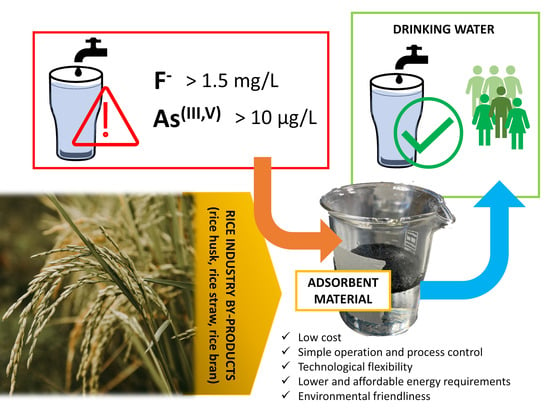
Rice Industry By-Products as Adsorbent Materials for Removing Fluoride and Arsenic from Drinking Water—A Review
Abstract
:1. Introduction
- i.
- Reviewing the literature concerning the potential use of rice industry by-products (rice straws, rice husk, and rice husk ash) for the removal of fluoride and arsenic in drinking water.
- ii.
- Assessing main strengths and limitations in a large-scale implementation of rice industry by-products as an adsorption material in the water treatment sector.
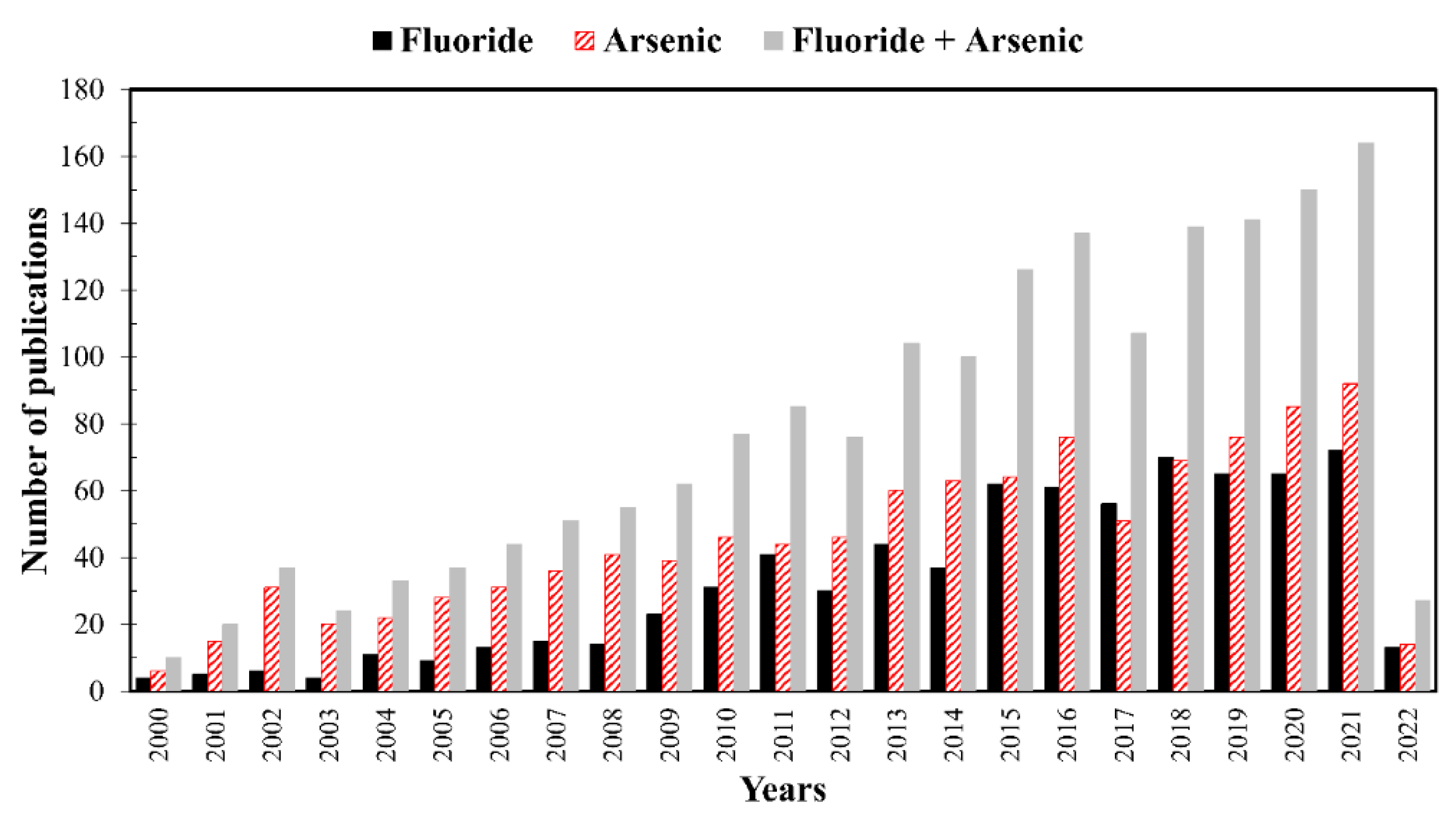
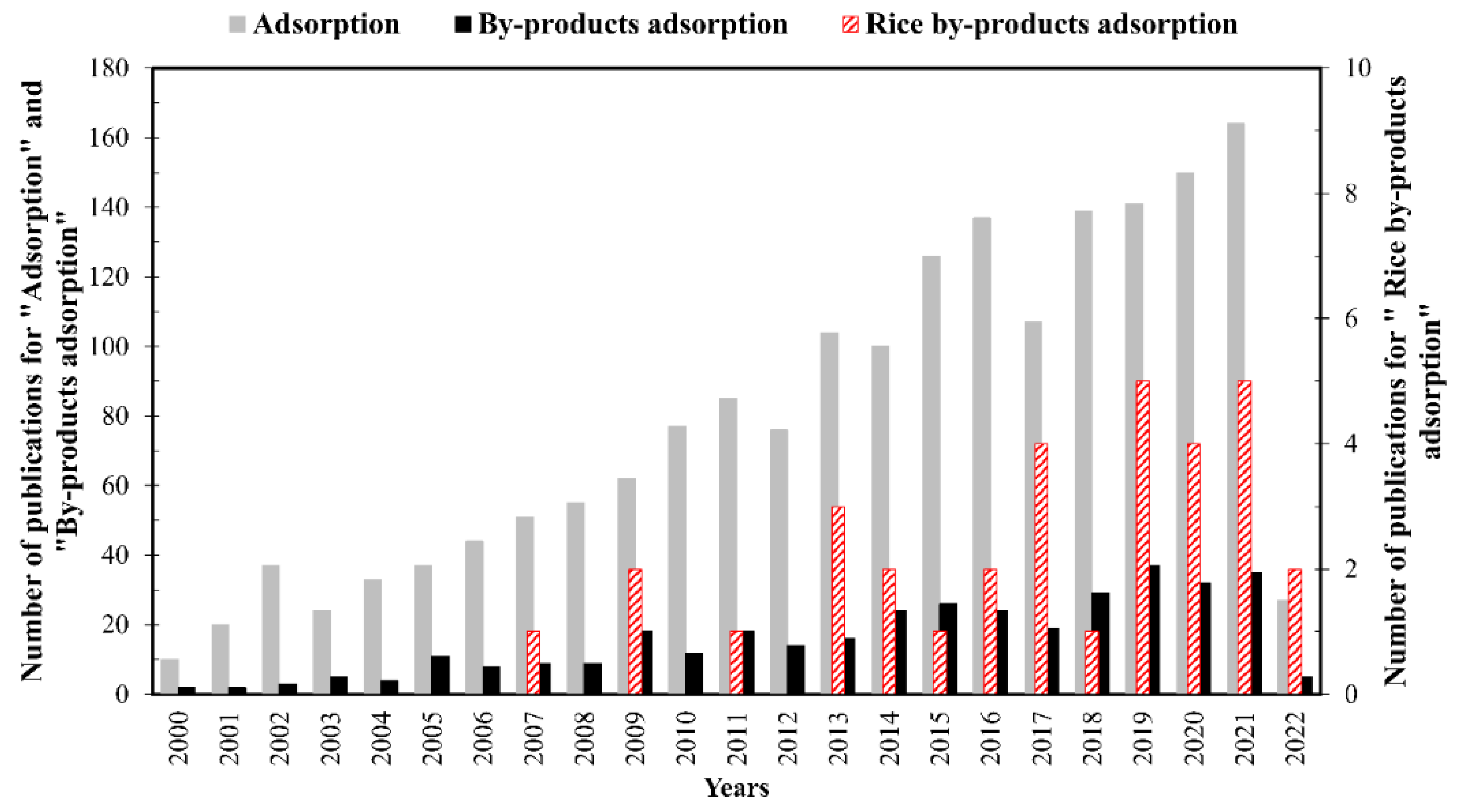
2. Structure and Bibliometric Analysis
3. Rice Industry By-Products
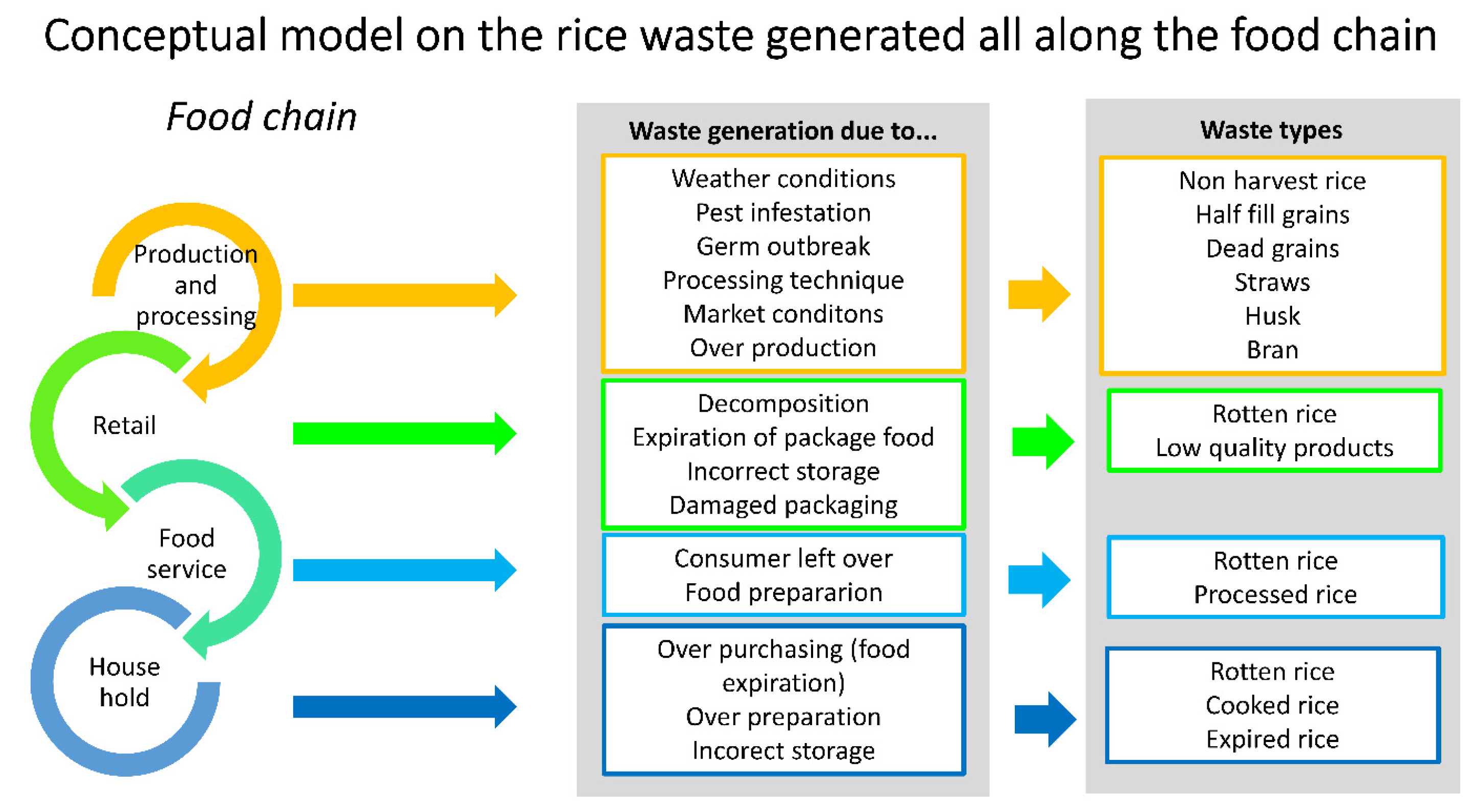
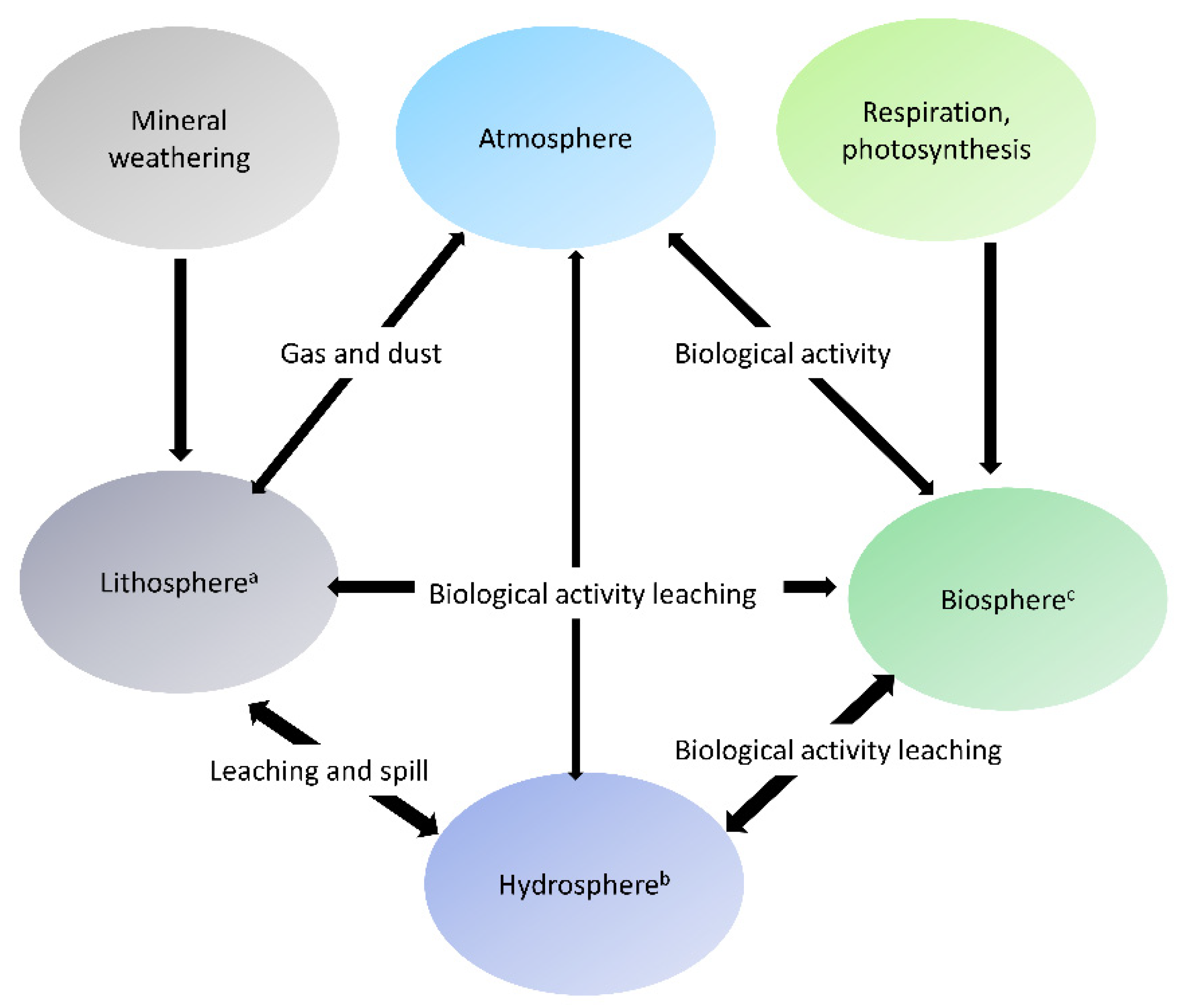
3.1. Rice Industry Value Chain
3.2. Properties of Rice Husk and Rice Husk Ash
Adsorption on Rice Husk
3.3. Properties of Rice Straws
Adsorption on Rice Straw
3.4. Properties of Rice Bran/Rice Polish
Adsorption on Rice Bran
4. Fluoride Removal
- i.
- Environmental Factors: weathering, dissolution of fluoride-containing minerals by rainwater, and continuous evaporation of groundwater can affect a gradual increase of fluoride concentration.
- ii.
- Anthropogenic activities: mining, various industries like glass and coke production, semiconductors, metal smelting, electroplating, photovoltaic activities, etc., release high fluoride concentrated wastewater which is mixed with surface water resulting in fluoride entering groundwater.
4.1. Rice Husk and Rice Husk Ash as Adsorbents for the Removal of Fluoride
| Type of Adsorbent | Type of Water | Experimental Conditions | Adsorption Capacity (mg/g) | References |
|---|---|---|---|---|
| Rice husk | Synthetic | Time = 50 min | 15.2 | [104] |
| Adsorbent = 0.5 mg/100 mL | ||||
| pH = 6.5 | ||||
| Initial concentration = 5 mg/L | ||||
| Stirring rate = 150 rpm | ||||
| Temperature = 298 K | ||||
| Rice husk | Synthetic | Time = 180 min | (Maximum removal: 83%) | [105] |
| Adsorbent = 6 g/L | ||||
| pH = 2 | ||||
| Initial concentration = 5 mg/L | ||||
| Stirring rate = 60 rpm | ||||
| Temperature = 302 K | ||||
| Nanosized rice husk biochar | Synthetic | Time = 60 min | 12.6 | [106] |
| Adsorbent = 1 g/L | ||||
| pH = 7 | ||||
| Initial concentration = 5 mg/L | ||||
| Temperature = 298 K | ||||
| Rice husk activated carbon | Synthetic | Time = 3 h | 7.9 | [78] |
| Adsorbent = 5 g/L | ||||
| pH = 4 | ||||
| Initial concentration = 23 mg/L | ||||
| Stirring rate = 60 rpm | ||||
| Temperature = 298 K | ||||
| Silica nano adsorbent modified by rice husk | Synthetic | Time = 60 min | 12 | [101] |
| Adsorbent = 4 g | ||||
| pH = 8 | ||||
| Initial concentration = 10 mg/L | ||||
| Rice husk treated with Al(OH)3 | Synthetic | Time = 60 min | 15.1 | [100] |
| pH = 5 | ||||
| Initial concentration = 5 mg/L | ||||
| Temperature = 300 K | ||||
| Rice husk treated with NaOH and activated carbon | Synthetic | pH = 4 | 7.9 | [78] |
| Iron oxyhydroxide coated with rice husk | Synthetic | Time = 45 min | 26 | [107] |
| Adsorbent = 0.8 g/L | ||||
| pH = 4 | ||||
| Initial concentration = 10 mg/L | ||||
| Stirring rate = 400 rpm | ||||
| Temperature = 303 K | ||||
| Rice husk-derived silica nanoparticles doped on calcium peroxide | Synthetic | Time = 65 min | 55 | [108] |
| Adsorbent = 1.1 g/L | ||||
| pH = 6.5 | ||||
| Initial concentration = 10 mg/L | ||||
| Rice husk ash | Synthetic | pH = 8 | 2.9 | [109] |
4.2. Rice Straw as Adsorbent for Removal of Fluoride
5. Removal of Arsenic
5.1. Rice Husk as Adsorbent for Removal of Arsenic
| Type of Adsorbent | Type of Water | Type of Arsenic | Experimental Conditions | Maximum Removal (%) | Adsorption Capacity | References |
|---|---|---|---|---|---|---|
| Rice husk biochar | Aqueous solution | As(V) | Temperature = 298 K | 25 | 0.00259 mg/g | [128] |
| Reaction time = 24 h | ||||||
| Initial concentration = 90 μg/L | ||||||
| Surface area = 155 m2/g | ||||||
| Adsorbent = 8 g | ||||||
| Rice husk biochar | Aqueous solution | As(V) | Temperature = 298 K | - | 0.35 mg/g | [129] |
| pH = 9.5 | ||||||
| Initial concentration = 0–200 mg/L | ||||||
| Surface area = 23.2 m2/g | ||||||
| Adsorbent = 2 g | ||||||
| Rice husk biochar | Aqueous solution | As(III) | Temperature = 298 K | - | 19.3 mg/g | [27] |
| pH = 8 | ||||||
| Initial concentration = 3–300 mg/L | ||||||
| Surface area = 25.1 m2/g | ||||||
| Adsorbent = 5 g/L | ||||||
| Rice husk biochar | Aqueous solution | As(V) | Temperature = 298 K | - | 7.1 mg/g | [27] |
| pH = 6 | ||||||
| Initial concentration = 3–300 mg/L | ||||||
| Surface area = 25.1 m2/g | ||||||
| Adsorbent = 5 g/L | ||||||
| Macromolecule carbonized rice husks | Aqueous solution | As(V) | pH = 6 | 85 | - | [120] |
| Time = 65 min | ||||||
| Initial concentration = 100 μg/L | ||||||
| Adsorbent = 0.2 mg | ||||||
| Rice husk (column bed method) | Contaminated groundwater | As(III)—60–90% | pH = 7.8 | 96 | - | [115] |
| Temperature = 298 K | ||||||
| Initial concentration = 270 μg/L | ||||||
| Particle size = 780 μm | ||||||
| Adsorbent = 12 g | ||||||
| Flow rate = 0.8 mL/min | ||||||
| Rice husk (column bed method) | Contaminated groundwater | As(III)—60–90% | pH = 7.6 | 96 | - | [115] |
| Temperature = 298 K | ||||||
| Initial concentration = 596 μg/L | ||||||
| Particle size = 780 μm | ||||||
| Adsorbent = 12 g | ||||||
| Flow rate = 0.8 mL/min | ||||||
| Rice husk (column bed method) | Aqueous solution | As(V) | pH = 8 | 90.7 | - | [121] |
| Temperature = 298 K | ||||||
| Initial concentration = 15 μg/L | ||||||
| Particle size = 710 μm | ||||||
| Adsorbent = 42.5 g | ||||||
| Flow rate = 7 mL/min | ||||||
| Column diameter = 5 cm | ||||||
| Bed height = 28 cm |
| Type of Adsorbent | Type of Water | Type of Arsenic | Experimental Conditions | Maximum Removal (%) | Adsorption Capacity | References |
|---|---|---|---|---|---|---|
| Rice husk–Fe biochar | Aqueous solution | As(III) | Initial concentration = 50 mg/L | - | 30.7 mg/g | [130] |
| Adsorbent = 5 g | ||||||
| pH = 6.5 | ||||||
| Iron oxide amended with rice husk nanoparticles | Aqueous solution | As(V) | Temperature = 303 K | 95 | 82 mg/g | [126] |
| Reaction time = 60 min | ||||||
| Initial concentration = 10 mg/L | ||||||
| Adsorbent = 2.5 g/L | ||||||
| pH = 10.75 | ||||||
| Calcium chloride impregnated rice husk carbon | Aqueous solution | As(III) | Temperature = 301 K | 85 | 0.0182 mg/g | [125] |
| Initial concentration = 1 mg/L | ||||||
| Surface area = 171 m2/g | ||||||
| Adsorbent = 40 g/L | ||||||
| pH = 5 | ||||||
| Iron–Manganese oxide incorporated active rice husk silica | Aqueous solution | As(V) | Temperature = 298 K | - | 11.9 mg/g | [127] |
| Reaction time = 24 h | ||||||
| Initial concentration = 5 mg/L | ||||||
| Surface area = 366 m2/g | ||||||
| Adsorbent = 0.3 g/L | ||||||
| pH = 5 | ||||||
| Iron–Manganese oxide incorporated active rice husk silica | Aqueous solution | As(III), As(V) | Temperature = 298 K | - | 19.1 mg/g, 20.3 mg/g | [127] |
| Reaction time = 24 h | ||||||
| Initial concentration = 2–40 mg/L | ||||||
| Surface area = 366 m2/g | ||||||
| Adsorbent = 0.4 g/L | ||||||
| pH = 7 |
5.2. Rice Straw and Rice Bran as Adsorbents for Removal of Arsenic
6. Final Remarks and Future Outlooks
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ricciardi, P.; Cillari, G.; Carnevale Miino, M.; Collivignarelli, M.C. Valorization of agro-industry residues in the building and environmental sector: A review. Waste Manag. Res. 2020, 38, 487–513. [Google Scholar] [CrossRef] [PubMed]
- Bundschuh, J.; Maity, J.P.; Mushtaq, S.; Vithanage, M.; Seneweera, S.; Schneider, J.; Bhattacharya, P.; Khan, N.I.; Hamawand, I.; Guilherme, L.R.G.; et al. Medical geology in the framework of the sustainable development goals. Sci. Total. Environ. 2017, 581–582, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.; Masuda, H.; Firdous, N. Toxic fluoride and arsenic contaminated groundwater in the Lahore and Kasur districts, Punjab, Pakistan and possible contaminant sources. Environ. Pollut. 2007, 145, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.K.; Choi, B.; Shinogi, Y.; Chikushi, J. Effect of pH conditions on actual and apparent fluoride adsorption by biochar in aqueous phase. Water. Air. Soil Pollut. 2012, 223, 3729–3738. [Google Scholar] [CrossRef]
- Cinti, D.; Vaselli, O.; Poncia, P.P.; Brusca, L.; Grassa, F.; Procesi, M.; Tassi, F. Anomalous concentrations of arsenic, fluoride and radon in volcanic-sedimentary aquifers from central Italy: Quality indexes for management of the water resource. Environ. Pollut. 2019, 253, 525–537. [Google Scholar] [CrossRef]
- Alarcón-Herrera, M.T.; Martin-Alarcon, D.A.; Gutiérrez, M.; Reynoso-Cuevas, L.; Martín-Domínguez, A.; Olmos-Márquez, M.A.; Bundschuh, J. Co-occurrence, possible origin, and health-risk assessment of arsenic and fluoride in drinking water sources in Mexico: Geographical data visualization. Sci. Total Environ. 2020, 698, 134168. [Google Scholar] [CrossRef]
- Cao, H.; Xie, X.; Wang, Y.; Liu, H. Predicting geogenic groundwater fluoride contamination throughout China. J. Environ. Sci. 2022, 115, 140–148. [Google Scholar] [CrossRef]
- Podgorski, J.E.; Labhasetwar, P.; Saha, D.; Berg, M. Prediction Modeling and Mapping of Groundwater Fluoride Contamination throughout India. Environ. Sci. Technol. 2018, 52, 9889–9898. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Thakur, S.K.; Sarkar, A.; Shekhar, S. Worldwide contamination of water by fluoride. Environ. Chem. Lett. 2016, 14, 291–315. [Google Scholar] [CrossRef]
- Aravinthasamy, P.; Karunanidhi, D.; Subramani, T.; Srinivasamoorthy, K.; Anand, B. Geochemical evaluation of fluoride contamination in groundwater from Shanmuganadhi River basin, South India: Implication on human health. Environ. Geochem. Health 2020, 42, 1937–1963. [Google Scholar] [CrossRef]
- Francisca, F.M.; Carro Perez, M.E. Assessment of natural arsenic in groundwater in Cordoba Province, Argentina. Environ. Geochem. Health 2009, 31, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Podgorski, J.E.; Eqani, S.A.M.A.S.; Khanam, T.; Ullah, R.; Shen, H.; Berg, M. Extensive arsenic contamination in high-pH unconfined aquifers in the Indus Valley. Sci. Adv. 2017, 3, elP1700935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aullón Alcaine, A.; Schulz, C.; Bundschuh, J.; Jacks, G.; Thunvik, R.; Gustafsson, J.P.; Mörth, C.M.; Sracek, O.; Ahmad, A.; Bhattacharya, P. Hydrogeochemical controls on the mobility of arsenic, fluoride and other geogenic co-contaminants in the shallow aquifers of northeastern La Pampa Province in Argentina. Sci. Total Environ. 2020, 715, 136671. [Google Scholar] [CrossRef] [PubMed]
- Maity, J.P.; Chen, C.Y.; Bundschuh, J.; Bhattacharya, P.; Mukherjee, A.; Chang, Y.F. Hydrogeochemical reconnaissance of arsenic cycling and possible environmental risk in hydrothermal systems of Taiwan. Groundw. Sustain. Dev. 2017, 5, 1–13. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, R.; Zhao, J.; Ma, F.; Zhang, Y.; Meng, Q. Characterization of H3PO4-treated rice husk adsorbent and adsorption of copper(II) from aqueous solution. Biomed Res. Int. 2014, 2014, 8. [Google Scholar]
- Sud, D.; Mahajan, G.; Kaur, M.P. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions—A review. Bioresour. Technol. 2008, 99, 6017–6027. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Babbar, N.; Sandhu, S.K.; Dhaliwal, S.S.; Kaur, U.; Chadha, B.S.; Bhargav, V.K. Ethanol production from alkali-treated rice straw via simultaneous saccharification and fermentation using newly isolated thermotolerant Pichia kudriavzevii HOP-1. J. Ind. Microbiol. Biotechnol. 2012, 39, 557–566. [Google Scholar] [CrossRef]
- Adav, S.S.; Ravindran, A.; Sze, S.K. Quantitative proteomic analysis of lignocellulolytic enzymes by Phanerochaete chrysosporium on different lignocellulosic biomass. J. Proteom. 2012, 75, 1493–1504. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abb, A.; Caccamo, F.M.; Miino, M.C.; Durante, A.; Bellazzi, S.; Baldi, M.; Bertanza, G. How to Produce an Alternative Carbon Source for Denitrification by Treating and Drastically Reducing Biological Sewage Sludge. Membranes 2021, 11, 977. [Google Scholar] [CrossRef]
- Quansah, J.O.; Hlaing, T.; Lyonga, F.N.; Kyi, P.P.; Hong, S.H.; Lee, C.G.; Park, S.J. Nascent rice husk as an adsorbent for removing cationic dyes from textile wastewater. Appl. Sci. 2020, 10, 3437. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chowdhury, S.; Das Saha, P. Adsorption of Crystal Violet from aqueous solution onto NaOH-modified rice husk. Carbohydr. Polym. 2011, 86, 1533–1541. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saha, P. Das Artificial neural network (ANN) modeling of adsorption of methylene blue by NaOH-modified rice husk in a fixed-bed column system. Environ. Sci. Pollut. Res. 2013, 20, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Tsigie, Y.A.; Wang, C.Y.; Truong, C.T.; Ju, Y.H. Lipid production from Yarrowia lipolytica Po1g grown in sugarcane bagasse hydrolysate. Bioresour. Technol. 2011, 102, 9216–9222. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Templer, R.; Murphy, R.J. High-solids loading enzymatic hydrolysis of waste papers for biofuel production. Appl. Energy 2012, 99, 23–31. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.R.; Reshma, B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.; Jagadevan, S. Influence of torrefaction and pyrolysis on engineered biochar and its applicability in defluoridation: Insight into adsorption mechanism, batch adsorber design and artificial neural network modelling. J. Anal. Appl. Pyrolysis 2021, 154, 105015. [Google Scholar] [CrossRef]
- Samsuri, A.W.; Sadegh-Zadeh, F.; Seh-Bardan, B.J. Adsorption of As(III) and As(V) by Fe coated biochars and biochars produced from empty fruit bunch and rice husk. J. Environ. Chem. Eng. 2013, 1, 981–988. [Google Scholar] [CrossRef]
- Mohan, D.; Sharma, R.; Singh, V.K.; Steele, P.; Pittman, C.U. Fluoride removal from water using bio-char, a green waste, low-cost adsorbent: Equilibrium uptake and sorption dynamics modeling. Ind. Eng. Chem. Res. 2012, 51, 900–914. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Miino, M.C.; Torretta, V.; Rada, E.C.; Caccamo, F.M.; Sorlini, S. Adsorption of fluorides in drinking water by palm residues. Sustainability 2020, 12, 3786. [Google Scholar] [CrossRef]
- Ismail, Z.Z.; AbdelKareem, H.N. Sustainable approach for recycling waste lamb and chicken bones for fluoride removal from water followed by reusing fluoride-bearing waste in concrete. Waste Manag. 2015, 45, 66–75. [Google Scholar] [CrossRef]
- Araga, R.; Kali, S.; Sharma, C.S. Coconut-Shell-Derived Carbon/Carbon Nanotube Composite for Fluoride Adsorption from Aqueous Solution. Clean Soil Air Water 2019, 47, 1–9. [Google Scholar] [CrossRef]
- Abu Bakar, A.H.; Abdullah, L.C.; Mohd Zahri, N.A.; Alkhatib, M. Column Efficiency of Fluoride Removal Using Quaternized Palm Kernel Shell (QPKS). Int. J. Chem. Eng. 2019, 2019, 5743590. [Google Scholar] [CrossRef]
- Sorlini, S.; Palazzini, D.; Collivignarelli, C. Fluoride removal from drinking water in Senegal: Laboratory and pilot experimentation on bone char-based treatment. J. Water Sanit. Hyg. Dev. 2011, 1, 213–223. [Google Scholar] [CrossRef]
- Lu, L.; Yu, W.; Wang, Y.; Zhang, K.; Zhu, X.; Zhang, Y.; Wu, Y.; Ullah, H.; Xiao, X.; Chen, B. Application of Biochar-Based Materials in Environmental Remediation: From Multi-Level Structures to Specific Devices; Springer: Singapore, 2020; Volume 2, ISBN 0123456789. [Google Scholar]
- Zhang, W.; Geng, A. Improved ethanol production by a xylose-fermenting recombinant yeast strain constructed through a modified genome shuffling method. Biotechnol. Biofuels 2012, 5, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chovau, S.; Degrauwe, D.; Van Der Bruggen, B. Critical analysis of techno-economic estimates for the production cost of lignocellulosic bio-ethanol. Renew. Sustain. Energy Rev. 2013, 26, 307–321. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Gupta, V.K. Rice husk and its ash as low-cost adsorbents in water and wastewater treatment. Ind. Eng. Chem. Res. 2011, 50, 13589–13613. [Google Scholar] [CrossRef]
- Lang, J.; Matějová, L.; Cuentas-Gallegos, A.K.; Lobato-Peralta, D.R.; Ainassaari, K.; Gómez, M.M.; Solís, J.L.; Mondal, D.; Keiski, R.L.; Cruz, G.J.F. Evaluation and selection of biochars and hydrochars derived from agricultural wastes for the use as adsorbent and energy storage materials. J. Environ. Chem. Eng. 2021, 9, 105979. [Google Scholar] [CrossRef]
- Ashraf, H.M.; Al-sobhi, S.A.; El-naas, M.H. Mapping the desalination journal: A systematic bibliometric study over 54 years. Desalination 2022, 526, 115535. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Abbasi, S.; Pashaei, R.; Bogusz, A.; Oleszczuk, P. Investigating impact of physicochemical properties of microplastics on human health: A short bibliometric analysis and review. Chemosphere 2022, 289, 133146. [Google Scholar] [CrossRef]
- Alexandri, M.; López-Gómez, J.P.; Olszewska-Widdrat, A.; Venus, J. Valorising agro-industrial wastes within the circular bioeconomy concept: The case of defatted rice bran with emphasis on bioconversion strategies. Fermentation 2020, 6, 42. [Google Scholar] [CrossRef]
- Boris, Ć.; Tomislav, P.; Goran, K.; Neven, D.; Nataša, M.; Hrvoje, M.; Milan, V.; Robert, B. Database/Inventory of the CEREALS AWCB Value Chain; Agrocycle: Essen, Germany, 2016. [Google Scholar]
- Patsios, S.I.; Kontogiannopoulos, K.N.; Mitrouli, S.T.; Plakas, K.V.; Karabelas, A.J. Characterisation of Agricultural Waste Co- and By-Products; Agrocycle: Essen, Germany, 2016; pp. 1–243. [Google Scholar]
- Cu, T.T.T.; Nguyen, T.X.; Triolo, J.M.; Pedersen, L.; Le, V.D.; Le, P.D.; Sommer, S.G. Biogas production from Vietnamese animal manure, plant residues and organic waste: Influence of biomass composition on methane yield. Asian Australas. J. Anim. Sci. 2015, 28, 280–289. [Google Scholar] [CrossRef] [Green Version]
- Romano, J.S.; Miranda, M.S.; Oliveira, M.B.R.; Rodrigues, F.A. Biogenic cements and encapsulation of zinc. J. Clean. Prod. 2011, 19, 1224–1228. [Google Scholar] [CrossRef]
- Naziri, E.; Nenadis, N.; Mantzouridou, F.T.; Tsimidou, M.Z. Valorization of the major agrifood industrial by-products and waste from Central Macedonia (Greece) for the recovery of compounds for food applications. Food Res. Int. 2014, 65, 350–358. [Google Scholar] [CrossRef]
- Abbas, A.; Ansumali, S. Global Potential of Rice Husk as a Renewable Feedstock for Ethanol Biofuel Production. Bioenergy Res. 2010, 3, 328–334. [Google Scholar] [CrossRef]
- Acharya, J.; Kumar, U.; Mahammed Rafi, P. International Journal of Current Engineering and Technology Removal of Heavy Metal Ions from Wastewater by Chemically Modified Agricultural Waste Material as Potential Adsorbent-A Review. Int. J. Curr. Eng. Technol. 2018, 8, 526–530. [Google Scholar]
- Abdelwahab, O.; El Nemr, A.; Khaled, A. Use of rice husk for adsorption of direct dyes from aqueous solution: A case study of direct F. Scarlet Development of water Treatment System for Fish Farming View project Green synthesis of TiO2 nanoparticles and its toxicity View project. Egypt. J. Aquat. Res. 2005, 31, 1–11. [Google Scholar]
- Chuah, T.G.; Jumasiah, A.; Azni, I.; Katayon, S.; Thomas Choong, S.Y. Rice husk as a potentially low-cost biosorbent for heavy metal and dye removal: An overview. Desalination 2005, 175, 305–316. [Google Scholar] [CrossRef]
- Hlihor, R.M.; Gavrilescu, M. Removal of some environmentally relevant heavy metals using low-cost natural sorbents. Environ. Eng. Manag. J. 2009, 8, 353–372. [Google Scholar] [CrossRef]
- Babaso, P.N.; Sharanagouda, H. Rice Husk and Its Applications: Review. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1144–1156. [Google Scholar] [CrossRef]
- Pode, R. Potential applications of rice husk ash waste from rice husk biomass power plant. Renew. Sustain. Energy Rev. 2016, 53, 1468–1485. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Pramada, P.N. Rice husk ash as an adsorbent for methylene blue-effect of ashing temperature. Adsorption 2006, 12, 27–43. [Google Scholar] [CrossRef]
- Akhtar, M.; Bhanger, M.I.; Iqbal, S.; Hasany, S.M. Sorption potential of rice husk for the removal of 2,4-dichlorophenol from aqueous solutions: Kinetic and thermodynamic investigations. J. Hazard. Mater. 2006, 128, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Mansaray, K.G.; Ghaly, A.E. Thermal degradation of rice husks in nitrogen atmosphere. Bioresour. Technol. 1998, 65, 13–20. [Google Scholar] [CrossRef]
- Williams, P.T.; Nugranad, N. Comparison of products from the pyrolysis and catalytic pyrolysis of rice husks. Energy 2000, 25, 493–513. [Google Scholar] [CrossRef]
- Feng, Q.; Yamamichi, H.; Shoya, M.; Sugita, S. Study on the pozzolanic properties of rice husk ash by hydrochloric acid pretreatment. Cem. Concr. Res. 2004, 34, 521–526. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Pramada, P.N.; Praveen, L. Effect of organic acid treatment on the properties of rice husk silica. J. Mater. Sci. 2005, 40, 6535–6544. [Google Scholar] [CrossRef]
- Bondioli, F.; Andreola, F.; Barbieri, L.; Manfredini, T.; Ferrari, A.M. Effect of rice husk ash (RHA) in the synthesis of (Pr,Zr)SiO4 ceramic pigment. J. Eur. Ceram. Soc. 2007, 27, 3483–3488. [Google Scholar] [CrossRef]
- Rukzon, S.; Chindaprasirt, P.; Mahachai, R. Effect of grinding on chemical and physical properties of rice husk ash. Int. J. Miner. Metall. Mater. 2009, 16, 242–247. [Google Scholar] [CrossRef]
- Boldyrev, A. Infrared Spectra of Minerals; Nedra: Moscow, Russia, 1976. [Google Scholar]
- Pokrovskaya, E.N.; Sidorov, V.I.; Melnikova, I.N.; Prihodko, P.L.; Kireev, V.V. Hydrophobization of wood with phosphorus and organosilicon compounds. Wood Chem 1990, 1, 90–96. [Google Scholar]
- O’connor, R.T.; Dupré, E.F.; Mitcham, D. Applications of Infrared Absorption Spectroscopy to Investigations of Cotton and Modified Cottons:Part I: Physical and Crystalline Modifications and Oxidation. Text. Res. J. 1958, 28, 382–392. [Google Scholar] [CrossRef]
- Liu, C.F.; Sun, R.C.; Zhang, A.P.; Ren, J.L.; Wang, X.A.; Qin, M.H.; Chao, Z.N.; Luo, W. Homogeneous modification of sugarcane bagasse cellulose with succinic anhydride using a ionic liquid as reaction medium. Carbohydr. Res. 2007, 342, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Yoo, D.l.; Shin, Y.; Seo, G. FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide. Carbohydr. Res. 2005, 340, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Labbé, N.; Rials, T.; Kelley, S.; Cheng, Z.M.; Kim, J.Y.; Li, Y. FT-IR imaging and pyrolysis-molecular beam mass spectrometry: New tools to investigate wood tissues. Wood Sci. Technol. 2005, 39, 61–76. [Google Scholar] [CrossRef]
- Ang, T.N.; Ngoh, G.C.; Chua, A.S.M.; Lee, M.G. Elucidation of the effect of ionic liquid pretreatment on rice husk via structural analyses. Biotechnol. Biofuels 2012, 5, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, Y.S.; Chiang, C.C.; Hsu, Y.C. Sorption kinetics for dye removal from aqueous solution using activated clay. Sep. Sci. Technol. 2001, 36, 2473–2488. [Google Scholar] [CrossRef]
- Matsumura, Y.; Minowa, T.; Yamamoto, H. Amount, availability, and potential use of rice straw (agricultural residue) biomass as an energy resource in Japan. Biomass Bioenergy 2005, 29, 347–354. [Google Scholar] [CrossRef]
- Drake, D.J.; Nader, G.; Forero, L. Feeding Rice Straw to Cattle; UC ANR Publications: Davis, CA, USA, 2002. [Google Scholar]
- Abraham, A.; Mathew, A.K.; Sindhu, R.; Pandey, A.; Binod, P. Potential of rice straw for bio-refining: An overview. Bioresour. Technol. 2016, 215, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Mirmohamadsadeghi, S.; Karimi, K. Recovery of Silica from Rice Straw and Husk; Elsevier B.V.: Amsterdam, The Netherlands, 2020; ISBN 9780444643216. [Google Scholar]
- Yu, C.T.; Chen, W.H.; Men, L.C.; Hwang, W.S. Microscopic structure features changes of rice straw treated by boiled acid solution. Ind. Crops Prod. 2009, 29, 308–315. [Google Scholar] [CrossRef]
- Van Soest, P.J. Rice straw, the role of silica and treatments to improve quality. Anim. Feed Sci. Technol. 2006, 130, 137–171. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Y.; Zeng, G.; Xu, W.; Li, T.; Xia, W. Characterization of Cr(VI) removal from aqueous solutions by a surplus agricultural waste-Rice straw. J. Hazard. Mater. 2008, 150, 446–452. [Google Scholar] [CrossRef]
- Goodman, B.A. Utilization of waste straw and husks from rice production: A review. J. Bioresour. Bioprod. 2020, 5, 143–162. [Google Scholar] [CrossRef]
- Gebrewold, B.D.; Kijjanapanich, P.; Rene, E.R.; Lens, P.N.L.; Annachhatre, A.P. Fluoride removal from groundwater using chemically modified rice husk and corn cob activated carbon. Environ. Technol. 2019, 40, 2913–2927. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, P.; Bhaduri, D.; Adak, T.; Munda, S.; Satapathy, B.S.; Dash, P.K.; Padhy, S.R.; Pattanayak, A.; Routray, S.; Chakraborti, M.; et al. Characterization of rice straw from major cultivars for best alternative industrial uses to cutoff the menace of straw burning. Ind. Crops Prod. 2020, 143, 111919. [Google Scholar] [CrossRef]
- Li, J.; Lu, J.; Li, X.; Ren, T.; Cong, R.; Zhou, L. Dynamics of potassium release and adsorption on rice straw residue. PLoS ONE 2014, 9, e90440. [Google Scholar] [CrossRef]
- Zhou, N.; Guo, X.; Ye, C.; Yan, L.; Gu, W.; Wu, X.; Zhou, Q.; Yang, Y.; Wang, X.; Cheng, Q. Enhanced fluoride removal from drinking water in wide pH range using La/Fe/Al oxides loaded rice straw biochar. Water Supply 2022, 22, 779–794. [Google Scholar] [CrossRef]
- Cao, W.; Wang, Z.; Zeng, Q.; Shen, C. 13 C NMR and XPS characterization of anion adsorbent with quaternary ammonium groups prepared from rice straw, corn stalk and sugarcane bagasse. Appl. Surf. Sci. 2016, 389, 404–410. [Google Scholar] [CrossRef]
- Spears, J.K.; Grieshop, C.M.; Fahey, G.C. Evaluation of stabilized rice bran as an ingredient in dry extruded dog diets. J. Anim. Sci. 2004, 82, 1122–1135. [Google Scholar] [CrossRef]
- Sayre, R.N.; Earl, L.; Kratzer, F.H.; Saunders, R.M. Nutritional qualities of stabilized and raw rice bran for chicks. Poult. Sci. 1987, 66, 493–499. [Google Scholar] [CrossRef]
- Rosniyana, A.; Hazila, K.; Abdullah, S. Characteristics of local rice flour (MR 220) produced by wet and dry milling methods. J. Trop. Agric. Food Sci. 2016, 44, 147–155. [Google Scholar]
- Resurrection, A.P.; Juliano, B.O.; Tanaka, Y. Nutrient content and distribution in milling fractions of rice grain. J. Sci. Food Agric. 1979, 30, 475–481. [Google Scholar] [CrossRef]
- Singh, K.K.; Rastogi, R.; Hasan, S.H. Removal of Cr(VI) from wastewater using rice bran. J. Colloid Interface Sci. 2005, 290, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Montanher, S.F.; Oliveira, E.A.; Rollemberg, M.C. Removal of metal ions from aqueous solutions by sorption onto rice bran. J. Hazard. Mater. 2005, 117, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.A.; Montanher, S.F.; Andrade, A.D.; Nóbrega, J.A.; Rollemberg, M.C. Equilibrium studies for the sorption of chromium and nickel from aqueous solutions using raw rice bran. Process Biochem. 2005, 40, 3485–3490. [Google Scholar] [CrossRef]
- Kumagai, M.; Takahashi, T.; Takahashi, H.; Ogawa, N.; Toeda, K. NIR analysis of rice bran depending on different percentages of rice polishing. Int. J. Soc. Mater. Eng. Resour. 2006, 13, 35–38. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, A.; Kumar, E.; Sillanpää, M. Fluoride removal from water by adsorption-A review. Chem. Eng. J. 2011, 171, 811–840. [Google Scholar] [CrossRef]
- Gai, W.Z.; Deng, Z.Y. A comprehensive review of adsorbents for fluoride removal from water: Performance, water quality assessment and mechanism. Environ. Sci. Water Res. Technol. 2021, 7, 1362–1386. [Google Scholar] [CrossRef]
- Mohapatra, M.; Anand, S.; Mishra, B.K.; Giles, D.E.; Singh, P. Review of fluoride removal from drinking water. J. Environ. Manag. 2009, 91, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Gai, W.Z.; Deng, Z.Y.; Shi, Y. Fluoride removal from water using high-activity aluminum hydroxide prepared by the ultrasonic method. RSC Adv. 2015, 5, 84223–84231. [Google Scholar] [CrossRef]
- Khandare, D.; Mukherjee, S. A review of metal oxide nanomaterials for fluoride decontamination from water environment. Mater. Today Proc. 2019, 18, 1146–1155. [Google Scholar] [CrossRef]
- Tanvir Arfin, S.S.W. Fluoride Removal from Water By Calcium Materials: A State-Of-The-Art Review. Int. J. Innov. Res. Sci. Eng. Technol. 2015, 4, 8090–8102. [Google Scholar] [CrossRef]
- Daffalla, S.B.; Mukhtar, H.; Shaharun, M.S. Characterization of adsorbent developed from rice husk: Effect of surface functional group on phenol adsorption. J. Appl. Sci. 2010, 10, 1060–1067. [Google Scholar] [CrossRef] [Green Version]
- Noor Syuhadah, S.; Rohasliney, H. Rice Husk as biosorbent: Areview. Heal. Environ. J. 2012, 3, 89–95. [Google Scholar]
- Environment, N.; Waghmare, M.D. Investigation on Sorption of Fluoride in Water Using Rice Husk as an Adsorbent. Nat. Environ. Pollut. Technol. 2015, 8, 217–223. [Google Scholar]
- Ganvir, V.; Das, K. Removal of fluoride from drinking water using aluminum hydroxide coated rice husk ash. J. Hazard. Mater. 2011, 185, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Pillai, P.; Dharaskar, S.; Shah, M.; Sultania, R. Determination of fluoride removal using silica nano adsorbent modified by rice husk from water. Groundw. Sustain. Dev. 2020, 11, 100423. [Google Scholar] [CrossRef]
- Tembhurkar, A.R.; Dongre, S. Studies on fluoride removal using adsorption process. J. Environ. Sci. Eng. 2006, 48, 151–156. [Google Scholar] [PubMed]
- Tomar, V.; Kumar, D. A critical study on efficiency of different materials for fluoride removal from aqueous media. Chem. Cent. J. 2013, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Vijila, B.; Gladis, E.H.E.; Jose, J.M.A.; Sharmila, T.M.; Joseph, J. Removal of fluoride with rice husk derived adsorbent from agro waste materials. Mater. Today Proc. 2021, 45, 2125–2129. [Google Scholar] [CrossRef]
- Vardhan, C.M.V.; Karthikeyan, J. Removal of fluoride from water using low-cost materials. In Proceedings of the Fifteenth International Water Technology Conference, IWTC-15 2011, Alexandria, Egypt, 28–31 May 2011 ; pp. 1–14. [Google Scholar]
- Goswami, R.; Kumar, M. Removal of fluoride from aqueous solution using nanoscale rice husk biochar. Groundw. Sustain. Dev. 2018, 7, 446–451. [Google Scholar] [CrossRef]
- Pillai, P.; Lakhtaria, Y.; Dharaskar, S.; Khalid, M. Synthesis, characterization, and application of iron oxyhydroxide coated with rice husk for fluoride removal from aqueous media. Environ. Sci. Pollut. Res. 2020, 27, 20606–20620. [Google Scholar] [CrossRef]
- Pillai, P.; Dharaskar, S.; Pandian, S. Rice husk derived silica nano doped on calcium peroxide for fluoride: Performance, characterization, kinetic, isotherm, and groundwater treatment. Environ. Technol. Innov. 2020, 19, 100901. [Google Scholar] [CrossRef]
- Bibi, S.; Farooqi, A.; Yasmin, A.; Kamran, M.A.; Niazi, N.K. Arsenic and fluoride removal by potato peel and rice husk (PPRH) ash in aqueous environments. Int. J. Phytoremediat. 2017, 19, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Daifullah, A.A.M.; Yakout, S.M.; Elreefy, S.A. Adsorption of fluoride in aqueous solutions using KMnO4-modified activated carbon derived from steam pyrolysis of rice straw. J. Hazard. Mater. 2007, 147, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Maheshwari, P.H.; Tripathy, S.S.; Waseem, S.; Dhakate, S.R. Processing of rice straw to derive carbon with efficient de-fluoridation properties for drinking water treatment. J. Water Process Eng. 2020, 34, 101136. [Google Scholar] [CrossRef]
- Baig, S.A.; Sheng, T.; Hu, Y.; Xu, J.; Xu, X. Arsenic Removal from Natural Water Using Low Cost Granulated Adsorbents: A Review. Clean Soil Air Water 2015, 43, 13–26. [Google Scholar] [CrossRef]
- Matschullat, J. Arsenic in the geosphere A review. Sci. Total Environ. 2000, 249, 297–312. [Google Scholar] [CrossRef]
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Amin, N.; Kaneco, S.; Kitagawa, T.; Begum, A.; Katsumata, H.; Suzuki, T.; Ohta, K. Removal of arsenic in aqueous solutions by adsorption onto waste rice husk. Ind. Eng. Chem. Res. 2006, 45, 8105–8110. [Google Scholar] [CrossRef]
- Shih, M.C. An overview of arsenic removal by pressure-driven membrane processes. Desalination 2005, 172, 85–97. [Google Scholar] [CrossRef]
- Langdon, C.J.; Piearce, T.G.; Meharg, A.A.; Semple, K.T. Interactions between earthworms and arsenic in the soil environment: A review. Environ. Pollut. 2003, 124, 361–373. [Google Scholar] [CrossRef]
- Hu, S.; Lu, J.; Jing, C. A novel colorimetric method for field arsenic speciation analysis. J. Environ. Sci. 2012, 24, 1341–1346. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Pham, T.H.; Nguyen Thi, H.T.; Nguyen, T.N.; Nguyen, M.V.; Tran Dinh, T.; Nguyen, M.P.; Do, T.Q.; Phuong, T.; Hoang, T.T.; et al. Synthesis of Iron-Modified Biochar Derived from Rice Straw and Its Application to Arsenic Removal. J. Chem. 2019, 2019. [Google Scholar] [CrossRef]
- Babazad, Z.; Kaveh, F.; Ebadi, M.; Mehrabian, R.Z.; Juibari, M.H. Efficient removal of lead and arsenic using macromolecule-carbonized rice husks. Heliyon 2021, 7, e06631. [Google Scholar] [CrossRef] [PubMed]
- Asif, Z.; Chen, Z. Removal of arsenic from drinking water using rice husk. Appl. Water Sci. 2017, 7, 1449–1458. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.P.; Fu, P.L.K. Treatment of arsenic (V)-containing water by the activated carbon process. J. Water Pollut. Control Fed. 1984, 56, 233–242. [Google Scholar]
- Wu, Y.; Ma, X.; Feng, M.; Liu, M. Behavior of chromium and arsenic on activated carbon. J. Hazard. Mater. 2008, 159, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, L.; van Deventer, J.S.J.; Landi, W.M. Factors affecting the mechanism of the adsorption of arsenic species on activated carbon. Miner. Eng. 1995, 8, 557–569. [Google Scholar] [CrossRef]
- Mondai, P.; Majumder, C.B.; Mohanty, B. Removal of trivalent arsenic (As(III)) from contaminated water by calcium chloride (CaCl2)-impregnated rice husk carbon. Ind. Eng. Chem. Res. 2007, 46, 2550–2557. [Google Scholar] [CrossRef]
- Pillai, P.; Kakadiya, N.; Timaniya, Z.; Dharaskar, S.; Sillanpaa, M. Removal of arsenic using iron oxide amended with rice husk nanoparticles from aqueous solution. Mater. Today Proc. 2019, 28, 830–835. [Google Scholar] [CrossRef]
- Bui, T.H.; Pham, V.S.; Thanh-Nho, N.; Trieu, Q.A. Removal of Arsenic from Water Using a Composite of Iron–Manganese Oxide Incorporated Active Rice Husk Silica. Clean Soil Air Water 2021, 49, 1–8. [Google Scholar] [CrossRef]
- Agrafioti, E.; Kalderis, D.; Diamadopoulos, E. Arsenic and chromium removal from water using biochars derived from rice husk, organic solid wastes and sewage sludge. J. Environ. Manag. 2014, 133, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Sari, N.A.; Ishak, C.F.; Bakar, R.A. Characterization of oil palm empty fruit bunch and rice husk biochars and their potential to adsorb arsenic and cadmium. Am. J. Agric. Biol. Sci. 2014, 9, 450–456. [Google Scholar] [CrossRef] [Green Version]
- Vithanage, M.; Herath, I.; Joseph, S.; Bundschuh, J.; Bolan, N.; Ok, Y.S.; Kirkham, M.B.; Rinklebe, J. Interaction of arsenic with biochar in soil and water: A critical review. Carbon N. Y. 2017, 113, 219–230. [Google Scholar] [CrossRef]
- Ranjan, D.; Talat, M.; Hasan, S.H. Rice polish: An alternative to conventional adsorbents for treating arsenic bearing water by up-flow column method. Ind. Eng. Chem. Res. 2009, 48, 10180–10185. [Google Scholar] [CrossRef]
- Mukherjee, S.; Thakur, A.K.; Goswami, R.; Mazumder, P.; Taki, K.; Vithanage, M.; Kumar, M. Efficacy of agricultural waste derived biochar for arsenic removal: Tackling water quality in the Indo-Gangetic plain. J. Environ. Manag. 2021, 281, 111814. [Google Scholar] [CrossRef]
- Kwon, G.; Bhatnagar, A.; Wang, H.; Kwon, E.E.; Song, H. A review of recent advancements in utilization of biomass and industrial wastes into engineered biochar. J. Hazard. Mater. 2020, 400, 123242. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Yang, Y.; Tsang, Y.F.; Sarkar, B.; Hou, D.; Cao, X.; Meers, E.; Rinklebe, J.; Kim, K.H.; Ok, Y.S. Occurrence of contaminants in drinking water sources and the potential of biochar for water quality improvement: A review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 549–611. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, Y.; Liu, S.; Jiang, L.; Tan, X.; Zeng, G.; Li, M.; Liu, S.; Tian, S.; Fang, Y. Activated magnetic biochar by one-step synthesis: Enhanced adsorption and coadsorption for 17β-estradiol and copper. Sci. Total Environ. 2018, 639, 1530–1542. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z.; Hu, Z.; Zhang, J. Recent advances in biochar application for water and wastewater treatment: A review. PeerJ 2020, 8, e9164. [Google Scholar] [CrossRef]
- Luyen, N.T.; Linh, H.X.; Huy, T.Q. Preparation of Rice Husk Biochar-Based Magnetic Nanocomposite for Effective Removal of Crystal Violet. J. Electron. Mater. 2020, 49, 1142–1149. [Google Scholar] [CrossRef]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Lübken, M. Biochar for wastewater treatment-conversion technologies and applications. Appl. Sci. 2020, 10, 3492. [Google Scholar] [CrossRef]
- Greiner, B.G.; Shimabuku, K.K.; Summers, R.S. Influence of biochar thermal regeneration on sulfamethoxazole and dissolved organic matter adsorption. Environ. Sci. Water Res. Technol. 2018, 4, 169–174. [Google Scholar] [CrossRef]
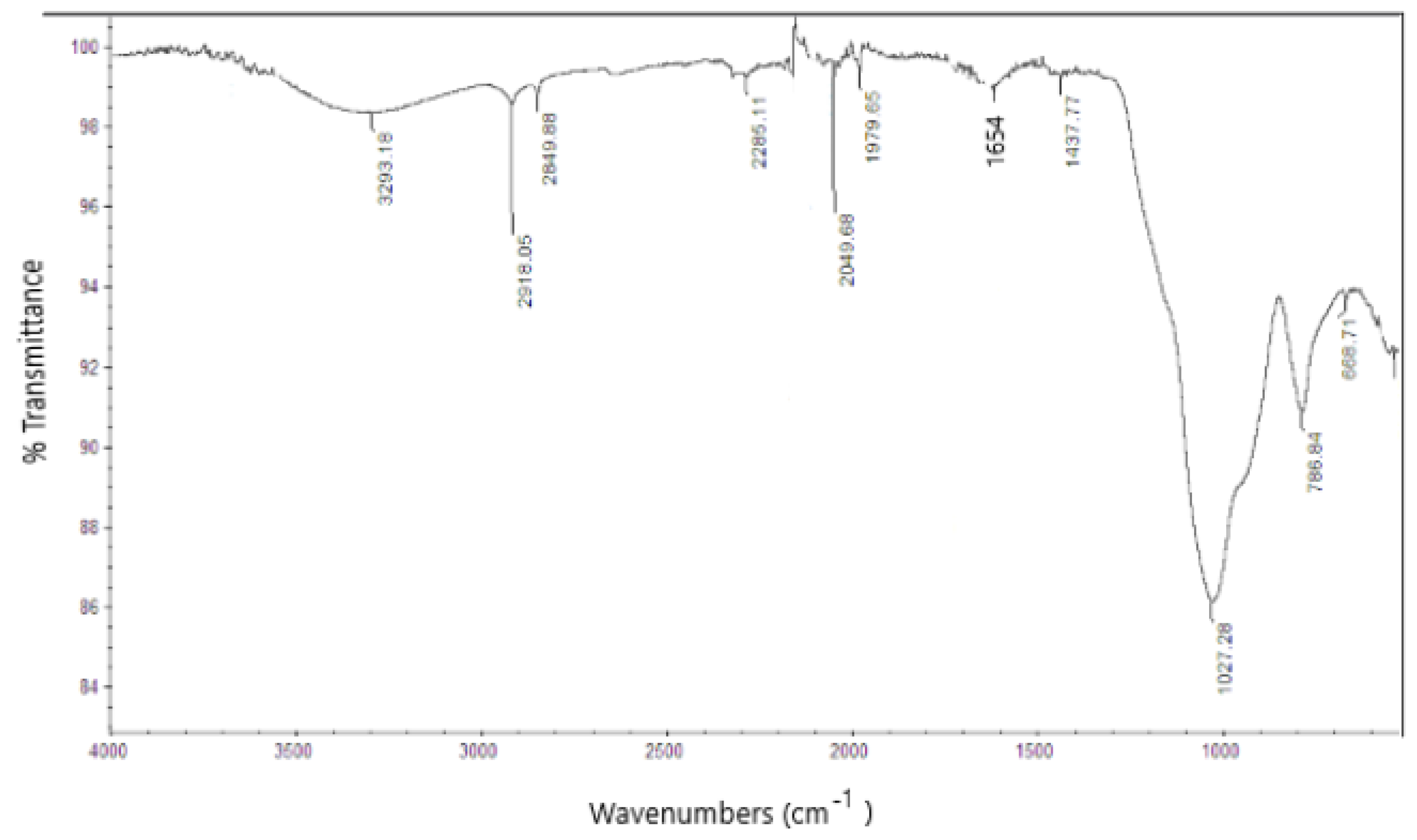
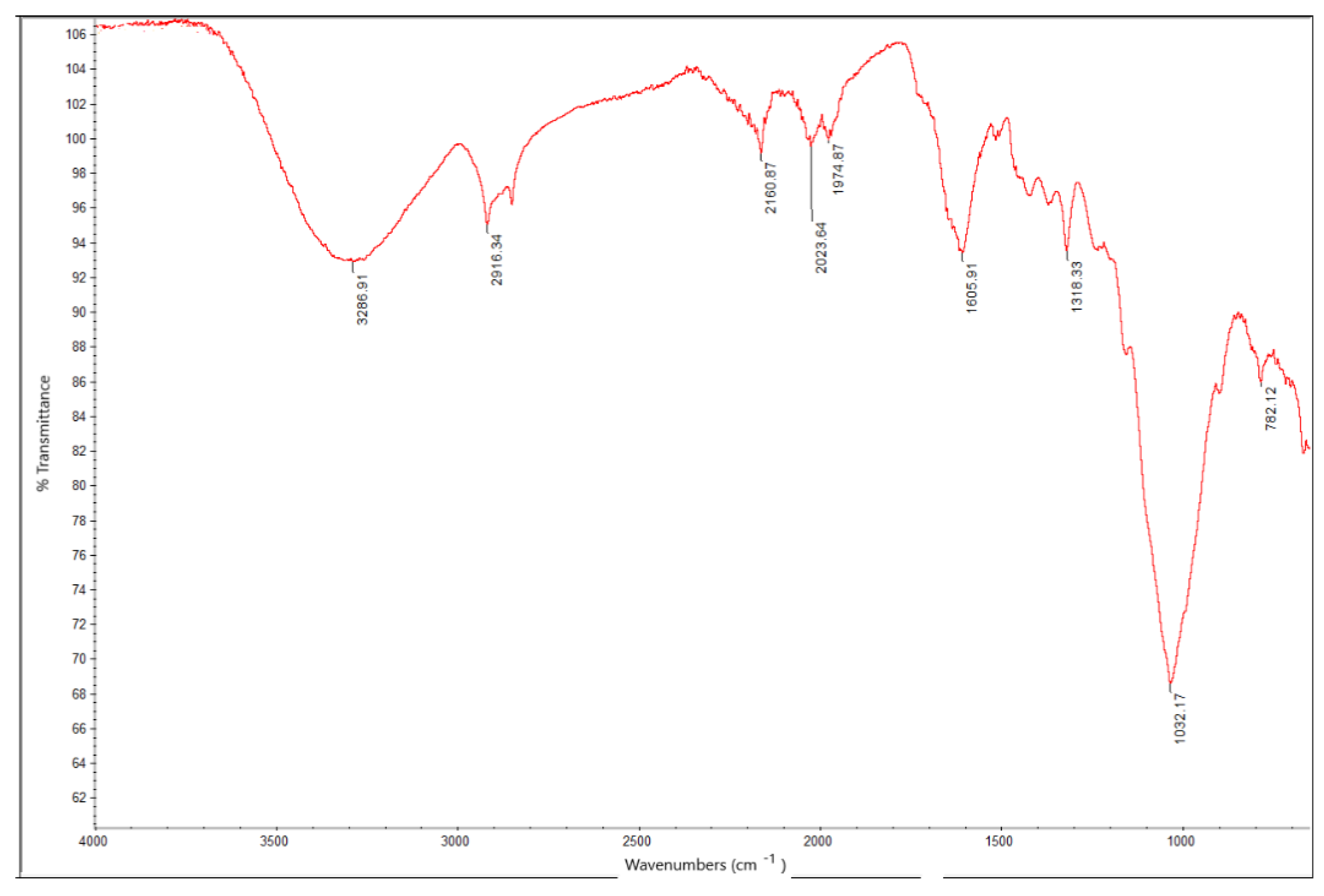

| Elemental Composition (Average wt %) | |
|---|---|
| Carbon | 28.22 |
| Oxygen | 53.53 |
| Silicon | 17.00 |
| Potassium | 0.60 |
| Magnesium | 0.05 |
| Calcium | 0.45 |
| Sulphur | 0.11 |
| Chlorine | 0.03 |
| Chemical Component | Composition (mg/g) | |||
|---|---|---|---|---|
| [56] | [56] | [57] | [37] | |
| Cellulose | 334.7 | 292 | 344 | 322.4 |
| Hemicellulose | 210.3 | 201 | 293 | 213.4 |
| Lignin | 267 | 307 | 192 | 214.4 |
| Extractives | 18.2 | |||
| Water | 81.1 | |||
| Mineral ash | 150.5 | |||
| Component | Composition (mg/g) | |||||
|---|---|---|---|---|---|---|
| [58] | [59] | [54] | [60] | [61] | [37] | |
| SiO2 | 924 | 946.4 | 884.7 | 810.9 | 920 | 945 |
| Al2O3 | 3 | 0.5 | 2.9 | |||
| Fe2O3 | 4 | 2.3 | 4 | 1.4 | 1 | <5 |
| CaO | 7 | 18.9 | 18 | 10.7 | 12.8 | 2.5 |
| MgO | 3 | 9.6 | 7.1 | 7.5 | 3.7 | 2.3 |
| Na2O | 0.7 | 3.9 | 2.6 | 0.5 | 7.8 | |
| K2O | 25.4 | 5.8 | 25 | 13.9 | 21.9 | 11.8 |
| SO3 | 14.5 | 9.4 | 6 | |||
| loss on ignition, LOI | 23.1 | 87.3 | 34.3 | |||
| Zn (ppm) | 18.2 | 32.28 | ||||
| Mn (ppm) | 52.24 | 56.44 | ||||
| Cu (ppm) | 32.17 | 16.98 | ||||
| Cd (ppm) | 0.48 | 0.49 | ||||
| Cellulose | Hemicellulose | Lignin | Ash | |
|---|---|---|---|---|
| Rice Straw | 320–386 | 197–357 | 135–223 | 100–170 |
| Elemental Composition (mg/g) | |||||
|---|---|---|---|---|---|
| Carbon | Oxygen | Silicon | Potassium | Chlorine | Calcium |
| 470.84 | 467.9 | 15.4 | 33.1 | 9.9 | 2.9 |
| Proximate Analysis | |
|---|---|
| Moisture | 83 |
| Volatile matter | 431.2 |
| Fixed carbon | 301.4 |
| Ash (oxides of Ca, Mn, Si, Fe, Mg, etc.) | 184.4 |
| Elemental Composition (wt %) | |||||||
|---|---|---|---|---|---|---|---|
| Carbon | Oxygen | Silicon | Potassium | Magnesium | Phosphorus | Sulphur | Chlorine |
| 45.41 | 47.84 | 1.57 | 1.97 | 0.80 | 2.15 | 0.21 | 0.05 |
| Type of Adsorbent | Type of Water | Experimental Conditions | Adsorption Capacity (mg/g) | References |
|---|---|---|---|---|
| Rice straw biochar | Aqueous solution | pH = 3 | 10.9 | [81] |
| Temperature = 298 K | ||||
| Initial concentration = 60–160 mg/L | ||||
| Surface area = 2.59 m2/g | ||||
| Adsorbent = 1 g | ||||
| Activated carbon-derived Mn treated rice straw- activated carbon | Aqueous solution | pH = 2.0 | 15.9 | [110] |
| Temperature = 298 K | ||||
| Initial concentration = 20.0 mg/L | ||||
| Surface area = 123 m2/g | ||||
| Alumina impregnated onto the activated carbon derived from rice straw | Aqueous solution | pH = 6.1 | 10 | [111] |
| Initial concentration = 50.0 mg/L | ||||
| Surface area = 151 m2/g | ||||
| Adsorbent = 4 g | ||||
| La/Fe/Al oxides loaded rice straw biochar | Aqueous solution | pH = 3–11 | 111.1 | [81] |
| Temperature = 298 K | ||||
| Initial concentration = 60–160 mg/L | ||||
| Surface area = 95.36 m2/g | ||||
| Adsorbent = 1 g |
| Type of Adsorbent | Type of Water | Type of Arsenic | Experimental Conditions | Maximum Removal (%) | Adsorption Capacity | References |
|---|---|---|---|---|---|---|
| Rice straw biochar | Aqueous solution | As(V) | pH = 6.5 | - | 25.6 μg/g | [132] |
| Temperature = 298 K | ||||||
| reaction time = 120 min | ||||||
| Initial concentration = 100 μg/L | ||||||
| Surface area = 133 m2/g | ||||||
| Adsorbent = 0.2 g/L | ||||||
| Iron-modified biochar derived from rice straw | Aqueous solution | As(V) | pH = 5 | 91.5 | 28.5 mg/g | [119] |
| Temperature = 295 K | ||||||
| Reaction time = 120 min | ||||||
| Initial concentration = 4 mg/L | ||||||
| Iron-modified rice straw derived from biochar | Aqueous solution | As(V) | Temperature = 298 K | - | 30.6 μg/g | [132] |
| Surface area = 68.9 m2/g | ||||||
| Pristine/rice straw-derived biochar | Aqueous solution | As(V) | Temperature = 298 K | - | 25.6 μg/g | [132] |
| Surface area = 68.9 m2/g | ||||||
| Rice polish/rice bran (column bed method) | Aqueous solution | As(III) | pH = 7 | - | 67 μg/g | [131] |
| Temperature = 298 K | ||||||
| Initial concentration = 1000 μg/L | ||||||
| Flow rate = 1.66 mL/min | ||||||
| Column diameter = 2 cm | ||||||
| Bed height = 25 cm | ||||||
| Rice polish/rice bran (column bed method) | Aqueous solution | As(V) | pH = 4 | - | 79 μg/g | [131] |
| Temperature = 298 K | ||||||
| Initial concentration = 1000 μg/L | ||||||
| Flow rate = 1.66 mL/min | ||||||
| Column diameter = 2 cm | ||||||
| Bed height = 25 cm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collivignarelli, M.C.; Sorlini, S.; Milanese, C.; Illankoon, W.A.M.A.N.; Caccamo, F.M.; Calatroni, S. Rice Industry By-Products as Adsorbent Materials for Removing Fluoride and Arsenic from Drinking Water—A Review. Appl. Sci. 2022, 12, 3166. https://doi.org/10.3390/app12063166
Collivignarelli MC, Sorlini S, Milanese C, Illankoon WAMAN, Caccamo FM, Calatroni S. Rice Industry By-Products as Adsorbent Materials for Removing Fluoride and Arsenic from Drinking Water—A Review. Applied Sciences. 2022; 12(6):3166. https://doi.org/10.3390/app12063166
Chicago/Turabian StyleCollivignarelli, Maria Cristina, Sabrina Sorlini, Chiara Milanese, W. A. M. A. N. Illankoon, Francesca Maria Caccamo, and Silvia Calatroni. 2022. "Rice Industry By-Products as Adsorbent Materials for Removing Fluoride and Arsenic from Drinking Water—A Review" Applied Sciences 12, no. 6: 3166. https://doi.org/10.3390/app12063166
APA StyleCollivignarelli, M. C., Sorlini, S., Milanese, C., Illankoon, W. A. M. A. N., Caccamo, F. M., & Calatroni, S. (2022). Rice Industry By-Products as Adsorbent Materials for Removing Fluoride and Arsenic from Drinking Water—A Review. Applied Sciences, 12(6), 3166. https://doi.org/10.3390/app12063166