The Novel Strain of Gluconobacter oxydans H32 Isolated from Kombucha as a Proposition of a Starter Culture for Sour Ale Craft Beer Production
Abstract
:1. Introduction
2. Materials and Methods
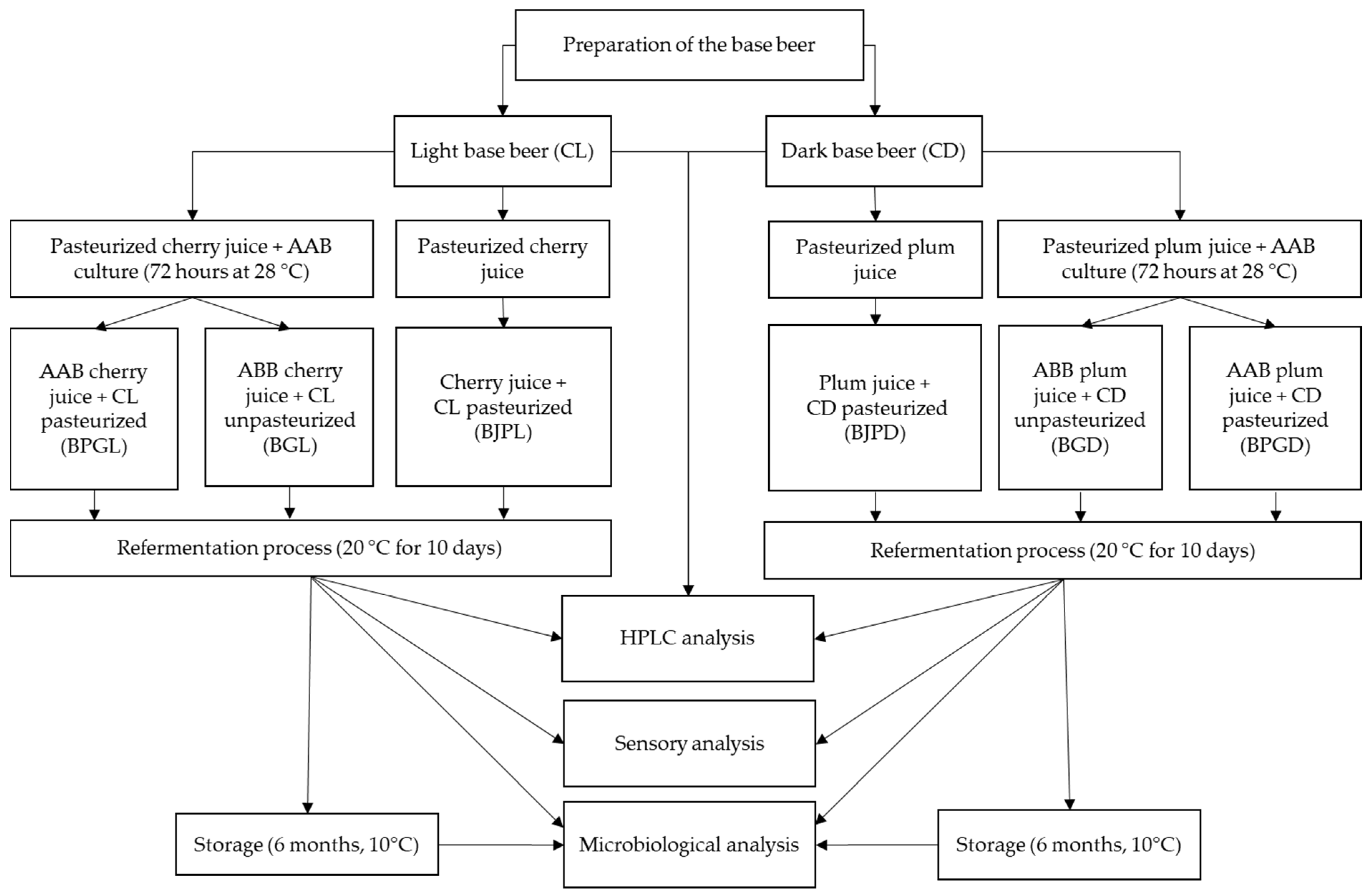
2.1. Preparation of Gluconobacter oxydans H32 Starter Culture
2.2. Beer Technology and Sampling Procedures
2.2.1. Preparation of Basic Beer Beverage
2.2.2. Preparation of AAB Beer
2.3. Microbiological Analyses
2.4. Physicochemical Analyses
2.4.1. Determination Content of the Organic Acids
2.4.2. Determination of Sugars and Ethanol Content
2.4.3. Determination of Vitamin C Content
2.4.4. pH Measurement
2.5. Sensory Analyses
2.6. Statistical Analysis
3. Results and Discussion
3.1. Microbiological Analyses and Survival of AAB Starter Culture in Beer Beverages
3.2. The Content of Organic Acids, Sugars, Ethanol, and Vitamin C
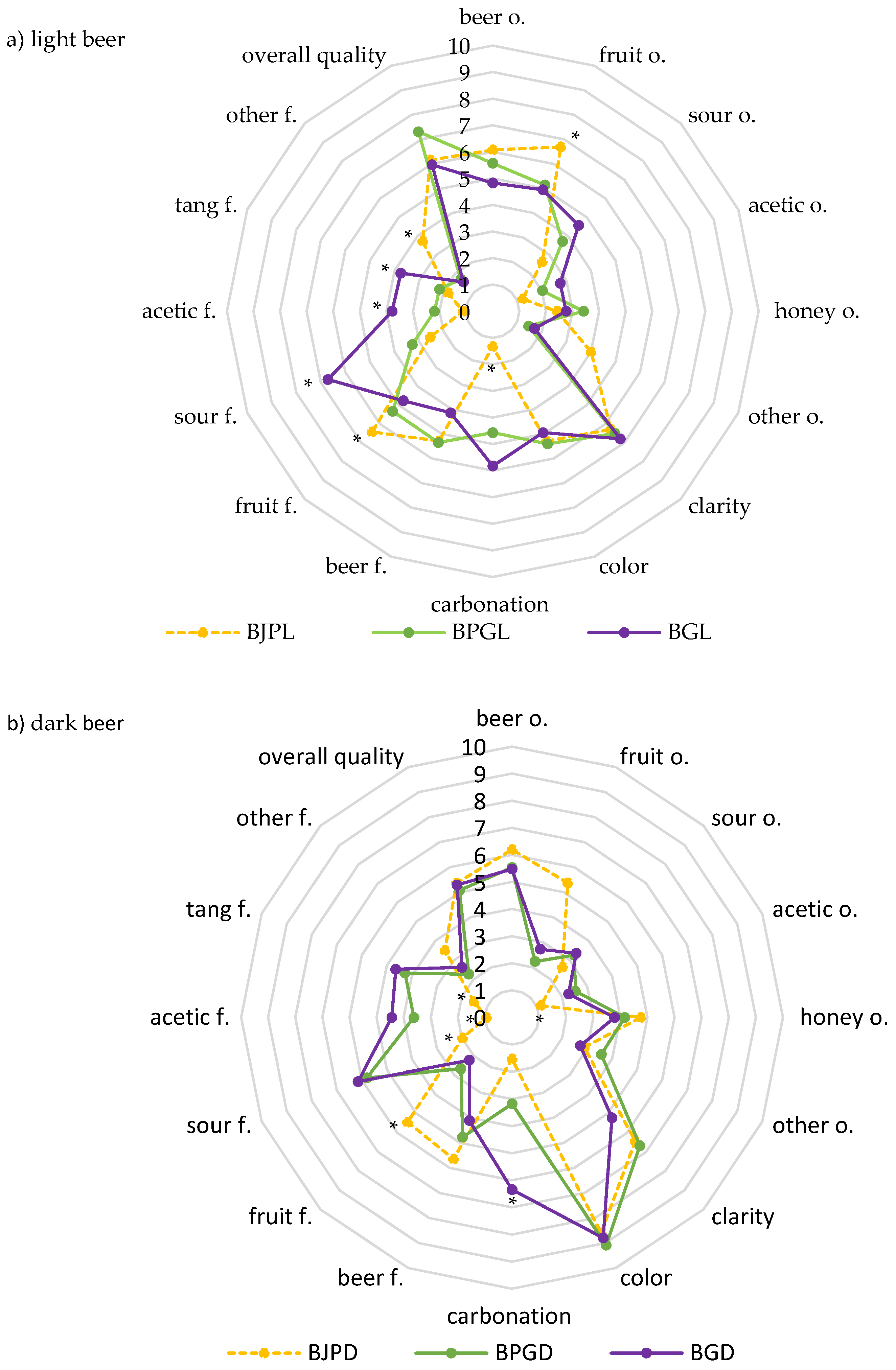
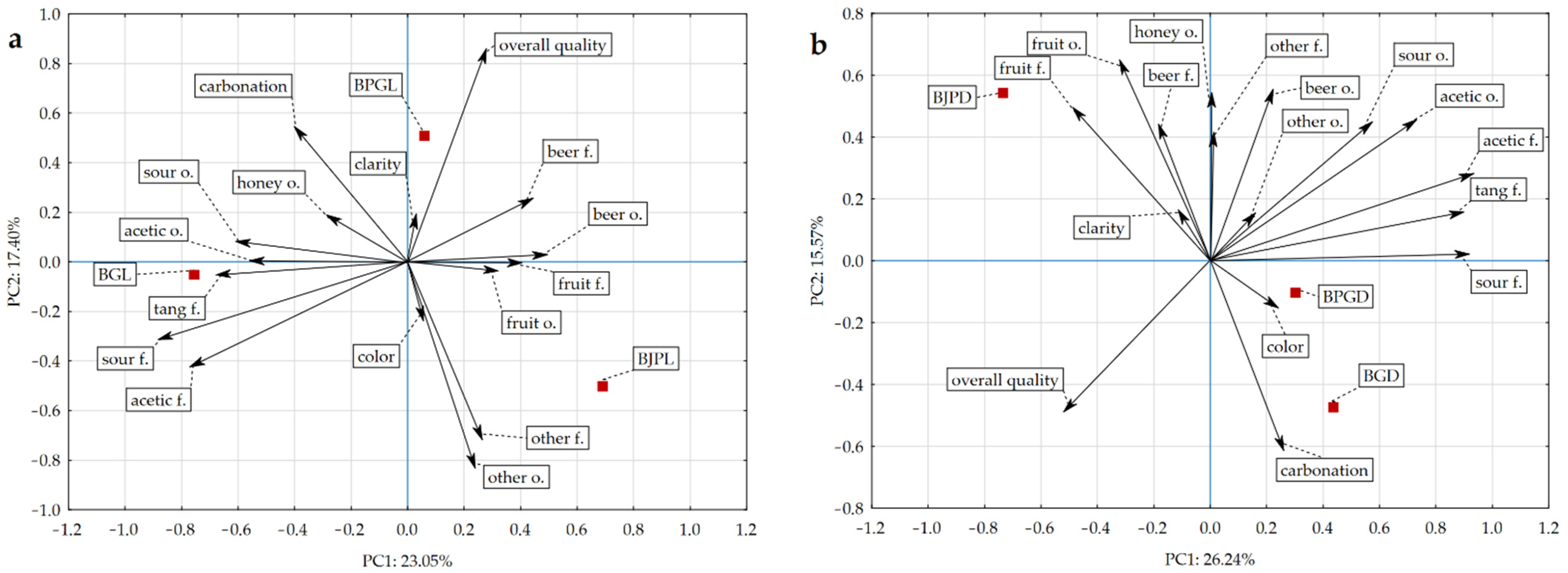
3.3. Sensory Quality
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alcine Chan, M.Z.; Chua, J.Y.; Toh, M.; Liu, S.-Q. Survival of Probiotic Strain Lactobacillus Paracasei L26 during Co-Fermentation with S. Cerevisiae for the Development of a Novel Beer Beverage. Food Microbiol. 2019, 82, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, L.; Angeloni, G.; Masella, P.; Calamai, L.; Parenti, A. A Technological Solution to Modulate the Aroma Profile during Beer Fermentation. Food Bioprocess. Technol. 2018, 11, 1259–1266. [Google Scholar] [CrossRef]
- Hindy, S. Craft Beer Revolution, The: How a Band of Microbrewers Is Transforming the World’s Favorite Drink; St. Martin’s Griffin: New York, NY, USA, 2015; ISBN 978-1-137-28012-1. [Google Scholar]
- Silva, L.C.; de Souza Lago, H.; Rocha, M.O.T.; de Oliveira, V.S.; Laureano-Melo, R.; Stutz, E.T.G.; de Paula, B.P.; Martins, J.F.P.; Luchese, R.H.; Guerra, A.F.; et al. Craft Beers Fermented by Potential Probiotic Yeast or Lacticaseibacilli Strains Promote Antidepressant-Like Behavior in Swiss Webster Mice. Probiotics. Antimicrob. Proteins. 2021, 13, 698–708. [Google Scholar] [CrossRef]
- Beer Judge Certification Program. In Beer Judge Certyfication Program 2015 Style Guidelines—Beer Style Guidelines; BJCP Inc.: St. Louis Park, MN, USA, 2015.
- Krennhuber, K.; Kahr, H.; Jäger, A. Suitability of Beer as an Alternative to Classical Fitness Drinks. Curr. Res. Nutr. Food Sci. 2016, 4, 26–31. [Google Scholar] [CrossRef]
- Redondo, N.; Nova, E.; Díaz-Prieto, L.E.; Marcos, A. Effects of Moderate Beer Consumption on Health. Nutr. Hosp. 2018, 35, 41–44. [Google Scholar] [CrossRef]
- Horn, P.A.; Pedron, N.B.; Junges, L.H.; Rebelo, A.M.; da Silva Filho, H.H.; Zeni, A.L.B. Antioxidant Profile at the Different Stages of Craft Beers Production: The Role of Phenolic Compounds. Eur. Food Res. Technol. 2021, 247, 439–452. [Google Scholar] [CrossRef]
- Baiano, A. Craft Beer: An Overview. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1829–1856. [Google Scholar] [CrossRef]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The Soul of Beer’s Aroma—A Review of Flavour-Active Esters and Higher Alcohols Produced by the Brewing Yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira, G.A.V.; Silva, J.M.S.F. da Chemical equilibrium and enzymatic kinetics of α-amylase interaction with phenolic compounds found in beer. Química. Nova. 2017, 40, 726–732. [Google Scholar]
- Yeo, H.Q.; Liu, S.-Q. An Overview of Selected Specialty Beers: Developments, Challenges and Prospects. Int. J. Food Sci. Technol. 2014, 49, 1607–1618. [Google Scholar] [CrossRef]
- Silva, L.C.; Schmidt, G.B.; Alves, L.G.O.; Oliveira, V.S.; Laureano-Melo, R.; Stutz, E.; Martins, J.F.P.; Paula, B.P.; Luchese, R.H.; Guerra, A.F.; et al. Use of Probiotic Strains to Produce Beers by Axenic or Semi-Separated Co-Culture System. Food Bioprod. Processing 2020, 124, 408–418. [Google Scholar] [CrossRef]
- Haghshenas, B.; Nami, Y.; Abdullah, N.; Radiah, D.; Rosli, R.; Khosroushahi, A.Y. Anticancer Impacts of Potentially Probiotic Acetic Acid Bacteria Isolated from Traditional Dairy Microbiota. LWT Food Sci. Technol. 2015, 60, 690–697. [Google Scholar] [CrossRef]
- Haghshenas, B.; Nami, Y.; Abdullah, N.; Radiah, D.; Rosli, R.; Barzegari, A.; Khosroushahi, A.Y. Potentially Probiotic Acetic Acid Bacteria Isolation and Identification from Traditional Dairies Microbiota. Int. J. Food Sci. Technol. 2015, 50, 1056–1064. [Google Scholar] [CrossRef]
- Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Čanadanović-Brunet, J.M. Influence of Starter Cultures on the Antioxidant Activity of Kombucha Beverage. Food Chem. 2011, 127, 1727–1731. [Google Scholar] [CrossRef]
- Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; Food and Agriculture Organization of the United Nations; World Health Organization (Eds.) FAO food and nutrition paper; Food and Agriculture Organization of the United Nations; World Health Organization: Rome, Italy, 2006; ISBN 978-92-5-105513-7. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Food Safety Authority. Introduction of a Qualified Presumption of Safety (QPS) Approach for Assessment of Selected Microorganisms Referred to EFSA—Opinion of the Scientific Committee. EFSA J. 2007, 5, 587. [Google Scholar] [CrossRef]
- Lynch, K.M.; Zannini, E.; Wilkinson, S.; Daenen, L.; Arendt, E.K. Physiology of Acetic Acid Bacteria and Their Role in Vinegar and Fermented Beverages. Compr. Rev. Food Sci. Food Saf. 2019, 18, 587–625. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority. Opinion of the Scientific Committee on a Request from EFSA Related to a Generic Approach to the Safety Assessment by EFSA of Microorganisms Used in Food/Feed and the Production of Food/Feed Additives. EFSA J. 2005, 3, 226. [Google Scholar] [CrossRef]
- Ramachandran, S.; Fontanille, P.; Pandey, A.; Larroche, C. Gluconic Acid: Properties, Applications and Microbial Production. Food Technol. Biotechnol. 2006, 44, 185–195. [Google Scholar]
- Panel, E.B.; Koutsoumanis, K.; Allende, A.; Alvarez-Ordonez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F.; et al. Updated List of QPS-Recommended Biological Agents for Safety Risk Assessments Carried out by EFSA. EFSA J. 2022, 20, e07045. [Google Scholar] [CrossRef]
- Neffe-Skocińska, K.; Dybka-St, K.; Antolak, H. Isolation and identification of acetic acid bacteria with potential prohealth properties. Żywność. Nauka Technologia Jakość 2019, 26, 183–195. [Google Scholar] [CrossRef]
- Flores, P.; Hellín, P.; Fenoll, J. Determination of Organic Acids in Fruits and Vegetables by Liquid Chromatography with Tandem-Mass Spectrometry. Food Chem. 2012, 132, 1049–1054. [Google Scholar] [CrossRef]
- Usenik, V.; Fabčič, J.; Štampar, F. Sugars, Organic Acids, Phenolic Composition and Antioxidant Activity of Sweet Cherry (Prunus avium L.). Food Chem. 2008, 107, 185–192. [Google Scholar] [CrossRef]
- Monošík, R.; Magdolen, P.; Stredanský, M.; Šturdík, E. Monitoring of Monosaccharides, Oligosaccharides, Ethanol and Glycerol during Wort Fermentation by Biosensors, HPLC and Spectrophotometry. Food Chem. 2013, 138, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Chebrolu, K.K.; Jayaprakasha, G.K.; Yoo, K.S.; Jifon, J.L.; Patil, B.S. An Improved Sample Preparation Method for Quantification of Ascorbic Acid and Dehydroascorbic Acid by HPLC. LWT 2012, 47, 443–449. [Google Scholar] [CrossRef]
- ISO 13299; 2016 Sensory Analysis-Methodology-General Guidance for Establishing a Sensory Profile. ISO: Geneva, Switzerland, 2016.
- ISO 8586; 2012 Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. ISO: Geneva, Switzerland, 2012.
- Calumba, K.F.; Reyes, V.; Bonilla, F.; Villasmil, E.; Sathivel, S. Ale Beer Containing Free and Immobilized Lactobacillus Brevis, a Potential Delivery System for Probiotics. Food Prod. Process Nutr. 2021, 3, 8. [Google Scholar] [CrossRef]
- Narvhus, J.A.; Gadaga, T.H. The Role of Interaction between Yeasts and Lactic Acid Bacteria in African Fermented Milks: A Review. Int. J. Food Microbiol. 2003, 86, 51–60. [Google Scholar] [CrossRef]
- Suharja, A.A.S.; Henriksson, A.; Liu, S.-Q. Impact of Saccharomyces Cerevisiae on Viability of Probiotic Lactobacillus Rhamnosus in Fermented Milk under Ambient Conditions. J. Food Processing Preserv. 2014, 38, 326–337. [Google Scholar] [CrossRef]
- Borah, T.; Gogoi, B.; Khataniar, A.; Gogoi, M.; Das, A.; Borah, D. Probiotic Characterization of Indigenous Bacillus Velezensis Strain DU14 Isolated from Apong, a Traditionally Fermented Rice Beer of Assam. Biocatal. Agric. Biotechnol. 2019, 18, 101008. [Google Scholar] [CrossRef]
- Antolak, H.; Kręgiel, D. Acetic acid bacteria—Taxonomy, ecology, and industrial application. Food Sci. Technol. Qual. 2015, 4, 21–22. [Google Scholar] [CrossRef]
- Wang, B.; Shao, Y.; Chen, F. Overview on Mechanisms of Acetic Acid Resistance in Acetic Acid Bacteria. World J. Microbiol. Biotechnol. 2015, 31, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Almonacid, S.F.; Nájera, A.L.; Young, M.E.; Simpson, R.J.; Acevedo, C.A. A Comparative Study of Stout Beer Batch Fermentation Using Free and Microencapsulated Yeasts. Food Bioprocess Technol. 2012, 5, 750–758. [Google Scholar] [CrossRef]
- Walker, G.M.; Stewart, G.G. Saccharomyces Cerevisiae in the Production of Fermented Beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Raspor, P.; Goranovic, D. Biotechnological Applications of Acetic Acid Bacteria. Crit. Rev. Biotechnol. 2008, 28, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Sengun, I.; Karabiyikli, S. Importance of Acetic Acid Bacteria in Food Industry. Food Control 2011, 22, 647–656. [Google Scholar] [CrossRef]
- Prust, C.; Hoffmeister, M.; Liesegang, H.; Wiezer, A.; Fricke, W.F.; Ehrenreich, A.; Gottschalk, G.; Deppenmeier, U. Complete Genome Sequence of the Acetic Acid Bacterium Gluconobacter Oxydans. Nat. Biotechnol. 2005, 23, 195–200. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Vitamin C. EFSA J. 2013, 11, 3418. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Liu, F. Changes in Organic Acids during Beer Fermentation. J. Am. Soc. Brew. Chem. 2015, 73, 275–279. [Google Scholar] [CrossRef]
- Bae, H.; Yun, S.K.; Yoon, I.K.; Nam, E.Y.; Kwon, J.H.; Jun, J.H. Assessment of Organic Acid and Sugar Composition in Apricot, Plumcot, Plum, and Peach during Fruit Development. J. Appl. Bot. Food Qual. 2014, 87, 25–29. [Google Scholar] [CrossRef]
- Sokół-Łętowska, A.; Kucharska, A.Z.; Hodun, G.; Gołba, M. Chemical Composition of 21 Cultivars of Sour Cherry (Prunus Cerasus) Fruit Cultivated in Poland. Molecules 2020, 25, 4587. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Molina, M.; Muñoz-Garach, A.; Tinahones, F.J.; Moreno-Indias, I. A New Perspective on the Health Benefits of Moderate Beer Consumption: Involvement of the Gut Microbiota. Metabolites 2019, 9, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tozetto, L.M.; do Nascimento, R.F.; de Oliveira, M.H.; Van Beik, J.; Canteri, M.H.G. Production and Physicochemical Characterization of Craft Beer with Ginger (Zingiber Officinale). Food Sci. Technol. 2019, 39, 962–970. [Google Scholar] [CrossRef] [Green Version]
- Ashtavinayak, P.; Elizabeth, H.A. Review: Gram Negative Bacteria in Brewing. Adv. Microbiol. 2016, 6, 195–209. [Google Scholar] [CrossRef] [Green Version]
| Types of the Base Beer | Designation of Samples | Description of Research Sample | Abbreviation of Research Sample | ||
|---|---|---|---|---|---|
| Pasteurization Process | Juice Addition | AAB Inoculum | |||
| Light “L” | + | - | - | Control 1: pasteurized light beer without juice and AAB | CL |
| + | + | - | Control 2: pasteurized light beer only with juice | BJPL | |
| + | + | + | Pasteurized light beer with juice and AAB | BPGL | |
| - | + | + | Unpasteurized light beer with juice and AAB | BGL | |
| Dark “D” | + | - | - | Control 1: pasteurized dark beer without juice and AAB | CD |
| + | + | - | Control 2: pasteurized dark beer only with juice | BJPD | |
| + | + | + | Pasteurized dark beer with juice and AAB | BPGD | |
| - | + | + | Unpasteurized dark beer with juice and AAB | BGD | |
| Sample | Yeast | AAB | pH | |||
|---|---|---|---|---|---|---|
| Storage (Months) | ||||||
| 0 | 6 | 0 | 6 | 0 | 6 | |
| CL | nd aA | nd aA | nd aA | nd aA | 4.10 aA ± 0.02 | 4.12 aA ± 0.03 |
| BJPL | nd aA | nd aA | nd aA | nd aA | 3.71 bA ± 0.02 | 3.75 bA ± 0.04 |
| BPGL | nd aA | nd aA | 8.14 bA | 6.39 bB | 3.54 cA ± 0.04 | 3.42 cA ± 0.03 |
| BGL | 5.45 bA | 2.74 bB | 8.04 bA | 5.06 cB | 3.39 dA ± 0.01 | 3.34 cdA ± 0.03 |
| CD | nd aA | nd aA | nd aA | nd aA | 4.06 aA ± 0.03 | 4.09 aA ± 0.04 |
| BJPD | nd aA | nd aA | nd aA | nd aA | 3.75 bA ± 0.01 | 3.75 bA ± 0.04 |
| BPGD | nd aA | nd aA | 8.17 bA | 4.20 cB | 3.26 dA ± 0.02 | 3.31 dA ± 0.04 |
| BGD | 2.01 cA | nd aB | 7.91 bB | 7.19 dB | 3.48 cA ± 0.04 | 3.45 cA ± 0.03 |
| Sample | Ethanol [mL 100 mL−1] | Vitamin C [mg 100 mL−1] | Glucose | Fructose | Maltose | Sucrose |
|---|---|---|---|---|---|---|
| [g 100 mL−1] | ||||||
| BJPL | 2.82 a ± 0.04 | 1.03 a ± 0.06 | 1.13 a ± 0.01 | 0.83 a ± 0.01 | 0.27 a ± 0.01 | 0.18 a ± 0.02 |
| BPGL | 2.72 ab ± 0.03 | 2.83 bf ± 0.06 | 0.34 b ± 0.02 | 0.03 b ± 0.01 | 0.23 b ± 0.01 | nd b |
| BGL | 3.14 c ± 0.04 | 2.03 c ± 0.06 | nd c | 0.01 cg ± 0.00 | 0.24 b ± 0.01 | 0.03 c ± 0.00 |
| CL | 3.79 d ± 0.09 | nd d | 0.05 d ± 0.01 | nd cd | 0.33 c ± 0.01 | nd b |
| BJPD | 2.53 b ± 0.02 | 1.57 e ± 0.06 | 0.65 e ± 0.02 | 0.02 bc ± 0.01 | 0.39 d ± 0.01 | nd b |
| BPGD | 2.94 ac ± 0.03 | 2.43 f ± 0.06 | 0.99 f ± 0.02 | 0.67 e ± 0.01 | 0.23 b ± 0.01 | 0.03 c ± 0.00 |
| BGD | 3.22 c ± 0.13 | 2.60 bf ± 0.20 | nd c | 0.09 f ± 0.01 | 0.39 d ± 0.01 | nd b |
| CD | 3.28 c ± 0.08 | nd d | 0.03 cd ± 0.01 | nd dg | 0.52 e ± 0.01 | nd b |
| Sample | Malic Acid | Acetic Acid | Gluconic Acid | Fumaric Acid | Lactic Acid | Pyruvic Acid |
|---|---|---|---|---|---|---|
| [mg 100 mL−1] | ||||||
| BJPL | 242.43 a ± 1.29 | 9.60 a ± 0.10 | nd a | 2.50 ad ± 0.10 | 26.53 ad ± 0.35 | 6.83 a ± 0.06 |
| BPGL | 259.40 b ± 2.36 | 46.93 b ± 0.65 | 69.07 b ± 0.45 | 3.13 b ± 0.06 | 30.97 b ± 0.50 | 8.30 b ± 0.10 |
| BGL | 257.80 b ± 0.70 | 37.00 c ± 1.49 | 44.93 c ± 1.15 | 3.00 bc ± 0.10 | 42.50 c ± 0.96 | 7.50 c ± 0.10 |
| CL | 54.13 c ± 1.08 | 12.93 d ± 0.76 | nd a | 2.27 af ± 0.12 | 40.87 cd ± 0.67 | 10.8 d ± 0.35 |
| BJPD | 251.77 d ± 1.42 | 10.83 ad ± 0.40 | nd a | 2.67 acd ± 0.15 | 27.43 a ± 0.57 | 7.77 bc ± 0.15 |
| BPGD | 239.60 a ± 0.85 | 32.17 e ± 0.59 | 15.17 d ± 0.38 | 3.50 e ± 0.10 | 39.67 d ± 1.06 | 7.43 ac ± 0.21 |
| BGD | 262.93 b ± 4.03 | 28.07 f ± 0.50 | 8.60 e ± 0.10 | 3.33 be ± 0.12 | 31.63 b ± 1.00 | 8.13 bc ± 0.25 |
| CD | 65.30 e ± 1.20 | 20.60 g ± 1.05 | nd a | 2.53 adf ± 0.12 | 34.67 e ± 1.14 | 12.73 e ± 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neffe-Skocińska, K.; Kruk, M.; Ścibisz, I.; Zielińska, D. The Novel Strain of Gluconobacter oxydans H32 Isolated from Kombucha as a Proposition of a Starter Culture for Sour Ale Craft Beer Production. Appl. Sci. 2022, 12, 3047. https://doi.org/10.3390/app12063047
Neffe-Skocińska K, Kruk M, Ścibisz I, Zielińska D. The Novel Strain of Gluconobacter oxydans H32 Isolated from Kombucha as a Proposition of a Starter Culture for Sour Ale Craft Beer Production. Applied Sciences. 2022; 12(6):3047. https://doi.org/10.3390/app12063047
Chicago/Turabian StyleNeffe-Skocińska, Katarzyna, Marcin Kruk, Iwona Ścibisz, and Dorota Zielińska. 2022. "The Novel Strain of Gluconobacter oxydans H32 Isolated from Kombucha as a Proposition of a Starter Culture for Sour Ale Craft Beer Production" Applied Sciences 12, no. 6: 3047. https://doi.org/10.3390/app12063047
APA StyleNeffe-Skocińska, K., Kruk, M., Ścibisz, I., & Zielińska, D. (2022). The Novel Strain of Gluconobacter oxydans H32 Isolated from Kombucha as a Proposition of a Starter Culture for Sour Ale Craft Beer Production. Applied Sciences, 12(6), 3047. https://doi.org/10.3390/app12063047









