Featured Application
This paper investigates the effectiveness of using an automatic NDT trainer for the clinical rehabilitation of stroke patients.
Abstract
This paper presents a clinical rehabilitation protocol for stroke patients using a movable trainer, which can automatically execute a neurodevelopmental treatment (NDT) intervention based on key gait events. The trainer consists of gait detection and motor control systems. The gait detection system applied recurrent neural networks (RNNs) to recognize important gait events in real time to trigger the motor control system to repeat the NDT intervention. This paper proposes a modified intervention method that simultaneously improves the user’s gait symmetry and pelvic rotation. We recruited ten healthy subjects and had them wear a rehabilitation gaiter on one knee joint to mimic stroke gaits for verification of the effectiveness of the trainer. We used the RNN model and a modified intervention method to increase the trainer’s effectiveness in improving gait symmetry and pelvic rotation. We then invited ten stroke patients to participate in the experiments, and we found improvement in gait symmetry in 80% and 90% of the patients during and after the training, respectively. Similarly, pelvic rotation improved in 80% of the patients during and after the training. These findings confirmed that the movable NDT trainer could improve gait performance for the rehabilitation of stroke patients.
Keywords:
gait; stroke; rehabilitation; NDT; trainer; inertial measurement unit; deep learning; neural network 1. Introduction
Stroke has been reported as the second leading cause of death in the last 15 years [1]. The most common disorder caused by stroke is motor impairment, which typically affects the control of movement on one side of the body and is seen in about 80% of patients [2]. Although 65% to 85% of stroke survivors could recover independent walking by six months post-stroke, gait abnormalities persist through the chronic stages [3]. Only 30–50% of stroke survivors achieve community mobility, which is an essential indicator of the activities and participation domains according to the International Classification of Functioning, Disability, and Health [4]. People with stroke may have an impaired ability to adapt to environmental challenges. They have trouble walking across obstacles, increasing the risk of tripping or navigating around more significant obstacles (e.g., another person approaching in the opposite direction). Long-term rehabilitation is usually required for these patients to help them recover their ability to live independently and walk on their own. As stroke recovery progresses, stroke survivors usually develop better gross motor skills in trunk balance and greater leg strength. Despite spasticity and the inability to selectively activate individual muscles, most stroke survivors will eventually walk. Therefore, gait training is essential for stroke patients [3].
Poststroke gait deficits may be primary or secondary gait deviations [5]. The former is related to the disruption of neural pathways controlling gait, while the latter is the adaptive compensations following the primary deviation. Compared to stroke patients, relatively strict symmetric coordination of the lower limbs is a characteristic of bipedal walking in people with intact central nervous and musculoskeletal systems [6]. In contrast to other features of poststroke gait patterns, gait asymmetry appears to be more resistant to intervention. The majority (>79%) of people with subacute stroke will not significantly improve gait asymmetry during inpatient rehabilitation, despite improving other features of walking and balance [7]. Traditional gait rehabilitation methods use hands-on techniques to guide the patient’s body movements and gait patterns [8,9,10]. Among these techniques, neurodevelopmental treatment (NDT) is used to give the patients the feeling of walking with minimal intervention. Hence, patients can intentionally drive their center of gravity (COG) forward and balance themselves using striding steps. However, this conventional rehabilitation practice places high demands on therapists. Therefore, many rehabilitation devices have been proposed to help patients improve their walking abilities with minimal therapist intervention.
Colombo et al. [11] developed a gait orthosis that could move the user’s legs on a treadmill. Schmidt et al. [12] proposed programmable footplates that could control the patients’ feet to simulate walking patterns. Wang et al. [13] presented an active gait trainer that used motors to guide the user’s ankles. Unlike these devices, which guide the users’ feet according to preset movements, NDT training stimulates the patients’ joints at key time points and lets the patients intentionally drive their bodies, so that the patients can elicit positive brain reorganization and regain control of their feet by motor training. However, despite its effectiveness in stroke rehabilitation, traditional NDT training can also be very labor intensive and time consuming for therapists. Therefore, Wang et al. [14,15] designed a stationary NDT trainer that could automatically repeat the therapist’s intervention patterns. They then developed a movable NDT trainer [16], which allowed the users to walk at their preferred speeds while receiving visual feedback.
Correct identification of gait events is crucial for rehabilitation intervention. For example, Wang et al. [14,15] found that therapists tended to cue the subject’s anterior superior iliac spine (ASIS) when observing the subject’s heel strike (HS) on the opposite side during clinical NDT training. Therefore, automatic NDT trainers were designed to repeat this intervention pattern at the key gait events, and many methods have been proposed to identify these important gait events. For instance, optical motion capture systems, such as the VZ4000 [17] and VICON [18], are frequently applied to measure motions. Much research has also applied artificial intelligence to detect gait events based on gait information obtained by inertial measurement units (IMUs) [19,20]. For example, Williamson and Andrews [21] applied machine learning to detect real-time gait events using accelerometer signals, whereas Aminian et al. [20] estimated stride length and velocity using gyroscope data. Mazilu [22] proposed multi-feature fusion to recognize freezing gaits, and Sun et al. [23] applied wearable IMUs to recognize elderly users. Wang et al. [16] developed a gait detection algorithm to identify important gait events based on IMU data. The algorithm could automatically update parameters in real time for different users with various walking patterns. However, updating the parameters took several steps (in a few seconds) and might have degraded the rehabilitation effects. Therefore, a recurrent neural network (RNN) model was applied to the gait detection system [24], and this allowed detection of HS events in real time based on the IMU data from two legs. The average accuracy was more than 98%, and the average latency was less than 0.036 s for different subjects, including elderly subjects, stroke patients, and patients with Parkinson’s disease (PD). Therefore, in this paper, we applied the RNN model to detect HS events.
The currently available NDT trainer can repeat the therapist’s intervention pattern by cuing the subject’s ASIS when observing the HS on the opposite side. However, we noted that the present intervention method improves the subject’s longitudinal symmetry but does not improve pelvic rotation. The locomotion of the pelvis is closely related to walking stability [25,26]. For example, Wagenaar et al. [27] showed the contribution of pelvic rotation to lengthening the stride, especially when walking at fast speeds. Energy conservation is crucial during gait, and the vertical displacement of the body is minimized by a number of factors known as the determinants of gaits. These determinants operate independently and simultaneously to produce a smooth sinusoidal vertical and horizontal path, which has one vertical peak and trough for each step. One of the determinants of gait is the rotation of the pelvis, which reduces center of mass (COM) movement, thereby conserving energy [28,29]. Therefore, we analyzed the subjects’ pelvic motions and the therapists’ interventions during clinical NDT rehabilitation and proposed a modified method to improve the pelvic rotation. We implemented the modified intervention method and recruited 10 healthy subjects who wore a rehabilitation gaiter to mimic stoke gaits to verify the effectiveness of the trainer in improving gait performance and pelvic rotation. We also invited 10 stroke patients to participate in the experiments, and their results confirmed that the proposed intervention method simultaneously improved both pelvic rotation and gait symmetry.
This paper is organized as follows. Section 2 describes the movable trainer, which can automatically conduct NDT rehabilitation intervention. The trainer consists of a gait detection system and a motor control system. The gait detection system employs an RNN model to identify HS events in real time, so that the motor system can be activated for NDT intervention at the key time. Section 3 introduces a modified intervention method that can simultaneously improve the subjects’ gait symmetry and pelvic rotation. We recruited 10 healthy subjects and had them wear a rehabilitation gaiter to imitate stroke gaits to test the trainer. The results confirmed the effectiveness of the trainer. Section 4 presents the results for 10 stroke patients invited to participate the experiments and shows that the NDT trainer can improve both gait symmetry and pelvic rotation in stroke patients. Finally, we draw conclusions in Section 5.
2. The Movable NDT Trainer: Experimental Design and Data Collection
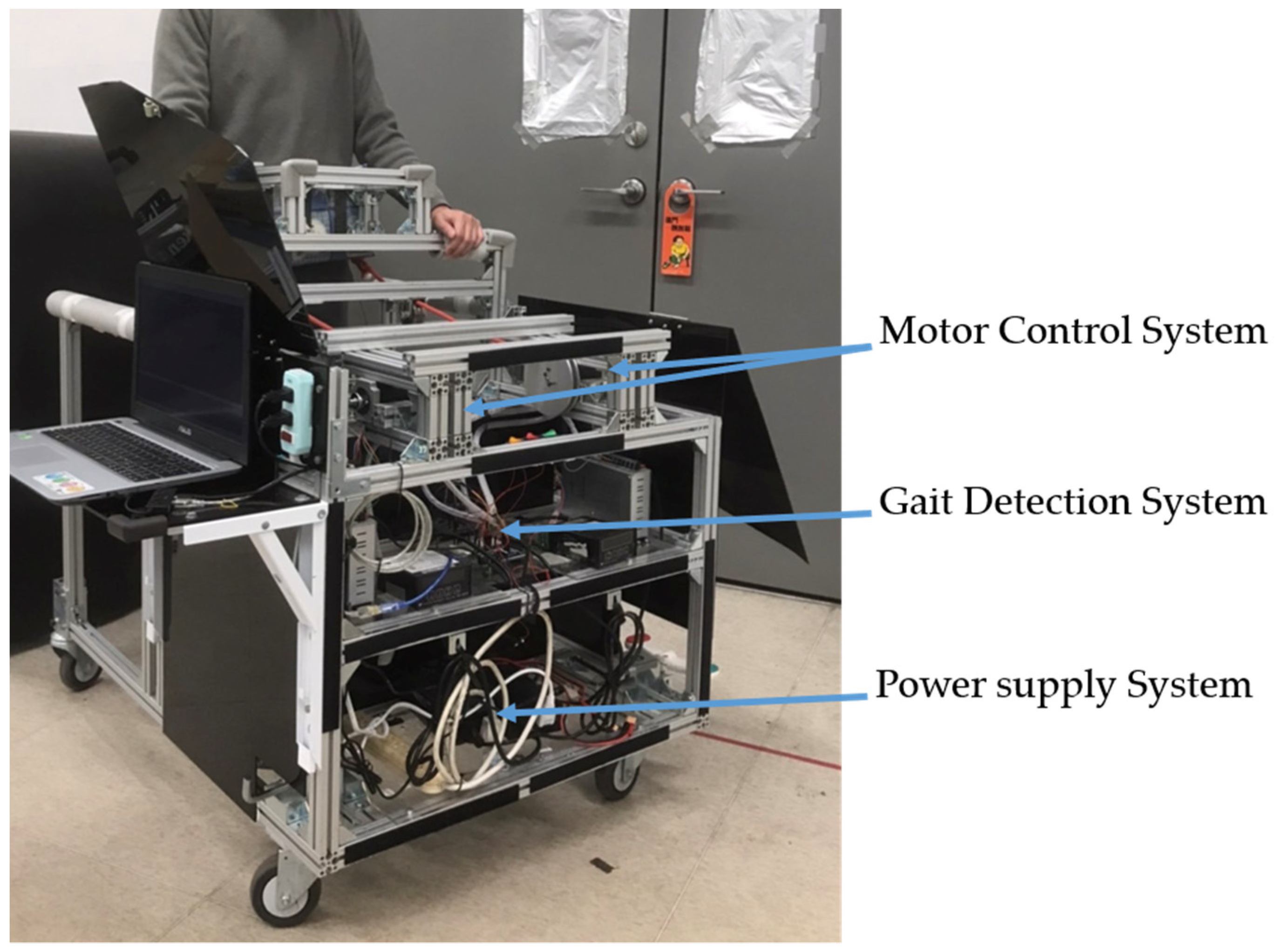
This section describes the movable trainer [16], which automatically repeats the NDT intervention. The trainer consists of a gait detection system and a motor control system, as shown in Figure 1, which equips with an adjustable handle for safety. The gait detection system analyzed the gait information obtained from two IMUs attached to the user’s shanks. It could automatically recognize the HS events and send triggering signals to the motor control system to imitate the therapists’ intervention. The motor control system could repeat the therapists’ intervention patterns by stimulating the users through ropes. The ropes connected the motors and the user’s pelvis to cue the user as the traditional NDT training. Load cells were implemented between the ropes and the motors to measure the applied forces for feedback control. Previous studies [14,15,16] showed that the therapists tended to cue the subject’s right (left) pelvis when the subject’s left (right) foot struck the ground. Therefore, an RNN model [24] was applied to the gait detection system, which detects the HS in real time to activate the motor control system. The motor control system then repeats the NDT intervention after receiving the triggering signals from the gait detection system [14,15,16].
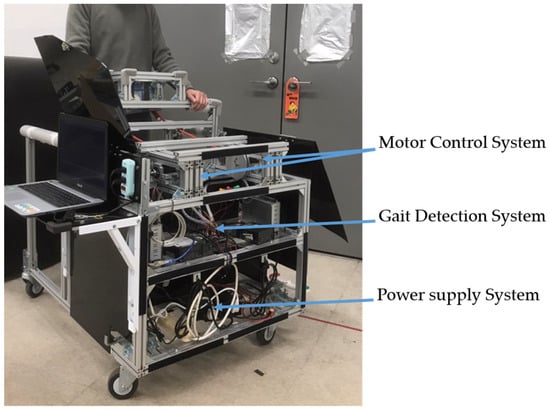
Figure 1.
The movable NDT trainer.
Stroke patients tend to have uneven gaits due to hemiparesis, and stroke patients have their own individual walking patterns and speeds. Therefore, we developed an RNN model to detect HS events for subjects who might have varied walking patterns and speeds [24]. We applied the APDM OPAL system [30] to measure each subject’s kinematic data with a sampling rate of 25 Hz. Two IMUs were attached to the subject’s legs, as shown in Figure 2, to measure gait data. The specifications of the IMU are illustrated in Table 1.

Figure 2.
Two IMUs for gait detection.

Table 1.
Specifications of the APDM OPAL IMU [30].
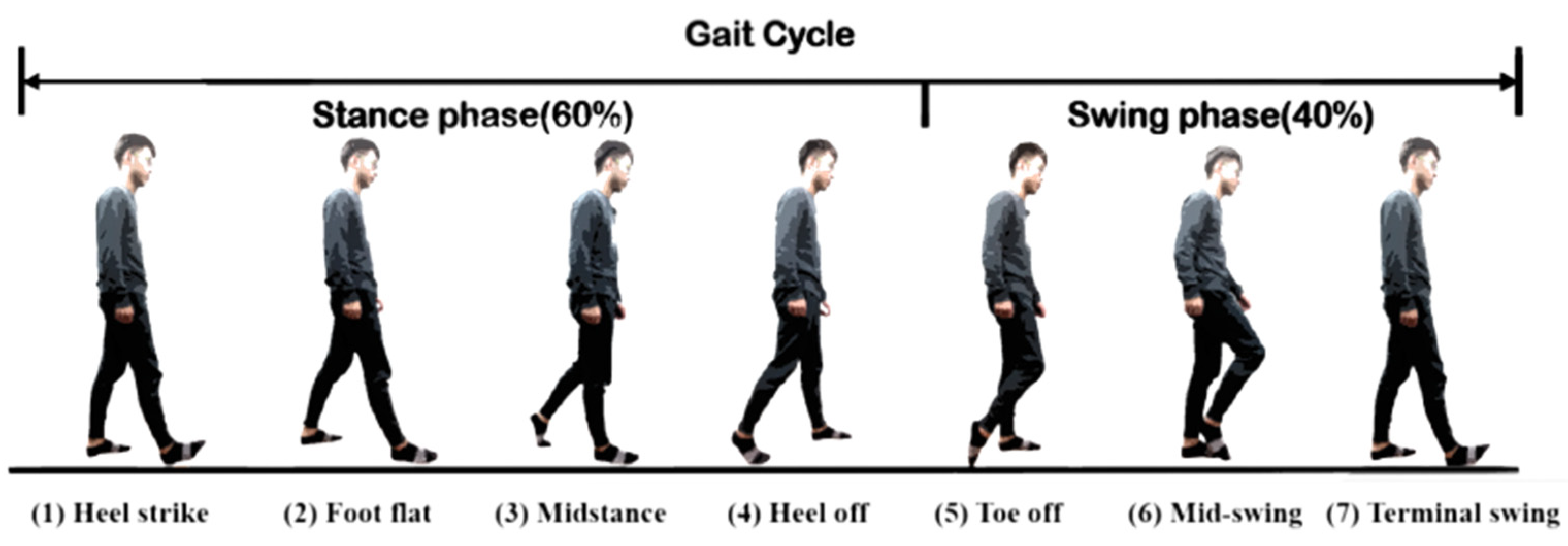
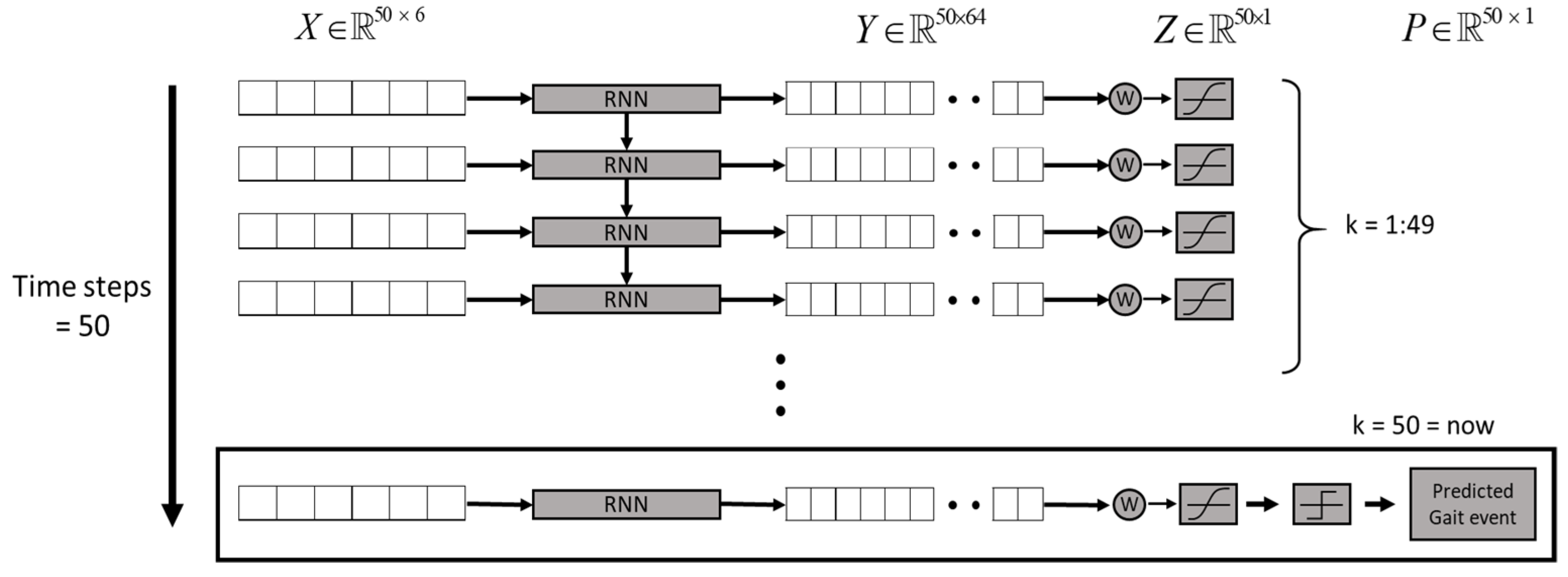
Human gaits are regularly periodic. A complete gait cycle, as illustrated in Figure 3, contains three important gait events: mid-swing, HS, and toe-off. We applied the six-axial IMU data and marked the HS events to build an RNN model [24], as shown in Figure 4. The model was trained by the gait data measured in five healthy subjects who wore a rehabilitation gaiter with a 2 kg mass to limit their joint movement on one knee to increase gait varieties (see Figure 5). We segmented the gait data with a sliding window, as illustrated in Figure 4, which consisted of 50 samples of six-axial IMU data as the model input. The window was then moved by one sample each time, with an overlap of 98%. The model output was marked as 1 for an HS event or 0 for a non-HS event. We applied the leave-one-out cross validation method [31] to train the RNN model. We then applied the model to three groups of subjects: healthy elderly subjects, stroke patients, and patients with PD. The results illustrated in Table 2 confirm that the RNN model successfully recognized the HS events with an average accuracy of 99.65% and an average delay of 0.0256 s. Therefore, we applied the RNN model to detect the HS and to activate the NDT intervention in real time.
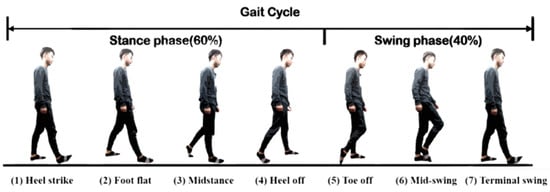
Figure 3.
A standard gait cycle.
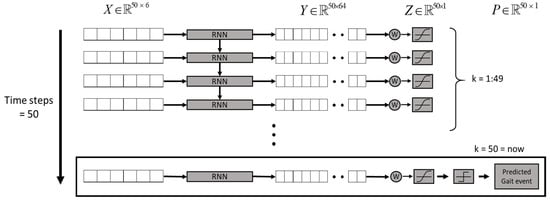
Figure 4.
The RNN model for HS detection [24].

Figure 5.
Rehab gaiter and IMUs’ location.

Table 2.
Testing results of the RNN model [24].
3. Modified NDT Intervention
The motor control system can repeat the NDT intervention after receiving the triggering signals from the gait detection system. In previous studies [14,15,16], we concluded that the therapists cued a subject’s right (left) pelvis when they observed the subject’s left (right) HS during the NDT rehabilitation, as illustrated in Appendix A. Therefore, we designed the motor control system to automatically repeat this pattern. When detecting a HS, the detection system sent a triggering signal to the motor control system on the opposite side, which tracked the following force command:
where and represent the maximum and the minimum forces, respectively, while f is the frequency. Based on previous experiences [14,15,16], we set , , and f =1 Hz in the experiments.
Since gait symmetry is critical for walking rehabilitation, we analyzed the asymmetry of the swing phase [32], defined as follows:
where and represent the proportion of the swing phase on the paretic side and the non-paretic side, respectively. Since stroke patients tend to have hemiparesis and a longer swing time on the paretic side, we applied to evaluate the rehabilitation effectiveness and regarded the training as effective if approached zero.
We recruited 10 healthy subjects for the experiments. The subjects’ data are provided in Table 3. A rehabilitation gaiter was attached to one knee joint on each subject to limit the joint movements and to mimic stroke gaits. Each subject received the tests by the procedures, where represented the before-treatment, during-treatment, and after-treatment periods, respectively. The subjects first walked on their own once down a 20 m corridor (A). They then received NDT training twice by the trainer on the 20 m corridor (B), and they finally walked on their own down the 20-m corridor once (). Their gait data were recorded, as illustrated in Appendix B, to evaluate the training effects.

Table 3.
Information of the healthy subjects.
The experimental results are shown in Table 4, where imp% represents the percentage improvement of the training compared to before the training (stage A). As shown in Table 4, the asymmetry of the swing phases of 9 of the 10 subjects was improved after the treatment ( stage). A possible reason is that subject H9 had a light body weight and may have had insufficient muscle strength to push the movable trainer. Generally, the NDT intervention is effective in improving .

Table 4.
The asymmetry of the swing phase.
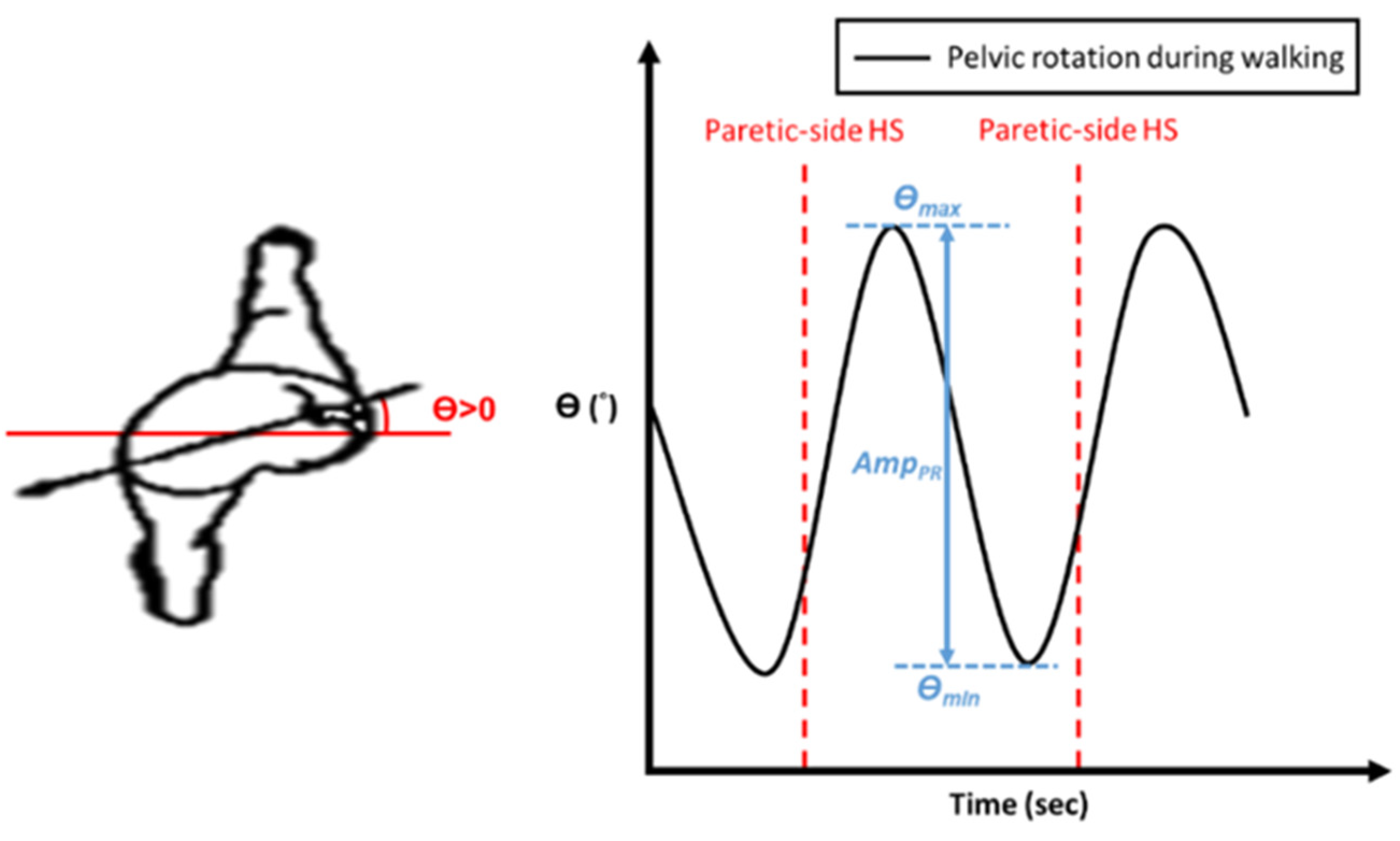
Apart from the gait symmetry, the locomotion of the pelvis is also important for maintaining walking stability [25,26,27]. During each step, the pelvis rotates forward on the side of the swinging limb. The axis of this rotation is the hip joint of the stance leg, which undergoes internal rotation. As the pelvis forms a bridge between the two hips, it reduces the angle of intersection of the thighs to reduce the vertical descent of the trunk. As mentioned previously, pelvis rotation conserves energy during the normal gait and allows people to walk at a comfortable pace. Therefore, we further analyzed the subjects’ pelvic rotation during the NDT training. The amplitude of pelvic rotation is defined as the maximum rotation angle between two consecutive HS events on the paretic side, as follows:
where and represent the maximum and minimum pelvic angles during a complete gait cycle, respectively, as shown in Figure 6. Since stroke patients’ muscles are usually weakened on the paretic side, their pelvic rotation is limited and less than in healthy persons. Therefore, we can evaluate the effectiveness of the NDT rehabilitation by , and we regard the training as effective if is increased. We attached an IMU to the subjects’ waists (see Figure 5) to record their pelvic rotations. The results are illustrated in Table 5; only 6 of the 10 subjects had an improvement in during the treatment (B stage) and after the treatment ( stage). That is, the current intervention method can improve the subjects’ gait symmetry but not their pelvic rotation. Therefore, we analyzed the therapists’ interventions during the clinical NDT training in [14,15,16], as those interventions were manually conducted by the therapists. The analyses are illustrated in Appendix C and show that the therapist tended to increase the intervention forces on the non-paretic side when the subject’s pelvic rotation was small. Based on that analysis, we proposed a modified NDT intervention algorithm that can improve the gait asymmetry and the pelvic rotation simultaneously.
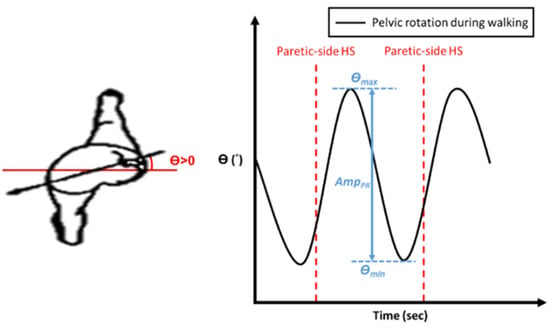
Figure 6.
Definition of the amplitude of pelvic rotation .

Table 5.
The amplitude of pelvic rotation () using the original intervention.
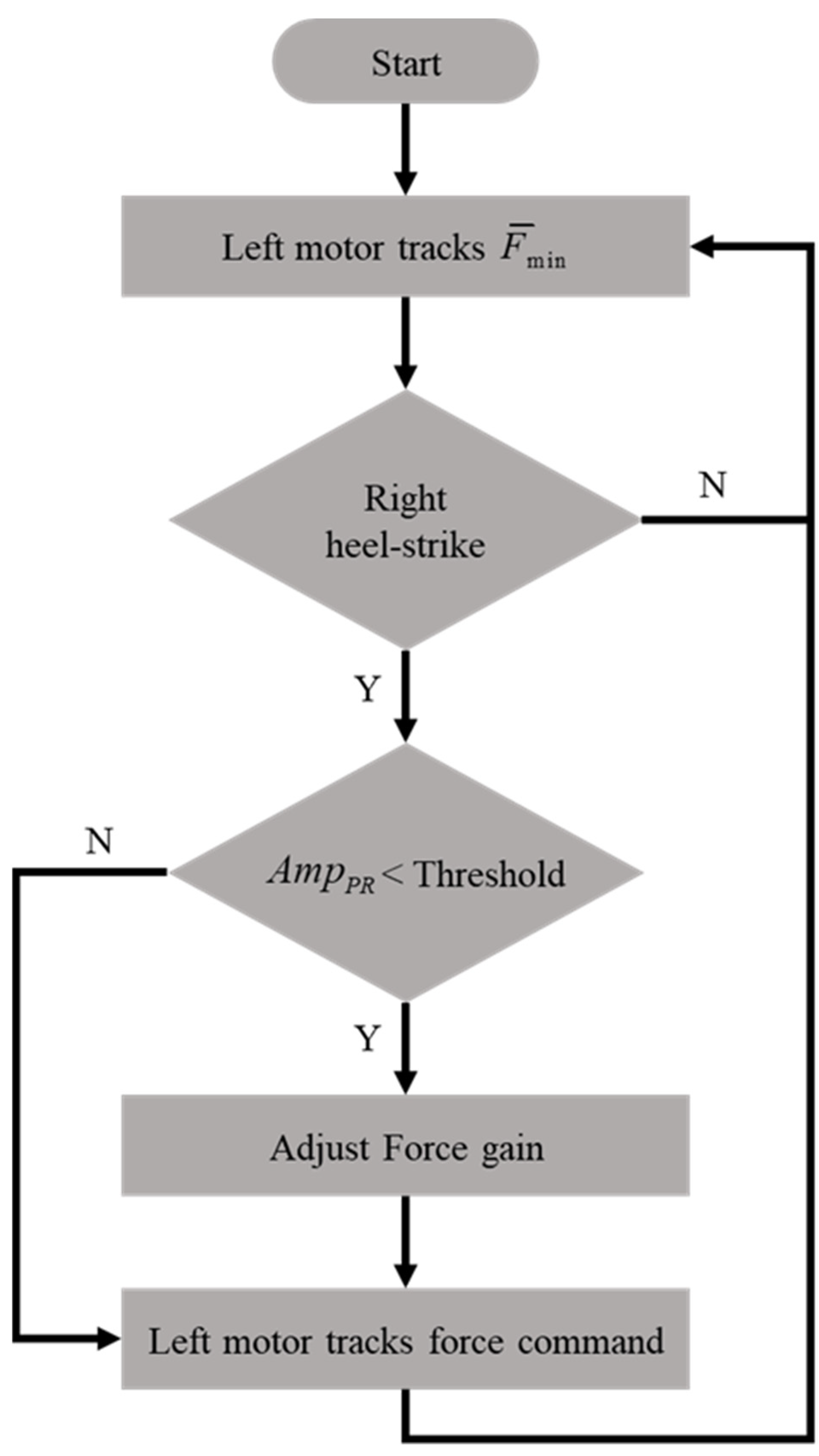
The modified intervention method is shown in Figure 7, where the maximum intervention forces on the non-paretic side are increased by 50% if the pelvic rotation is less than a given threshold. For example, we applied the rehabilitation gaiter to the subjects’ right knee so that the left motor was controlled according to the following procedures:
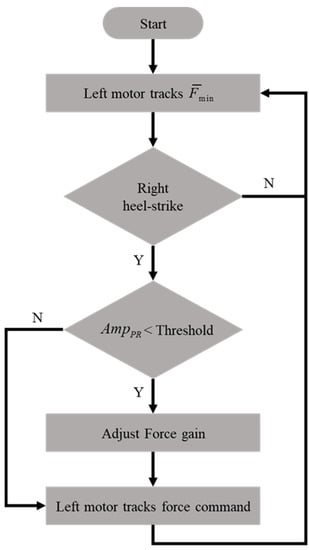
Figure 7.
The modified intervention method (on the non-paretic side).
- i.
- The motor tracked a minimum force of to keep the rope straight.
- ii.
- The motor was activated to follow the intervention force pattern of (2), when the right HS was detected.
- iii.
- The maximum intervention force was increased if the pelvic rotation was less than a threshold value.
We implemented the modified intervention method with the movable NDT trainer and repeated the experiments. In this paper, we increased the maximum intervention force by 50% when the pelvic rotation was less than a threshold of . The analysis results are shown in Table 6. First, the modified intervention improved the pelvic rotation in 7 of 10 subjects during the training and in 9 of 10 subjects after the training. That is, the modified method can provide greater improvement in pelvic rotation compared with the original method (see Table 5). Second, the modified method also improved the gait symmetry in 7 of 10 subjects during the training and in 9 of 10 subjects after the training. Compared with the data shown in Table 4, the modified method increased pelvic rotation without sacrificing the improvement in gait symmetry. That is, the modified method can simultaneously improve the subject’s pelvic rotation and gait symmetry. However, factors still might limit the improvement in gait symmetry or pelvis rotation. One is that healthy participants still have normal muscle strength to exert a force against external load, so they display a less significant pelvis rotation. By contrast, the paretic side of a stroke patient is relatively weak and less resistant. Another possibility is that the healthy individual’s restricted leg can exert more force when extra weight is applied. Therefore, their gait pattern may not be as asymmetric as that of stroke patients before intervention and no improvement may be evident. Therefore, we applied the modified NDT intervention in clinical trials on stroke patients.

Table 6.
Results of the experiments employing the modified intervention method.
4. Clinical NDT Rehabilitation with Stroke Patients
We recruited 10 stroke patients to participate in the experiments. The following restrictions were set on the selection of subjects: (i) the Mini-Mental State Examination (MMSE) score [33] was higher than 24 and the subject could cooperate with orders; (ii) the Brunnstrom Stage (BS) [34] on the paretic lower extremity was stage 3 to 4; (iii) the Functional Ambulation Category (FAC) [35] was stage 3 to 4; (iv) subjects could walk 10 m indoors with or without aid devices; and (v) subjects could stand up on their own using a handrail and aids. The patients’ data are provided in Table 7. All subjects signed informed consent forms approved by the Human Subject Research Ethics Committee of the Institutional Review Board (IRB number: (870)110-16, Cheng-Hsin General Hospital, Taipei, Taiwan, ClinicalTrials.gov Identifier: NCT04968418).

Table 7.
Information of stroke participants.
We attached three IMUs onto each subject’s legs and waist and conducted clinical experiments in the hospital, as shown in Figure 8. Each subject underwent the tests by the procedures, as demonstrated in Appendix D. The subjects walked forth and back with the trainer on a straight line of about 11 m. At stage A (before treatment), the subjects walked three times (about 66 m) with the motors off. At stage B (during treatment), the trainer’s motors were switched on, and the subjects walked six times (about 132 m). Finally, at stage (after treatment), the subject walked three times (about 66 m) on their own with the motors off. No break was given between the procedures, but the subjects were free to rest at any time during the experiments.

Figure 8.
Clinical rehabilitation training by the NDT trainer.
The experimental results are shown in Table 8. The gait symmetry was improved in 8 of the 10 patients during the training and in 9 of the 10 patients after the training. The average percentage improvements (imp%) were 13.04% and 22.01% during and after the training, respectively. The pelvic rotation was improved in 8 of the 10 patients both during and after the training. The average percentage improvements (imp%) were 24.46% and 8.19% during and after the training, respectively. The trainer helped improve the rehabilitation effects in stroke patients in general. However, several factors may have contributed to the lack of improvement during this intervention. One was that patients with poor hand function of the hemiparetic upper limb were able to hold the handrails only with the unaffected hand. Thus, body sway during walking was common and could have limited the effect of pelvis rotation. Second, spasticity of the lower limbs causes an abnormal synergy pattern of movement, including knee extension and ankle plantarflexion and eversion, which may interfere with the coordination of pelvis rotation. Third, in this study, the patients’ heights varied. Therefore, not all NDT interventions could precisely induce pelvic rotation since the cuing force would be a projection of the applied force. For example, taller patients may have experienced downward plus forward force rather than forward force alone to induce pelvic rotation due to their higher pelvic position. Last, the current trainer’s induction force is only 100% or 150%. Therefore, this device is not customized for patients with different physical characteristics or ambulation functions. Due to these technical limitations, a future study will optimize the device by adding vertical adjustability and providing fasteners for the participants’ hands on the handrails to decrease asymmetry during the intervention.

Table 8.
Experimental results of the stroke patients.
Overall, based on the clinical experimental results, the movable NDT trainer employing the RNN gait detection model and the modified NDT intervention was deemed to help improve stroke patients’ rehabilitation effects.
5. Conclusions
This paper demonstrated the results of clinical stroke rehabilitation using a movable trainer employed during NDT intervention. The trainer consisted of a gait detection system and a motor control system. We implemented an RNN model in the gait detection system and a modified NDT intervention in the motor control system. The RNN model can recognize the HS events in real time to activate the NDT intervention at critical moments. The users can feel walking and intentionally drive their body to regain gait control by motor training. The aim of using the modified NDT intervention method was to simultaneously improve the user’s gait symmetry and pelvic rotation. We recruited ten healthy subjects to verify the effectiveness of the trainer. The results showed that the trainer could simultaneously improve the subjects’ gait symmetry and pelvic rotation. The findings from 10 stroke patients invited to participate in the experiments showed that gait symmetry improved in 80% of the patients during the training and 90% after the training. The pelvic rotation was improved in 80% both during and after the training. Based on these results, we conclude that the NDT trainer can potentially help stroke patients’ rehabilitation. Some patients did not show improvements, possibly due to difficulty in holding handrails with the paretic hand or variations in the participants’ heights that affected accurate pelvic rotation force and limited induction force options (we only included 100% and 150%). Therefore, in future studies, we plan to fasten the participants’ hands onto the handrails for the participants’ hands to decrease asymmetry during the intervention. We will also modify the trainer design, for example, by applying a suitable mechanism to automatically adjust the intervention positions for patients with different heights and body weights or by adding motor wheels to reduce the burden of pushing the trainer.
Author Contributions
Conceptualization, F.-C.W. and S.-F.C.; methodology, F.-C.W., S.-F.C., Y.-C.L. and A.-C.L.; software, Y.-C.L. and C.-J.S.; validation, F.-C.W., S.-F.C. and C.-J.S.; formal analysis, F.-C.W., Y.-C.L. and C.-J.S.; investigation, F.-C.W., S.-F.C., A.-C.L. and T.-T.L.; resources, F.-C.W., S.-F.C., A.-C.L. and T.-T.L.; data curation, F.-C.W., Y.-C.L. and C.-J.S.; writing—original draft preparation, F.-C.W., Y.-C.L. and C.-J.S.; writing—review and editing, F.-C.W., S.-F.C. and A.-C.L.; visualization, F.-C.W., Y.-C.L. and C.-J.S.; supervision, F.-C.W. and S.-F.C.; project administration, F.-C.W. and S.-F.C.; funding acquisition, F.-C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by in part by the Ministry of Science and Technology of Taiwan (Grands MOST 107-2634-F-002-018-, MOST 108-2634-F-002-016-, MOST 109-2634-F-002-027-, MOST 110-2634-F-002-042-) and National Taiwan University, Center for Artificial Intelligence & Advanced Robotics.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Cheng Hsin General Hospital (IRB number: (870)110-16, Cheng-Hsin General Hospital, Taipei, Taiwan, ClinicalTrials.gov Identifier: NCT04968418).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The dataset of gaits applied in this paper is available at http://140.112.14.7/~sic/PaperMaterial/Experiment Data.zip (accessed on 1 March 2022).
Acknowledgments
This research was also financially supported in part by the Ministry of Science and Technology of Taiwan (Grands MOST 107-2634-F-002-018, MOST 108-2634-F-002-016-, MOST 109-2634-F-002-027-) and National Taiwan University, Center for Artificial Intelligence & Advanced Robotics.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A. NDT Intervention for Improving Gait Symmetry
We invited therapists to conduct clinical NDT training and observed their actions on the test subjects [14,15,16]. The therapists guided each subject’s movements using the ropes attached to the subject’s ASIS. We analyzed the therapists’ applied forces and the subject’s motions and concluded that the therapists’ intervention patterns were as follows:
The therapist applied forces on the subject’s opposite ASIS approximately when the subject’s heel struck the ground,
as shown in Figure A1. In addition, the intervention force patterns were approximated as sinusoidal waves, as illustrated in (2). Therefore, we were able to control the motors to track the force commands when detecting the HS on the opposite side. Although these parameters varied for different individuals, we set them as , , and f = 1 Hz in this paper for simplification.
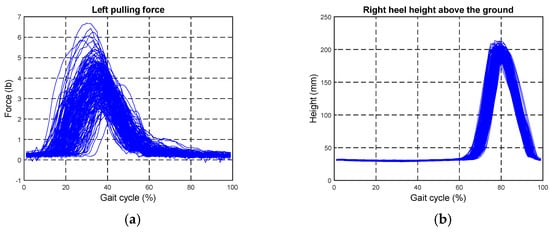
Figure A1.
Analyses of the intervention patterns. (a) Applied force on the left ASIS; (b) heights of the right heel.
Figure A1.
Analyses of the intervention patterns. (a) Applied force on the left ASIS; (b) heights of the right heel.
Appendix B. IMU Data
The gait data in this paper are available at: http://140.112.14.7/~sic/PaperMaterial/Experiment Data.zip (accessed on 1 March 2022).
Appendix C. NDT Intervention for Improving Pelvic Rotation
Since the original intervention method did not improve the subjects’ pelvic rotation (see Table 7), we further analyzed the therapists’ intervention during the clinical NDT rehabilitation in [14,15,16]. The results are illustrated in Table A1, where the pelvic rotation, in general, was improved in all subjects during the treatment (stage B) and the effects remained after the treatment (stage ). Therefore, we analyzed the relationship between the therapists’ interventions and the subjects’ pelvic rotations in the following three scenarios: (1) the applied forces when the pelvic rotation was better; (2) the pelvic rotation when the applied forces were larger; and (3) the influences of larger forces on the asymmetry of the swing phases. (Figures: 20% forces and 20% pelvic rotation).

Table A1.
Pelvic rotation from the clinical NDT rehabilitation by therapists.
Table A1.
Pelvic rotation from the clinical NDT rehabilitation by therapists.
| Subject | A | ||||
|---|---|---|---|---|---|
| P1 | 8.9 | 16.7 | (87) | 10.0 | (12) |
| P2 | 18.0 | 23.1 | (28) | 18.7 | (4) |
| P3 | 11.3 | 16.3 | (45) | 11.4 | (2) |
| P4 | 12.1 | 16.9 | (39) | 15.8 | (31) |
| P5 | 19.9 | 26.5 | (34) | 22.6 | (14) |
| P6 | 17.3 | 21.6 | (25) | 15.1 | (−12) |
| P7 | 21.9 | 35.9 | (65) | 24.4 | (12) |
| P8 | 15.6 | 31.6 | (103) | 17.9 | (15) |
| P9 | 9.4 | 25.9 | (175) | 17.7 | (87) |
| P10 | 9.2 | 18.2 | (98) | 12.8 | (39) |
- (1)
- The applied forces when the pelvic rotation was better: we analyzed the applied forces when the pelvic rotation was better. For example, Figure A2 illustrates the intervention forces when the pelvic rotation was the largest (20%). We noted that:
- (i)
- the average pelvic rotation is , where the average applied force was ;
- (ii)
- for the largest (20%), the average pelvic rotation was and the average applied force was .
That is, the therapists’ intervention forces were greater when the pelvic rotation was larger.
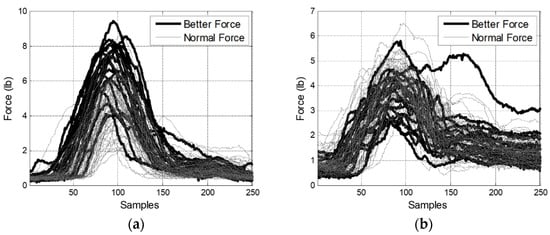
Figure A2.
The intervention forces. (a) on the left pelvic; (b) on the right pelvic.
Figure A2.
The intervention forces. (a) on the left pelvic; (b) on the right pelvic.
- (2)
- The pelvic rotation when the applied forces were larger: we analyzed the impacts of the magnitudes of applied forces on pelvic rotation, as follows:
- (i)
- the average applied force was , where the average pelvic rotation was ;
- (ii)
- when the applied forces were the largest (20%), the average applied force was and the average pelvic rotation was .
That is, the pelvic rotation was larger when the intervention forces were larger.
- (3)
- The influences of larger forces on the asymmetry of the swing phases: we analyzed the influences of the magnitudes of the applied forces on the gait symmetry . The results shown in Table A2 indicate that the magnitudes of the applied forces had no significant influence on the gait symmetry index . That is, the modified intervention method had no significant influence on the gait symmetry.

Table A2.
Comparison of the asymmetry of swing phases.
Table A2.
Comparison of the asymmetry of swing phases.
| Subject | All Steps | Steps with the Largest 20% Forces |
|---|---|---|
| P1 | 17.15 | 19.94 |
| P2 | 36.78 | 36.50 |
| P3 | 38.67 | 35.07 |
| P4 | 34.87 | 37.49 |
| P5 | 38.02 | 55.09 |
| P6 | 33.79 | 32.68 |
| P7 | 17.30 | 13.34 |
| P8 | 33.39 | 38.10 |
| P9 | 4.36 | −8.00 |
| P10 | 13.35 | −13.34 |
From the above analyses, changing the magnitudes of applied forces can improve the pelvic rotation without influencing the gait symmetry . Therefore, we propose the modified intervention method as follows:
increasing the intervention forceswhen the pelvic rotationis less than a threshold of, as illustrated in Figure 7
Appendix D. Demonstration Video
The demonstration video of the experiments is available at: http://140.112.14.7/~sic/PaperMaterial/experiment video.zip (accessed on 1 March 2022).
References
- The Top 10 Causes of Death in 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 8 January 2022).
- Hatem, S.M.; Saussez, G.; Della Faille, M.; Prist, V.; Zhang, X.; Dispa, D.; Bleyenheuft, Y. Rehabilitation of motor function after stroke: A multiple systematic review focused on techniques to stimulate upper extremity recovery. Front. Hum. Neurosci. 2016, 10, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jørgensen, H.S.; Nakayama, H.; Raaschou, H.O.; Olsen, T.S. Recovery of walking function in stroke patients: The Copenhagen Stroke Study. Arch. Phys. Med. Rehabil. 1995, 76, 27–32. [Google Scholar] [CrossRef]
- Selves, C.; Stoquart, G.; Lejeune, T. Gait rehabilitation after stroke: Review of the evidence of predictors, clinical outcomes and timing for interventions. Acta Neurol. Belg. 2020, 120, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Barclay, R.E.; Stevenson, T.J.; Poluha, W.; Ripat, J.; Nett, C.; Srikesavan, C.S. Interventions for improving community ambulation in individuals with stroke. Cochrane Database Syst. Rev. 2015, 3, CD010200. [Google Scholar] [CrossRef]
- Patterson, K.K.; Nadkarni, N.K.; Black, S.E.; McIlroy, W.E. Gait symmetry and velocity differ in their relationship to age. Gait Posture 2012, 35, 590–594. [Google Scholar] [CrossRef] [Green Version]
- Patterson, K.K.; Mansfield, A.; Biasin, L.; Brunton, K.; Inness, E.L.; McIlroy, W.E. Longitudinal changes in poststroke spatiotemporal gait asymmetry over inpatient rehabilitation. Neurorehabilit. Neural Repair 2015, 29, 153–162. [Google Scholar] [CrossRef]
- Gerbrands, T.A.; Pisters, M.; Vanwanseele, B. Individual selection of gait retraining strategies is essential to optimally reduce medial knee load during gait. Clin. Biomech. 2014, 29, 828–834. [Google Scholar] [CrossRef]
- Gao, C.; Liu, J.; Tan, Y.; Chen, S. Freezing of gait in Parkinson’s disease: Pathophysiology, risk factors and treatments. Transl. Neurodegener. 2020, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Hornby, T.G.; Reisman, D.S.; Ward, I.G.; Scheets, P.L.; Miller, A.; Haddad, D.; Fox, E.J.; Fritz, N.E.; Hawkins, K.; Henderson, C.E.; et al. Clinical practice guideline to improve locomotor function following chronic stroke, incomplete spinal cord injury, and brain injury. J. Neurol. Phys. Ther. 2020, 44, 49–100. [Google Scholar] [CrossRef]
- Colombo, G.; Joerg, M.; Schreier, R.; Dietz, V. Treadmill training of paraplegic patients using a robotic orthosis. J. Rehabil. Res. Dev. 2000, 37, 693–700. [Google Scholar]
- Schmidt, H.; Werner, C.; Bernhardt, R.; Hesse, S.; Krüger, J. Gait rehabilitation machines based on programmable footplates. J. Neuroeng. Rehabil. 2007, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.-C.; Yu, C.-H.; Chou, T.-Y. Design and implementation of robust controllers for a gait trainer. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2009, 223, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-C.; Lin, Y.-Y.; Li, Y.-C.; Chen, P.-Y.; Yu, C.-H. Development of an Automated Assistive Trainer Inspired by Neuro-developmental Treatment. Sens. Mater. 2020, 32, 3019–3037. [Google Scholar] [CrossRef]
- Wang, F.-C.; Lin, Y.-Y.; Yu, C.-H. Robust Loop-Shaping Control Design and Implementation for an Automatic Neuro-Developmental-Treatment Device. In Proceedings of the 2018 European Control Conference, Limassol, Cyprus, 12–15 June 2018. [Google Scholar]
- Wang, F.-C.; Li, Y.-C.; Wu, K.-L.; Chen, P.-Y.; Fu, L.-C. Online Gait Detection with an Automatic Mobile Trainer Inspired by Neuro-Developmental Treatment. Sensors 2020, 20, 3389. [Google Scholar] [CrossRef] [PubMed]
- VZ4000 Tracker. Available online: http://www.ptiphoenix.com/?prod-trackers-post=vz4000 (accessed on 8 January 2022).
- VICON Products. Available online: https://www.vicon.com/ (accessed on 8 January 2022).
- Rebula, J.R.; Ojeda, L.V.; Adamczyk, P.G.; Kuo, A.D. Measurement of foot placement and its variability with inertial sensors. Gait Posture 2013, 38, 974–980. [Google Scholar] [CrossRef] [Green Version]
- Aminian, K.; Najafi, B.; Büla, C.; Leyvraz, P.-F.; Robert, P. Spatio-temporal parameters of gait measured by an ambulatory system using miniature gyroscopes. J. Biomech. 2002, 35, 689–699. [Google Scholar] [CrossRef]
- Williamson, R.; Andrews, B.J. Gait event detection for FES using accelerometers and supervised machine learning. IEEE Trans. Rehabil. Eng. 2000, 8, 312–319. [Google Scholar] [CrossRef]
- Mazilu, S.; Hardegger, M.; Zhu, Z.; Roggen, D.; Tröster, G.; Plotnik, M.; Hausdorff, J.M. Online detection of freezing of gait with smartphones and machine learning techniques. In Proceedings of the 2012 6th International Conference on Pervasive Computing Technologies for Healthcare (PervasiveHealth) and Workshops, San Diego, CA, USA, 21–24 May 2012. [Google Scholar]
- Sun, F.; Zang, W.; Gravina, R.; Fortino, G.; Li, Y. Gait-based identification for elderly users in wearable healthcare systems. Inf. Fusion 2020, 53, 134–144. [Google Scholar] [CrossRef]
- Wang, F.-C.; Li, Y.-C.; Kuo, T.-Y.; Chen, S.-F.; Lin, C.-H. Real-Time Detection of Gait Events by Recurrent Neural Networks. IEEE Access 2021, 9, 134849–134857. [Google Scholar] [CrossRef]
- McGibbon, C.A.; Krebs, D.E. Age-related changes in lower trunk coordination and energy transfer during gait. J. Neurophysiol. 2001, 85, 1923–1931. [Google Scholar] [CrossRef] [Green Version]
- Dubey, L.; Karthikbabu, S.; Mohan, D. Effects of pelvic stability training on movement control, hip muscles strength, walking speed and daily activities after stroke: A randomized controlled trial. Ann. Neurosci. 2018, 25, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, R.C.; Beek, W. Hemiplegic gait: A kinematic analysis using walking speed as a basis. J. Biomech. 1992, 25, 1007–1015. [Google Scholar] [CrossRef]
- Della Croce, U.; Riley, P.O.; Lelas, J.L.; Kerrigan, D.C. A refined view of the determinants of gait. Gait Posture 2001, 14, 79–84. [Google Scholar] [CrossRef]
- Taylor, N.; Goldie, P.; Evans, O. Angular movements of the pelvis and lumbar spine during self-selected and slow walking speeds. Gait Posture 1999, 9, 88–94. [Google Scholar] [CrossRef]
- Opal User Guide. Available online: https://www.apdm.com/ (accessed on 8 January 2022).
- Kohavi, R. A study of cross-validation and bootstrap for accuracy estimation and model selection. In Proceedings of the IJCAI, Montreal, QC, Canada, 20–25 August 1995. [Google Scholar]
- Chen, G.; Patten, C.; Kothari, D.H.; Zajac, F.E. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture 2005, 22, 51–56. [Google Scholar] [CrossRef]
- Mungas, D. In-office mental status testing: A practical guide. Geriatrics 1991, 46, 54–58. [Google Scholar]
- The Brunnstrom Stages of Stroke Recovery. Available online: https://www.saebo.com/the-stages-of-stroke-recovery/ (accessed on 8 January 2022).
- Holden, M.K.; Gill, K.M.; Magliozzi, M.R. Gait assessment for neurologically impaired patients: Standards for outcome assessment. Phys. Ther. 1986, 66, 1530–1539. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).