Interfacial Properties and Antioxidant Activity of Whey Protein-Phenolic Complexes: Effect of Phenolic Type and Concentration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Whey Protein Modified with Oxidized Phenolic Compounds
2.3. Determination of Free Amino Group Content
2.4. Determination of Total Phenolic Content
2.5. Determination of Surface Hydrophobicity
2.6. Determination of Foam Properties
2.7. Determination of Emulsion Properties
2.8. Determination of Antioxidative Activities
2.9. Statistical Analysis
3. Results and Discussion
3.1. Free Amino Group Content
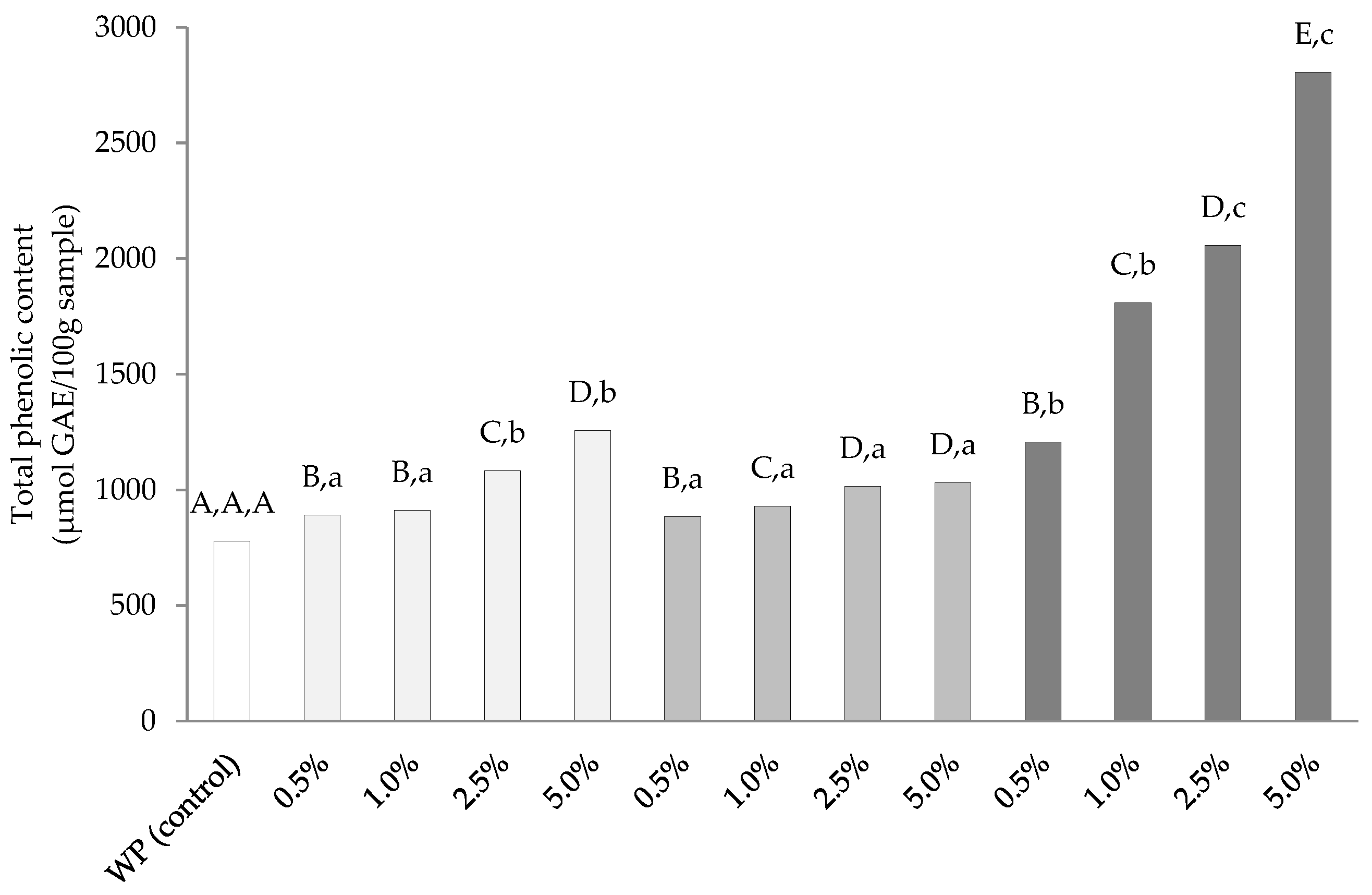
3.2. Total Phenolic Content
3.3. Surface Hydrophobicity
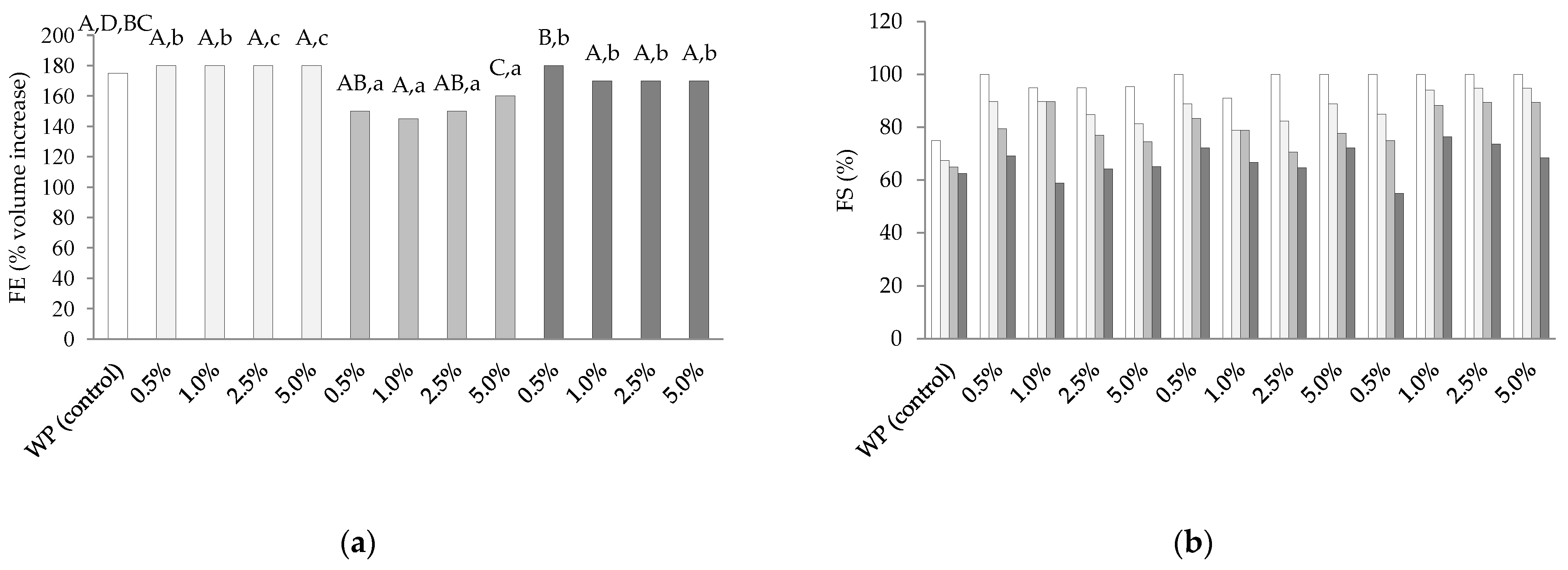
3.4. Foam Properties
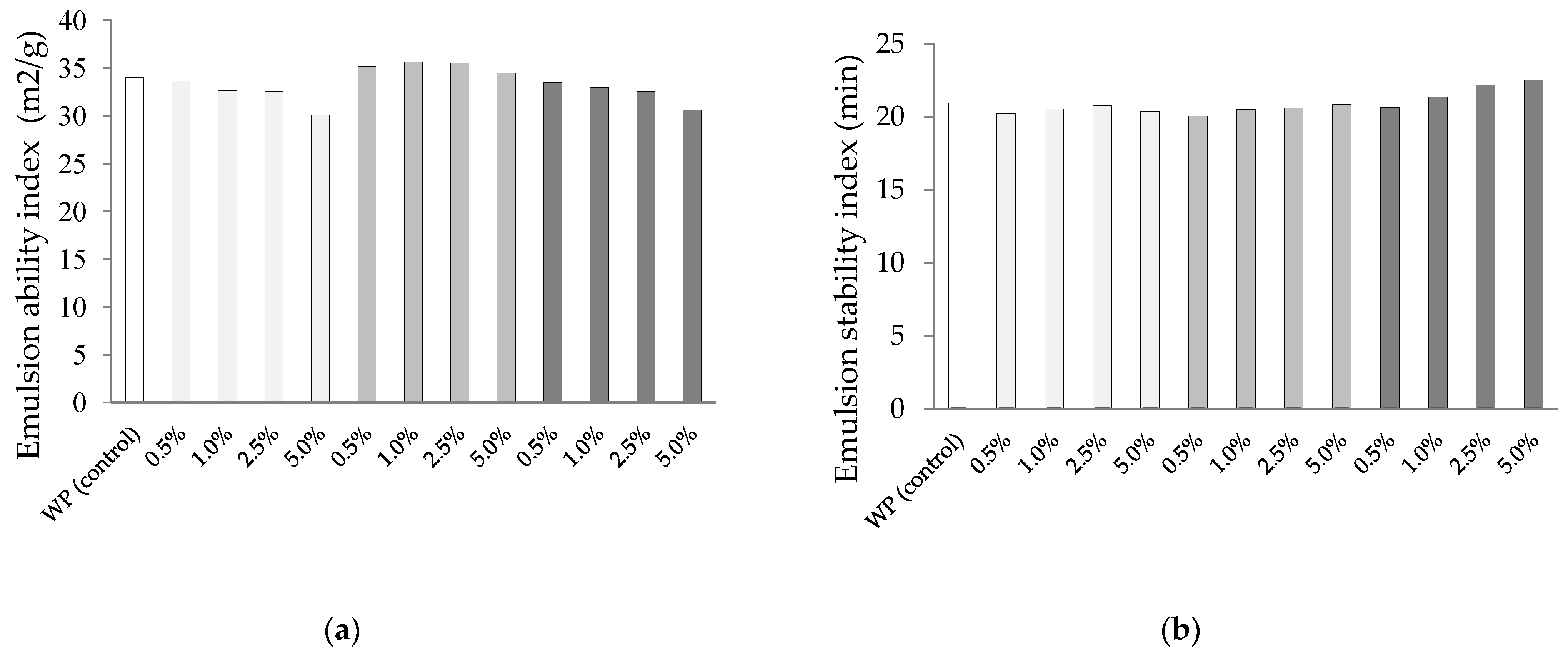
3.5. Emulsion Properties
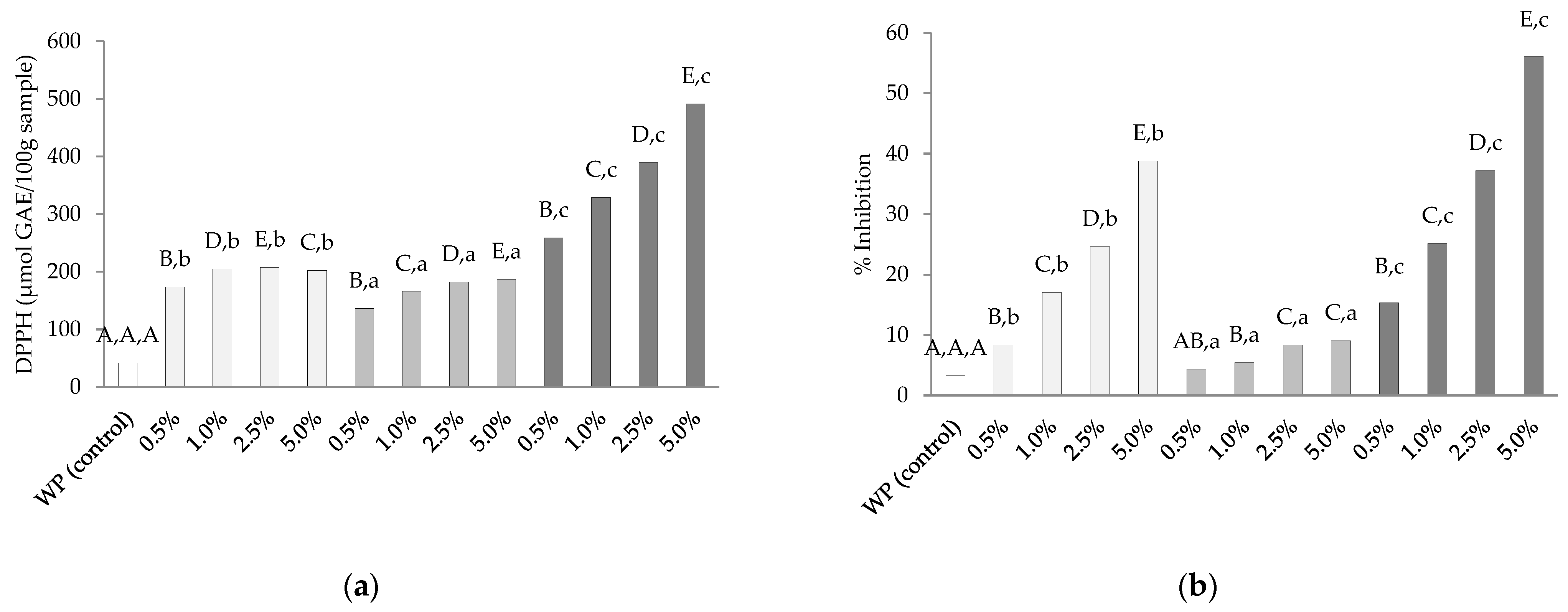
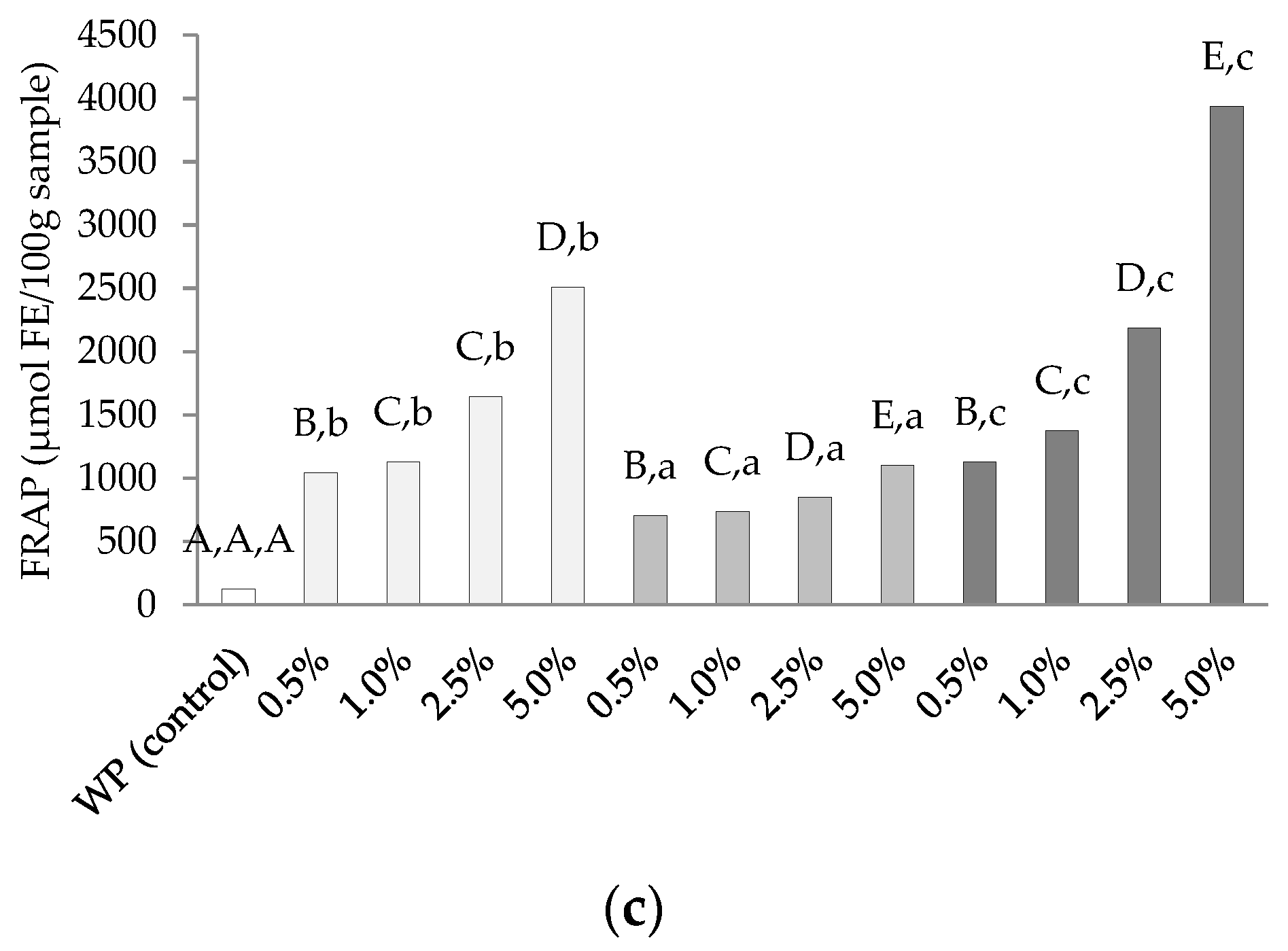
3.6. Antioxidative Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, C.; Chen, N.; Ashaolu, T.J. Whey proteins and peptides in health-promoting functions—A review. Int. Dairy J. 2021, 126, 105269. [Google Scholar] [CrossRef]
- Minj, S.; Anand, S. Whey proteins and its derivatives; Bioactivity, functionality, and current application. Dairy 2020, 1, 233–258. [Google Scholar] [CrossRef]
- Huppertz, T.; Vasiljevic, T.; Zisu, B.; Deeth, H. Novel processing technologies: Effects on whey protein structure and func-tionality. In Whey Proteins; Academic Press: London, UK, 2019; pp. 281–334. [Google Scholar]
- Quan, T.H.; Benjakul, S.; Sae-Leaw, T.; Balange, A.K.; Maqsood, S. Protein-polyphenol conjugates: Antioxidant property, functionalities and their applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Ali, M. Chemical, structural and functional properties of whey proteins covalently modified with phytochemical compounds. J. Food Meas. Charact. 2019, 13, 2970–2979. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, J.; Yi, X.; Ding, B.; Sun, W.; Yan, F.; Wei, S.; Li, Z. Interactions and emulsifying properties of ovalbumin with tannic acid. LWT 2018, 95, 282–288. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Z. Effects of gelatin-polyphenol and gelatin-genipin cross-linking on the structure of gelatin hydrogels. Int. J. Food Prop. 2017, 20, 2822–2832. [Google Scholar] [CrossRef]
- Tosif, M.M.; Najda, A.; Bains, A.; Krishna, T.C.; Chawla, P.; Dyduch-Siemińska, M.; Klepacka, J.; Kaushik, R. A comprehensive review on the interaction of milk protein concentrates with plant-based polyphenolics. Int. J. Mol. Sci. 2021, 22, 13548. [Google Scholar] [CrossRef]
- Aewsiri, T.; Benjakul, S.; Visessanguan, W.; Eun, J.B.; Wierenga, P.A.; Gruppen, H. Antioxidative activity and emulsifying properties of cuttlefish skin gelatin modified by oxidsed phenolic compounds. Food Chem. 2009, 117, 160–168. [Google Scholar] [CrossRef]
- Benjakul, S.; Morrissey, M. Protein hydrolysis from Pacific whiting solid wastes. J. Agric. Food Chem. 1997, 45, 3423–3430. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analyses; automation and comparison with manual method. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Benjakul, S.; Seymour, T.A.; Morrissey, M.T.; An, H. Physicochemical changes in Pacific whiting muscle proteins during iced storage. J. Food Sci. 1997, 62, 729–733. [Google Scholar] [CrossRef]
- Shahidi, F.; Han, X.Q.; Synowiechi, J. Production and characteristics of protein hydrolysates from capelin (Mallotus villosus). Food Chem. 1995, 53, 285–293. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins; evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Binsan, W.; Benjakul, S.; Visessanguan, W.; Roytrakul, S.; Tanaka, M.; Kishimura, H. Antioxidative activity of mungoong, an extract paste, from the cephalothorax of white shrimp (Litopenaeus vannamei). Food Chem. 2008, 106, 4185–4193. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.E.F.; Stain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay, Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubeau, S.; Samson, G.; Tajmir-Riahi, H.A. Dual effect of milk on the antioxidant capacity of green, Darjeeling and English breakfast teas. Food Chem. 2010, 122, 539–545. [Google Scholar] [CrossRef]
- Lowry, Q.H.; Rosebrough, N.J.; Farr, L.A.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 256–275. [Google Scholar] [CrossRef]
- Rohn, S.; Rawel, H.M.; Kroll, J. Reactions with phenolic substances can induce change in some physico-chemical properties and activities of bromelain-the consequences for supplementary food products. Int. J. Food Sci. Technol. 2005, 40, 771–782. [Google Scholar] [CrossRef]
- Rawel, H.M.; Czajka, D.; Rohn, S.; Kroll, J. Interactions of different phenolic acids and flavonoids with soy proteins. Int. J. Biol. Macromol. 2002, 30, 137–150. [Google Scholar] [CrossRef]
- Kroll, J.; Rawel, H.M.; Rohn, S. Reactions of plant phenolics with food proteins and enzymes under special consideration of covalent bonds. Food Sci. Technol. Res. 2003, 9, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Damodaran, S. Protein-stabilized foams and emulsions. In Food Proteins and Their Application; Damodaran, S., Paraf, A., Eds.; Marcel Dekker: New York, NY, USA, 1997; pp. 57–110. [Google Scholar]
- Bandyopadhyay, P.; Ghosh, A.K.; Ghosh, C. Recent developments on polyphenol-protein interactions; effects in tea and coffee tate, antioxidant properties and the digestive system. Food Funct. 2012, 3, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein-phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Davis, J.P.; Foegeding, E.A. Foaming and interfacial properties of polymerized whey protein isolate. J. Food Sci. 2004, 69, C404–C410. [Google Scholar] [CrossRef]
- Kuan, Y.H.; Bhat, R.; Karim, A.A. Emulsifying and foaming properties of ultraviolet-irradiated egg white protein and sodium caseinate. J. Agric. Food Chem. 2011, 59, 4111–4118. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Nakai, S. Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins. Biochim. Biophys. Acta 1980, 624, 13–20. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Gao, L.; Zhang, Y.; Yi, J. Oxidative stability and in vitro digestion of menhaden oil emulsions with whey protein: Effects of EGCG conjugation and interfacial cross-linking. Food Chem. 2018, 265, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Cai, H.; Wang, H.; Li, C.; Liu, S. Improved oxidative stability of fish oil emulsion by grafted ovalbumin-catechin con-jugates. Food Chem. 2018, 241, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Peng, N.; Chang, C.; McClements, D.J.; Su, Y.; Yang, Y. Fabrication of surface-active antioxidant food biopolymers: Conjugation of catechin polymers to egg white protein. Food Biophys. 2017, 12, 198–210. [Google Scholar] [CrossRef]
- Moure, A.; Domínguez, H.; Parajó, J.C. Antioxidant properties of ultrafiltration-recovered soy protein fractions from industrial effluents and their hydrolysates. Process Biochem. 2006, 41, 447–456. [Google Scholar] [CrossRef]

| Concentrations of Oxidized Phenolic Compounds | ANS (S0) | ||
|---|---|---|---|
| WP-OGA | WP-OFA | WP-OTA | |
| 0 (control) | 1172.5 | 1172.5 | 1172.5 |
| 0.5 | 905.8 | 1722.5 | 845.0 |
| 0.1 | 897.5 | 1730.0 | 632.5 |
| 2.5 | 540.0 | 1742.5 | 578.3 |
| 5 | 315.0 | 1197.5 | 110.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongzai, H.; Matan, N.; Ganesan, P.; Aewsiri, T. Interfacial Properties and Antioxidant Activity of Whey Protein-Phenolic Complexes: Effect of Phenolic Type and Concentration. Appl. Sci. 2022, 12, 2916. https://doi.org/10.3390/app12062916
Thongzai H, Matan N, Ganesan P, Aewsiri T. Interfacial Properties and Antioxidant Activity of Whey Protein-Phenolic Complexes: Effect of Phenolic Type and Concentration. Applied Sciences. 2022; 12(6):2916. https://doi.org/10.3390/app12062916
Chicago/Turabian StyleThongzai, Hataikan, Narumol Matan, Palanivel Ganesan, and Tanong Aewsiri. 2022. "Interfacial Properties and Antioxidant Activity of Whey Protein-Phenolic Complexes: Effect of Phenolic Type and Concentration" Applied Sciences 12, no. 6: 2916. https://doi.org/10.3390/app12062916






