Universal Soil Loss Equation. Nat. Hazards Earth Syst. Sci. 2019, 19, 757–774. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Zhao, T.; Huang, X.; Zhao, P. Ecological restoration of highway slope by covering with straw-mat and seeding with grass–legume mixture. Ecol. Eng. 2016, 90, 68–76. [Google Scholar] [CrossRef]
- Rahimi, A.; Rahardjo, H.; Leong, E. Effect of antecedent rainfall patterns on rainfall-induced slope failure. J. Geotech. Geoenviron. 2011, 137, 483–491. [Google Scholar] [CrossRef]
- Pourmalekshah, A.A.M.A.; Moayeri, M.H.; Parsakhoo, A. Effect of the root biotechnical characteristics of Alnus subcordata, Paulownia fortunei and Populus deltoides on the soil mechanics. J. For. Sci. 2019, 65, 283–290. [Google Scholar] [CrossRef]
- Shao, Q.; Gu, W.; Dai, Q.; Makoto, S.; Liu, Y. Effectiveness of geotextile mulches for slope restoration in semi-arid northern China. Catena 2014, 116, 1–9. [Google Scholar] [CrossRef]
- Tang, Y.; Bossard, C.; Reidhead, J. Effects of percent cover of Japanese cedar in forests on slope slides in Sichuan, China. Ecol. Eng. 2015, 74, 42–47. [Google Scholar] [CrossRef]
- Xiong, D.W.; Chen, F.Q.; Tan, X.Q.; Li, S.Y.; Huang, Y.W. Performance of Festuca rubra L. Population Vegetation Concrete in Soil Conservation and Slope Reinforcement under Experimental Conditions. Mt. Res. 2021, 39, 207–217. (In Chinese) [Google Scholar] [CrossRef]
- Xu, T.; Liu, C.Y.; Hu, X.S.; Xu, Z.W.; Shen, Z.Y.; Yu, D.M. Mechanical effects of vegetation protection on slope under loading conditions in loess areas of Xining Basin. Trans. Chin. Soc. Agric. Eng. 2021, 37, 142–151. (In Chinese) [Google Scholar] [CrossRef]
- Wang, H.X. Numerical Analysis of Plant Roots on Stability of Subgrade Slope. Master’s Degree Thesis, Northeast Forestry University, Harbin, China, 2018. [Google Scholar]
- Yang, J.; Duan, S.; Li, Q.; Liu, C. A review of flexible protection in rockfall protection. Nat. Hazards 2019, 99, 71–89. [Google Scholar] [CrossRef]
- Fu, H.; Zha, H.; Zeng, L.; Chen, C.; Jia, C.; Bian, H. Research progress on ecological protection technology of highway slope: Status and challenges. Transp. Saf. Environ. 2020, 2, 3–17. [Google Scholar] [CrossRef]
- Romano, N.; Lignola, G.P.; Brigante, M.; Bosso, L.; Chirico, G.B. Residual life and degradation assessment of wood elements used in soil bioengineering structures for slope protection. Ecol. Eng. 2016, 90, 498–509. [Google Scholar] [CrossRef]
- Zhao, B.Q.; Xia, Z.Y.; Xu, W.N.; Yang, S.; Xia, D.; Wang, Z.G. Review on research of slope eco-restoration technique for engineering disturbed area. Water Resour. Hydropower Eng. 2017, 48, 130–137. (In Chinese) [Google Scholar] [CrossRef]
- Cao, S.; Xu, C.; Ye, H.; Zhan, Y.; Gong, C. The use of air bricks for planting roadside vegetation: A new technique to improve landscaping of steep roadsides in China’s Hubei Province. Ecol. Eng. 2010, 36, 697–702. [Google Scholar] [CrossRef]
- Álvarez-Mozos, J.; Abad, E.; Giménez, R.; Campo, M.A.; Goñi, M.; Arive, M.; Casalí, J.; Díez, J.; Diego, I. Evaluation of erosion control geotextiles on steep slopes. Part 1: Effects on runoff and soil loss. Catena 2014, 118, 168–178. [Google Scholar] [CrossRef]
- Xu, Y.; Su, C.; Zhu, Q.; Yang, C.; Yang, Y. Field study of a new ecological slope protection method applied to expansive soil slope. Arab. J. Geosci. 2021, 14, 1–13. [Google Scholar] [CrossRef]
- Su, H.; Wu, D.; Lu, Y.; Peng, X.; Wang, X.; Chen, W.; Wang, S. Experimental and numerical study on stability performance of new ecological slope protection using bolt-hinge anchored block. Ecol. Eng. 2021, 172, 106409. [Google Scholar] [CrossRef]
- Ni, J.; Leung, A.K.; Ng, C.W. Unsaturated hydraulic properties of vegetated soil under single and mixed planting conditions. Géotechnique 2019, 69, 554–559. [Google Scholar] [CrossRef]
- Meijer, G.J.; Bengough, A.G.; Knappett, J.A.; Loades, K.W.; Nicoll, B.C. In situ measurement of root reinforcement using corkscrew extraction method. Can. Geotech. J. 2018, 55, 1372–1390. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X.; Yu, D.; Zhu, H.; Li, G. Influence of the roots of mixed-planting species on the shear strength of saline loess soil. J. Mt. Sci. 2021, 18, 806–818. [Google Scholar] [CrossRef]
- Chiatante, D.; Beltotto, M.; Onelli, E.; Di Iorio, A.; Montagnoli, A.; Scippa, S.G. New branch roots produced by vascular cambium derivatives in woody parental roots of Populus nigra L. Plant Biosyst. 2010, 144, 420–433. [Google Scholar] [CrossRef]
- Zegeye, A.D.; Langendoen, E.J.; Tilahun, S.A.; Mekuria, W.; Poesen, J.; Steenhuis, T.S. Root reinforcement to soils provided by common Ethiopian highland plants for gully erosion control. Ecohydrology 2018, 11, e1940. [Google Scholar] [CrossRef]
- Osman, N.; Barakbah, S.S. Parameters to predict slope stability—Soil water and root profiles. Ecol. Eng. 2006, 28, 90–95. [Google Scholar] [CrossRef]
- Fan, C.; Chen, Y. The effect of root architecture on the shearing resistance of root-permeated soils. Ecol. Eng. 2010, 36, 813–826. [Google Scholar] [CrossRef]
- He, H.Y.; Li, P.F.; Hu, Y.G.; Wang, Y.L.; Lei, Y.K.; Tian, G.X. Study on Root System Features of Herbaceous Plants at Different Aspects of Highway Slope. Southwest China J. Agric. Sci. 2017, 30, 915–922. (In Chinese) [Google Scholar] [CrossRef]
- Chen, H.; Zhang, H.L.; Tian, K.L.; Zhong, P.W. Experimental Study on the Effects of Amorpha Fruticosa Root System on Loess Slope Reinforcement. Yellow River 2017, 39, 84–88. (In Chinese) [Google Scholar] [CrossRef]
- Waldron, L.J. The shear resistance of root-permeated homogeneous and stratified soil. Soil Sci. Soc. Am. J. 1977, 41, 843–849. [Google Scholar] [CrossRef]
- Zhou, Y.Y. Study on Mechanism of Soil Reinforcement by Roots and Slope Protection Technology. Ph.D. Thesis, China University of Geosciences, Wuhan, China, 2010. (In Chinese). [Google Scholar]
- Operstein, V.; Frydman, S. The influence of vegetation on soil strength. Proc. Inst. Civ. Eng. Ground Improv. 2000, 4, 81–89. [Google Scholar] [CrossRef]
- Mickovski, S.B.; Stokes, A.; Van Beek, R.; Ghestem, M.; Fourcaud, T. Simulation of direct shear tests on rooted and non-rooted soil using finite element analysis. Ecol. Eng. 2011, 37, 1523–1532. [Google Scholar] [CrossRef]
- Ji, X.D.; Xu, C.; Dai, X.Q.; Zhang, A.; Chen, L.H. Studying the mechanical properties of the soil-root interface using the pullout test method. J. Mt. Sci. 2018, 15, 882–893. [Google Scholar] [CrossRef]
- Li, S.C.; Sun, H.L. Developing Trend and Situations of Technique to Stabilize Rock Slope with Vegetative Cover in China. Resour. Sci. 2004, 8, 62–66. (In Chinese) [Google Scholar] [CrossRef]
- Flannery, R.L.; Busscher, W.J. Use of a synthetic polymer in potting soils to improve water holding capacity. Commun. Soil Sci. Plant Anal. 1982, 13, 103–111. [Google Scholar] [CrossRef]
- Ran, Y.L.; Wang, Y.Q.; Zhang, R.X.; Zhu, F.H.; Liu, J. Research on the mechanism of super absorbent polymer to soil water-holding characteristic. Agric. Res. Arid Areas 2015, 33, 101–107. (In Chinese) [Google Scholar] [CrossRef]
- Yang, L.; Yang, Y.; Chen, Z.; Guo, C.; Li, S. Influence of super absorbent polymer on soil water retention, seed germination and plant survivals for rocky slopes eco-engineering. Ecol. Eng. 2014, 62, 27–32. [Google Scholar] [CrossRef]
- Agaba, H.; Baguma Orikiriza, L.J.; Osoto Esegu, J.F.; Obua, J.; Kabasa, J.D.; Hüttermann, A. Effects of hydrogel amendment to different soils on plant available water and survival of trees under drought conditions. Clean–Soil Air Water 2010, 38, 328–335. [Google Scholar] [CrossRef]
- Ni, B.; Liu, M.; Lü, S.; Xie, L.; Zhang, X.; Wang, Y. Novel slow-release multielement compound fertilizer with hydroscopicity and moisture preservation. Ind. Eng. Chem. Res. 2010, 49, 4546–4552. [Google Scholar] [CrossRef]
- Li, Y.Q.; Wu, H.Y.; Song, G.L.; Zhao, B.; Li, Y.W.; Xia, Y.; Sun, S.N.; Liang, Y.L. Effect of slope gradient on root morphological characteristics of Lespedeza bicolor and Amorpha fruticosa. Grassl. Turf 2020, 40, 23–29. (In Chinese) [Google Scholar] [CrossRef]
- Ji, X.L.; Xia, G.H.; Zhang, H.Y. Effects of Amorpha fruticosa Root System on Slope Soil and Water Conservation. Hubei For. Sci. Technol. 2016, 45, 16–19. (In Chinese) [Google Scholar] [CrossRef]
- Cucchi, V.; Meredieu, C.; Stokes, A.; Berthier, S.; Bert, D.; Najar, M.; Denis, A.; Lastennet, R. Root anchorage of inner and edge trees in stands of Maritime pine (Pinus pinaster Ait.) growing in different podzolic soil conditions. Trees 2004, 18, 460–466. [Google Scholar] [CrossRef]
- Li, S.; Sun, H.; Yang, Z.; Xiong, W.; Cui, B. Root anchorage of Vitex negundo L. on rocky slopes under different weathering degrees. Ecol. Eng. 2007, 30, 27–33. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, X.; Li, Y.; Luo, H.; Xu, Q. Preliminary study of tractive effect of lateral roots of Pinus yunnanensis on shallow soil mass. Chin. J. Plant Ecol. 1999, 23, 458. (In Chinese) [Google Scholar]
- Mcgeehan, S.L.; Naylor, D.V. Automated instrumental analysis of carbon and nitrogen in plant and soil samples. Commun. Soil Sci. Plant Anal. 1988, 19, 493–505. [Google Scholar] [CrossRef]
- Shi, R.H.; Bao, S.D. Soil Agriculture Chemistry Analysis; China Agriculture Press: Beijing, China, 1980; pp. 191–197. (In Chinese) [Google Scholar]
- Syers, J.K.; Williams, J.; Campbell, A.S.; Walker, T.W. The significance of apatite inclusions in soil phosphorus studies. Soil Sci. Soc. Am. J. 1967, 31, 752–756. [Google Scholar] [CrossRef]
- Bodner, G.; Leitner, D.; Kaul, H. Coarse and fine root plants affect pore size distributions differently. Plant Soil 2014, 380, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Mimmo, T.; Del Buono, D.; Terzano, R.; Tomasi, N.; Vigani, G.; Crecchio, C.; Pinton, R.; Zocchi, G.; Cesco, S. Rhizospheric organic compounds in the soil–microorganism–plant system: Their role in iron availability. Eur. J. Soil Sci. 2014, 65, 629–642. [Google Scholar] [CrossRef]
- Pierret, A.; Maeght, J.; Clément, C.; Montoroi, J.; Hartmann, C.; Gonkhamdee, S. Understanding deep roots and their functions in ecosystems: An advocacy for more unconventional research. Ann. Bot. 2016, 118, 621–635. [Google Scholar] [CrossRef]
- Jin, K.; Shen, J.; Ashton, R.W.; White, R.P.; Dodd, I.C.; Parry, M.A.; Whalley, W.R. Wheat root growth responses to horizontal stratification of fertiliser in a water-limited environment. Plant Soil 2015, 386, 77–88. [Google Scholar] [CrossRef]
- Badhon, F.F.; Islam, M.S.; Islam, M.A.; Arif, M.; Uddin, Z. A simple approach for estimating contribution of vetiver roots in shear strength of a soil–root system. Innov. Infrastruct. Solut. 2021, 6, 1–13. [Google Scholar] [CrossRef]
- Liu, C.; Lei, F.; Li, P.; Jiang, J.; Wang, K. Borax crosslinked fenugreek galactomannan hydrogel as potential water-retaining agent in agriculture. Carbohyd. Polym. 2020, 236, 116100. [Google Scholar] [CrossRef]
- Hou, X.Q.; Li, R.; He, W.S.; Ma, K.; Dai, X.H. Effects of Super Absorbent Dosages on Physicochemical Properties of Dryland Soil and Potato Growth. J. Soil Water Conserv. 2015, 29, 325–330. (In Chinese) [Google Scholar] [CrossRef]
- Mei, Y.N.; Zhao, S.M.; Zhao, M.Q.; Kong, D.H.; Shi, L.F. Effects of super absorbent polymers’ dosages on soil nutrient and quality of flue-cured tobacco in Western Henan arid region. Agric. Res. Arid Areas 2018, 36, 149–155. (In Chinese) [Google Scholar] [CrossRef]
- Li, J.; Min, Q.; Li, W.; Bai, Y.; Gc, D.B.; Yuan, Z. Spatial Variability Analysis of Soil Nutrients Based on GIS and Geostatistics: A Case Study of Yisa Township, Yunnan, China. J. Resour. Ecol. 2014, 5, 348–355. [Google Scholar] [CrossRef]
- Mzuku, M.; Khosla, R.; Reich, R.; Inman, D.; Smith, F.; Macdonald, L. Spatial variability of measured soil properties across site-specific management zones. Soil Sci. Soc. Am. J. 2005, 69, 1572–1579. [Google Scholar] [CrossRef]
- He, X.S.; Liao, Z.W.; Huang, P.Z.; Duan, X.X.; Ge, R.S.; Li, H.B.; Zhao, J.H. Research advances in slow/controlled-release water-storing fertilizers. Trans. Chin. Soc. Agric. Eng. 2006, 22, 184–190. (In Chinese) [Google Scholar] [CrossRef]
- Ma, Z.; Li, Q.; Yue, Q.; Gao, B.; Xu, X.; Zhong, Q. Synthesis and characterization of a novel super-absorbent based on wheat straw. Bioresour. Technol. 2011, 102, 2853–2858. [Google Scholar] [CrossRef]
- Du, J.J.; Ge, C.L.; Cui, Y.D.; Qu, D. Effects of Water Retaining Agent on Ammonia Volatilization and Nutrient Leaching Loss from N, P and K Fertdizers. J. Agro-Environ. Sci. 2007, 24, 1296–1301. (In Chinese) [Google Scholar] [CrossRef]
- Mehtab, A.; Jiang, Y.; Su, L.; Shamsher, S.; Li, J.; Mahfuzur, R. Scaling the Roots Mechanical Reinforcement in Plantation of Cunninghamia R. Br in Southwest China. Forests 2021, 12, 33. [Google Scholar] [CrossRef]
- Fan, C.; Lu, J.Z.; Chen, H.H. The pullout resistance of plant roots in the field at different soil water conditions and root geometries. Catena 2021, 207, 105593. [Google Scholar] [CrossRef]
- Li, X.; Jiang, J.; Song, C.W.; Min, S.J.; Zhang, H.; Jiang, Y.W. Effect of super absorbent polymer on the growth and root morphology of Haloxylon ammodendron seedlings. Acta Prataculturae Sin. 2012, 21, 51–56. (In Chinese) [Google Scholar]
- Hou, X.; Li, R.; He, W.; Dai, X.; Ma, K.; Liang, Y. Superabsorbent polymers influence soil physical properties and increase potato tuber yield in a dry-farming region. J. Soils Sediments 2018, 18, 816–826. [Google Scholar] [CrossRef]
- Yue, W.J.; Zhang, F.C.; Li, Z.J.; Wu, L.F. Effects of Water and Nitrogen Coupling on Root Growth and Single Fruit Weight of Greenhouse Muskmelon. Sci. Agric. Sin. 2015, 48, 1996–2006. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.X.; Shi, G.Y. Effects of Cellulose Aquasorb on Properties of Substrate and Growth of Cucumber Seedling. Trans. Chin. Soc. Agric. Mach. 2016, 47, 162–169. (In Chinese) [Google Scholar] [CrossRef]
- Long, F.; Sun, H.; Li, S. Influence of rocky slope gradient on root anchorage of Vitex negundo L. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2011, 145, 532–539. [Google Scholar] [CrossRef]
- Sun, H.; Li, S.; Xiong, W.; Yang, Z.; Cui, B. Influence of slope on root system anchorage of Pinus yunnanensis. Ecol. Eng. 2008, 32, 60–67. [Google Scholar] [CrossRef]
- Lombardi, F.; Scippa, G.S.; Lasserre, B.; Montagnoli, A.; Tognetti, R.; Marchetti, M.; Chiatante, D. The influence of slope on Spartium junceum root system: Morphological, anatomical and biomechanical adaptation. J. Plant Res. 2017, 130, 515. [Google Scholar] [CrossRef]
- Jaffe, M.J. Thigmomorphogenesis: The effect of mechanical perturbation on the growth of plants, with special reference to anatomical changes, the role of ethylene, and interaction with other environmental stresses. In Stress Physiology in Crop Plants; John Wiley and Sons: Hoboken, NJ, USA, 1979. [Google Scholar]
- Di Iorio, A.; Lasserre, B.; Scippa, G.S.; Chiatante, D. Root system architecture of Quercus pubescens trees growing on different sloping conditions. Ann. Bot. 2005, 95, 351–361. [Google Scholar] [CrossRef]
- Danjon, F.; Caplan, J.S.; Fortin, M.; Meredieu, C. Descendant root volume varies as a function of root type: Estimation of root biomass lost during uprooting in Pinus pinaster. Front. Plant Sci. 2013, 4, 402. [Google Scholar] [CrossRef] [PubMed]
- Chiatante, D.; Scippa, S.G.; Di Iorio, A.; Sarnataro, M. The influence of steep slopes on root system development. J. Plant Growth Regul. 2003, 21, 247–260. [Google Scholar] [CrossRef]
- Scippa, G.S.; Di Michele, M.; Di Iorio, A.; Costa, A.; Lasserre, B.; Chiatante, D. The response of Spartium junceum roots to slope: Anchorage and gene factors. Ann. Bot. 2006, 97, 857–866. [Google Scholar] [CrossRef]
- De Zio, E.; Trupiano, D.; Montagnoli, A.; Terzaghi, M.; Chiatante, D.; Grosso, A.; Marra, M.; Scaloni, A.; Scippa, G.S. Poplar woody taproot under bending stress: The asymmetric response of the convex and concave sides. Ann. Bot. 2016, 118, 865–883. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Fei, B.H.; Yu, Y.; Cheng, H.T.; Wang, C.G. Influence of lignin content on tensile properties of single wood fiber. J. Beijing For. Univ. 2012, 34, 131–134. (In Chinese) [Google Scholar] [CrossRef]
- Genet, M.; Stokes, A.; Salin, F.; Mickovski, S.B.; Fourcaud, T.; Dumail, J.; Van Beek, R. The influence of cellulose content on tensile strength in tree roots. Plant Soil 2005, 278, 1–9. [Google Scholar] [CrossRef]
- Ye, C.; Guo, Z.; Li, Z.; Cai, C. The effect of Bahiagrass roots on soil erosion resistance of Aquults in subtropical China. Geomorphology 2017, 285, 82–93. [Google Scholar] [CrossRef]
- Li, C.; Jia, Z.; Peng, X.; Zhai, L.; Zhang, B.; Liu, X.; Zhang, J. Functions of mineral-solubilizing microbes and a water retaining agent for the remediation of abandoned mine sites. Sci. Total Environ. 2021, 761, 143215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, X.; Jiang, J.; Wei, Y.; Ma, J.; Hallett, P.D. Root moisture content influence on root tensile tests of herbaceous plants. Catena 2019, 172, 140–147. [Google Scholar] [CrossRef]
- Gall, R.; Landolt, W.; Schleppi, P.; Michellod, V.; Bucher, J.B. Water content and bark thickness of Norway spruce (Picea abies) stems: Phloem water capacitance and xylem sap flow. Tree Physiol. 2002, 22, 613–623. [Google Scholar] [CrossRef]
- Whitehead, F.H.; Luti, R. Experimental studies of the effect of wind on plant growth and anatomy. I. Zea mays. N. Phytol. 1962, 61, 56–58. [Google Scholar] [CrossRef]
- Crook, M.J.; Ennos, A.R. The anchorage mechanics of deep rooted larch, Larix europea× L. japonica. J. Exp. Bot. 1996, 47, 1509–1517. [Google Scholar] [CrossRef]
- Goodman, A.M.; Ennos, A.R. The effects of soil bulk density on the morphology and anchorage mechanics of the root systems of sunflower and maize. Ann. Bot. 1999, 83, 293–302. [Google Scholar] [CrossRef]
- Dupuy, L.; Fourcaud, T.; Stokes, A. A. A numerical investigation into the influence of soil type and root architecture on tree anchorage. In Eco-and Ground Bio-Engineering: The Use of Vegetation to Improve Slope Stability; Springer: Berlin/Heidelberg, Germany, 2007; pp. 175–189. [Google Scholar] [CrossRef]
- Moore, J.R. Differences in maximum resistive bending moments of Pinus radiata trees grown on a range of soil types. Forest Ecol. Manag. 2000, 135, 63–71. [Google Scholar] [CrossRef]
- Kamimura, K.; Kitagawa, K.; Saito, S.; Mizunaga, H. Root anchorage of hinoki (Chamaecyparis obtuse (Sieb. Et Zucc.) Endl.) under the combined loading of wind and rapidly supplied water on soil: Analyses based on tree-pulling experiments. Eur. J. For. Res. 2012, 131, 219–227. [Google Scholar] [CrossRef]
- Lei, Y.L. Research on Performance Analysis and Structure Optimization of Treedozer. Master’s Thesis, Jilin University, Changchun, China, 2016. [Google Scholar]

| CK | S30 | S60 | S90 | S120 | S150 | n | p | |
|---|---|---|---|---|---|---|---|---|
| Height (cm) | 197.07 ± 8.26 | 203.97 ± 9.42 | 211.87 ± 9.38 | 221.46 ± 9.77 | 229.48 ± 11.29 | 232.92 ± 12.33 | 5 | <0.01 |
| Base diameter (mm) | 27.32 ± 0.76 | 28.39 ± 0.68 | 29.05 ± 0.89 | 30.17 ± 0.81 | 31.39 ± 0.97 | 31.91 ± 1.07 | 5 | <0.01 |
| CK | S30 | S60 | S90 | S120 | S150 | n | p | |
|---|---|---|---|---|---|---|---|---|
| Thickness (cm) | 11.2 ± 0.71 | 11.1 ± 0.9 | 11.3 ± 0.5 | 11.5 ± 0.8 | 11.7 ± 0.6 | 11.6 ± 0.8 | 5 | 0.232 |
| Bulk weight (g·cm−3) | 1.31 ± 0.22 | 1.29 ± 0.55 | 1.26 ± 0.46 | 1.23 ± 0.28 | 1.21 ± 0.34 | 1.19 ± 0.57 | 5 | 0.402 |
| Natural soil water content (%) | 13.48 ± 0.76 | 16.53 ± 1.16 | 18.74 ± 1.11 | 20.57 ± 1.53 | 22.74 ± 1.78 | 24.18 ± 1.33 | 5 | <0.01 |
| Soil water content after 24 h of watering (%) | 25.46 ± 0.71 | 27.41 ± 1.22 | 30.33 ± 1.76 | 32.67 ± 1.55 | 35.18 ± 1.82 | 38.33 ± 1.76 | 5 | <0.01 |
| Total K (g/kg) | 6.62 ± 0.31 | 6.39 ± 0.34 | 5.87 ± 0.49 | 5.31 ± 0.56 | 5.12 ± 0.69 | 4.86 ± 0.44 | 5 | <0.01 |
| Total N (g/kg) | 2.93 ± 0.22 | 2.71 ± 0.26 | 2.48 ± 0.17 | 2.21 ± 0.27 | 2.06 ± 0.19 | 1.88 ± 0.15 | 5 | <0.01 |
| Total P (g/kg) | 2.33 ± 0.18 | 2.17 ± 0.12 | 2.05 ± 0.16 | 1.88 ± 0.09 | 1.71 ± 0.11 | 1.88 ± 0.15 | 5 | <0.01 |
| CK | S30 | S60 | S90 | S120 | S150 | p | |
|---|---|---|---|---|---|---|---|
| First-order lateral roots (FL) | |||||||
| Upslope | 9.33 ± 1.75 a | 9.34 ± 1.66 a | 9.29 ± 1.56 a | 9.29 ± 3.07 a | 9.37 ± 1.26 a | 9.30 ± 1.16 a | 0.309 |
| Downslope | 9.26 ± 2.11 a | 9.31 ± 2.31 a | 9.25 ± 1.48 a | 9.27 ± 2.28 a | 9.28 ± 1.58 a | 9.25 ± 1.39 a | 0.941 |
| Non-embedded secondary lateral roots (NESL) | |||||||
| Upslope | 13.39 ± 2.16 a | 13.39 ± 1.38 a | 13.41 ± 2.11 a | 13.41 ± 2.19 a | 13.41 ± 3.31 a | 13.44 ± 1.23 a | 0.858 |
| Downslope | 13.36 ± 1.67 a | 13.34 ± 1.29 a | 13.38 ± 2.16 a | 13.36 ± 1.47 a | 13.31 ± 2.27 a | 13.28 ± 1.36 a | 0.821 |
| Embedded secondary lateral roots (ESL) | |||||||
| Upslope | 14.12 ± 3.28 a | 14.56 ± 2.41 a | 15.13 ± 3.16 a | 15.87 ± 2.22 a | 16.25 ± 3.83 a | 16.78 ± 1.51 a | 0.849 |
| Downslope | 13.41 ± 2.56 a | 13.39 ± 2.67 a | 13.37 ± 3.08 a | 13.33 ± 1.25 a | 13.29 ± 2.28 a | 13.27 ± 1.49 a | 0.739 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, S.; Sun, H.; Zhou, Y. Influence of Super Absorbent Polymer on Root Characteristics and Anchorage of Amorpha fruticosa on Rocky Slope. Appl. Sci. 2022, 12, 2640. https://doi.org/10.3390/app12052640
Hou S, Sun H, Zhou Y. Influence of Super Absorbent Polymer on Root Characteristics and Anchorage of Amorpha fruticosa on Rocky Slope. Applied Sciences. 2022; 12(5):2640. https://doi.org/10.3390/app12052640
Chicago/Turabian StyleHou, Shujun, Hailong Sun, and Yinghua Zhou. 2022. "Influence of Super Absorbent Polymer on Root Characteristics and Anchorage of Amorpha fruticosa on Rocky Slope" Applied Sciences 12, no. 5: 2640. https://doi.org/10.3390/app12052640
APA StyleHou, S., Sun, H., & Zhou, Y. (2022). Influence of Super Absorbent Polymer on Root Characteristics and Anchorage of Amorpha fruticosa on Rocky Slope. Applied Sciences, 12(5), 2640. https://doi.org/10.3390/app12052640





