Potential for Coastal and Offshore Aquaculture in Portugal: Insights from Physico-Chemical and Oceanographic Conditions
Abstract
:1. Introduction
1.1. Aquaculture
1.2. Fish Aquaculture
1.3. Bivalve Aquaculture
1.4. Macroalgae Aquaculture
1.5. Objectives
2. Materials and Methods
2.1. Field Surveys
2.2. Biological and Physico-Chemical Analysis
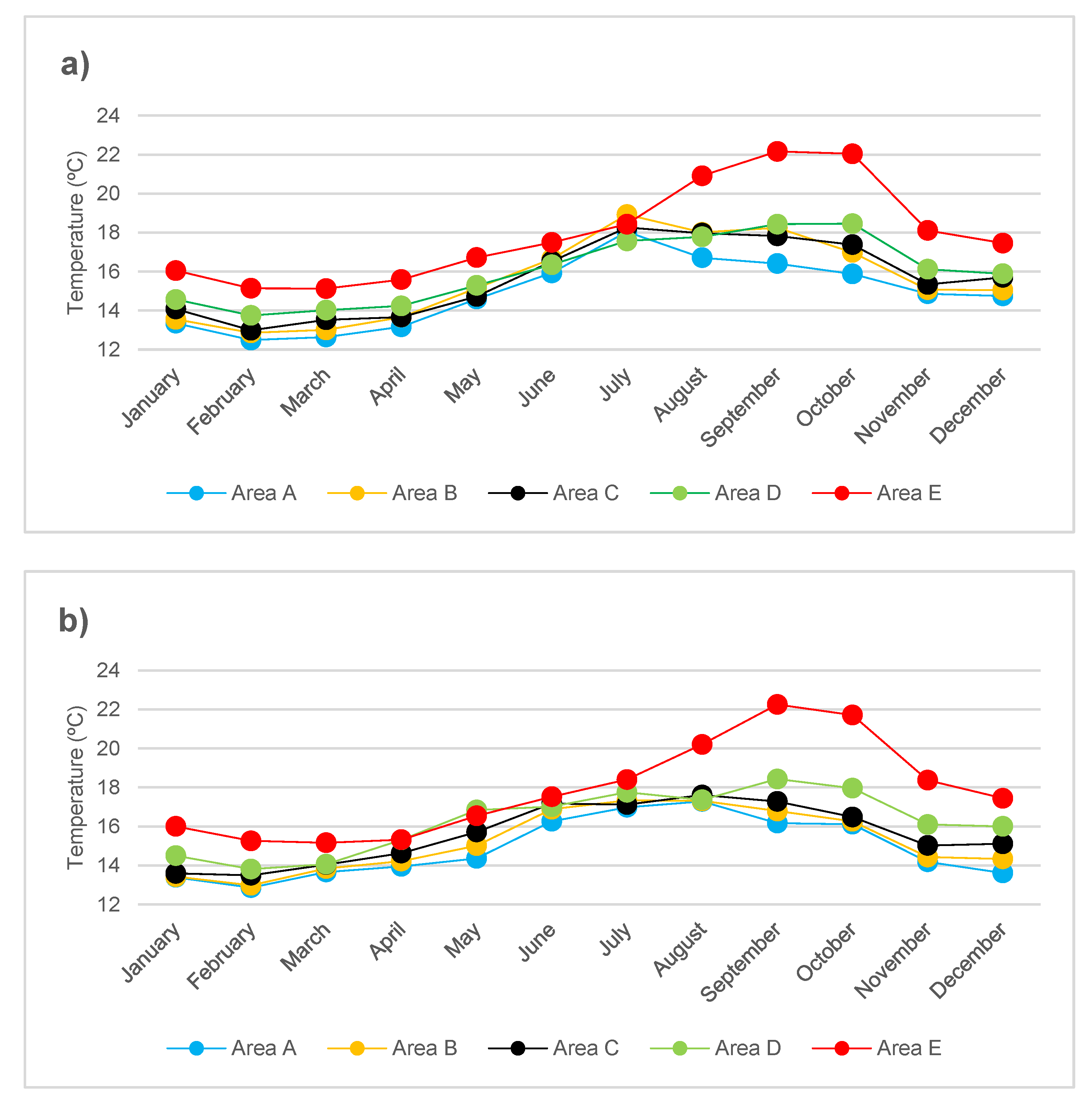
2.3. Mean Monthly Temperature
2.4. Waves
2.5. Currents
2.6. Species Selection
2.7. Statistical Analysis and Data Processing
3. Results
3.1. Biological and Physico-Chemical Parameters
3.2. Limit and Optimal Parameters for Production
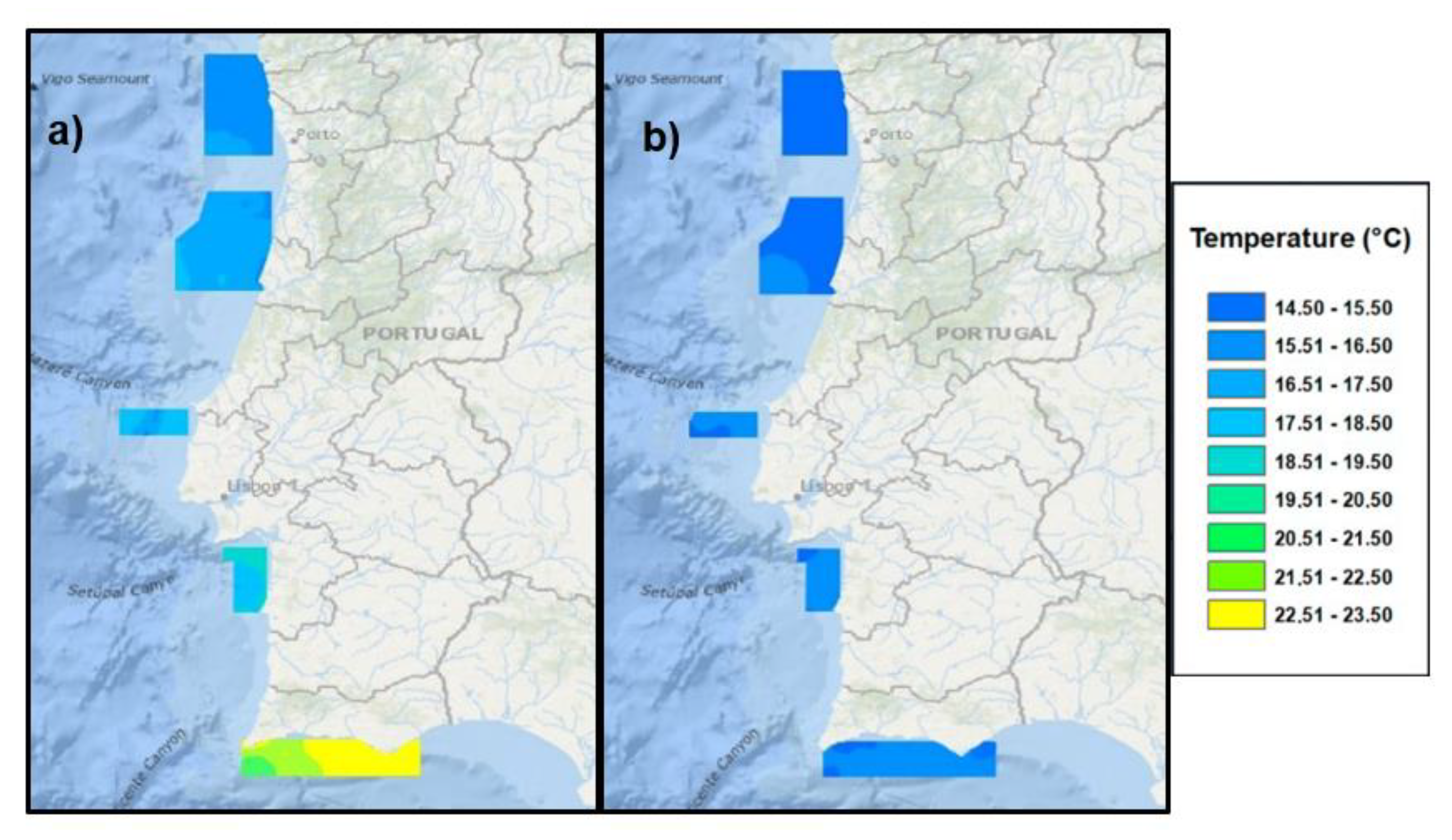
3.3. Sea State
3.4. Sea Currents
4. Discussion
4.1. Fish
4.1.1. Meagre Argyrosomus regius
4.1.2. Atlantic Cod Gadus morhua
4.1.3. Atlantic Salmon Salmo salar
4.1.4. Gilthead Sea Bream Sparus aurata
4.1.5. Sea Bass Dicentrarchus labrax
4.1.6. Sardine Sardinia pilchardus
4.1.7. Greater Amberjack Seriola dumerili
4.2. Bivalves
4.2.1. Mussel Mytilus edulis
4.2.2. Scallop Pecten maximus
4.2.3. Oyster Magallana gigas
4.2.4. Razor Clam Ruditapes decussatus
4.3. Macroalgae
4.3.1. Gracilaria gracilis
4.3.2. Porphyra umbilicalis
4.3.3. Undaria pinnatifida
4.4. Waves, Currents, and Equipment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020; pp. 21–36. [Google Scholar]
- Instituto Nacional de Estatística. Estatística da Pesca: 2020; INE: Lisbon, Portugal, 2021; Available online: https://www.ine.pt/xurl/pub/280980980 (accessed on 12 December 2021).
- Santos, P.M.; Albano, A.; Raposo, A.; Ferreira, S.M.F.; Costa, J.L.; Pombo, A. The effect of temperature on somatic and gonadal development of the sea urchin Paracentrotus lividus (Lamarck, 1816). Aquaculture 2020, 528, 735487. [Google Scholar] [CrossRef]
- Lourenço, S.; Cunha, B.; Raposo, A.; Neves, M.; Santos, P.M.; Gomes, A.S.; Tecelão, C.; Ferreira, S.M.F.; Baptista, T.; Gonçalves, S.C.; et al. Somatic growth and gonadal development of juvenile Paracentrotus lividus (Lamarck, 1816) fed with diets of different ingredient sources. Aquaculture 2021, 539, 736589. [Google Scholar] [CrossRef]
- Venâncio, E.; Félix, P.; Brito, A.C.; Sousa, J.; Silva, F.A.; Simões, T.; Narciso, L.; Amorim, A.; Dâmaso, L.; Pombo, A. Do broodstock diets influence viability and larval development of Holothuria mammata? Aquaculture 2021, 536, 736431. [Google Scholar] [CrossRef]
- Jansen, P.A.; Grøntvedt, R.; Tarpai, A.; Helgesen, K.O.; Horsberg, E. Surveillance of the Sensitivity towards Antiparasitic Bath-Treatments in the Salmon Louse (Lepeophteirus salmonis). PLoS ONE 2016, 11, e0149006. [Google Scholar] [CrossRef] [PubMed]
- Gentry, R.R.; Froehlich, H.E.; Grimm, D.; Kareiva, P.; Parke, M.; Rust, M.; Halpern, B.S. Mapping the global potential for marine aquaculture. Nat. Ecol. Evol. 2017, 1, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.R.; Clavelle, T.; Klinger, D.H.; Lester, S.E. The ecological and economic potential for offshore mariculture in the Caribbean. Nat. Sustain. 2019, 68, 219–225. [Google Scholar] [CrossRef]
- Primavera, H. Overcoming the impacts of aquaculture on the coastal zone. Ocean Coast. Manag. 2006, 49, 531–545. [Google Scholar] [CrossRef]
- McKindsey, C.W.; Thetmeyer, H.; Landry, T.; Silvert, W. Review of recent carrying capacity models for bivalve culture and recommendations for research and management. Aquaculture 2006, 261, 451–462. [Google Scholar] [CrossRef] [Green Version]
- Boyd, C.E.; Pillai, V. Water quality management in aquaculture. CMFRI Spec. Publ. 1985, 22, 44. [Google Scholar]
- Instituto Nacional de Estatística. Estatística da Pesca: 2019; INE: Lisbon, Portugal, 2020; Available online: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_publicacoes&PUBLICACOESpub_boui=435690295&PUBLICACOESmodo=2 (accessed on 27 December 2021).
- Ryan, J. Farming the Deep Blue; Irish Sea Fisheries Board (BIM) and Irish Marine Institute: Dublin, Ireland, 2004. [Google Scholar]
- Debnath, D.; Pal, A.K.; Sahu, N.P.; Baruah, K.; Yengkokpam, S.; Das, T.; Manush, S.M. Thermal tolerance and metabolic activity of yellowtail catfish Pangasius pangasius (Hamilton) advanced fingerlings with emphasis on their culture potential. Aquaculture 2006, 258, 606–610. [Google Scholar] [CrossRef]
- Sakamoto, K.; Miyazaki, A.; Taniguchi, N. Thermal tolerance traits of redfin velvetfish Paracentropogon rubripinnis evaluated using their caudal fin cells. Aquaculture 2010, 308, 124–127. [Google Scholar] [CrossRef]
- Person-Le Ruyet, J.; Buchet, V.; Vincent, B.; Le Delliou, H.; Quéméner, L. Effects of temperature on the growth of pollack (Pollachius pollachius) juveniles. Aquaculture 2006, 251, 340–345. [Google Scholar] [CrossRef] [Green Version]
- Deane, E.E.; Woo, N. Modulation of fish growth hormone levels by salinity, temperature, pollutants and aquaculture related stress: A review. Rev. Fish Biol. Fish. 2009, 19, 97–120. [Google Scholar] [CrossRef]
- Oliveira, J.; Castilho, F.; Cunha, A.; Pereira, M.J. Bivalve Harvesting and Production in Portugal: An Overview. J. Shellfish Res. 2013, 32, 911–924. [Google Scholar]
- Venugopal, V.; Gopakumar, K. Shellfish: Nutritive Value, Health Benefits, and Consumer Safety. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1219–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherifi, H.; Chebil, L.; Sadok, S. Nutritional value of the Tunisian mussel Mytilus galloprovincialis with a special emphasis on lipid quality. Food Chem. 2018, 268, 307–314. [Google Scholar] [CrossRef]
- Shumway, S.E.; Davis, C.; Downey, R.; Karney, R.; Kraeuter, J.; Parsons, J.; Rheault, R.; Wikfors, G. Shellfish aquaculture—in praise of sustainable economies and environments. World Aquac. 2003, 34, 15–17. [Google Scholar]
- Klinger, D.; Naylor, R. Searching for solutions in aquaculture: Charting a sustainable course. Annu. Rev. Environ. Resour. 2012, 37, 247–276. [Google Scholar] [CrossRef] [Green Version]
- Bostock, J.; Lane, A.; Hough, C.; Yamamoto, K. An assessment of the economic contribution of EU aquaculture production and the influence of policies for its sustainable development. Aquac. Int. 2016, 24, 699–733. [Google Scholar] [CrossRef] [Green Version]
- Mare, D. Study on Deepening Understanding of Potential Blue Growth in the EU Member States on Europe’s Atlantic Arc; DG Maritime Affairs and Fisheries: Rotterdam, The Netherlands; Brussels, Belgium, 2014. [Google Scholar]
- Makkar, H.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for livestock diets: A review. Anim. Feed Sci. Technol. 2014, 212, 1–17. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, S. Algal proteins: Extraction, application, and challenges concerning production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurd, C.L. Shaken and stirred: The fundamental role of water motion in resource acquisition and seaweed productivity. Perspect. Phycol. 2017, 4, 73–81. [Google Scholar] [CrossRef]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis; Fisheries Research Board of Canada: Ottawa, ON, Canada, 1972. [Google Scholar]
- Murphy, J.; Riley, J.P. A Modified Single Solution Method for the Determination of Phosphate in Natural Waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Koroleff, F. Determination of ammonia. In Methods of Seawater Analysis; Grasshoff, K., Ed.; Verlag Chemie: New York, NY, USA, 1976; pp. 126–158. [Google Scholar]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanzen. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Duncan, J.; Estévez, A.; Fernández-Palacios, H.; Gairin, I.; Hernández-Cruz, M.; Roo, J.; Schuchardt, D.; Vallés, R. Aquaculture production of meagre (Argyrosomus regius): Hatchery techniques, ongrowing and market. In Advances in Aquaculture Hatchery Technology; Allan, G., Burnell, G., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 519–541. [Google Scholar]
- Badiola, M.; Albaum, B.; Curtin, R.; Gartzia, I.; Mendiola, D. Land based on-growing of Atlantic cod (Gadus morhua) using Recirculating Aquaculture Systems: A case study from the Basque region (Northern Spain). Aquaculture 2017, 468, 428–441. [Google Scholar] [CrossRef]
- Sikveland, M.; Zhang, D. Determinants of capital structure in the Norwegian salmon aquaculture industry. Mar. Policy 2020, 119, 104061. [Google Scholar] [CrossRef]
- Føre, M.; Frank, K.; Norton, T.; Svendsen, E.; Alfredsen, J.; Dempster, T.; Berckmans, D. Precision fish farming: A new framework to improve production in aquaculture. Biosyst. Eng. 2018, 173, 176–193. [Google Scholar] [CrossRef]
- Islam, M.J.; Kunzmann, A.; Bögner, M.; Meyer, A.; Thiele, R.; James, M. Metabolic and molecular stress responses of European seabass, Dicentrarchus labrax at low and high temperature extremes. Ecol. Indic. 2020, 112, 106118. [Google Scholar] [CrossRef]
- Garrido, S.; Cristóvão, A.; Caldeira, C.; Ben-Hamadou, R.; Baylina, N.; Batista, H.; Saiz, E.; Peck, M.A.; Ré, P.; Santos, A.M.P. Effect of temperature on the growth, survival, development and foraging behaviour of Sardina pilchardus larvae. Mar. Ecol. Progr. Ser. 2016, 559, 131–145. [Google Scholar] [CrossRef] [Green Version]
- Sicuro, B.; Umberto Luzzana, U. The State of Seriola spp. Other Than Yellowtail (S. quinqueradiata) Farming in the World. Rev. Fish. Sci. Aquac. 2016, 24, 314–325. [Google Scholar] [CrossRef]
- Azpeitia, K.; Rios, Y.; Garcia, I.; Pagaldai, J.; Mendiola, D. A sensory and nutritional validation of open ocean mussels (Mytilus galloprovincialis Lmk.) cultured in SE Bay of Biscay (Basque Country) compared to their commercial counterparts from Galician Rias (Spain). Int. Aquat. Res. 2017, 9, 89–106. [Google Scholar] [CrossRef]
- Ansell, A.D.; Dao, J.; Mason, J. Three European scallops: Pecten maximus, Chlamys opercularis and C. (Chlamys) varia. In Scallops: Biology, Ecology and Aquaculture; Shumway, S.E., Parsons, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; pp. 715–751. [Google Scholar]
- Wang, Y.K.; He, H.L.; Wang, G.F.; Wu, H.; Zhou, B.C.; Chen, X.L.; Zhang, Y.Z. Oyster (Magallana gigas) hydrolysates produced on a plant scale have antitumor activity and immunostimulating effects in BALB/c mice. Mar. Drugs 2010, 8, 255–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojea, J.; Pazos, A.P.; Martínez, D.; Novoa, S.; García-Martínez, P.; Sánchez, J.L.; Abad, M. Effects of temperature regime on broodstock conditioning of Ruditapes decussatus. J. Shellfish Res. 2008, 27, 1093–1100. [Google Scholar] [CrossRef]
- Tseng, C.K. Algal biotechnology industries and research activities in China. J. Appl. Phycol. 2001, 13, 375–380. [Google Scholar] [CrossRef]
- Carmona, R.; Kraemer, P.; Yarish, C. Exploring Northeast American and Asian species of Porphyra for use in an integrated finfish–algal aquaculture system. Aquaculture 2006, 252, 54–65. [Google Scholar] [CrossRef]
- Gao, X.; Endo, H.; Nagaki, M.; Agatsuma, Y. Interactive effects of nutrient availability and temperature on growth and survival of different size classes of Saccharina japonica (Laminariales, Phaeophyceae). Phycologia 2017, 56, 253–260. [Google Scholar] [CrossRef]
- Chatzifotis, S.; Panagiotidou, M.; Papaioannou, N.; Pavlidis, M.; Nengas, I.; Mylonas, C.C. Effect of dietary lipid levels on growth, feed utilization, body composition and 13 serum metabolites of meagre (Argyrosomus regius) juveniles. Aquaculture 2010, 307, 65–70. [Google Scholar] [CrossRef]
- Antonopoulou, E.; Chatzigiannidou, I.; Feidantsis, K.; Kounna, C.; Chatzifotis, S. Effect of water temperature on cellular stress responses in meagre (Argyrosomus regius). Fish Physiol. Biochem. 2020, 46, 1075–1091. [Google Scholar] [CrossRef]
- Kir, M.; Topuz, M.; Sunar, M.C.; Topuz, H. Acute toxicity of ammonia in meagre (Argyrosomus regius Asso, 1801) at different temperatures. Aquac. Res. 2015, 47, 3593–3598. [Google Scholar] [CrossRef]
- Kir, M.; Topuz, H.; Sunar, M.C.; Topuz, M. Effect of temperature on acute toxicity of nitrite to meagre, (Argyrosomus regius Asso, 1801). J. World Aquac. Soc. 2015, 46, 564–568. [Google Scholar] [CrossRef]
- Jordaan, A.; Kling, L. Determining the Optimal Temperature Range for Atlantic Cod (Gadus morhua) during Early Life; The institute of Marine Research: Bergen, Norway, 2003; pp. 46–62. [Google Scholar]
- Kristjánson, T. Comparison of growth in Atlantic cod (Gadus morhua) originating from the northern and southern coast of Iceland reared under common conditions. Fish. Res. 2013, 139, 105–109. [Google Scholar] [CrossRef]
- Petersen, M.; Steffensen, J. Preferred temperature of juvenile Atlantic cod (Gadus morhua) with different haemoglobin genotypes at normoxia and moderate hypoxia. J. Exp. Biol. 2002, 206, 359–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Björnsson, B.; Steinarsson, A.; Árnason, T. Growth model for Atlantic cod (Gadus morhua): Effects of temperature and body weight on growth rate. Aquaculure 2007, 271, 216–226. [Google Scholar] [CrossRef]
- Thorarensen, H.; Imsland, A.K.D.; Gústavsson, A.; Gunnarsson, S.; Árnasond, J.; Steinarsson, A.; Björnsdóttir, R. Potential interactive effects of ammonia and CO2 on growth performance and feed utilization in juvenile Atlantic cod (Gadus morhua L.). Aquaculture 2018, 484, 272–276. [Google Scholar] [CrossRef]
- Siikavuopio, I.; Sæther, S. Effects of chronic nitrite exposure on growth in juvenile Atlantic cod, Gadus morhua. Aquaculture 2006, 255, 351–356. [Google Scholar] [CrossRef]
- Grande, M.; Andersen, S. Critical thermal maxima for young salmonids. J. Freshw. Ecol. 1991, 5, 275–279. [Google Scholar] [CrossRef]
- Finstad, A.G.; Naesje, T.F.; Forseth, T. Seasonal variation in the thermal performance of juvenile Atlantic salmon (Salmo salar). Freshw. Biol. 2004, 49, 1459–1467. [Google Scholar] [CrossRef]
- Oppedal, F.; Juell, J.; Tarranger, G.; Hansen, T. Artificial light and season affects vertical distribution and swimming behaviour of post-smolt Atlantic salmon in sea cages. J. Fish Biol. 2001, 58, 1570–1584. [Google Scholar] [CrossRef]
- Johansson, D.; Ruohonen, K.; Kiessling, A.; Oppedal, F.; Stiansen, J.; Kelly, M.; Juell, J. Effect of environmental factors on swimming depth preferences of Atlantic salmon (Salmo salar L.) and temporal and spatial variations in oxygen levels in sea cages at a fjord site. Aquaculture 2006, 254, 594–605. [Google Scholar] [CrossRef]
- Johansson, D.; Ruohonen, K.; Juell, J.; Oppedal, F. Swimming depth and thermal history of individual Atlantic salmon (Salmo salar L.) in production cages under different ambient temperature conditions. Aquaculture 2009, 290, 296–303. [Google Scholar] [CrossRef]
- Friedland, K.D.; Hansen, L.P.; Dunkley, D.A.; MacLean, J.C. Linkage between ocean climate, post-smolt growth and survival of Atlantic salmon (Salmo salar L.) in the North Sea area. ICES J. Mar. Sci. 2000, 57, 419–429. [Google Scholar] [CrossRef] [Green Version]
- Jonsson, B.; Jonsson, N. Factors affecting marine production of Atlantic salmon (Salmo salar). J. Fish. Aquacul. Sci. 2004, 61, 2369–2383. [Google Scholar] [CrossRef]
- Olsvik, P.; Vikeså, V.; Lie, K.; Hevrøy, E. Transcriptional responses to temperature and low oxygen stress in Atlantic salmon studied with next-generation sequencing technology. BMC Genom. 2013, 14, 817. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, S. Cardiac responses to elevated seawater temperature in Atlantic salmon. BMC Physiol. 2014, 14, 2. [Google Scholar] [CrossRef] [Green Version]
- Hevrøy, E.; Tipsmark, C.; Remø, S.; Hansen, T.; Fukuda, M.; Torgersen, T.; Vikeså, V.; Olsvik, P.; Waagbø, R.; Shimizu, M. Role of the GH-IGF-1 system in Atlantic salmon and 37 rainbow trout postsmolts at elevated water temperature. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 188, 127–138. [Google Scholar] [CrossRef]
- Elliott, J.M.; Elliott, J.A. Temperature requirements of atlantic salmon Salmo salar, brown trout Salmo trutta and arctic charr Salvelinus alpinus: Predicting the effects of climate change. J. Fish Biol. 2010, 77, 1793–1817. [Google Scholar] [CrossRef]
- Knoph, M.B. Acute toxicity of ammonia to Atlantic salmon (Salmo salar) parr. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1992, 101, 275–282. [Google Scholar] [CrossRef]
- Kolarevic, J.; Selset, R.; Felip, O.; Good, C.; Snekvik, K.; Takle, H.; Terjesen, B.F. Influence of long term ammonia exposure on Atlantic salmon (Salmo salar L.) parr growth and welfare. Aquac. Res. 2012, 44, 1649–1664. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Kolarevic, J.; Takle, H.; Baeverfjord, G.; Ytteborg, E.; Fyhn, B. Effects of chronic sub-lethal nitrite exposure at high water chloride concentration on Atlantic salmon (Salmo salar, Linnaeus 1758) parr. Aquac. Res. 2019, 50, 2687–2697. [Google Scholar] [CrossRef]
- Muller-Feuga, A. Modilisation dela Croissance des Poisons en Levage; Rapports Scientifiques et Techniques de l’Ifremer; Institut Français de Recherche pour l’Exploitation de la Mer, Archimer: Plouzané, France, 1990; Volume 21, pp. 26–28. [Google Scholar]
- Bovo, G.; Borghesan, F.; Comuzzi, M.; Ceschias, G.; Giorgetti, G. ‘‘Winter disease’’ in orati di allevamento: Observazioni preliminary. Boll. Soc. Ital. 1995, 17, 2–11. [Google Scholar]
- Ibarz, A.; Padrós, F.; Gallardo, M.Á. Low-temperature challenges to gilthead sea bream culture: Review of cold-induced alterations and ‘Winter Syndrome’. Rev. Fish Biol. Fish. 2010, 20, 539–556. [Google Scholar] [CrossRef]
- Tort, L.; Rotllant, J.; Rovira, L. Immunological suppression in gilthead sea bream Sparus aurata of the North-West Mediterranean at low temperatures. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 120, 175–179. [Google Scholar] [CrossRef]
- Seginer, I. Growth models of gilthead sea bream (Sparus aurata L.) for aquaculture: A review. Aquac. Eng. 2016, 70, 15–32. [Google Scholar] [CrossRef]
- Guinot, D.; Ureña, R.; Pastor, A.; Varó, I.; Ramo, J.; Torreblanca, A. Long-term effect of temperature on bioaccumulation of dietary metals and metallothionein induction in Sparus aurata. Chemosphere 2012, 87, 1215–1221. [Google Scholar] [CrossRef] [Green Version]
- Ravagnan, G. Valliculture du Loup et de la Daurade. In L’Aquaculture du Bar et des Sparid; Barnabé, G., Billard, R., Eds.; INRA Publication: Paris, France, 1984; pp. 361–372. [Google Scholar]
- Barnabé, G. Rearing bass and gilthead bream. Aquaculture 1991, 2, 647–686. [Google Scholar]
- Shields, R.J. Larviculture of marine finfish in Europe. Aquaculture 2001, 200, 55–88. [Google Scholar] [CrossRef]
- Person-Le Ruyet, J.; Chartois, H.; Quemener, L. Comparative acute ammonia toxicity in marine fish and plasma ammonia response. Aquaculture 1995, 136, 181–194. [Google Scholar] [CrossRef]
- Kir, M.; Sunar, M.C. Acute Toxicity of Ammonia and Nitrite to Sea Bream, Sparus aurata (Linnaeus, 1758), in Relation to Salinity. J. World Aquac. Soc. 2017, 49, 516–522. [Google Scholar] [CrossRef]
- Claridge, P.N.; Potter, I.C. Movements, abundance, age composition and growth of bass, Dicentrarchus labrax, in the Severn Estuary and Inner Bristol Channel. J. Mar. Biol. Assoc. 1983, 63, 871–879. [Google Scholar] [CrossRef] [Green Version]
- Kousoulaki, K.; Sæther, B.S.; Albrektsen, S.; Noble, C. Review on European sea bass (Dicentrarchus labrax, Linnaeus, 1758) nutrition and feed management: A practical guide for optimizing feed formulation and farming protocols. Aquacul. Nutr. 2015, 21, 129–151. [Google Scholar] [CrossRef]
- Person-Le Ruyet, J.; Mahé, K.; Le Bayon, N.; Le Delliou, H. Effects of temperature on growth and metabolism in a Mediterranean population of European sea bass, Dicentrarchus labrax. Aquaculture 2004, 237, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Dulger, N.; Kumlu, M.; Türkmen, S.; Ölçülü, A.; Eroldoğan, O.; Yilmaz, H.; Öçal, N. Thermal tolerance of European Sea Bass (Dicentrarchus labrax) juveniles acclimated to three temperature levels. J. Therm. Biol. 2012, 37, 79–82. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, C.; Fan, K.; Liu, Y.; Liu, P. Metabolomics analysis of the effects of temperature on the growth and development of juvenile European seabass (Dicentrarchus labrax). Sci. Total Environ. 2021, 769, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Saroglia, M.G.; Scarano, G.; Tibaldi, E. Acute toxicity of nitrite to sea bass (Dicentrarchus labrax) and european eel (Anguilla anguilla). J. World Maric. Soc. 1981, 12, 121–126. [Google Scholar] [CrossRef]
- Ettahiri, O.; Berraho, A.; Houssa, R.; Ramzi, A.; Somoue, L.; Zizah, S.; Machu, E. Characteristics of the spawning habitats of sardine, Sardina pilchardus, off the Moroccan Atlantic coast (21° N–26° N). Pech. Aquac. 2012, 18, 157–186. [Google Scholar]
- Baloi, M.; Carvalho, C.V.A.; Sterzelecki, F.C.; Passini, G.; Cerqueira, V.R. Effects of feeding frequency on growth, feed efficiency and body composition of juveniles brazilian sardine, Sardinella brasiliensis (steindacher 1879). Aquac. Res. 2017, 47, 554–560. [Google Scholar] [CrossRef]
- Fernández-Montero, A.; Caballero, M.J.; Torrecillas, S.; Izquierdo, M.S.; Montero, D. Effect of temperature on growth performance and immunological parameters of greater amberjack Seriola dumerili juveniles. Fish Shellfish Immunol. 2016, 53, 105. [Google Scholar] [CrossRef] [Green Version]
- Abbink, W.; Blanco Garcia, A.; Roques, J.A.C.; Partridge, G.J.; Kloet, K.; Schneider, O. The effect of temperature and pH on the growth and physiological response of juvenile yellowtail kingfish Seriola lalandi in recirculating aquaculture systems. Aquaculture 2012, 330, 130–135. [Google Scholar] [CrossRef] [Green Version]
- Widdows, J. Combined effects of body size, food concentration and season on the physiology of Mytilus edulis. J. Mar. Biol. Assoc. 1978, 58, 109–124. [Google Scholar] [CrossRef]
- Bayne, B.L.; Bayne, C.J.; Carefoot, T.C.; Thompson, R.J. The physiological ecology of Mytilus californianus Conrad. Metabolism and energy balance. Oceon 1976, 22, 211–228. [Google Scholar]
- Almada-Villela, P.C.; Davenport, J.; Gruffydd, L.L.D. The effects of temperature on the shell growth of young Mytilus edulis L. J. Exp. Mar. Biol. Ecol. 1982, 59, 275–288. [Google Scholar] [CrossRef]
- Kittner, C.; Risgaard, H. Effect of temperature on filtration rate in the mussel Mytilus edulis: No evidence for temperature compensation. Mar. Ecol. Prog. Ser. 2005, 305, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Riisgard, H.U.; Lassen, J.; Kittner, C. Valve-gape response times in mussels (Mytilus edulis) Effects of laboratory preceding-feeding conditions and in situ tidally induced variation in phytoplankton biomass. J. Shellfish Res. 2006, 25, 901–911. [Google Scholar]
- Pascoe, P.L.; Parrym, H.E.; Hawkins, A.J.S. Observations on the measurement and interpretation of clearance rate variations in suspension-feeding bivalve shellfish. Aquat. Biol. 2009, 6, 181–190. [Google Scholar] [CrossRef]
- Brynjelsen, E.; Strand, O. Test production of king scallop in intermediate culture 1995–1996. Aquac. Int. 1996, 18, 34. [Google Scholar]
- Laing, I. Effect of temperature and ration on growth and condition of king scallop (Pecten maximus) spat. Aquaculture 2000, 183, 325–334. [Google Scholar] [CrossRef]
- Davenport, J.; Gruffydd, D.; Beaumont, A.R. An apparatus to supply water of fluctuating salinity and its use in a study of the salinity tolerance of larvae of the scallop Pecten maximus L. J. Mar. Biol. 1975, 55, 391–409. [Google Scholar] [CrossRef]
- Hawkins, L.E.; Hutchinson, S.; Laing, I. The effects of temperature and food ration on metabolite concentrations in newly settled king scallop (Pecten maximus) spat. Aquaculture 2005, 250, 841–848. [Google Scholar] [CrossRef]
- Bergvik, M.; Stensås, L.; Handå, A.; Reitan, K.I.; Strand, Ø.; Olsen, Y. Incorporation of Feed and Fecal Waste from Salmon Aquaculture in Great Scallops (Pecten maximus) Co-fed by Different Algal Concentrations. Adv. Mar. Sci. 2019, 5. [Google Scholar] [CrossRef] [Green Version]
- Fabioux, C.; Huvet, A.; Le Souchu, P.; Le Pennec, M.; Pouvreau, S. Temperature and photoperiod drive Magallana gigas reproductive internal clock. Aquaculture 2005, 250, 458–470. [Google Scholar] [CrossRef] [Green Version]
- Moreira, A.; Figueira, E.; Libralato, G.; Soares, A.M.V.M.; Guida, M.; Freitas, R. Comparative sensitivity of Magallana angulata and Magallana gigas embryo-larval development to as under varying salinity and temperature. Mar. Environ. Res. 2018, 140, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Portela, T. Distribuição e Recrutamento da Ostra-Portuguesa, Crassostrea angulata (Lamarck, 1819), No Estuário do Sado. Master’s Thesis, Universidade de Lisboa, Lisbon, Portugal, 2016. [Google Scholar]
- Qiu, T.; Liu, Y.; Zheng, J.; Zhang, T.; Qi, J. A feeding model of oyster larvae (Magallana angulata). Physiol. Behav. 2015, 147, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Albentosa, M.; Camacho, A.P.; Beiras, R. The effect of food concentration on the scope for growth and growth performance of Ruditapes decussatus (L.) seed reared in an open-flow system. Aquac. Nutr. 1996, 2, 213–220. [Google Scholar] [CrossRef]
- Matias, D.; Joaquim, S.; Leitão, A.; Massapina, C. Effect of geographic origin, temperature and timing of broodstock collection on conditioning, spawning success and larval viability of Ruditapes decussatus (Linné, 1758). Aqua. Int. 2009, 17, 257–271. [Google Scholar] [CrossRef]
- Sobral, P.; Fernandes, S. Physiological responses and scope for growth of Ruditapes decussatus from Ria Formosa, southern Portugal, exposed to increased ambient ammonia. Sci. Mar. 2004, 68, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Aranda-Burgos, J.A.; da Costa, F.; Nóvoa, S.; Ojea, J.; Martínez-Patiño, D. Effects of microalgal diet on growth, survival, biochemical and fatty acid composition of Ruditapes decussatus larvae. Aquaculture 2014, 420, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Rebello, J.; Ohno, M.; Critchley, A.T.; Sawamura, M. Growth Rates and Agar Quality of Gracilaria gracilis (Stackhouse) Steentoft from Namibia, Southern Africa. Bot. Mar. 1996, 39, 273–280. [Google Scholar] [CrossRef]
- Raikar, S.; Lima, M.; Fujita, Y. Effect of temperature, sannity and light intensity on the growth of Gracilaria spp. (Gracilariales, Rhodophyta) from Japan, Malaysia and India. Indian J. Sci. 2001, 30, 98–104. [Google Scholar]
- Engledow, H.R.; Bolton, J.J. Environmental tolerances in culture and agar content of Gracilaria verrucosa (Hudson) Papenfuss (Rhodophyta, Gigartinales) from Saldanha Bay. S. Afr. J. Bot. 1992, 58, 263–267. [Google Scholar] [CrossRef] [Green Version]
- Green, L.A.; Neefus, C.D. Effects of temperature, light level, and photoperiod on the physiology of Porphyra umbilicalis Kützing from the Northwest Atlantic, a candidate for aquaculture. J. Appl. Phycol. 2016, 28, 1815–1826. [Google Scholar] [CrossRef]
- Kraemer, G.; Yarish, C. A preliminary comparison of the mariculture potential of Porphyra purpurea and Porphyra umbilicalis. J. Appl. Phycol. 1999, 11, 473–477. [Google Scholar] [CrossRef]
- Kim, J.K.; Kraemer, G.P.; Neefus, C.D.; Chung, I.K.; Yarish, C. Effects of temperature and ammonium on growth, pigment production and nitrogen uptake by four species of Porphyra (Bangiales, Rhodophyta) native to the New England coast. J. Appl. Phycol. 2007, 19, 431–440. [Google Scholar] [CrossRef]
- Hafting, J.T. Effect of tissue nitrogen and phosphorus quota on growth of Porphyra yezoensis blades in suspension cultures. In Proceedings of the Sixteenth International Seaweed Symposium, Cebu City, Philippines, 12–17 April 1998; Springer: Dordrecht, The Netherlands, 1999; pp. 305–314. [Google Scholar]
- Endo, H.; Okumura, Y.; Sato, Y.; Agatsuma, Y. Interactive effects of nutrient availability, temperature, and irradiance on photosynthetic pigments and color of the brown alga Undaria pinnatifida. J. Appl. Phycol. 2016, 29, 1683–1693. [Google Scholar] [CrossRef]
- Saito, Y. Practical significance of algae in Japan: Undaria. In Advance of Phycology in Japan; Tokida, J., Hirose, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1975; pp. 304–320. [Google Scholar]
- Salas-Leiton, E.; Vieira, L.R.; Guilhermino, L. Sustainable Fishing and Aquaculture Activities in the Atlantic Coast of the Portuguese North Region: Multi-Stakeholder Views as a Tool for Maritime Spatial Planning. Sustainability 2021, 13, 663. [Google Scholar] [CrossRef]
- Mota, V.; Nilsen, T.; Gerwins, J.; Gallo, M.; Ytteborg, E.; Baeverfjord, G.; Terjesen, B.F. The effects of carbon dioxide on growth performance, welfare, and health of Atlantic salmon post-smolt (Salmo salar) in recirculating aquaculture systems. Aquaculture 2018, 498, 578–586. [Google Scholar] [CrossRef]
- Kounna, C.; Fountoulaki, E.; Miliou, H.; Chatzifotis, S. Water temperature effects on growth performance, proximate body and tissue composition, morphometric characteristics and gastrointestinal evacuation processes of juvenile meagre, Argyrosomus regius (Asso 1801). Aquaculture 2021, 540, 736683. [Google Scholar] [CrossRef]
- Sales, R.; Galafat, A.; Vizcaíno, A.; Sáez, M.; Martínez, T.; Cerón-García, M.; Alarcón, J. Effects of dietary use of two lipid extracts from the microalga Nannochloropsis gaditana (Lubián, 1982) alone and in combination on growth and muscle composition in juvenile gilthead seabream, Sparus aurata. Algal Res. 2021, 53, 102162. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Z.; Zhang, L.; Liu, Y.; Liu, P. Effects of temperature on growth performance and metabolism of juvenile sea bass (Dicentrarchus labrax). Aquaculture 2021, 537, 736458. [Google Scholar] [CrossRef]
- Bendiksen, E.Å.; Jobling, M.; Arnesen, A.M. Feed intake of Atlantic salmon parr Salmo salar L. In relation to temperature and feed composition. J. Aquac. Res. Dev. 2002, 33, 525–532. [Google Scholar] [CrossRef]
- Handeland, S.O.; Imsland, A.K.; Stefansson, S.O. The effect of temperature and fish size on growth, feed intake, food conversion efficiency and stomach evacuation rate of Atlantic salmon post-smolts. Aquaculture 2008, 283, 36–42. [Google Scholar] [CrossRef]
- Shapawi, R.; Abdullah, F.; Senoo, S.; Mustafa, S. Nutrition, growth and resilience of tiger grouper (Epinephelus fuscoguttatis) × giant grouper (E. lanceolatus) hybrid—A review. Rev. Aquac. 2018, 11, 1285–1296. [Google Scholar] [CrossRef]
- Quéméner, L. Le Maigre Commun (Argyrosomus regius): Biologie, Pêche, Marché et Potentiel Aquacole; IFREMER: Plouzané, France, 2002. [Google Scholar]
- Morales-Nin, B.; Geffen, A.J.; Pérez-Mayol, S.; Palmer, M.; González-Quirós, R.; Grau, A. Seasonal and ontogenic migrations of meagre (Argyrosomus regius) determined by otolith geochemical signatures. Fish. Res. 2012, 127–128, 154–165. [Google Scholar] [CrossRef]
- Ibarz, A.; Fernández-Borràs, J.; Blasco, J.; Gallardo, M.A.; Sánchez, J. Oxygen consumption and feeding rates of gilthead sea bream (Sparus aurata) reveal lack of acclimation to cold. Fish Physiol. Biochem. 2003, 29, 313–321. [Google Scholar] [CrossRef]
- Kır, M. Thermal tolerance and standard metabolic rate of juvenile gilthead seabream (Sparus aurata) acclimated to four temperatures. J. Therm. Biol. 2020, 93, 102739. [Google Scholar] [CrossRef]
- Bandarra, N.; Marçalo, A.; Cordeiro, A.; Pousão-Ferreira, P. Sardine (Sardina pilchardus) lipid composition: Does it change after one year in captivity? Food Chem. 2018, 244, 408–413. [Google Scholar] [CrossRef]
- Marçalo, A.; Pousão-Ferreira, P.; Mateus, L.; Duarte Correia, J.; Stratoudakis, Y. Sardine early survival, physical condition and stress after introduction to captivity. J. Fish Biol. 2008, 72, 103–120. [Google Scholar] [CrossRef]
- Monge-Ortiz, R.; Martínez-Llorens, S.; Lemos-Neto, M.; Falcó-Giaccaglia, S.; Pagán, M.; Godoy-Olmos, S.; Tomás-Vidal, A. Growth, sensory and chemical characterization of Mediterranean yellowtail (Seriola dumerili) fed diets with partial replacement of fish meal by other protein sources. Aquac. Rep. 2020, 18, 100466. [Google Scholar] [CrossRef]
- Jerez, S.; Samper, M.; Santamaría, F.J.; Villamandos, J.E.; Cejas, J.R.; Felipe, B.C. Natural spawning of greater amberjack (Seriola dumerili) kept in captivity in the Canary Islands. Aquaculure 2006, 252, 199–207. [Google Scholar] [CrossRef]
- Weir, I.; Fawcett, S.; Smith, S.; Walker, D.; Bornman, T.; Fietz, S. Winter biogenic silica and diatom distributions in the Indian sector of the Southern Ocean. Deep Sea Res. Part A Oceanogr. Res. Pap. 2020, 166, 103421. [Google Scholar] [CrossRef]
- Pernet, F.; Malet, N.; Pastoureaud, A.; Vaquer, A.; Quéré, C.; Dubroca, L. Marine diatoms sustain growth of bivalves in a Mediterranean lagoon. J. Sea Res. 2012, 68, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Miossec, L.; Le Deuff, R.M.; Goulletquer, P. Alien Species Alert: Magallana gigas (Pacific Oyster); ICES Cooperative Research Report No. 299; International Council for the Exploration of the Sea: Copenhagen, Denmark, 2009. [Google Scholar]
- Malouf, R.E.; Breese, W.P. Seasonal changes in the effects of temperature and water flow rate on the growth of juvenile Pacific oysters, Magallana gigas (Thunberg). Aquaculture 1977, 12, 1–13. [Google Scholar] [CrossRef]
- Peinado, I.; Girón, J.; Koutsidis, G.; Ames, J.M. Chemical composition, antioxidant activity and sensory evaluation of five different species of brown edible seaweeds. Food Res. Int. 2014, 66, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Capillo, G.; Sanfilippo, M.; Aliko, V.; Spano, N.; Spinelli, A.; Manganaro, A. Gracilaria gracilis, Source of Agar: A Short Review. Curr. Org. Chem. 2017, 21, 380–386. [Google Scholar]
- Fortes, M.D.; Lüning, K. Growth rates of North Sea macroalgae in relation to temperature, irradiance and photoperiod. Helgol. Meeresunters 1980, 34, 15–29. [Google Scholar] [CrossRef] [Green Version]
- Blouin, N.; Xiugeng, F.; Peng, J.; Yarish, C.; Brawley, S.H. Seeding nets with neutral spores of the red alga Porphyra umbilicalis (L.) Kützing for use in integrated multitrophic aquaculture (IMTA). Aquaculture 2007, 270, 77–91. [Google Scholar] [CrossRef]
- Fairhead, V.A.; Cheshire, A.C. Seasonal and depth related variation in the photosynthesis-irradiance response of Ecklonia radiata (Phaeophyta, Laminariales) at West Island, South Australia. Mar. Biol. 2004, 145, 415–426. [Google Scholar] [CrossRef]
- Gao, X.; Endo, H.; Taniguchi, K.; Agatsuma, Y. Genetic differentiation of high-temperature tolerance in the kelp Undaria pinnatifida sporophytes from geographically separated populations along the Pacific coast of Japan. J. Appl. Phycol. 2012, 25, 567–574. [Google Scholar] [CrossRef]
- Goseberg, N.; Chambers, M.D.; Heasman, K.; Fredricksson, D.; Fredheim, A.; Schlurmann, T. Technological approaches to longline and cage-based aquaculture in the open ocean. In Aquaculture Perspective of Multi-Use Sites in the Open Ocean; Buck, B.H., Langan, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 71–96. [Google Scholar]
- Moe, H.; Fredheim, A.; Hopperstad, O.S. Structural analysis of aquaculture net cages in current. J. Fluids Struct. 2010, 26, 503–516. [Google Scholar] [CrossRef]
- Kristiansen, T.; Faltinsen, O.M. Experimental and numerical study of an aquaculture net cage with floater in waves and current. J. Fluids Struct. 2015, 54, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.J.; Yeh, P.H.; Huang, C.C.; Yang, R.Y. Numerical study of the mooring system failure of aquaculture net cages under irregular waves and current. Ocean Eng. 2020, 216, 108110. [Google Scholar] [CrossRef]
- Juell, J.; Fosseidengen, J. Use of artificial light to control swimming depth and fish density of Atlantic salmon (Salmo salar) in production cages. Aquaculture 2004, 233, 269–282. [Google Scholar] [CrossRef]
- Dempster, T.; Korsøen, Ø.; Folkedal, O.; Juell, J.; Oppedal, F. Submergence of Atlantic salmon (Salmo salar L.) in commercial scale sea-cages: A potential short-term solution to poor surface conditions. Aquaculture 2009, 288, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Araújo, J.; Soares, F.; Medeiros, A.; Bandarra, N.M.; Freire, M.; Falcão, M.; Pousão-Ferreira, P. Depth effect on growth and fatty acid profile of Mediterranean mussel (Mytilus galloprovincialis) produced on a longline off south Portugal. Aquac. Int. 2020, 28, 927–946. [Google Scholar] [CrossRef]

| Areas | A | B | C | D | E | Total | |
|---|---|---|---|---|---|---|---|
| Year | |||||||
| 2018 | 55 | 65 | 12 | 23 | 110 | 265 | |
| 2019 | 45 | 59 | 18 | 22 | 105 | 249 | |
| Area | 2018 | 2019 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ammonia NH3 (µmol L−1) | Nitrite NO2 (µmol L−1) | Nitrate NO3 (µmol L−1) | Chlorophyll a (µg L−1) | Silicate SIO2 (µmol L−1) | Phosphate PO4 (µmol L−1) | pH | Dissolved Oxygen (mg-O2 L−1) | Salinity (PSU) | Ammonia NH3 (µmol L−1) | Nitrite NO2 (µmol L−1) | Nitrate NO3 (µmol L−1) | Chlorophyll a (µg L−1) | Silicate SIO2 (µmol L−1) | Phosphate PO4 (µmol L−1) | pH | Dissolved Oxygen (mg-O2 L−1) | Salinity (PSU) | |
| A | <1.00 | 0.19 ± 0.15 | 1.27 ± 1.31 a | 2.03 ± 1.05 a | 2.34 ± 2.35 a | 0.21 ± 0.14 a | 8.04 ± 0.06 a | 7.66 ± 0.68 a* | 35.52 ± 0.19 a | <1.00 | 0.11 ± 0.13 a | 1.80 ± 2.30 a | 5.12 ± 3.64 a | 1.91 ± 1.49 a | <0.20 | 8.11 ± 0.08 a | 9.23 ± 0.74 a* | 35.53 ± 0.25 a |
| B | <1.00 | <0.10 | 0.55 ± 0.57 a | 0.94 ± 0.66 a | 2.23 ± 1.64 a | <0.20 | 8.10 ± 0.03 a | 7.86 ± 0.56 a | 35.75 ± 0.07 a | <1.00 | <0.10 | 0.75 ± 0.92 a | 1.26 ± 1.23 b | 1.20 ± 0.64 a | <0.20 | 8.10 ± 0.07 a | 8.54 ± 0.59 a | 35.65 ± 0.10 a |
| C | <1.00 | <0.10 | 0.74 ± 1.28 a | 0.85 ± 0.35 a | 3.04 ± 1.76 a | <0.20 | 8.08 ± 0.08 a | 7.82 ± 0.40 a | 35.95 ± 0.04 a | <1.00 | <0.10 | 1.18 ± 0.60 a | 1.06 ± 0.70 b | 2.17 ± 1.45 a | <0.20 | 8.12 ± 0.05 a | 8.59 ± 0.69 a | 35.80 ± 0.06 a |
| D | <1.00 | <0.10 | 0.53 ± 0.51 a | 0.62 ± 0.49 a | 1.58 ± 0.32 a | 0.21 ± 0.14 a | 8.10 ± 0.07 a | 7.85 ± 0.39 a | 36.11 ± 0.06 ab | <1.00 | <0.10 | 0.77 ± 0.55 a | 1.35 ± 1.08 b | 1.50 ± 0.55 a | <0.20 | 8.14 ± 0.04 a | 8.76 ± 0.45 a | 35.94 ± 0.03 ab |
| E | <1.00 | <0.10 | 0.90 ± 1.31 a | 0.30 ± 0.21 b | 2.35 ± 1.64 a | 0.24 ± 0.29 a | 8.12 ± 0.05 a | 7.29 ± 0.83 a | 36.48 ± 0.16 b | <1.00 | 0.15 ± 0.12 a | 1.80 ± 0.13 a | 2.34 ± 1.99 ab | 1.24 ± 0.65 a | <0.20 | 8.07 ± 0.07 a | 8.58 ± 0.49 a | 36.09 ± 0.06 b |
| Fish | Temperature (°C) | Ammonia (NH3) | Nitrite (NO2) | ||
|---|---|---|---|---|---|
| Minimum | Maximum | Ideal | |||
| Meagre (Argyrosomus regius) | 13.0 °C [29] 14.0 °C [46] | 28.0 °C [29] 26.0 °C [46] | 20.0–21.0 °C (Larvae) [29] 26 °C [47] | Lc 50 *—41.1 µmol L−1 (3.0 ± 0.9 g; 96 h; 30 PSU; 22 °C; pH: 8.2) [48] | Lc 50 *—849.6 µmol L−1 (3.2 ± 0.6 g; 96 h; 30 PSU; 22 °C; pH: 8.0) [49] |
| Atlantic Cod (Gadus morhua) | −1.5 °C [50] | 17.0 °C (Juvenile) [51] | 7.0 °C [51] 15.4 ± 1.1 °C [52] 8.2 ± 1.5 °C [52] 9.0 °C [53] | >5.9 µmol L−1 - Slow growth of juveniles, [54] | >20 µmol L−1 - Slow growth (7.0 ± 1.9 g; 96 days; 33 PSU; pH: 8.0) [55] |
| Atlantic Salmon (Salmo salar) | 0.0–7.0 °C (Parr + smolt) [56,57] 14.0 °C [58,59,60] 8.0 °C (Post-smolt) [61,62] | 22.0–28.0 °C (Parr + smolt) [56,57] 18.0 °C [58,59,60] 12.0 °C (Post-smolt) [61,62] | 13.0 °C [63,64,65] 12.0–18.0 °C [66] | Lc 50 *—5.34 µmol L−1 (4.8–9.2 cm; 96 h; 12.5 °C; pH: 6.05; [67]) >2.06 µmol L−1—Slow growth (17.4 ± 0.1 g; 35 PSU; pH: 7.2) [68] | >74 µmol L−1 - Slow growth (Smolt) [69] |
| Gilthead bream (Sparus aurata) | 11.0 °C [70] 11.0–12.0 °C [71] 13.0 °C [72] >12.0 °C [73] >12.0 °C [74] | 32.9 °C [70] 30.0 °C [75] 30.0 °C [74] | 24.0–26.0 °C [76,77] 24.0 °C (Larvae) [78] 22.0 °C [75] 23.0 °C [73] 25.0 °C [74] | Lc 50 *—146.8 µmol L−1 (160 g; 96 h; 34 PSU; 18 °C; pH 8,1) [79] Lc 50—44.7 µmol L−1 (1.1 ± 0.1 g; 96 h; 30 PSU; 20 °C) [80] | - |
| Sea bass (Dicentrarchus labrax) | 5.0 °C [81] 2.0–3.0 °C [77] 5.0 °C [82] 11.0–15.0 °C (Larvae) [77] | 28.0 °C [81] 30.0–32.0 °C [77] 26.0 °C (Larvae) [83] 28.0 °C [82] 30.0–32.0 °C (Larvae) [77] | 22.0–24.0 °C [81] 22.0–25.0 °C [77] 25.0 °C [84] 20.0–22.0 °C (Larvae) [77] 20.0 °C [85] | Lc 50 *—99.8 µmol L−1 (11 g; 96 h; 37 PSU; 21.8 °C; pH 8.0) [79] | Lc 50 *—1035 µmol L−1 (15 cm; 96 h; 36 PSU; 23 °C; pH 8.1–8.4) [86] |
| Sardine (Sardina pilchardus) | 13.0 °C (Larvae) [37] | 22.0 °C (Larvae) [37] | 15.5–17.5 °C [87] | Lc 50 *—43.45 µmol L−1 (Sardinella brasiliensis; 1.04 ± 0.20 g; 96 h; 33.2 PSU; 19.2 °C) [88] | - |
| Greater amberjack (Seriola dumerili) | 17.0 °C (Juvenile) [89] | - | 26.5 °C (Seriola lalandi) [90] 26.0 °C (Juvenile) [89] | - | - |
| Bivalves | Temperature (°C) | Ammonia (NH3) | Nitrite (NO2) | Phytoplankton (Chlorophyll a or Concentration) | ||
|---|---|---|---|---|---|---|
| Minimum | Maximum | Ideal | ||||
| Blue mussel (Mytilus edulis) | 5.0 °C [91] | 28.2 °C [91] | 13.0–22.0 °C (Mytilus californianus) [92] 15.0–20.0 °C [93] 11.0–18.0 °C [94] | Lc 50 *—1.47 µmol L−1 (22 ± 2.5 mm; 21 days; 15.9 ± 0.5 °C; pH: 7.94 ± 0.07; 29.7± 0.03 PSU) [94] | - | Min: 0.5 µg L−1–0.9 µg L−1 Ideal: 6.3 µg L−1–10 µg L−1 (Chlorophyll a) [95,96] |
| Scallop (Pecten maximus) | 4.0 °C [97] 6.5 °C [98] 5.0–8.0 °C [99] | 22.9 °C [98] | 16.0–17.0 °C [98] 10.0–14.0 °C [100] | - | - | Fast growth: 300 µg L−1 (Rhodomonas baltica) [101] |
| Osyter (Magallana gigas) | 8.0–10.0 °C [102] | 32.0 °C [103] | 18.0–20.0 °C (Magallana angulata) [104] | - | - | Fast growth: 25 cells/hour/larvae (Magallana angulata) [105] |
| Razor clam (Ruditapes decussatus) | 10.0 °C [106] | 28.0 °C (Larvae D) [106] | 20.0 ± 1.0 °C (Nothern region of the Iberian peninsula) [107] 22.0 ± 1.0 °C (Southern region of the Iberian peninsula) [107] | Slow growth: 99.8 µmol L−1 [108] | Fast growth: 70.000 cells mL−1 (Isochrysis galbana, Pavlova lutheri and Chaetoceros muelleri (1:1:2)) [109] | |
| Macroalgae | Temperature (°C) | Phosphate (P) | Nitrogen Compounds (N) | ||
|---|---|---|---|---|---|
| Minimum) | Maximum | Ideal | |||
| Gracilaria (Gracilaria gracilis) | 10.0 °C [110] | 28.0 °C [110] | 18.0–23.0 °C [110] 20–25 °C (Gracilaria sp.) [111] 25 °C (Gracilaria verrucos) [112] | - | - |
| Nori (Porphyra umbilicalis) | 3 °C (Porphyra yezoensi) [113] 8 °C (Porphyra haitanensi) [113] | 20.0 °C [114] | 10.0–15.0 °C [113] 10.0 °C [115] | Fast growth: 19.50 µmol L−1 [116] | Fast growth: 35 µmol L−1 (Ammonia) [115] |
| Wakame (Undaria pinnatifida) | 5 °C [117] | 25.0 °C [117] | 12.0–13.0 °C [118] 15.0 °C [117] | - | - |
| Sea State | ||||
|---|---|---|---|---|
| Areas | Hm0 (m) Minimum | Hm0 (m) Maximum | Hm0 (m) Mean | Predominant Wave Direction |
| A, B and C | 0.30 | 9.70 | 2.10 | NW |
| D | 0.28 | 9.79 | 1.72 | NW |
| E | 0.14 | 6.97 | 0.96 | WSW |
| Sea Currents | ||
|---|---|---|
| Areas | Intensity (m s−1) Maximum | Current Direction |
| A and B | 0.40–0.50 (5–15 m depth) | SSW |
| C | No data | No data |
| D | 0.36 (5 m depth) | NNW—SSE |
| E | 0.75 (5 m depth) | ENE—WSW |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosqueira, M.; Pombo, A.; Borges, C.; Brito, A.C.; Zacarias, N.; Esteves, R.; Palma, C. Potential for Coastal and Offshore Aquaculture in Portugal: Insights from Physico-Chemical and Oceanographic Conditions. Appl. Sci. 2022, 12, 2742. https://doi.org/10.3390/app12052742
Mosqueira M, Pombo A, Borges C, Brito AC, Zacarias N, Esteves R, Palma C. Potential for Coastal and Offshore Aquaculture in Portugal: Insights from Physico-Chemical and Oceanographic Conditions. Applied Sciences. 2022; 12(5):2742. https://doi.org/10.3390/app12052742
Chicago/Turabian StyleMosqueira, Miguel, Ana Pombo, Carlos Borges, Ana C. Brito, Nuno Zacarias, Rita Esteves, and Carla Palma. 2022. "Potential for Coastal and Offshore Aquaculture in Portugal: Insights from Physico-Chemical and Oceanographic Conditions" Applied Sciences 12, no. 5: 2742. https://doi.org/10.3390/app12052742
APA StyleMosqueira, M., Pombo, A., Borges, C., Brito, A. C., Zacarias, N., Esteves, R., & Palma, C. (2022). Potential for Coastal and Offshore Aquaculture in Portugal: Insights from Physico-Chemical and Oceanographic Conditions. Applied Sciences, 12(5), 2742. https://doi.org/10.3390/app12052742








