Development and Characterization of Bioadsorbents Derived from Different Agricultural Wastes for Water Reclamation: A Review
Abstract
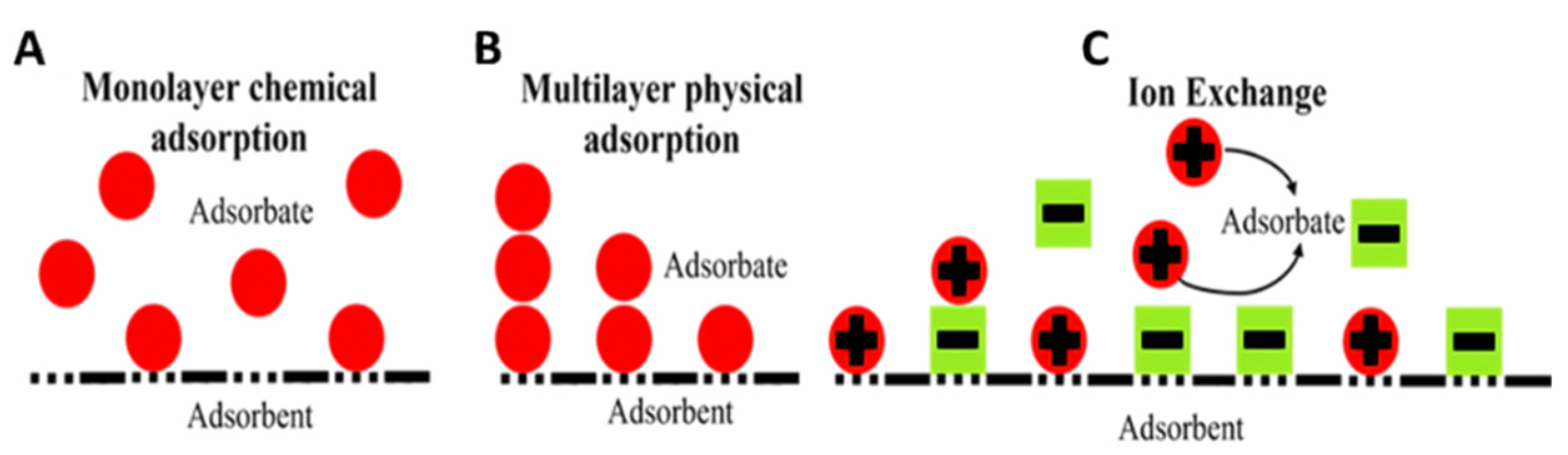
:1. Introduction
2. Methods
3. Obtaining Waste-Based Bioadsorbents
3.1. Activated Carbon Production
3.2. Raw Wastes as Bioadsorbents
4. Characterization of Bioadsorbents
5. Water Treatment Employing Bioadsorbents
5.1. Bioadsorbents Based on Grains and Seeds
5.1.1. Nutrients and Heavy Metals
5.1.2. Industrial Contaminants
5.1.3. Pharmaceuticals
5.2. Bioadsorbents Based on Fruits and Vegetables
5.2.1. Heavy Metals
5.2.2. Industrial Contaminants
5.2.3. Nutrients
5.2.4. Pharmaceuticals
5.3. Bioadsorbents Based on Herbage and Forage
5.3.1. Heavy Metals
5.3.2. Industrial Contaminants
5.3.3. Nutrients and Pharmaceuticals
6. Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bhatnagar, A.; Sillanpää, M.; Witek-Krowiak, A. Agricultural waste peels as versatile biomass for water purification–A review. Chem. Eng. J. 2015, 270, 244–271. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Water Pollution from Agriculture: A Global Review. Executive Summary. Available online: https://www.fao.org/3/i7754e/i7754e.pdf (accessed on 21 August 2021).
- Bhattacharjee, C.; Dutta, S.; Saxena, V.K. A review on biosorptive removal of dyes and heavy metals from wastewater using watermelon rind as biosorbent. Environ. Adv. 2020, 2, 100007. [Google Scholar] [CrossRef]
- Karim, M.A.H.; Aziz, K.H.H.; Omer, K.M.; Salih, Y.M.; Mustafa, F.; Rahman, K.O.; Mohammad, Y. Degradation of aqueous organic dyes pollutants by heterogeneous photo-assisted Fenton-like process using natural mineral activator: Parameter optimization and degradation kinetics. IOP Conf. Ser. Earth Environ. Sci. 2021, 958, 012011. [Google Scholar] [CrossRef]
- Abdulla, S.M.; Jamil, D.M.; Aziz, K.H.H. Investigation in heavy metal contents of drinking water and fish from Darbandikhan and Dokan Lakes in Sulaimaniyah Province—Iraqi Kurdistan Region. IOP Conf. Ser. Earth Environ. Sci. 2020, 612, 012023. [Google Scholar] [CrossRef]
- Pesqueria, J.F.J.R.; Pereira, M.F.R.; Silva, A.M.T. Environmental impact assessment of advanced urban wastewater treatment technologies for the removal of priority substances and contaminants of emerging concern: A review. J. Clean. Prod. 2020, 261, 121078. [Google Scholar] [CrossRef]
- Ramírez-Malule, H.; Quiñones-Murillo, D.H.; Manotas-Duque, D. Emerging contaminants as global environmental hazards. A bibliometric analysis. Emerg. Contam. 2020, 6, 179–193. [Google Scholar] [CrossRef]
- Intisar, A.; Ramzan, A.; Sawaira, T.; Kareem, A.T.; Hussain, N.; Din, M.I.; Bilal, M.; Iqbal, H.M.N. Occurrence, toxic effects, and mitigation of pesticides as emerging environmental pollutants using robust nanomaterials—A review. Chemosphere 2022, 293, 133538. [Google Scholar] [CrossRef]
- World Water Assessment Programme (UNESCO WWAP). The United Nations World Water Development Report 2015: Water for a Sustainable World. Available online: http://www.unesco.org/new/en/natural-sciences/environment/water/wwap/wwdr/2015-water-for-a-sustainable-world (accessed on 8 November 2021).
- Bhatnagar, A.; Sillanpää, M. Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment—A review. Chem. Eng. J. 2010, 157, 277–296. [Google Scholar] [CrossRef]
- Sanchez-Silva, J.M.; González-Estrada, R.R.; Blancas-Benitez, F.J.; Fonseca-Cantabrana, N. Utilización de subproductos agroindustriales para la bioadsorción de metales pesados. TIP 2020, 23, 1–18. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.; Reshma, B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef]
- Volesky, B. Biosorption and me. Water Res. 2007, 41, 4017–4029. [Google Scholar] [CrossRef] [PubMed]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef] [Green Version]
- Rudi, N.N.; Muhamad, M.S.; te Chuan, L.; Alipal, J.; Omar, S.; Hamidon, N.; Abdul Hamid, N.H.; Mohamed Sunar, N.; Ali, R.; Harun, H. Evolution of adsorption process for manganese removal in water via agricultural waste adsorbents. Heliyon 2020, 6, e05049. [Google Scholar] [CrossRef] [PubMed]
- Amer, H.; El-Gendy, A.; El-Haggar, S. Removal of lead (II) from aqueous solutions using rice straw. Water Sci. Technol. 2017, 76, 1011–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafiq, M.; Alazba, A.A.; Amin, M.T. Removal of Heavy Metals from Wastewater using Date Palm as a Biosorbent: A Comparative Review. Sains Malays. 2018, 47, 35–49. [Google Scholar] [CrossRef]
- De Andrade, J.R.; Oliveira, M.F.; da Silva, M.G.C.; Vieira, M.G.A. Adsorption of Pharmaceuticals from Water and Wastewater Using Nonconventional Low-Cost Materials: A Review. Ind. Eng. Chem. Res. 2018, 57, 3103–3127. [Google Scholar] [CrossRef]
- Salman, A.; Ibrahim, I.; Tarek, M.; Abbas, S. Biosorption of heavy metals: A review. J. Chem. Sci. Technol. 2014, 3, 74–102. [Google Scholar]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Kaur, S.; Rani, S.; Mahajan, R.K.; Asif, M.; Gupta, V.K. Synthesis and adsorption properties of mesoporous material for the removal of dye safranin: Kinetics, equilibrium, and thermodynamics. J. Ind. Eng. Chem. 2015, 22, 19–27. [Google Scholar] [CrossRef]
- Piccin, J.S.; Dotto, G.L.; Pinto, A.A. Adsorption isotherms and thermochemical data of FD&C red n° 40 binding by chitosan. Braz. J. Chem. Eng. 2011, 28, 295–304. [Google Scholar] [CrossRef]
- Ray, S.S.; Gusain, R.; Kumar, N. Adsorption equilibrium isotherms, kinetics and thermodynamics. In Carbon Nanomaterial-Based Adsorbents for Water Purification Fundamentals and Applications, 1st ed.; Ray, S.S., Gusain, R., Neeraj, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 101–118. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Jones, A.N.; Bridgeman, J. A fluorescence-based assessment of the fate of organic matter in water treated using crude/purified Hibiscus seeds as coagulant in drinking water treatment. Sci. Total Environ. 2019, 646, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda Yamaguchi, N.; Cusioli, L.F.; Quesada, H.B.; Camargo Ferreira, M.E.; Fagundes-Klen, M.R.; Salcedo Vieira, A.M.; Bergamasco, R. A review of Moringa oleifera seeds in water treatment: Trends and future challenges. Process. Saf. Environ. Prot. 2021, 147, 405–420. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and application of Granular activated carbon from biomass waste materials for water treatment: A review. J. Bioresour. Bioprod. 2021, 4, 292–322. [Google Scholar] [CrossRef]
- Edathil, A.A.; Shittu, I.; Hisham Zain, J.; Banat, F.; Haija, M.A. Novel magnetic coffee waste nanocomposite as effective bioadsorbent for Pb(II) removal from aqueous solutions. J. Environ. Chem. Eng. 2018, 6, 2390–2400. [Google Scholar] [CrossRef]
- Sciban, M.; Antov, M.G.; Klasnja, M. Extraction and partial purification of coagulation active components from common bean seed. Acta Period. Technol. 2006, 37, 37–43. [Google Scholar] [CrossRef]
- Bodlund, I.; Pavankumar, A.R.; Chelliah, R.; Kasi, S.; Sankaran, K.; Rajarao, G.K. Coagulant proteins identified in mustard: A potential water treatment agent. Int. J. Environ. Sci. Technol. 2014, 11, 873–880. [Google Scholar] [CrossRef] [Green Version]
- Foo, K.Y.; Hameed, B.H. Preparation and characterization of activated carbon from sunflower seed oil residue via microwave assisted K2CO3 activation. Bioresour. Technol. 2011, 102, 9794–9799. [Google Scholar] [CrossRef]
- Jayasree, R.; Kumar, P.S.; Saravanan, A.; Hemavathy, R.V.; Yaashikaa, P.R.; Arthi, P.; Choi, K.C. Sequestration of toxic Pb(II) ions using ultrasonic modified agro waste: Adsorption mechanism and modelling study. Chemosphere 2021, 285, 131502. [Google Scholar] [CrossRef]
- Aguayo-Villarreal, I.A.; Bonilla-Petriciolet, A.; Muñiz-Valencia, R. Preparation of activated carbons from pecan nutshell and their application in the antagonistic adsorption of heavy metal ions. J. Mol. Liq. 2017, 230, 686–695. [Google Scholar] [CrossRef]
- Williams, P.T.; Reed, A.R. Development of activated carbon pore structure via physical and chemical activation of biomass fibre waste. Biomass Bioenergy 2006, 30, 144–152. [Google Scholar] [CrossRef]
- Marsh, H.; Rodríguez-Reinoso, F. Activation Processes (Chemical). In Activated Carbon, 1st ed.; Marsh, H., Rodríguez-Reinoso, F., Eds.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2006; pp. 322–365. [Google Scholar] [CrossRef]
- Yi, H.; Nakabayashi, K.; Yoon, S.-H.; Miyawaki, J. Pressurized physical activation: A simple production method for activated carbon with a highly developed pore structure. Carbon 2021, 183, 735–742. [Google Scholar] [CrossRef]
- Anirudhan, T.; Sreekumari, S. Adsorptive removal of heavy metal ions from industrial effluents using activated carbon derived from waste coconut buttons. J. Environ. Sci. 2011, 23, 1989–1998. [Google Scholar] [CrossRef]
- Shin, J.; Kwak, J.; Lee, Y.G.; Kim, S.; Choi, M.; Bae, S.; Chon, K. Competitive adsorption of pharmaceuticals in lake water and wastewater effluent by pristine and NaOH-activated biochars from spent coffee wastes: Contribution of hydrophobic and π-π interactions. Environ. Pollut. 2021, 270, 116244. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Alwi, S.R.W.; Webb, C.; Ghasemi, N.; Muhamad, I.I. Treatment of lead-contaminated water using activated carbon adsorbent from locally available papaya peel biowaste. J. Clean. Prod. 2016, 118, 210–222. [Google Scholar] [CrossRef]
- Arris, S.; Bencheikh Lehocine, M.; Meniai, A.H. Sorption study of chromium sorption from wastewater using cereal by-products. Int. J. Hydrogen Energy 2014, 41, 10299–10310. [Google Scholar] [CrossRef]
- Soliman, A.M.; Elwy, H.M.; Thiemann, T.; Majedi, Y.; Labata, F.T.; Al-Rawashdeh, N.A. Removal of Pb(II) ions from aqueous solutions by sulphuric acid-treated palm tree leaves. J. Taiwan Inst. Chem. Eng. 2016, 58, 264–273. [Google Scholar] [CrossRef]
- Garg, D.; Kumar, S.; Sharma, K.; Majumder, C.B. Application of waste peanut shells to form activated carbon and its utilization for the removal of Acid Yellow 36 from wastewater. Groundw. Sustain. Dev. 2019, 8, 512–519. [Google Scholar] [CrossRef]
- Rahman, A.; Hango, H.J.; Daniel, L.S.; Uahengo, V.; Jaime, S.J.; Bhaskaruni, S.V.H.S.; Jonnalagadda, S.B. Chemical preparation of activated carbon from Acacia erioloba seed pods using H2SO4 as impregnating agent for water treatment: An environmentally benevolent approach. J. Clean. Prod. 2019, 237, 117689. [Google Scholar] [CrossRef]
- Jung, K.W.; Choi, B.H.; Hwang, M.J.; Jeong, T.U.; Ahn, K.H. Fabrication of granular activated carbons derived from spent coffee grounds by entrapment in calcium alginate beads for adsorption of acid orange 7 and methylene blue. Bioresour. Technol. 2016, 219, 185–195. [Google Scholar] [CrossRef]
- Pereira, R.G.; Veloso, C.M.; da Silva, N.M.; de Sousa, L.F.; Bonomo, R.C.F.; de Souza, A.O.; da Guarda Souza, M.O.; da Costa Ilhéu Fontan, R. Preparation of activated carbons from cocoa shells and siriguela seeds using H3PO4 and ZnCl2 as activating agents for BSA and α-lactalbumin adsorption. Fuel Process. Technol. 2014, 126, 476–486. [Google Scholar] [CrossRef]
- Namasivayam, C.; Kavitha, D. Removal of Congo red from water by adsorption onto activated carbon prepared from coir pith, an agricultural solid waste. Dye. Pigm. 2002, 54, 47–58. [Google Scholar] [CrossRef]
- Namasivayam, C.; Radhika, R.; Suba, S. Uptake of dyes by a promising locally available agricultural solid waste: Coir pith. J. Waste Manag. 2001, 21, 381–387. [Google Scholar] [CrossRef]
- Mahamad, M.N.; Zaini, M.A.A.; Zakaria, Z.A. Preparation and characterization of activated carbon from pineapple waste biomass for dye removal. Int. Biodeterior. Biodegrad. 2015, 102, 274–280. [Google Scholar] [CrossRef]
- Yadav, D.; Kapur, M.; Kumar, P.; Mondal, M.K. Adsorptive removal of phosphate from aqueous solution using rice husk and fruit juice residue. Process. Saf. Environ. Prot. 2015, 94, 402–409. [Google Scholar] [CrossRef]
- Isiuku, B.O.; Enyoh, C.E.; Duru, C.E.; Ibe, F.C. Phosphate ions removal from aqueous phase by batch adsorption on activated (activation before carbonization) biochar derived from rubber pod husk. Curr. Res. Green Sustain. Chem. 2021, 4, 100136. [Google Scholar] [CrossRef]
- De Luna, M.D.G.; Murniati; Budianta, W.; Rivera, K.K.; Arazo, R.O. Removal of sodium diclofenac from aqueous solution by adsorbents derived from cocoa pod husks. J. Environ. Chem. Eng. 2017, 5, 1465–1474. [Google Scholar] [CrossRef]
- El Mouchtari, E.M.; Daou, C.; Rafqah, S.; Najjar, F.; Anane, H.; Piram, A.; Hamade, A.; Briche, S.; Wong-Wah-Chung, P. TiO2 and activated carbon of Argania Spinosa tree nutshells composites for the adsorption photocatalysis removal of pharmaceuticals from aqueous solution. J. Photochem. Photobiol. A Chem. 2020, 388, 112183. [Google Scholar] [CrossRef]
- Chakraborty, P.; Banerjee, S.; Kumar, S.; Sadhukhan, S.; Halder, G. Elucidation of ibuprofen uptake capability of raw and steam activated biochar of Aegle marmelos shell: Isotherm, kinetics, thermodynamics and cost estimation. Process. Saf. Environ. Prot. 2018, 118, 10–23. [Google Scholar] [CrossRef]
- Cabrita, I.; Ruiz, B.; Mestre, A.; Fonseca, I.; Carvalho, A.; Ania, C. Removal of an analgesic using activated carbons prepared from urban and industrial residues. Chem. Eng. J. 2010, 163, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Mora Alvarez, N.M.; Pastrana, J.M.; Lagos, Y.; Lozada, J.J. Evaluation of mercury (Hg2+) adsorption capacity using exhausted coffee waste. Sustain. Chem. Pharm. 2018, 10, 60–70. [Google Scholar] [CrossRef]
- Araújo, C.; Melo, E.; Alves, V.; Coelho, N.M.M. Moringa oleifera Lam. Seeds as a Natural Solid Adsorbent for Removal of AgI in Aqueous Solutions. J. Braz. Chem. Soc. 2010, 21, 1727–1732. [Google Scholar] [CrossRef]
- Çelekli, A.; Al-Nuaimi, A.I.; Bozkurt, H. Adsorption kinetic and isotherms of Reactive Red 120 on Moringa oleifera seed as an eco-friendly process. J. Mol. Struct. 2019, 1195, 168–178. [Google Scholar] [CrossRef]
- Obeng, G.Y.; Amoah, D.Y.; Opoku, R.; Sekyere, C.K.; Adjei, E.A.; Mensah, E. Coconut wastes as bioresource for sustainable energy: Quantifying wastes, calorific values and emissions in Ghana. Energies 2020, 13, 2178. [Google Scholar] [CrossRef]
- Parab, H.; Joshi, S.; Shenoy, N.; Lali, A.; Sarma, U.S.; Sudersanan, M. Determination of kinetic and equilibrium parameters of the batch adsorption of Co(II), Cr(III) and Ni(II) onto coir pith. Process. Biochem. 2006, 41, 609–615. [Google Scholar] [CrossRef]
- Memon, J.R.; Memon, S.Q.; Bhanger, M.I.; Memon, G.Z.; El-Turki, A.; Allen, G.C. Characterization of banana peel by scanning electron microscopy and FT-IR spectroscopy and its use for cadmium removal. Colloids Surf. B Biointerfaces 2008, 66, 260–265. [Google Scholar] [CrossRef]
- Memon, J.R.; Memon, S.Q.; Bhanger, M.I.; El-Turki, A.; Hallam, K.R.; Allen, G.C. Banana peel: A green and economical sorbent for the selective removal of Cr(VI) from industrial wastewater. Colloids Surf. B Biointerfaces 2009, 70, 232–237. [Google Scholar] [CrossRef]
- Liu, C.; Ngo, H.H.; Guo, W. Watermelon Rind: Agro-waste or Superior Biosorbent? Appl. Biochem. Biotechnol. 2012, 167, 1699–1715. [Google Scholar] [CrossRef]
- Liu, C.; Ngo, H.H.; Guo, W.; Tung, K.L. Optimal conditions for preparation of banana peels, sugarcane bagasse and watermelon rind in removing copper from water. Bioresour. Technol. 2012, 119, 349–354. [Google Scholar] [CrossRef]
- Banerjee, K.; Ramesh, S.T.; Gandhimathi, R.; Nidheesh, P.V.; Bharathi, K.S. A novel agricultural waste adsorbent, watermelon shell for the removal of copper from aqueous solutions. Iran. J. Energy Environ. 2012, 3, 143–156. [Google Scholar] [CrossRef]
- Gupta, H.; Gogate, P.R. Intensified removal of copper from waste water using activated watermelon based biosorbent in the presence of ultrasound. Ultrason. Sonochem. 2016, 30, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.A.; Lakshmipathy, R.; Sarada, N.C. Application of Citrullus lanatus rind as biosorbent for removal of trivalent chromium from aqueous solution. Alex. Eng. J. 2014, 53, 969–975. [Google Scholar] [CrossRef] [Green Version]
- Annadurai, G.; Juang, R.S.; Lee, D.J. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 2002, 92, 263–274. [Google Scholar] [CrossRef]
- Mondal, N.K.; Roy, A. Potentiality of a fruit peel (banana peel) toward abatement of fluoride from synthetic and underground water samples collected from fluoride affected villages of Birbhum district. Appl. Water Sci. 2018, 8, 90. [Google Scholar] [CrossRef]
- Baldikova, E.; Politi, D.; Maderova, Z.; Pospiskova, K.; Sidiras, D.; Safarikova, M.; Safarik, I. Utilization of magnetically responsive cereal by-product for organic dye removal. J. Sci. Food Agric. 2015, 96, 2204–2214. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Khan, F.; Ahmad, A. Cauliflower Leave, an Agricultural Waste Biomass Adsorbent, and Its Application for the Removal of MB Dye from Aqueous Solution: Equilibrium, Kinetics, and Thermodynamic Studies. Int. J. Anal. Chem. 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Paredes-Laverde, M.; Silva-Agredo, J.; Torres-Palma, R.A. Removal of norfloxacin in deionized, municipal water and urine using rice (Oryza sativa) and coffee (Coffea arabica) husk wastes as natural adsorbents. J. Environ. Manag. 2018, 213, 98–108. [Google Scholar] [CrossRef]
- Etim, U.; Umoren, S.; Eduok, U. Coconut coir dust as a low cost adsorbent for the removal of cationic dye from aqueous solution. J. Saudi Chem. Soc. 2016, 20, S67–S76. [Google Scholar] [CrossRef] [Green Version]
- ŠćIban, M.; Klašnja, M.; ŠKrbić, B. Adsorption of copper ions from water by modified agricultural by-products. Desalination 2008, 229, 170–180. [Google Scholar] [CrossRef]
- Hameed, B.H.; Hakimi, H. Utilization of durian (Durio zibethinus Murray) peel as low cost sorbent for the removal of acid dye from aqueous solutions. Biochem. Eng. J. 2008, 39, 338–343. [Google Scholar] [CrossRef]
- Saeed, A.; Sharif, M.; Iqbal, M. Application potential of grapefruit peel as dye sorbent: Kinetics, equilibrium and mechanism of crystal violet adsorption. J. Hazard. Mater. 2010, 179, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.H. Removal of cationic dye from aqueous solution using jackfruit peel as non-conventional low-cost adsorbent. J. Hazard. Mater. 2009, 162, 344–350. [Google Scholar] [CrossRef]
- Al-Qahtani, K.M. Water purification using different waste fruit cortexes for the removal of heavy metals. J. Taibah Univ. Sci. 2016, 10, 700–770. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, M.; Saeed, A.; Zafar, S.I. FTIR spectrophotometry, kinetics and adsorption isotherms modeling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste. J. Hazard. Mater. 2009, 164, 161–171. [Google Scholar] [CrossRef]
- Phanthuwongpakdee, J.; Babel, S.; Laohhasurayotin, K.; Sattayaporn, S.; Kaneko, T. Anthocyanin based agricultural wastes as bio-adsorbents for scavenging radioactive iodide from aqueous environment. J. Environ. Chem. Eng. 2020, 8, 104147. [Google Scholar] [CrossRef]
- Namasivayam, C.; Muniasamy, N.; Gayatri, K.; Rani, M.; Ranganathan, K. Removal of dyes from aqueous solutions by cellulosic waste orange peel. Bioresour. Technol. 1996, 57, 37–43. [Google Scholar] [CrossRef]
- Arami, M.; Limaee, N.Y.; Mahmoodi, N.M.; Tabrizi, N.S. Removal of dyes from colored textile wastewater by orange peel adsorbent: Equilibrium and kinetic studies. J. Colloid Interface. Sci. 2005, 288, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Aryee, A.A.; Dovi, E.; Shi, X.; Han, R.; Li, Z.; Qu, L. Zirconium and iminodiacetic acid modified magnetic peanut husk as a novel adsorbent for the sequestration of phosphates from solution: Characterization, equilibrium and kinetic study. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126260. [Google Scholar] [CrossRef]
- Argun, M.E.; Güclü, D.; Karatas, M. Adsorption of Reactive Blue 114 dye by using a new adsorbent: Pomelo peel. J. Ind. Eng. Chem. 2014, 20, 1079–1084. [Google Scholar] [CrossRef]
- Gupta, N.; Kushwaha, A.K.; Chattopadhyaya, M. Application of potato (Solanum tuberosum) plant wastes for the removal of methylene blue and malachite green dye from aqueous solution. Arab. J. Chem. 2016, 9, S707–S716. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhou, X.; Chen, X.; Dai, C.; Zhang, J.; Zhang, Y. Biosorption of clofibric acid and carbamazepine in aqueous solution by agricultural waste rice straw. J. Environ. Sci. 2013, 25, 2384–2395. [Google Scholar] [CrossRef]
- Soliman, E.M.; Ahmed, S.A.; Fadl, A.A. Reactivity of sugar cane bagasse as a natural solid phase extractor for selective removal of Fe(III) and heavy-metal ions from natural water samples. Arab. J. Chem. 2011, 4, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Esfandiar, N.; Nasernejad, B.; Ebadi, T. Removal of Mn(II) from groundwater by sugarcane bagasse and activated carbon (a comparative study): Application of response surface methodology (RSM). J. Ind. Eng. Chem. 2014, 20, 3726–3736. [Google Scholar] [CrossRef]
- Ahmed, S.; El-Roudi, A.M.; Salem, A.A. Removal of Mn(II) from Ground Water by Solid Wastes of Sugar Industry. J. Environ. Sci. Technol. 2015, 8, 338–351. [Google Scholar] [CrossRef] [Green Version]
- Feizi, M.; Jalali, M. Removal of heavy metals from aqueous solutions using sunflower, potato, canola and walnut shell residues. J. Taiwan Inst. Chem. Eng. 2015, 54, 125–136. [Google Scholar] [CrossRef]
- Amarasinghe, B.; Williams, R. Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. Chem. Eng. J. 2007, 132, 299–309. [Google Scholar] [CrossRef]
- Ahsan, M.A.; Katla, S.K.; Islam, M.T.; Hernandez-Viezcas, J.A.; Martinez, L.M.; Díaz-Moreno, C.A.; Lopez, J.; Singamaneni, S.R.; Banuelos, J.; Gardea-Torresdey, J.; et al. Adsorptive removal of methylene blue, tetracycline and Cr(VI) from water using sulfonated tea waste. Environ. Technol. Innov. 2018, 11, 23–40. [Google Scholar] [CrossRef]
- Badrealam, S.; Darrell, V.C.; Dollah, Z.; Mohamed-Latiff, M.F.P.; Handan, R. Adsorption of manganese and zinc in synthetic wastewater by tea waste (TW) as a low cost adsorbent. J. Phys. Conf. Ser. 2019, 1349, 012061. [Google Scholar] [CrossRef]
- Cherdchoo, W.; Nithettham, S.; Charoenpanich, J. Removal of Cr(VI) from synthetic wastewater by adsorption onto coffee ground and mixed waste tea. Chemosphere 2019, 221, 758–767. [Google Scholar] [CrossRef]
- Solangi, N.H.; Kumar, J.; Mazari, S.A.; Ahmed, S.; Fatima, N.; Mujawar, N.M. Development of fruit waste derived bio-adsorbents for wastewater treatment: A review. J. Hazard. Mater. 2021, 416, 125848. [Google Scholar] [CrossRef]
- Sivaraj, R.; Namasivayam, C.; Kadirvelu, K. Orange peel as an adsorbent in the removal of acid violet 17 (acid dye) from aqueous solutions. Waste Manag. 2001, 21, 105–110. [Google Scholar] [CrossRef]
- Hou, S.X. Adsorption properties of pomelo peels against methylene blue in dye wastewater. Adv. Mater. Res. 2013, 634, 178–181. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Atarod, M.; Sajjadi, M.; Sajadi, S.M.; Issaabadi, Z. Plant-Mediated Green Synthesis of Nanostructures: Mechanisms, Characterization, and Applications. Interface Sci. Technol. 2019, 28, 199–322. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental methods in chemical engineering: Specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Yan, Y. Tribology and tribo-corrosion testing and analysis of metallic biomaterials. In Metals for Biomedical Devices: Woodhead Publishing Series in Biomaterials, 1st ed.; Niinomi, M., Ed.; Woodhead Publishing Ltd.: Sawston, UK, 2010; pp. 178–201. [Google Scholar] [CrossRef]
- Ahsan, M.A.; Islam, M.T.; Imam, M.A.; Hyder, A.G.; Jabbari, V.; Dominguez, N.; Noveron, J.C. Biosorption of bisphenol A and sulfamethoxazole from water using sulfonated coffee waste: Isotherm, kinetic and thermodynamic studies. J. Environ. Chem. Eng. 2018, 6, 6602–6611. [Google Scholar] [CrossRef]
- Chiang, C.H.; Chen, J.; Lin, J.H. Preparation of pore-size tunable activated carbon derived from waste coffee grounds for high adsorption capacities of organic dyes. J. Environ. Chem. Eng. 2020, 8, 103929. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Ben-Ali, S.; Jaouali, I.; Souissi-Najar, S.; Ouederni, A. Characterization and adsorption capacity of raw pomegranate peel biosorbent for copper removal. J. Clean. Prod. 2017, 142, 3809–3821. [Google Scholar] [CrossRef]
- Kumar, G.V.S.R.; Rao, K.S.; Yadav, A.; Kumar, M.L.; Sarathi, T.V.N. Biosorption of copper(II) and manganese(II) from waste water using low cost bio adsorbents. J. Indian Chem. Soc. 2018, 95, 1–8. [Google Scholar]
- Baccar, R.; Sarrà, M.; Bouzid, J.; Feki, M.; Blánquez, P. Removal of pharmaceutical compounds by activated carbon prepared from agricultural by-product. Chem. Eng. J. 2012, 211, 310–317. [Google Scholar] [CrossRef]
- Masoudian, N.; Rajabi, M.; Ghaedi, M. Titanium oxide nanoparticles loaded onto activated carbon prepared from bio-waste watermelon rind for the efficient ultrasonic-assisted adsorption of congo red and phenol red dyes from wastewaters. Polyhedron 2019, 173, 114105. [Google Scholar] [CrossRef]
- Ismail, Z.Z. Kinetic study for phosphate removal from water by recycled date-palm wastes as agricultural by-products. Int. J. Environ. Stud. 2012, 69, 135–149. [Google Scholar] [CrossRef]
- Ye, M.; Sun, M.; Chen, X.; Feng, Y.; Wan, J.; Liu, K.; Tian, D.; Liu, M.; Wu, J.; Schwab, A.P.; et al. Feasibility of sulfate-calcined eggshells for removing pathogenic bacteria and antibiotic resistance genes from landfill leachates. Waste Manag. 2017, 63, 275–283. [Google Scholar] [CrossRef]
- Liu, Z.; Tran, K.-Q. A review on disposal and utilization of phytoremediation plants containing heavy metals. Ecotoxicol. Environ. Saf. 2021, 226, 112821. [Google Scholar] [CrossRef]
- El Gamal, M.; Mousa, H.A.; El-Naas, M.H.; Zacharia, R.; Judd, S. Bio-regeneration of activated carbon: A comprehensive review. Sep. Purif. Technol. 2018, 197, 345–359. [Google Scholar] [CrossRef]
- Reza, M.S.; Hasan, A.B.M.; Afroze, S.; Abu Bakar, M.; Taweekun, J.; Azad, A. Analysis on Preparation, Application, and Recycling of Activated Carbon to Aid in COVID-19 Protection. Int. J. Integr. Eng. 2020, 12, 233–244. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, T.; Yao, Y.; Jiang, T.; Wang, B.; Wang, M. Recycling supercapacitor activated carbons for adsorption of silver (I) and chromium (VI) ions from aqueous solutions. Chemosphere 2020, 238, 124638. [Google Scholar] [CrossRef]
- Hassan, M.; Naidu, R.; Du, J.; Liu, Y.; Qi, F. Critical review of magnetic biosorbents: Their preparation, application, and regeneration for wastewater treatment. Sci. Total Environ. 2020, 702, 134893. [Google Scholar] [CrossRef]
- Gao, Q.; Blum, K.M.; Gago-Ferrero, P.; Wiberg, K.; Ahrens, L.; Andersson, P.L. Impact of on-site wastewater infiltration systems on organic contaminants in groundwater and recipient waters. Sci. Total Environ. 2019, 651, 1670–1679. [Google Scholar] [CrossRef]
- Moreau, M.; Hadfield, J.; Hughey, J.; Sanders, F.; Lapworth, D.J.; White, D.; Civil, W. A baseline assessment of emerging organic contaminants in New Zealand groundwater. Sci. Total Environ. 2019, 686, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Llamas-Dios, M.I.; Vadillo, I.; Jiménez-Gavilán, P.; Candela, L.; Corada-Fernández, C. Assessment of a wide array of contaminants of emerging concern in a Mediterranean water basin (Guadalhorce river, Spain): Motivations for an improvement of water management and pollutants surveillance. Sci. Total Environ. 2021, 788, 147822. [Google Scholar] [CrossRef] [PubMed]
- Riva, F.; Castiglioni, S.; Fattore, E.; Manenti, A.; Davoli, E.; Zuccato, E. Monitoring emerging contaminants in the drinking water of Milan and assessment of the human risk. Int. J. Hyg. Environ. Health 2018, 221, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Zhao, J.L.; Liu, Y.S.; Liu, W.R.; Zhang, Q.Q.; Yao, L.; Hu, L.X.; Zhang, J.N.; Jiang, Y.X.; Ying, G.G. Pharmaceuticals and personal care products (PPCPs) and artificial sweeteners (ASs) in surface and ground waters and their application as indication of wastewater contamination. Sci. Total Environ. 2018, 616, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-L.; Kim, K.Y.; Hamm, S.-Y.; Kim, M.S.; Kim, H.K.; Oh, J.-E. Occurrence and distribution of pharmaceutical and personal care products, artificial sweeteners, and pesticides in groundwater from an agricultural area in Korea. Sci. Total Environ. 2019, 659, 168–176. [Google Scholar] [CrossRef]
- Sharma, B.J.; Bečanová, J.; Scheringer, M.; Sharma, A.; Bharat, G.K.; Whitehead, P.G.; Klánová, J.; Nizzetto, L. Health and ecological risk assessment of emerging contaminants (pharmaceuticals, personal care products, and artificial sweeteners) in surface and groundwater (drinking water) in the Ganges River Basin, India. Sci. Total Environ. 2019, 646, 1459–1467. [Google Scholar] [CrossRef]
- Alquwaizany, A.S.; Alfadul, S.M.; Khan, M.A.; Alabdulaaly, A.I. Occurrence of organic compounds in groundwater of Saudi Arabia. Environ. Monit. Assess. 2019, 191, 601. [Google Scholar] [CrossRef]
- Hou, L.; Jiang, J.; Gan, Z.; Dai, Y.Y.; Yang, P.; Yan, Y.; Ding, S.; Su, S.; Bao, X. Spatial Distribution of Organophosphorus and Brominated Flame Retardants in Surface Water, Sediment, Groundwater, and Wild Fish in Chengdu, China. Arch. Environ. Contam. Toxicol. 2019, 77, 279–290. [Google Scholar] [CrossRef]
- Close, M.E.; Humphries, B.; Northcott, G. Outcomes of the first combined national survey of pesticides and emerging organic contaminants (EOCs) in groundwater in New Zealand 2018. Sci. Total Environ. 2021, 754, 142005. [Google Scholar] [CrossRef]
- Huang, H.; Liu, H.; Xiong, S.; Zeng, F.; Bu, J.; Zhang, B.; Liu, W.; Zhou, H.; Qi, S.; Xu, L.; et al. Rapid transport of organochlorine pesticides (OCPs) in multimedia environment from karst area. Sci. Total Environ. 2021, 775, 145698. [Google Scholar] [CrossRef]
- Thompson, T.J.; Briggs, M.A.; Phillips, P.J.; Blazer, V.S.; Smalling, K.L.; Kolpin, D.W.; Wagner, T. Groundwater discharges as a source of phytoestrogens and other agriculturally derived contaminants to streams. Sci. Total Environ. 2021, 755, 142873. [Google Scholar] [CrossRef]
| Biowaste | Pretreatment | Characterization | Contaminant | Ref. |
|---|---|---|---|---|
| Acacia erioloba seed | Chemical activation (H2SO4), Pyrolysis (600 °C) | SEM, XRD, BET, FTIR, EDX | MB, iodine | [43] |
| Aegle marmelos shell | Carbonization (650 °C), Steam activation (800 °C) | SEM, BET, FTIR, PZC | IBU | [53] |
| Argania spinosa tree nutshells | Chemical activation (H3PO4), Carbonization (500 °C)—TiO2 Impregnation | FTIR, SEM-EDS, TGA, XRD, BET | CBZ, SMX, DCF | [52] |
| Cacao pod husk | Chemical activation (H2SO4), Carbonization (600 °C) | SEM, FTIR, EDX | SD | [51] |
| Cacao shells and Siriguela seeds | Chemical activation (ZnCl2, H3PO4), Carbonization (500 °C, N2) | FTIR, DTA/TG, BET, BJH | α-Lac I, BSA | [45] |
| Cereal byproducts | Carbonization (600 °C) | N.R | Cr(VI) | [40] |
| Coconut buttons | Chemical activation (H2SO4), Steam carbonization (400 °C) | FTIR, XRD, SEM, TGA, PZC, BET | Pb(II), Hg(II), Cu(II) | [37] |
| Coir pith | Carbonization (700 °C) | N.R | CR, RB, AV | [46,47] |
| Palm tree leaves | Chemical activation (H2SO4), Carbonization (250–450 °C) | FTIR, SEM-EDX, BET | Pb(II) | [41] |
| Papaya Peel | Carbonization (450 °C), H3PO4 Oxidation | FESEM, SEM-EDX, FTIR, BET, XRD | Pb(II) | [39] |
| Peach stones | Chemical activation (K2CO3), Carbonization (700 °C) | N2 and CO2 adsorption, PZC, thermal analysis | ACP | [54] |
| Peanut Shell | Chemical activation (H3PO4), Pyrolysis (650 °C, N2) | TGA, FESEM, EDS, BET, FTIR | AY-36 | [42] |
| Pineapple waste | Chemical activation (ZnCl2), Pyrolysis (500 °C) | BET, BJH, FTIR, SEM | MB | [48] |
| Rice husk and Lemon juice residue | Chemical activation (NaOH, H2SO4), Carbonization (650 °C) | FTIR, BET, BJH | Phosphates | [49] |
| Rubber pod husk | Chemical activation (H3PO4), Pyrolysis (500 °C) | BET, SEM, EDX, FTIR, PZC | Phosphates | [50] |
| Spent Coffee | Chemical activation (NaOH), Pyrolysis (800 °C) | UHR-SEM, FTIR | NPX, DCF, IBU | [38] |
| Spent Coffee (Granular) | Chemical activation (KOH), Pyrolysis (700 °C), Granulation | SEM, FTIR, BET, BJH, Horvath-Kawazoe | AO7, MB | [44] |
| Biowaste | Pretreatment | Characterization | Contaminant | Ref. |
|---|---|---|---|---|
| Banana peel | No treatment, Esterification (MeOH-HCl) | FTIR, BET, SEM, PZC, EDX | MO, MB, RB, CR, MV, AB-10, F, Cd(II), Cr(VI) | [60,61,67,68] |
| Barley straw | No treatment, Citric acid–NaOH treatment, Magnetic modification | FTIR, SEM | BBY, CV, MB, SO | [69] |
| Cauliflower leaf | No pretreatment | FTIR, SEM | MB | [70] |
| Coffee husk | No pretreatment | BET, FTIR, PZC, SEM-EDS | NFX | [71] |
| Coconut coir dust | No pretreatment | FTIR | MB | [72] |
| Coir pith | No pretreatment | N.R | Co(II), Cr(III), Ni(II) | [59] |
| Corn cob and stalk | No treatment, Formaldehyde, NaOH-H2SO4 treatment | N.R | Cu, Ni, Cd, Pb | [73] |
| Durian peels | HCl treatment | N.R | AG25 | [74] |
| Grapefruit peel | No pretreatment | FTIR | CV | [75] |
| Jackfruit peel | No pretreatment | N.R | MB | [76] |
| Kiwi and Tangerine peels | NaOH treatment | N.R | Cd(II), Cr(III), Zn(II) | [77] |
| Mango peel | No pretreatment | PZC, FTIR, SEM, EDX | Cd(II), Pb(II) | [78] |
| Mangosteen pericarps | No Pretreatment | SEM, FTIR, EDX, XPS, XAS | I− | [79] |
| Moringa oleifera seeds | No pretreatment | FTIR, SEM | RR-120, Ag(I) | [56,57] |
| Orange peel | No pretreatment | BET, SEM | CR, PO, RB, MO, MB, MV, AB-10, DR23, DR80 | [67,80,81] |
| Passion fruit rinds | No Pretreatment | SEM, FTIR, EDX, XPS, XAS | I− | [79] |
| Peanut husk | Chemical modification (Fe3O4-IA-Zr) | BET, SEM, FTIR, XPS, VSM, XRD | Phosphates | [82] |
| Pomelo peel | No pretreatment | ZP, FESEM, FTIR, BET | RB-114 | [83] |
| Potato leaf/stem | No pretreatment | FTIR, SEM | MB, MG | [84] |
| Red onion and Red dragon fruit peels | No Pretreatment | SEM, FTIR, EDX, XPS, XAS | I− | [79] |
| Rice husk | No Pretreatment | BET, FTIR, PZC, SEM-EDS | NFX | [71] |
| Rice straw | No Pretreatment | FTIR, BET, FTIR, SEM, EDX | CFA, CBZ, Pb(II) | [16,85] |
| Soybean and wheat straw | No treatment, Formaldehyde, NaOH and H2SO4 treatment. | N.R | Cu, Ni, Cd, Pb | [73] |
| Spent ground coffee | No treatment, Magnetic modification (Fe3O4 NPs) | BET, XRD, FTIR, SEM-EDX, ZP | Hg(II), Pb(II) | [28,55] |
| Sugarcane bagasse (SCB) | No treatment, NaOH, HCl treatments | FTIR, SEM | Fe(III), Cu(II), Pb(II), Zn(II), Cd(II), Co(II), Mn(II) | [86,87] |
| SCB and beet pulp | NaOH treatment | FTIR, SEM | Mn(II) | [88] |
| Sunflower leaf/stalk | No treatment, NaOH activation (Ni removal) | PZC, SEM | Fe, Mn, Zn, Ni, Cu, Cd | [89] |
| Tamarind seeds | H2SO4 treatment., Ultrasonic modification | FTIR, SEM | Pb(II) | [32] |
| Tea waste (Black tea) | No treatment, Sulfonation (H2SO4) | BET, BSLLD, SEM-EDX, FTIR, TGA, Raman, XPS, ESR, SAXS | Cu, Pb, MB, Tet, Cr(VI) | [90,91] |
| Tea waste (mixed tea) | No pretreatment | SEM, TEM, EDS, BET, FTIR, XPS | Mn(II), Zn(II), Cr(VI) | [92,93] |
| Watermelon rinds | No treatment, Ca(OH)2, Citric acid treatments | SEM-EDX, BET, MP, PZC, FTIR | Zn, Pb, Cu(II), Cr(III) | [62,63,64,65,66] |
| Biowaste | Contaminant | Isothermal Model Adjusted | Kinetic Model Adjusted | qm/qe | Removal Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| Argania Spinosa tree nutshells (TiO2 composite AC) | DFC | Langmuir | Pseudo-first order | 153.8 mg/g | 100.00 | [52] |
| CBZ | Langmuir | Pseudo-first order | 105.3 mg/g | 85.00 | ||
| SMX | Langmuir | Pseudo-first order | 125.0 mg/g | 67.00 | ||
| Cacao pod husk | SD | Freundlich | Pseudo-second order | 5.53 mg/g | 93.6 | [51] |
| Cacao shell (H3PO4-activated) | α-Lac | Toth | Pseudo-second order | 179.91 mg/g | 91.67 | [45] |
| BSA | Langmuir | Pseudo-second order | 41.02 mg/g | 21.25 | ||
| Cacao shell (ZnCl2-activated) | α-Lac | Toth | Pseudo-second order | 141.69 mg/g | 70.33 | [45] |
| BSA | Langmuir | Pseudo-second order | 147.84 mg/g | 86.86 | ||
| Coffee husk | NFX | Langmuir and Redlich-Peterson | Pseudo-second order | 33.56 mg/g | 99.66 | [71] |
| Exhausted coffee | Hg(II) | Langmuir | Pseudo-second order | 31.75 mg/g | 97.69 | [55] |
| Magnetic coffee waste (Fe3O4 NPs) | Pb(II) | Langmuir | Pseudo-second order | 48.78 mg/g | 99.56 | [28] |
| Moringa oleifera seeds | Ag(I) | Langmuir | N.R | 23.13 mg/g | 99.00 | [56] |
| RR-120 | Langmuir and Freundlich | Pseudo-second order | 413.32 mg/g | N.R | [57] | |
| Peanut husk (Magnetic husk) | Phosphates | Freundlich | Elovich | 13.2 mg/g | 88.5 | [82] |
| Peanut shell | AY-36 | Langmuir and Freundlich | Pseudo-second order | 66.7 mg/g | 98.55 | [42] |
| Raw tamarind seeds | Pb(II) | Langmuir | Pseudo-first order | 16.0 mg/g | 99.52 | [32] |
| Rice husk | NFX | Langmuir and Redlich-Peterson | Pseudo-second order | 20.12 mg/g | 96.95 | [71] |
| Rubber pod husk | Phosphates | Freundlich | Pseudo-second order | 39.98 mg/g | N.R | [50] |
| Siriguela seeds (ZnCl2-activated) | α-Lac | Toth | Pseudo-second order | 173.05 mg/g | 87.42 | [45] |
| BSA | Langmuir | Pseudo-second order | 188.29 mg/g | 92.29 | ||
| Siriguela seeds (H3PO4-activated) | α-Lac | Toth | Pseudo-second order | 193.54 mg/g | 96.67 | [45] |
| BSA | Langmuir | Pseudo-second order | 130.31 mg/g | 81.67 | ||
| Spent coffee ground (Calcium-alginate beads) | AO7 | Sips | Pore diffusion | 665.9 mg/g | 99.90 | [44] |
| MB | Sips | Pore diffusion | 986.8 mg/g | 100 | ||
| Spent coffee waste biochar (NaOH-activated) | NPΧLK | Langmuir | Pseudo-second order | 269.01 μmol/g | 30.7–97.1 | [38] |
| DCFLK | Langmuir | Pseudo-second order | 97.17 μmol/g | |||
| IBULK | Langmuir | Pseudo-second order | 76.10 μmol/g | |||
| NPΧWW | Langmuir | Pseudo-second order | 263.34 μmol/g | |||
| DCFWW | Langmuir | Pseudo-second order | 97.12 μmol/g | |||
| IBUWW | Langmuir | Pseudo-second order | 74.07 μmol/g | |||
| Spent coffee waste biochar (Pristine-activated) | NPΧLK | Freundlich | Pseudo-second order | 107.53 μmol/g | 7.5–10.3 | [38] |
| DCFLK | Freundlich | Pseudo-second order | 91.74 μmol/g | |||
| IBULK | Freundlich | Pseudo-second order | 86.21 μmol/g | |||
| NPΧWW | Freundlich | Pseudo-second order | 344.48 μmol/g | |||
| DCFWW | Freundlich | Pseudo-second order | 202.92 μmol/g | |||
| IBUWW | Freundlich | Pseudo-second order | 124.14 μmol/g | |||
| Sulfonated coffee waste | SMX | Langmuir and Temkin | Pseudo-second order | 256 mg/g | N.R | [100] |
| BPA | Temkin | Pseudo-second order | 271 mg/g | N.R | ||
| Sunflower seed hulls | MB | Langmuir | Pseudo-second order | 473.44 mg/g | N.R | [31] |
| AB-15 | Langmuir | Pseudo-second order | 430.37 mg/g | N.R | ||
| Tamarind seeds (H2SO4-modified) | Pb(II) | Langmuir | Pseudo-first order | 18.34 mg/g | 99.52 | [32] |
| Tamarind seeds (Ultrasound-modified) | Pb(II) | Langmuir | Pseudo-first order | 18.86 mg/g | 99.52 | [32] |
| Walnut shell | Mn(II) | Langmuir | Pseudo-second order | 28.6 mg/g | 96.5 | [89] |
| Zn | Langmuir | Pseudo-second order | 33.3 mg/g | N.R | ||
| Fe | Langmuir | Pseudo-second order | 62.6 mg/g | N.R | ||
| Cd | Langmuir | Pseudo-second order | 76.9 mg/g | N.R | ||
| Cu | Langmuir | Pseudo-second order | 38.8 mg/g | N.R | ||
| Ni | Langmuir | Pseudo-second order | 29.4 mg/g | N.R | ||
| Walnut shell (NaOH-modified) | Ni | Langmuir | Pseudo-second order | 38.9 mg/g | N.R | [89] |
| Waste coffee grounds (CO2-activated carbon) | MB | Langmuir | Pseudo-second order | 678 mg/g | N.R | [101] |
| MO | Langmuir | Pseudo-second order | 612 mg/g | N.R |
| Biowaste | Contaminant | Isothermal Model Adjusted | Kinetic Model Adjusted | qm/Q° | Removal Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| Aegle marmelos shell | IBU | Freundlich | Pseudo-second order | 5 mg/g | 90 | [53] |
| Aegle marmelos shell (Steam-activated) | IBU | Langmuir | Pseudo-second order | 12.65 mg/g | 95 | [53] |
| Banana peel | MO | Freundlich | Pseudo-first order | 17.2 mg/g | N.R | [10,67,68] |
| MB | Freundlich | Pseudo-first order | 15.9 mg/g | N.R | ||
| RB | Freundlich | Pseudo-first order | 13.2 mg/g | N.R | ||
| CR | Freundlich | Pseudo-first order | 11.2 mg/g | N.R | ||
| MV | Freundlich | Pseudo-first order | 7.9 mg/g | N.R | ||
| AB-10B | Freundlich | Pseudo-first order | 7.9 mg/g | N.R | ||
| Fluoride | N.R | N.R | 8.15 mg/g | 86.5 | ||
| Banana peel (MeOH/HCl-treated) | Cd(II) | Langmuir | Pseudo-first order | 35.52 mg/g | 95 | [60,61] |
| Cr(VI) | Langmuir and Dubinin-Radushkevic | Pseudo-first order | 131.56 mg/g | 98 | ||
| Beet Pulp (NaOH-treated) | Mn(II) | N.R | Pseudo-second order | N.R | 86.36 | [88] |
| Coconut buttons (H2SO4-treated) | Pb(II) | Freundlich | Pseudo-second order | 97.72 mg/g | 98.7 | [37] |
| Hg(II) | Freundlich | Pseudo-second order | 78.84 mg/g | 95.8 | ||
| Cu(II) | Freundlich | Pseudo-second order | 73.6 mg/g | 90.6 | ||
| Coconut coir pith | Co(II) | Langmuir | Pseudo-second order | 12.82 mg/g | 49.64–85.4 | [10,46,47,59] |
| Cr(III) | Langmuir | Pseudo-second order | 11.56 mg/g | 46.08–98.2 | ||
| Ni(II) | Langmuir | Pseudo-second order | 15.95 mg/g | 62.88–98 | ||
| RB | Langmuir and Freundlich | Pseudo-first order | 2.56 mg/g | 43.6–79.4 | ||
| AV | Langmuir and Freundlich | Pseudo-first order | 8.06 mg/g | 47.0–78.7 | ||
| CR | Langmuir | Pseudo-second order | 6.72 mg/g | 30.5–66.5 | ||
| Coconut coir | MB | Langmuir and Temkin | Pseudo-second order | 29.5 mg/g | 99.5 | [72] |
| Corn cob | Mn(II) | Langmuir | Pseudo-second order | 6.24 mg/g | 99.8 | [73,104] |
| Cu(II) | Langmuir | Pseudo-second order | 6.24 mg/g | 99.7 | ||
| Cu | N.R | N.R | 0.034 mmol/g | 22 | ||
| Cd | N.R | N.R | 0.096 mmol/g | 58 | ||
| Ni | N.R | N.R | 0.097 mmol/g | 60 | ||
| Pb | N.R | N.R | 0.019 mmol/g | 12 | ||
| Durian peel (HCl-treated) | AG25 | Langmuir | Pseudo-second order | 63.29 mg/g | N.R | [74] |
| Grapefruit peel | CV | Langmuir | Pseudo-second order | 254.16 mg/g | 96 | [75] |
| Jackfruit peel | MB | Type 2 Langmuir | Pseudo-second order | 285.71 mg/g | 58.2–89.8 | [76] |
| Kiwi peel (NaOH-treated) | Cd(II) | Langmuir | Pseudo-second order | 15.87 mg/g | 78 | [77,94] |
| Cr(III) | Langmuir | Pseudo-second order | 41.66 mg/g | 98 | ||
| Zn(II) | Langmuir | Pseudo-second order | 37.03 mg/g | 57 | ||
| Mango Peel | Cd(II) | Langmuir | Pseudo-second order | 68.92 mg/g | 90.56 | [10,78] |
| Pb(II) | Langmuir | Pseudo-second order | 99.05 mg/g | 92.5 | ||
| Mangosteen pericarps (ABR) | Iodide (I−) | Langmuir | Pseudo-second order | 79.4 mg/g | 100 | [79] |
| Olive waste (H3PO4-activated) | IBU | Langmuir | Pseudo-second order | 12.6 mg/g | 79 | [105] |
| NPX | Langmuir | Pseudo-second order | 39.5 mg/g | 95 | ||
| KTP | Langmuir | Pseudo-second order | 24.7 mg/g | 90 | ||
| DCF | Langmuir | Pseudo-second order | 56.2 mg/g | 96 | ||
| Orange peel | V-17 | Langmuir and Freundlich | N.R | 19.88 mg/g | 87 | [10,80,81,95] |
| CR | Langmuir and Freundlich | Pseudo-first order | 22.44 mg/g | 76.6 | ||
| PO | Langmuir and Freundlich | Pseudo-first order | 1.3 mg/g | 49 | ||
| RB | Langmuir and Freundlich | Pseudo-first order | 3.23 mg/g | 59.0–67.5 | ||
| DR23 | Langmuir | Pseudo-second order | 10.72 mg/g | 92 | ||
| DR80 | Langmuir | Pseudo-second order | 21.05 mg/g | 91 | ||
| Papaya Peel (H3PO4-activated) | Pb(II) | Langmuir | Pseudo-second order | 38.31 mg/g | 93.22 | [39] |
| Passion fruit rinds (ABR) | Iodide (I−) | Langmuir | Pseudo-second order | 6.67 mg/g | 87.5 | [79] |
| Peach stones (K2CO3-activated) | ACE | Langmuir | Pseudo-second order | 204 mg/g | 82 | [54] |
| Pineapple waste (ZnCl2-activated) | MB | Langmuir | N.R | 288.34 mg/g | 67–76 | [48] |
| Pomegranate peel | Cu(II) | Langmuir, Freundlich, Dubinin Radushkevich and Temkin | Pseudo-second order | 30.12 mg/g | 78.85 | [103] |
| Pomelo peel | MB | Langmuir and Temkin | Pseudo-second order | 133 mg/g | 83 | [1,83,96] |
| RB-114 | Langmuir | Pseudo-second order | 16.3 mg/g | 89 | ||
| Red dragon fruit peels (ABR) | Iodide (I−) | Freundlich | Pseudo-second order | 68.6 mg/g | 68.4 | [79] |
| Red onion peels (ABR) | Iodide (I−) | Langmuir | Pseudo-second order | 75.8 mg/g | 92 | [79] |
| Rice husk and fruit juice residue | Phosphate | Freundlich | Pseudo-first order | 13.89 mg/g | 95.85 | [49] |
| Tangerine peel (NaOH-treated) | Cd(II) | Langmuir | Pseudo-second order | 17.54 mg/g | 73 | [77,94] |
| Cr(III) | Langmuir | Pseudo-second order | 47.61 mg/g | 96 | ||
| Zn(II) | Langmuir | Pseudo-second order | 38.41 mg/g | 52 | ||
| Watermelon rinds | Cu(II) | Langmuir | Pseudo-second order | 5.7–111.1 mg/g | 58.4–88 | [3] |
| Ni(II) | N.R | N.R | 18.4–38.9 mg/g | 69–70 | ||
| Zn(II) | N.R | N.R | 22.5 mg/g | 52.4–90.3 | ||
| Pb(II) | Langmuir, Thomas and Yoon–Nelson | Pseudo-second order | 19.33–116.2 mg/g | 72–99.9 | ||
| Cd(II) | Langmuir | Pseudo-second order | 40.16–63.29 mg/g | 80 | ||
| Cr(III) | Langmuir | Pseudo-second order | 172.6 mg/g | 91 | ||
| Tl(I) | Langmuir and Temkin | Pseudo-second order | 178.4–1123 mg/g | 98–98.5 | ||
| As(III) | Langmuir | Pseudo-second order | NR | 99 | ||
| As(V) | Langmuir | Pseudo-second order | NR | 98 | ||
| Fe(II) | N.R | N.R | 4.98 mg/g | 98.3 | ||
| Mn(II) | N.R | N.R | 1.37 mg/g | 98.9 | ||
| Co(II) | N.R | N.R | 23.3 mg/g | 57 | ||
| MB | Langmuir | Pseudo-second order | 188.6–489.8 mg/g | 83–99 | ||
| BG | Langmuir | Pseudo-second order | 188.6 mg/g | 98 | ||
| RBBR | Freundlich | Pseudo-second order | 333.33 mg/g | 92–97 | ||
| CR | Langmuir | Pseudo-second order | 17 mg/g | 101.45 | ||
| BR2 | Extended Langmuir | Pseudo-first order | 125.79 mg/g | 75 | ||
| OG | Extended Langmuir | Pseudo-first order | 27.24 mg/g | 85 |
| Biowaste | Contaminant | Isothermal Model Adjusted | Kinetic Model Adjusted | qm/Qe | Removal Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| Barley straw | BBY | Langmuir | Pseudo-second order | 124.3 mg/g | N.R | [69] |
| CV | Langmuir | Pseudo-second order | 95.8 mg/g | N.R | ||
| MB | Langmuir | Pseudo-second order | 86.5 mg/g | N.R | ||
| SO | Langmuir | Pseudo-second order | 99.7 mg/g | N.R | ||
| Barley straw (CA/NaOH-modified) | BBY | Langmuir | Pseudo-second order | 524.3 mg/g | N.R | [69] |
| CV | Langmuir | Pseudo-second order | 473.2 mg/g | N.R | ||
| MB | Langmuir | Pseudo-second order | 498.1 mg/g | N.R | ||
| SO | Langmuir | Pseudo-second order | 296.6 mg/g | N.R | ||
| Barley straw (Magnetic) | BBY | Langmuir | Pseudo-second order | 137.6 mg/g | N.R | [69] |
| CV | Langmuir | Pseudo-second order | 96.1 mg/g | N.R | ||
| MB | Langmuir | Pseudo-second order | 94.1 mg/g | N.R | ||
| SO | Langmuir | Pseudo-second order | 102 mg/g | N.R | ||
| Barley straw (Magnetic CA/NaOH-modified) | BBY | Langmuir | Pseudo-second order | 520.3 mg/g | N.R | [69] |
| CV | Langmuir | Pseudo-second order | 410.8 mg/g | N.R | ||
| MB | Langmuir | Pseudo-second order | 455.8 mg/g | N.R | ||
| SO | Langmuir | Pseudo-second order | 460.7 mg/g | N.R | ||
| Black tea | Cu | Langmuir | Pseudo-second order | 48 mg/g | 77 | [90] |
| Pb | Freundlich | Pseudo-second order | 65 mg/g | 94 | ||
| Cauliflower leaf powder | MB | Freundlich | Pseudo-second order | 149.22 mg/g | 88.1 | [70] |
| Corn stalks | Cu | N.R | N.R | 0.059 mmol/g | 35 | [73] |
| Cd | N.R | N.R | 0.046 mmol/g | 30 | ||
| Ni | N.R | N.R | 0.009 mmol/g | 8 | ||
| Pb | N.R | N.R | 0.029 mmol/g | 20 | ||
| Date palm wastes (Surface fibres) | Phosphates | N.R | N.R | N.R | 85 | [107] |
| Date palm wastes (Date stones) | Phosphates | N.R | N.R | N.R | 87 | [107] |
| Date palm tree leaves (H2SO4-activated) | Pb | Langmuir | Pseudo-second order | 88.61 mg/g | 98.6 | [41] |
| Mixed tea waste | Cr(VI) | Freundlich | Pseudo-second order | 94.34 mg/g | ~97 | [93] |
| Potato Stem powder | MB | Langmuir and Freundlich | Pseudo-second order | 41.6 mg/g | 82 | [84] |
| MG | Langmuir and Freundlich | Pseudo-second order | 27.0 mg/g | 67 | ||
| Potato Leaves powder | MB | Langmuir and Freundlich | Pseudo-second order | 52.6 mg/g | 87 | [84] |
| MG | Langmuir and Freundlich | Pseudo-second order | 33.3 mg/g | 75 | ||
| Raw wheat straw | Cu | N.R | N.R | 0.070 mmol/g | 42 | [73] |
| Cd | N.R | N.R | 0.089 mmol/g | 55 | ||
| Ni | N.R | N.R | 0.051 mmol/g | 30 | ||
| Pb | N.R | N.R | 0.015 mmol/g | 10 | ||
| Rice straw | Pb(II) | Langmuir | N.R | 42.55 mg/g | 94 | [16,85] |
| CA | Freundlich | Pseudo-second order | 126.3 mg/g | 42.5 | ||
| CBZ | Freundlich | Pseudo-second order | 40.0 mg/g | 75.3 | ||
| Soybean straws | Cu | N.R | N.R | 0.085 mmol/g | 60 | [73] |
| Cd | N.R | N.R | 0.018 mmol/g | 10 | ||
| Ni | N.R | N.R | 0.007 mmol/g | 5 | ||
| Pb | N.R | N.R | 0.033 mmol/g | 25 | ||
| Sugarcane bagasse | Mn(II) | N.R | Pseudo-second order | N.R | 62.5 | [87,88] |
| Mn(II) | Langmuir | Pseudo-second order | 0.676 mg/g | 63 | ||
| Sugarcane bagasse (HCl-treated) | Mn(II) | Freundlich | Pseudo-second order | 1.897 mg/g | 99 | [87] |
| Sugarcane bagasse (NaOH-treated) | Fe(III) | Langmuir | Pseudo-second order | 331.1 μmol/g | >95.0 | [86] |
| Co(II) | N.R | N.R | 15.5 μmol/g | N.R | ||
| Cu(II) | N.R | N.R | 86 μmol/g | N.R | ||
| Cd(II) | N.R | N.R | 70 μmol/g | N.R | ||
| Pb(II) | N.R | N.R | 87 μmol/g | N.R | ||
| Zn(II) | N.R | N.R | 81 μmol/g | N.R | ||
| Sulfonated tea waste | Cr(VI) | Langmuir | Pseudo-second order | 438.18 mg/g | 96 | [91] |
| MB | Langmuir | Pseudo-second order | 1007.61 mg/g | >99 | ||
| Tet | Langmuir | Pseudo-second order | 380.97 mg/g | 97 | ||
| Sunflower | Mn(II) | Langmuir | Pseudo-second order | 47.6 mg/g | 81.6 | [89] |
| Cd | Langmuir | Pseudo-second order | 83.3 mg/g | N.R | ||
| Cu | Langmuir | Pseudo-second order | 30.3 mg/g | N.R | ||
| Zn | Langmuir | Pseudo-second order | 45.4 mg/g | N.R | ||
| Fe | Langmuir | Pseudo-second order | 71.4 mg/g | N.R | ||
| Ni | Langmuir | Pseudo-second order | 27 mg/g | N.R | ||
| Sunflower (NaOH-modified) | Ni | Langmuir | Pseudo-second order | 41.7 mg/g | N.R | [89] |
| Tea waste | Mn(II) | Langmuir | Pseudo-second order | 0.157 mg/g | 95.5 | [92] |
| Zn(II) | Langmuir | Pseudo-second order | 0.278 mg/g | 99.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Rosero, J.; Urbina-López, M.E.; Rodríguez-González, B.E.; León-Villegas, S.X.; Luna-Cruz, I.E.; Cárdenas-Chávez, D.L. Development and Characterization of Bioadsorbents Derived from Different Agricultural Wastes for Water Reclamation: A Review. Appl. Sci. 2022, 12, 2740. https://doi.org/10.3390/app12052740
Aguilar-Rosero J, Urbina-López ME, Rodríguez-González BE, León-Villegas SX, Luna-Cruz IE, Cárdenas-Chávez DL. Development and Characterization of Bioadsorbents Derived from Different Agricultural Wastes for Water Reclamation: A Review. Applied Sciences. 2022; 12(5):2740. https://doi.org/10.3390/app12052740
Chicago/Turabian StyleAguilar-Rosero, Julián, María E. Urbina-López, Blanca E. Rodríguez-González, Sol X. León-Villegas, Itza E. Luna-Cruz, and Diana L. Cárdenas-Chávez. 2022. "Development and Characterization of Bioadsorbents Derived from Different Agricultural Wastes for Water Reclamation: A Review" Applied Sciences 12, no. 5: 2740. https://doi.org/10.3390/app12052740
APA StyleAguilar-Rosero, J., Urbina-López, M. E., Rodríguez-González, B. E., León-Villegas, S. X., Luna-Cruz, I. E., & Cárdenas-Chávez, D. L. (2022). Development and Characterization of Bioadsorbents Derived from Different Agricultural Wastes for Water Reclamation: A Review. Applied Sciences, 12(5), 2740. https://doi.org/10.3390/app12052740









