Novel Chitosan Derivatives and Their Multifaceted Biological Applications
Abstract
:1. Introduction
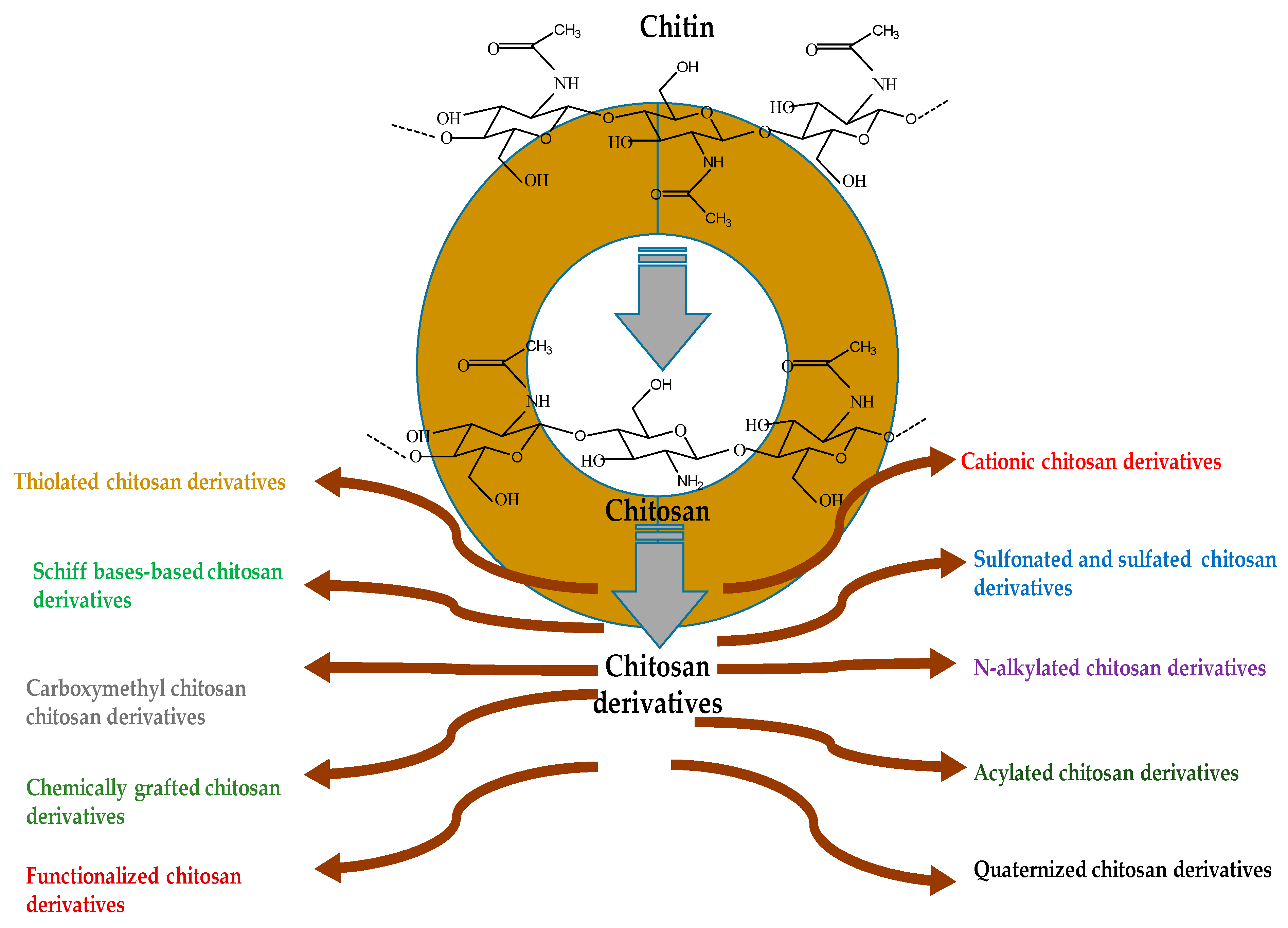
2. Novel ChDs
3. Biological Applications of ChDs
3.1. Antimicrobial Applications
3.2. Antioxidant Applications
3.3. Miscellaneous Bio-Applications
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Grotz, E.; Tateosian, N.; Amiano, N.; Cagel, M.; Bernabeu, E.; Chiappetta, D.A.; Moretton, M.A. Nanotechnology in tuberculosis: State of the art and the challenges ahead. Pharm. Res. 2018, 35, 213. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.L.; Tan, Y.C.; Liang, H.F.; Sung, H.W. In vivo biocompatibility and degradability of a novel injectable-chitosan-based implant. Biomaterials 2002, 23, 181–191. [Google Scholar] [CrossRef]
- Tamer, M.T.; Valachova, K.; Hassan, M.A.; Omer, A.M.; El-Shafeey, M.; Mohy Eldin, M.S.; Soltes, L. Chitosan/hyaluronan/edaravone membranes for anti-inflammatory wound dressing: In vitro and in vivo evaluation studies. Mater. Sci. Eng. C 2018, 90, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Omer, A.; Tamer, T.; Hassan, M.; Rychter, P.; Eldin, M.M.; Koseva, N. Development of amphoteric alginate/aminated chitosan coated microbeads for oral protein delivery. Int. J. Biol. Macromol. 2016, 92, 362–370. [Google Scholar] [CrossRef]
- Yildirim-Aksoy, M.; Beck, B.H. Antimicrobial activity of chitosan and a chitosan oligomer against bacterial pathogens of warmwater fish. J. Appl. Microbiol. 2017, 122, 1570–1578. [Google Scholar] [CrossRef]
- Valachová, K.; Tamer, T.M.; Eldin, M.M.; Šoltés, L. Radical-Scavenging activity of glutathione, chitin derivatives and their combination. Chem. Pap. 2016, 70, 820–827. [Google Scholar] [CrossRef]
- Xie, F.; Ding, R.L.; He, W.F.; Liu, Z.J.; Fu, S.Z.; Wu, J.B.; Yang, L.L.; Lin, S.; Wen, Q.L. In vivo antitumor effect of endostatin-loaded chitosan nanoparticles combined with paclitaxel on Lewis lung carcinoma. Drug Deliv. 2017, 24, 1410–1418. [Google Scholar] [CrossRef] [Green Version]
- Archana, D.; Dutta, J.; Dutta, P.K. Evaluation of chitosan nano dressing for wound healing: Characterization, in vitro and in vivo study. Int. J. Biol. Macromol. 2013, 57, 193–203. [Google Scholar] [CrossRef]
- Tamer, T.M.; Collins, M.N.; Valachová, K.; Hassan, M.A.; Omer, A.M.; Eldin, M.S.M.; Švík, K.; Jurčík, R.; Ondruška, Ľ.; Biró, C.; et al. MitoQ loaded chitosan-hyaluronan composite membranes for wound healing. Materials 2018, 11, 569. [Google Scholar] [CrossRef] [Green Version]
- Morsy, R.; Ali, S.S.; El-Shetehy, M. Development of hydroxyapatite-chitosan gel sunscreen combating clinical multidrug-resistant bacteria. J. Mol. Struct. 2017, 1143, 251–258. [Google Scholar] [CrossRef]
- Mahmoudzadeh, M.; Fassihi, A.; Dorkoosh, F.; Heshmatnejad, R.; Mahnam, K.; Sabzyan, H.; Sadeghi, A. Elucidation of molecular mechanisms behind the self-assembly behavior of chitosan amphiphilic derivatives through experiment and molecular modeling. Pharm. Res. 2015, 32, 3899–3915. [Google Scholar] [CrossRef] [PubMed]
- Rwei, S.P.; Lien, C.C. Synthesis and Rheological Characterization of Sulfonated Chitosan Solutions. Colloid Polym. Sci. 2014, 292, 785–795. [Google Scholar] [CrossRef]
- Afshar, H.A.; Ghaee, A. Preparation of aminated chitosan/alginate scaffold containing hallosyte nanotubes with improved cell attachment. Carbohydr. Polym. 2016, 151, 1120–1131. [Google Scholar] [CrossRef]
- Bukzem, A.L.; Signini, R.; Dos Santos, D.M.; Liao, L.M.; Ascheri, D.P. Optimization of carboxymethyl chitosan synthesis using response surface methodology and desirability function. Int. J. Biol. Macromol. 2016, 85, 615–624. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Reis, R.L.; Mano, J.F. Graft copolymerized chitosan—Present status and applications. Carbohydr. Polym. 2005, 62, 142–158. [Google Scholar] [CrossRef] [Green Version]
- Tamer, T.M.; Omer, A.M.; Hassan, M.A.; Hassan, M.E.; Sabet, M.M.; Eldin, M.M. Development of thermo-sensitive poly N-isopropyl acrylamide grafted chitosan derivatives. J. Appl. Pharm. Sci. 2015, 5, 1–6. [Google Scholar]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef]
- Sahariah, P.; Maásson, M. Antimicrobial chitosan and chitosan derivatives: A review of the structure–activity relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Sashiwa, H.; Aiba, S. Chemically modified chitin and chitosan as biomaterials. Prog. Polym. Sci. 2004, 29, 887–908. [Google Scholar] [CrossRef]
- Chen, C.; Tao, S.; Qiu, X.; Ren, X.; Hu, S. Long-Alkane-Chain modified N-phthaloyl chitosan membranes with controlled permeability. Carbohydr. Polym. 2013, 91, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Meng, X.; Xing, R.; Liu, S.; Chen, X.; Qin, Y.; Yu, H.; Li, P. Design, synthesis and antimicrobial activity of 6- N -substituted chitosan derivatives. Bioorgan. Med. Chem. Lett. 2016, 26, 4548–4551. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, F.; Yang, R. Synthesis and characterization of chitosan derivatives with dual-antibacterial functional groups. Int. J. Biol. Macromol. 2015, 75, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Kurita, K.; Sugita, K.; Kodaira, N.; Hirakawa, M.; Yang, J. Preparation and evaluation of trimethylsilylated chitin as a versatile precursor for facile chemical modifications. Biomacromolecules 2005, 6, 1414–1418. [Google Scholar] [CrossRef]
- Benediktsdottir, B.E.; Gaware, V.S.; Runarsson, O.V.; Jonsdottir, S.; Jensen, K.J.; Masson, M. Synthesis of N,N,N-trimethyl chitosan homopolymer and highly substituted N-alkyl-N,N-dimethyl chitosan derivatives with the aid of di-tert-butyldimethylsilyl chitosan. Carbohydr. Polym. 2011, 86, 1451–1460. [Google Scholar] [CrossRef]
- Jain, A.; Gulbake, A.; Shilpi, S.; Jain, A.; Hurkat, P.; Jain, S.K. A new horizon in modifcations of chitosan: Syntheses and applications. Crit. Rev. Ther. Drug Carrier Syst. 2013, 30, 91–181. [Google Scholar] [CrossRef]
- Sieval, A.B.; Thanou, M.; Kotzé, A.F.; Verhoef, J.C.; Brussee, J.; Junginger, H.E. Preparation and NMR characterization of highly substituted N-trimethyl chitosan chloride. Carbohydr. Polym. 1998, 36, 157–165. [Google Scholar] [CrossRef]
- Verheul, R.J.; Amidi, M.; van der Wal, S.; van Riet, E.; Jiskoot, W.; Hennink, W.E. Synthesis, characterization and in vitro biological properties of O-methyl free N,N,N-trimethylated chitosan. Biomaterials 2008, 29, 3642–3649. [Google Scholar] [CrossRef]
- Hamman, J.H.; Stander, M.; Kotze, A.F. Effect of the degree of quaternisation of N-trimethyl chitosan chloride on absorption enhancement: In vivo evaluation in rat nasal epithelia. Int. J. Pharm. 2002, 232, 235–242. [Google Scholar] [CrossRef]
- Thanou, M.; Verhoef, J.C.; Marbach, P.; Junginger, H.E. Intestinal absorption of octreotide: N-trimethyl chitosan chloride (TMC) ameliorates the permeability and absorption properties of the somatostatin analogue in vitro and in vivo. J. Pharm. Sci. 2000, 89, 951–957. [Google Scholar] [CrossRef]
- Thanou, M.; Nihot, M.T.; Jansen, M.; Verhoef, J.C.; Junginger, H.E. Mono-N-carboxymethyl chitosan (MCC), a polyampholytic chitosan derivative, enhances the intestinal absorption of low molecular weight heparin across intestinal epithelia in vitro and in vivo. J. Pharm. Sci. 2001, 90, 38–46. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization and potential application of chitosan, chitosan derivatives and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.M.; Du, Y.M.; Huang, R.H.; Gao, L.P. Preparation and modification of N-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan chloride nanoparticle as a protein carrier. Biomaterials 2003, 24, 5015–5022. [Google Scholar] [CrossRef]
- Tomita, M.; Hayashi, M.; Horie, T.; Ishizawa, T.; Awazu, S. Enhancement of colonic drug absorption by transcellular permeation route. Pharm. Res. 1988, 5, 786–789. [Google Scholar] [CrossRef]
- Tien, C.L.; Lacroix, M.; Ispas-Szabo, P.; Mateescu, M.A. N-Acylated chitosan: Hydrophobic matrices for controlled drug release. J. Control. Release 2003, 93, 1–13. [Google Scholar] [CrossRef]
- Sonia, T.A.; Sharma, C.P. Chitosan and Its Derivatives for Drug Delivery Perspective. Adv. Polym. Sci. 2011, 243, 23–54. [Google Scholar]
- Schnurch, A.B.; Hornof, M.; Zoidl, T. Thiolated polymers–thiomers: Synthesis and in vitro evaluation of chitosan-2-iminothiolane conjugates. Int. J. Pharm. 2003, 260, 229–237. [Google Scholar] [CrossRef]
- Hornof, M.D.; Kast, C.E.; Bernkop-Schnürch, A. In Vitro Evaluation of the Viscoelastic Properties of Chitosan-Thioglycolic Acid Conjugates. Eur. J. Pharm. Biopharm. 2003, 55, 185–190. [Google Scholar] [CrossRef]
- Roldo, M.; Hornof, M.; Caliceti, P.; Bernkop-Schnürch, A. Mucoadhesive thiolated chitosans as platforms for oral controlled drug delivery: Synthesis and in vitro evaluation. Eur. J. Pharm. Biopharm. 2004, 57, 115–121. [Google Scholar] [CrossRef]
- Yin, L.; Ding, J.; He, C.; Cui, L.; Tang, C.; Yin, C. Biomaterials Drug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanoparticles in oral insulin delivery. Biomaterials 2009, 30, 5691–5700. [Google Scholar] [CrossRef]
- Ubaidulla, U.; Khar, R.K.; Ahmad, F.J.; Sultana, Y.; Panda, A.K. Development and characterization of chitosan succinate microspheres for the improved oral bioavailability of insulin. J. Pharm. Sci. 2007, 96, 3010–3023. [Google Scholar] [CrossRef] [PubMed]
- Ubaidulla, U.; Sultana, Y.; Ahmed, F.J.; Khar, R.K.; Panda, A.K. Chitosan phthalate microspheres for oral delivery of insulin: Preparation, characterization, and in vitro evaluation. Drug Deliv. 2007, 14, 19–23. [Google Scholar] [CrossRef]
- Rekha, M.R.; Sharma, C.P. Synthesis and evaluation of lauryl succinyl chitosan particles towards oral insulin delivery and absorption. J. Control. Release 2009, 135, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Casettari, L.; Castagnino, E.; Stolnik, S.; Lewis, A.; Howdle, S.M.; Illum, L. Surface Characterisation of Bioadhesive PLGA/Chitosan Microparticles Produced by Supercritical Fluid Technology. Pharm. Res. 2011, 28, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- Tamer, A.M.; Hassan, M.A.; Omer, M.A.; Baset, W.M.A.; Hassan, M.E.; El-Shafeey, M.E.A.; Mohy Elding, M.S. Synthesis, characterization and antimicrobial evaluation of two aromatic chitosan Schiff base derivatives. Process Biochem. 2016, 51, 1721–1730. [Google Scholar] [CrossRef]
- Malik, S.; Nema, B. Antimicrobial activities of schiff bases: A review. Int. J. Theor. Appl. Phys. 2016, 8, 28–30. [Google Scholar]
- Rui, L.; Xie, M.; Hu, B.; Zhou, L.; Saeeduddin, M.; Zeng, X. Enhanced solubility and antioxidant activity of chlorogenic acid-chitosan conjugates due to the conjugation of chitosan with chlorogenic acid. Carbohydr. Polym. 2017, 170, 206–216. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Zhou, Y.-N.; Li, X.-Y.; Huang, J.; Wahid, F.; Zhong, C.; Chu, L.-Q. Continuous production of antibacterial carboxymethyl chitosan-zinc supramolecular hydrogel fiber using a double-syringe injection device. Int. J. Biol. Macromol. 2020, 156, 252–261. [Google Scholar] [CrossRef]
- Lei, M.; Huang, W.; Sun, J.; Shao, Z.; Duan, W.; Wu, T.; Wang, Y. Synthesis, characterization, and performance of carboxymethyl chitosan with different molecular weight as additive in water-based drilling fluid. J. Mol. Liq. 2020, 310, 113135. [Google Scholar] [CrossRef]
- Shieh, Y.; Chen, Y.; Don, T. Carboxymethyl chitosan has sensitive two-way CO2-responsive hydrophilic/hydrophobic feature. Carbohydr. Polym. 2020, 241, 116408. [Google Scholar] [CrossRef]
- Lyu, R.L.; Xia, T.; Liang, C.; Zhang, C.; Li, Z.Q.; Wang, L.C.; Wang, Y.; Wu, M.; Luo, X.G.; Ma, J.Y.; et al. MPEG grafted alkylated carboxymethyl chitosan as a high-efficiency demulsifier for O/W crude oil emulsions. Carbohydr. Polym. 2020, 241, 116309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, X.; Li, Y.; Qiao, C.; Wang, S.; Wang, X.; Xu, C.; Yang, H.; Li, T. Enhancement of a zwitterionic chitosan derivative on mechanical properties and antibacterial activity of carboxymethyl cellulosebased films. Int. J. Biol. Macromol. 2020, 159, 1197–1205. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Dutta, P.K.; Koh, J. A physico-chemical and biological study of novel chitosan-chloroquinoline derivative for biomedical applications. Int. J. Biol. Macromol. 2011, 49, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Patale, R.L.; Patravale, V.B. O,N-carboxymethyl chitosan-zinc complex: A novel chitosan complex with enhanced antimicrobial activity. Carbohyd. Polym. 2011, 85, 105–110. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Y.; Cheng, S.; Huang, N.; Leng, Y. Syntheses of Novel Chitosan Derivative with Excellent Solubility, Anticoagulation, and Antibacterial Property by Chemical Modification. J. Appl. Polym. Sci. 2012, 124, 2641–2648. [Google Scholar] [CrossRef]
- Kumar, S.; Koh, J. Physiochemical, optical and biological activity of chitosan-chromone derivative for biomedical applications. Int. J. Mol. Sci. 2012, 13, 6102–6116. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Koh, J.; Kim, H.; Gupta, M.K.; Dutta, P.K. A new chitosan-thymine conjugate: Synthesis, characterization and biological activity. Int. J. Biol. Macromol. 2012, 50, 493–502. [Google Scholar] [CrossRef]
- Niemczyk, A.; El Fray, M. Novel chitosan derivatives as films with an antimicrobial effect. Prog. Chem. Appl. Chitin Its Deriv. 2013, XVIII, 59–66. [Google Scholar]
- Mohamed, N.A.; Mohamed, R.R.; Seoudi, R.S. Synthesis and characterization of some novel antimicrobial thiosemicarbazone O-carboxymethyl chitosan derivatives. Int. J. Biol. Macromol. 2014, 63, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.; Rabea, E.I.; Taktak, N.E. Antimicrobial and inhibitory enzyme activity of N-(benzyl) and quaternary N-(benzyl) chitosan derivatives on plant pathogens. Carbohydr. Polym. 2014, 111, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, A.; Katas, H.; Samsudin, S.N.; Zin, N.M. Regioselective Sequential Modification of Chitosan via Azide-Alkyne Click Reaction: Synthesis, Characterization, and Antimicrobial Activity of Chitosan Derivatives and Nanoparticles. PLoS ONE 2015, 10, e0123084. [Google Scholar] [CrossRef] [Green Version]
- Abdelwahab, H.; Hassan, S.; Yacout, G.; Mostafa, M.; El Sadek, M. Synthesis and biological evaluation of new imine- and amino-chitosan derivatives. Polymers 2015, 7, 2690–2700. [Google Scholar] [CrossRef]
- Pathania, D.; Gupta, D.; Kothiyal, N.C.; Sharma, G.; Eldesoky, G.E.; Naushad, M. Preparation of a novel chitosan-g-poly(acrylamide)/Zn nanocomposite hydrogel and its applications for controlled drug delivery of ofloxacin. Int. J. Biol. Macromol. 2016, 84, 340–348. [Google Scholar] [CrossRef]
- Moghadas, B.; Dashtimoghadam, E.; Mirzadeh, H.; Seidi, F.; Hasani-Sadrabadi, M.M. Novel chitosan-based nanobiohybrid membranes for wound dressing applications. RSC Adv. 2016, 6, 7701–7711. [Google Scholar] [CrossRef]
- Zhu, D.; Cheng, H.; Li, J.; Zhang, W.; Shen, Y.; Chen, S.; Ge, Z.; Chen, S. Enhanced water-solubility and antibacterial activity of novel chitosan derivatives modified with quaternary phosphonium salt. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 79–84. [Google Scholar] [CrossRef]
- Dragostin, O.M.; Samal, S.K.; Dash, M.; Lupascu, F.; Pânzariu, A.; Tuchilus, C.; Ghetu, N.; Danciu, M.; Dubruel, P.; Pieptu, D.; et al. New antimicrobial chitosan derivatives for wound dressing applications. Carbohydr. Polym. 2016, 141, 28–40. [Google Scholar] [CrossRef]
- Martins, D.B.; Nasario, F.D.; Silva-Goncalves, L.C.; Oliveira Tiera, V.A.; Arcisio-Miranda, M.; Tiera, M.J.; Dos Santos Cabrera, M.P. Chitosan derivatives targeting lipid bilayers: Synthesis, biological activity and interaction with model membranes. Carbohydr. Polym. 2018, 181, 1213–1223. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.A.; Omer, A.M.; Abbas, E.; Baset, W.M.A.; Tamer, T.M. Preparation, physicochemical characterization and antimicrobial activities of novel two phenolic chitosan Schiff base derivatives. Sci. Rep. 2018, 8, 11416. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.S.; Kenawy, E.R.; Sonbol, F.I.; Sun, J.; Al-Etewy, M.; Ali, A.; Huizi, L.; El-Zawawy, A.N. Pharmaceutical Potential of a Novel Chitosan Derivative Schiff Base with Special Reference to Antibacterial, Anti-Biofilm, Antioxidant, Anti-Inflammatory, Hemocompatibility and Cytotoxic Activities. Pharm. Res. 2018, 36, 5. [Google Scholar] [CrossRef] [PubMed]
- Kritchenkov, A.S.; Egorov, A.R.; Kurasova, M.N.; Volkova, O.V.; Meledina, T.V.; Lipkan, N.A.; Tskhovrebov, A.G.; Kurliuk, A.V.; Shakola, T.V.; Dysin, A.P.; et al. Novel non-toxic high efficient antibacterial azido chitosan derivatives with potential application in food coatings. Food Chem. 2019, 301, 125247. [Google Scholar] [CrossRef] [PubMed]
- Gularte, M.S.; Anghinoni, J.M.; Abenante, L.; Voss, G.T.; de Oliveira, R.L.; Vaucher, R.A.; Luchese, C.; Wilhelm, E.A.; Lenardão, E.J.; Fajardo, A.R. Synthesis of chitosan derivatives with organoselenium and organosulfur compounds: Characterization, antimicrobial properties and application as biomaterials. Carbohydr. Polym. 2019, 219, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Saud, R.; Pokhrel, S.; Yadav, P.N. Synthesis, characterization and antimicrobial activity of maltol functionalized chitosan derivatives. J. Macromol. Sci. Part A 2019, 56, 375–383. [Google Scholar] [CrossRef]
- Salama, A.; Hasanin, M.; Hesemann, P. Synthesis and antimicrobial properties of new chitosan derivatives containing guanidinium groups. Carbohydr. Polym. 2020, 241, 116363. [Google Scholar] [CrossRef]
- Menazea, A.A.; Eid, M.M.; Ahmed, M.K. Synthesis, characterization, and evaluation of antimicrobial activity of novel chitosan/tigecycline composite. Int. J. Biol. Macromol. 2020, 147, 194–199. [Google Scholar] [CrossRef]
- Affes, S.; Maalej, H.; Aranaz, I.; Acosta, N.; Heras, Á.; Nasri, M. Enzymatic production of low-Mw chitosan-derivatives: Characterization and biological activities evaluation. Int. J. Biol. Macromol. 2020, 144, 279–288. [Google Scholar] [CrossRef]
- Sedghi, R.; Gholami, M.; Shaabani, A.; Saber, M.; Niknejad, H. Preparation of Novel Chitosan Derivative Nanofibers for Prevention of Breast Cancer Recurrence. Eur. Polym. J. 2020, 123, 109421. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Egorov, A.R.; Artemjev, A.A.; Kritchenkov, I.S.; Volkova, O.V.; Kiprushkina, E.I.; Zabodalova, L.A.; Suchkova, E.P.; Yagafarov, N.Z.; Tskhovrebov, A.G. Novel heterocyclic chitosan derivatives and their derived nanoparticles: Catalytic and antibacterial properties. Int. J. Biol. Macromol. 2020, 149, 682–692. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Egorov, A.R.; Artemjev, A.A.; Kritchenkov, I.S.; Volkova, O.V.; Kurliuk, A.V.; Shakola, T.V.; Rubanik, V.V.; Tskhovrebov, A.G.; Yagafarov, N.Z.; et al. Ultrasound-assisted catalyst-free thiol-yne click reaction in chitosan chemistry: Antibacterial and transfection activity of novel cationic chitosan derivatives and their based nanoparticles. Int. J. Biol. Macromol. 2020, 143, 143–152. [Google Scholar] [CrossRef]
- Hamed, A.A.; Abdelhamid, I.A.; Saad, G.R.; Elkady, N.A.; Elsabee, M.Z. Synthesis, characterization and antimicrobial activity of a novel chitosan schiff bases based on heterocyclic moieties. Int. J. Biol. Macromol. 2020, 153, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Nadia, Q.H.; Mohsin, O.M.; Luqman, E.M. Synthesis and Biological Evaluation of Three New Chitosan Schiff Base Derivatives. ACS Omega 2020, 5, 13948–13954. [Google Scholar]
- Iqbal, D.N.; Shafiq, S.; Khan, S.M.; Ibrahim, S.M.; Abubshait, S.A.; Nazir, A.; Abbas, M.; Iqbal, M. Novel chitosan/guar gum/PVA hydrogel: Preparation, characterization and antimicrobial activity evaluation. Int. J. Biol. Macromol. 2020, 164, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Pokhrel, S.; Yadav, P.N. Novel chitosan derivatives of 2-imidazolecarboxaldehyde and 2-thiophenecarboxaldehyde and their antibacterial activity. J. Macromol. Sci. A 2020, 57, 703–710. [Google Scholar] [CrossRef]
- Kandile, N.G.; Mohamed, H.M. New chitosan derivatives inspired on heterocyclic anhydride of potential bioactive for medical applications. Int. J. Biol. Macromol. 2021, 182, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.; Hou, Z.; Vikhe, Y.S.; Thappeta, K.R.V.; Marimuthu, K.; De, P.P.; Ng, O.T.; Li, P.; Zhu, Y.; Pethe, K.; et al. Antimicrobial effect of a novel chitosan derivative and its synergistic effect with antibiotics. ACS Appl. Mater. Interfaces 2021, 13, 3237–3245. [Google Scholar] [CrossRef]
- Wei, X.Y.; Xia, W.; Zhou, T. Antibacterial activity and action mechanism of a novel chitosan oligosaccharide derivative against dominant spoilage bacteria isolated from shrimp Penaeus vannamei. Lett. Appl. Microbiol. 2021, 74, 268–276. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Rabea, E.I.; Rogge, T.M.; Stevens, C.V.; Smagghe, G.; Steurbaut, W.; Hofte, M. Synthesis and fungicidal activity of new N,O-acyl chitosan derivatives. Biomacromolecules 2004, 5, 589–595. [Google Scholar] [CrossRef]
- Badawy, M.E. Chemical modification of chitosan: Synthesis and biological activity of new heterocyclic chitosan derivatives. Polym. Int. 2008, 57, 254–261. [Google Scholar] [CrossRef]
- Li, R.; Guo, Z.; Jiang, P. Synthesis, characterization, and antifungal activity of novel quaternary chitosan derivatives. Carbohydr. Res. 2010, 345, 1896–1900. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Xing, R.; Liu, S.; Li, K.; Meng, X.; Li, R.; Cui, J.; Li, B.; Li, P. Novel thiosemicarbazone chitosan derivatives: Preparation, characterization, and antifungal activity. Carbohydr. Polym. 2012, 87, 2664–2670. [Google Scholar] [CrossRef]
- Li, Q.; Ren, J.; Dong, F.; Feng, Y.; Gu, G.; Guo, Z. Synthesis and antifungal activity of thiadiazole-functionalized chitosan derivatives. Carbohydr. Res. 2013, 373, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Xing, R.; Liu, S.; Li, K.; Hu, L.; Yu, H.; Chen, X.; Li, P. Synthesis of chitosan derivative with diethyldithiocarbamate and its antifungal activity. Int. J. Biol. Macromol. 2014, 65, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, S.; Qin, Y.; Xing, R.; Chen, X.; Li, K.; Li, P. Synthesis of novel pyrimethanil grafted chitosan derivatives with enhanced antifungal activity. BioMed Res. Int. 2016, 2016, 8196960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Tan, W.; Zhang, C.; Gu, G.; Guo, Z. Synthesis of water soluble chitosan derivatives with halogeno-1,2,3-triazole and their antifungal activity. Int. J. Biol. Macromol. 2016, 91, 623–629. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, J.; Luan, F.; Wei, L.; Chen, Y.; Dong, F.; Li, Q.; Guo, Z. Design, synthesis of novel chitosan derivatives bearing quaternary phosphonium salts and evaluation of antifungal activity. Int. J. Biol. Macromol. 2017, 102, 704–711. [Google Scholar] [CrossRef]
- Tan, W.; Li, Q.; Dong, F.; Chen, Q.; Guo, Z. Preparation and characterization of novel cationic chitosan derivatives bearing quaternary ammonium and phosphonium salts and assessment of their antifungal properties. Molecules 2017, 22, 1438. [Google Scholar] [CrossRef] [Green Version]
- Fan, Z.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, K.; Li, P. Synthesis, characterization, and antifungal evaluation of diethoxyphosphoryl polyaminoethyl chitosan derivatives. Carbohydr. Polym. 2018, 190, 1–11. [Google Scholar] [CrossRef]
- Tan, W.; Li, Q.; Dong, F.; Zhang, J.; Luan, F.; Wei, L.; Chen, Y.; Guo, Z. Novel cationic chitosan derivative bearing 1,2,3-triazolium and pyridinium: Synthesis, characterization, and antifungal property. Carbohydr. Polym. 2018, 182, 180–187. [Google Scholar] [CrossRef]
- Yang, G.; Jin, O.; Xu, C.; Fan, S.; Wang, C.; Xie, P. Synthesis, characterization and antifungal activity of coumarin-functionalized chitosan derivatives. Int. J. Biol. Macromol. 2018, 106, 179–184. [Google Scholar] [CrossRef]
- Liu, W.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, K.; Li, P. Synthesis, characterization and antifungal efficacy of chitosan derivatives with triple quaternary ammonium groups. Int. J. Biol. Macromol. 2018, 114, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, Q.; Chen, Y.; Zhang, J.; Mi, Y.; Dong, F.; Lei, C.; Guo, Z. Enhanced antioxidant and antifungal activity of chitosan derivatives bearing 6-O-imidazole-based quaternary ammonium salts. Carbohydr. Polym. 2019, 206, 493–503. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, W.; Wei, L.; Chen, Y.; Mi, Y.; Sun, X.; Li, Q.; Dong, F.; Guo, Z. Synthesis of urea-functionalized chitosan derivatives for potential antifungal and antioxidant applications. Carbohydr. Polym. 2019, 215, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Tan, W.; Wang, G.; Li, Q.; Dong, F.; Guo, Z. The antioxidant and antifungal activity of chitosan derivatives bearing Schiff bases and quaternary ammonium salts. Carbohydr. Polym. 2019, 226, 115256. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, J.; Mi, Y.; Dong, F.; Li, Q.; Guo, Z. Enhanced Antifungal Activity of Novel Cationic Chitosan Derivative Bearing Triphenylphosphonium Salt via Azide-Alkyne Click Reaction. Int. J. Biol. Macromol. 2020, 165, 1765–1772. [Google Scholar] [CrossRef]
- Zhang, J.J.; Mi, Y.Q.; Sun, X.; Chen, Y.; Miao, Q.; Tan, W.Q.; Li, Q.; Dong, F.; Guo, Z.Y. Improved antioxidant and antifungal activity of chitosan derivatives bearing urea groups. Starch-Stärke 2020, 72, 5–6. [Google Scholar] [CrossRef]
- Mi, Y.; Zhang, J.; Chen, Y.; Sun, X.; Tan, W.; Li, Q.; Guo, Z. New synthetic chitosan derivatives bearing benzenoid/heterocyclic moieties with enhanced antioxidant and antifungal activities. Carbohydr. Polym. 2020, 249, 116847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tan, W.; Li, Q.; Dong, F.; Guo, Z. Synthesis and Characterization of N,N,N-trimethyl-O-(ureidopyridinium)acetyl Chitosan Derivatives with Antioxidant and Antifungal Activities. Mar. Drugs 2020, 18, 163. [Google Scholar] [CrossRef] [Green Version]
- Dragostin, O.M.; Samal, S.K.; Lupascu, F.; Pânzariu, A.; Dubruel, P.; Lupascu, D.; Tuchiluș, C.; Vasile, C.; Profire, L. Development and Characterization of Novel Films Based on Sulfonamide-Chitosan Derivatives for Potential Wound Dressing. Int. J. Mol. Sci. 2015, 16, 29843–29855. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Zhang, C.; Tan, W.; Gu, G.; Guo, Z. Novel Amino-Pyridine Functionalized Chitosan Quaternary Ammonium Derivatives: Design, Synthesis, and Antioxidant Activity. Molecules 2017, 22, 156. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Li, Q.; Tan, W.; Dong, F.; Luan, F.; Guo, Z. Synthesis, Characterization, and the Antioxidant Activity of Double Quaternized Chitosan Derivatives. Molecules 2017, 22, 501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Sun, X.; Gu, G.; Guo, Z. Novel water soluble chitosan derivatives with 1,2,3-triazolium and their free radical-scavenging activity. Mar. Drugs 2018, 16, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Tan, W.; Wei, L.; Dong, F.; Li, Q.; Guo, Z. Synthesis, Characterization, and Antioxidant Evaluation of Novel Pyridylurea-Functionalized Chitosan Derivatives. Polymers 2019, 11, 951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Zhang, J.; Chen, Y.; Mi, Y.; Tan, W.; Li, Q.; Dong, F.; Guo, Z. Synthesis, characterization, and the antioxidant activity of carboxymethyl chitosan derivatives containing thiourea salts. Polymers 2019, 11, 1810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.W.; Cheng, H.; Jiang, Q.X.; Xia, W.S. The characterization and biological activities of synthetic N, O-selenized chitosan derivatives. Int. J. Biol. Macromol. 2021, 173, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Su, W.; He, C.; Duan, C. Novel chitosan-based fluorescent materials for the selective detection and adsorption of Fe in water and consequent bio-imaging applications. Talanta 2012, 97, 456–461. [Google Scholar] [CrossRef]
- Sakai, S.; Yamada, Y.; Zenke, T.; Kawakami, K. Novel chitosan derivative soluble at neutral pH and in-situ gellable via peroxidase-catalyzed enzymatic reaction. J. Mater. Chem. 2009, 19, 230–235. [Google Scholar] [CrossRef]
- Rasad, M.S.B.A.; Halim, A.S.; Hashim, K.; Rashid, A.H.A.; Yusof, N.; Shamsuddin, S. In vitro evaluation of novel chitosan derivatives sheet and paste cytocompatibility on human dermal fibroblasts. Carbohydr. Polym. 2010, 79, 1094–1100. [Google Scholar] [CrossRef]
- Wang, L.; Xu, X.; Guo, S.; Peng, Z.; Tang, T. Novel water soluble phosphonium chitosan derivatives: Synthesis, characterization and cytotoxicity studies. Int. J. Biol. Macromol. 2011, 48, 375–380. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Shen, Y.; Guo, S. Synthesis and in vitro cellular evaluation of novel anti-tumor norcantharidin-conjugated chitosan derivative. Int. J. Biol. Macromol. 2013, 62, 418–425. [Google Scholar] [CrossRef]
- Samadi, F.Y.; Mohammadi, Z.; Yousefi, M.; Majdejabbari, S. Synthesis of raloxifene-chitosan conjugate: A novel chitosan derivative as a potential targeting vehicle. Int. J. Biol. Macromol. 2016, 82, 599–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakimi, S.; Mortazavian, E.; Mohammadi, Z.; Samadi, F.Y.; Samadikhah, H.; Taheritarigh, S.; Tehrani, N.R.; Rafiee-Tehranig, M. Thiolated methylated dimethylaminobenzyl chitosan: A novel chitosan derivative as a potential delivery vehicle. Int. J. Biol. Macromol. 2017, 95, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Packialakshmi, P.; Gobinath, P.; Ali, D.; Alarifi, S.; Alsaiari, N.S.; Idhayadhulla, A.; Surendrakumar, R. Synthesis and Characterization of Aminophosphonate Containing Chitosan Polymer Derivatives: Investigations of Cytotoxic Activity and in Silico Study of SARS-CoV-19. Polymers 2021, 13, 1046. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Zhang, C.; Ping, Q.; Yu, L. A series of novel chitosan derivatives: Synthesis, characterization and micellar solubilization of paclitaxel. Carbohydr. Polym. 2007, 68, 781–792. [Google Scholar] [CrossRef]
- Kim, C.H.; Choi, J.W.; Chun, H.J.; Choi, K.S. Synthesis of chitosan derivatives with quaternary ammonium salt and their antibacterial activity. Polym. Bull. 1997, 38, 387–393. [Google Scholar] [CrossRef]
- Morimoto, M.; Saimoto, H.; Usui, H.; Okamoto, Y.; Minami, S.; Shigemasa, Y. Biological activities of carbohydrate-branched chitosan derivatives. Biomacromolecules 2001, 2, 1133–1136. [Google Scholar] [CrossRef]
- Yin, T.; Liu, Y.; Yang, M.; Wang, L.; Zhou, J.; Huo, M. Novel Chitosan Derivatives with Reversible Cationization and Hydrophobicization for Tumor Cytoplasm-Specific Burst Co-delivery of siRNA and Chemotherapeutics. ACS Appl. Mater. Interfaces 2020, 12, 14770–14783. [Google Scholar] [CrossRef]
- Shakil, M.S.; Mahmud, K.M.; Sayem, M.; Niloy, M.S.; Halder, S.K.; Hossen, M.S.; Uddin, M.F.; Hasan, M.A. Using Chitosan or Chitosan Derivatives in Cancer Therapy. Polysaccharides 2021, 2, 795–816. [Google Scholar] [CrossRef]
- Salahuddin, N.; Elbarbary, A.A.; Salem, M.L.; Elksass, S. Antimicrobial and antitumor activities of 1,2,4-triazoles/polypyrrole chitosan core shell nanoparticles. J. Phys. Org. Chem. 2017, 30, e3702. [Google Scholar] [CrossRef]
- Cui, Z.; Ni, N.C.; Wu, J.; Du, G.Q.; He, S.; Yau, T.M.; Weisel, R.D.; Sung, H.W.; Li, R.K. Polypyrrole-Chitosan conductive biomaterialsynchronizes cardiomyocyte contraction and improves myocardial electrical impulse propagation. Theranostics 2018, 8, 2752–2764. [Google Scholar] [CrossRef]
- Jiang, M.; Ouyang, H.; Ruan, P.; Zhao, H.; Pi, Z.; Huang, S.; Yi, P.; Crepin, M. Chitosan derivatives inhibit cell proliferation andinduce apoptosis in breast cancer cells. Anticancer Res. 2011, 31, 1321–1328. [Google Scholar] [PubMed]
- Jiang, Z.; Han, B.; Li, H.; Yang, Y.; Liu, W. Carboxymethyl chitosan represses tumor angiogenesis in vitro and in vivo. Carbohydr. Polym. 2015, 129, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Mendis, E.; Rajapakse, N.; Kim, S.K. Strong electronic charge as an important factor for anticancer activity of chitooligosaccharides (COS). Life Sci. 2006, 78, 2399–2408. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, J.; Chen, X.; Tian, J.; Li, L.; Zhao, M.; Jiao, Y.; Zhou, C. Effect of chitooligosaccharides on cyclin D1, bcl-xl and bcl-2 mRNA expression in A549 cells using quantitative PCR. Chin. Sci. Bull. 2011, 56, 1629. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, H.S.; Yadav, P.N. Anticancer Activity of Chitosan, Chitosan Derivatives, and Their Mechanism of Action. Int. J. Biomater. 2018, 2018, 2952085. [Google Scholar] [CrossRef] [Green Version]
- Harish Prashanth, K.; Tharanathan, R. Depolymerized products of chitosan as potent inhibitors of tumor-induced angiogenesis. Biochim. Biophys. Acta Gen. Subj. 2005, 1722, 22–29. [Google Scholar] [CrossRef]
- Maeda, Y.; Kimura, Y. Antitumor effects of various low-molecular-weight chitosans are due to increased natural killer activity of intestinal intraepithelial lymphocytes in sarcoma 180-bearing mice. J. Nutr. 2004, 134, 945–950. [Google Scholar] [CrossRef]
- Qin, C.; Du, Y.; Xiao, L.; Gao, X. Enzymic preparation of water-soluble chitosan and their antitumor activity. Int. J. Biol. Macromol. 2002, 31, 111–117. [Google Scholar] [CrossRef]
- Wang, S.L.; Lin, H.T.; Liang, T.W.; Chen, Y.J.; Yen, Y.H.; Guo, S.P. Reclamation of chitinous materials by bromelain for the preparation of antitumor and antifungal materials. Bioresour. Technol. 2008, 99, 4386–4393. [Google Scholar] [CrossRef]
- Yamada, S.; Ganno, T.; Ohara, N.; Hayashi, Y. Chitosan monomer accelerates alkaline phosphatase activity on human osteoblastic cells under hypofunctional conditions. J. Biomed. Mater. Res. A 2007, 83, 290–295. [Google Scholar] [CrossRef]
| Novel Chitosan Derivative Type | Biological Application | Bio-Functionality | Reference |
|---|---|---|---|
| Chitosan–chloroquinoline derivative | Antibacterial (AB) | AB activity against Escherichia coli and Staphylococcus aureus, moderate AF activity against Candida albicans. | [55] |
| Antifungal (AF) | |||
| O,N-carboxymethyl chitosan–zinc complex chitosan–zinc complex | Antibacterial (AB) | AB activity against Escherichia coli and Staphylococcus aureus. | [56] |
| Hydroxyethyl chitosan | Antibacterial (AB) | AB activity against E coli and Enterococcus and anticoagulation | [57] |
| Anticoagulation | |||
| Chitosan-chromone derivative | Antibacterial (AB) | AB activity against Escherichia coli. non-toxic to mouse embryonic fibroblast cells. | [58] |
| Cytotoxicity | |||
| Chitosan–thymine conjugate | Antibacterial (AB) | AB activity against E coli and S aureus and AF activity against Aspergillus niger. no toxicity on mouse embryonic fibroblast cell line (NIH 3T3) but high toxicity on human liver cancer cell line (HepG2). | [59] |
| Antifungal (AF) | |||
| Cytotoxicity | |||
| Chitosan acylation with linoleic and dilinoleic acid | Antibacterial (AB) | AB assays in vitro exhibited AB effect in direct contact with the material. | [60] |
| p-methoxybenzaldehyde thiosemicarbazone/o-hydroxybenzaldehyde thiosemi-carbazone/p-chlorobenzaldehyde thiosemicarbazone O-Carboxymethyl-chitosan | Antibacterial (AB) | AB activity against Bacillus subtilis, E coli, and S aureus and AF activity against Aspergillus fumigatus, Candida albicans, and Geotrichum candidum. | [61] |
| Antifungal (AF) | |||
| N-(benzyl) chitosan derivatives (C1-C9) QuaternaryN-(benzyl) chitosan derivatives (QC1-QC9) | Antibacterial (AB) | QC derivatives had high AB activity against Agrobacterium tumefaciens and Erwinia carotovora and AF activity against Botrytis cinerea, Botryodiplodia theobromae, Fusarium oxysporum, and Phytophthora infestans. | [62] |
| Antifungal (AF) | |||
| O-quaternary ammonium N-acyl thiourea chitosan | Antibacterial (AB) | AB activity against Bacillus subtilis, E coli, Pseudomonas aeruginosa and Staphylococcus aureus | [23] |
| (Chitosan O-(adamantane) triazolylcarbamate, chitosan O-(benzoyl) triazolylcarbamate, chitosan O-(1-methylbenzene) triazolylcarbamate, chitosan O-(1-methyl phenyl sulphide) triazolylcarbamate, and chitosan O-((R) (1-methyl)-1-Boc-pyrrolidine) triazolylcarbamate) and their nanoparticles (NPs). | Antibacterial (AB) | AB activity, AF activity and non-toxic to fibroblast cell lines (V79) and human hepatic cell lines (WRL68). | [63] |
| Antifungal (AF) | |||
| cytotoxicity | |||
| Imine- and amino-chitosan derivatives | Antibacterial (AB) | AB activity against Gram (+) and Gram (−) bacteria, AF activity against Aspergillus fumigates and highly toxic to MCF-7 (breast cancer cells), HCT-116 (colon cancer cells) and HepG-2 (hepatocellular cancer cells). | [64] |
| Antifungal (AF) | |||
| Anticancer | |||
| Chitosan-g-poly(acrylamide)/Zn nanocomposite | Antibacterial (AB) | Inhibited the growth of E coli. controlled drug delivery. | [65] |
| Drug delivery | |||
| Nanohybrid films based on chitosan and biofunctionalized montmorillonite (MMT) with chitosan sulfate chains (SMMT) | Antibacterial (AB) | AB activity against E coli and no cytotoxicity to L929 fibroblasts cells. | [66] |
| Cytotoxicity | |||
| N-quaternary phosphonium chitosan derivatives (N-QPCS 11, 12, 14 and 21) | Antibacterial (AB) | AB activity against E coli and S aureus. | [67] |
| Chitosan-aminoacetyl-sulfamethoxydiazine Chitosan-aminoacetyl-sulfadiazine Chitosan-aminoacetyl-sulfadimethoxine Chitosan-aminoacetyl-sulfamethoxazole Chitosan-aminoacetyl-sulfamerazine Chitosan-aminoacetyl-sulfisoxazole | Antibacterial (AB) | AB activity against Bacillus subtilis, E coli, Sarcina lutea, and S aureus. AF against Candida albicans, Candida glabrata and Candida sake. low toxicity, against mouse fibroblasts (L929) cells, except chitosan-aminoacetyl-sulfamerazine. | [68] |
| Antifungal (AF) | |||
| Cytotoxicity | |||
| Methylated derivatives, CH30 and CH50 | Antibacterial (AB) | AB activity against Escherichia coli and Staphylococcus aureus and CH30 do not affect the viability of human cervical carcinoma cells, whereas CH50 had high toxicity to human cervical carcinoma cells. | [69] |
| Cytotoxicity | |||
| Phenolic chitosan Schiff base derivatives | Antibacterial (AB) | AB effect against Gram (+) bacteria than chitosan with 4-dimethylaminobenzaldehyde and AF activity against Candida albicans and low cytotoxicity against fibroblast cells. | [70] |
| Antifungal (AF) | |||
| Cytotoxicity | |||
| Methyl acrylate chitosan bearing p-nitrobenzaldehyde Schiff base | Antibacterial (AB) | AB effect against Gram (+) bacteria. anti-biofilm activity against MDR-PA-09 strain, excellent proteinase inhibitory activity (90.3%), antioxidant activity, low cytotoxicity, hemolytic activity | [71] |
| Anti-biofilm | |||
| Anti-inflammatory | |||
| Antioxidant | |||
| Cytotoxicity | |||
| Hemocompatibility | |||
| N-(2-azidoethyl)chitosans N-(3-azido-2-hydroxypropyl)chitosans | Antibacterial (AB) | AB against Escherichia coli and Staphylococcus aureus low cytotoxicity. | [72] |
| cytotoxicity | |||
| Schiff bases of chitosan derivatives containing organoselenium and organosulfur | Antibacterial (AB) | AB activity against Escherichia coli and Staphylococcus aureus and AF activity against Candida albicans. | [73] |
| Antifungal (AF) | |||
| Chitosan-maltol (CS-maltol) and CS-ethyl maltol derivatives | Antibacterial (AB) | AB activity against Escherichia coli. | [74] |
| N-guanidinium chitosan acetate/-guanidinium chitosan chloride/N-guanidinium chitosan (N,N′-dicyclohexyl) chloride, N-guanidinium chitosan (N-(3-dimethylaminopropyl)-N’-ethyl hydrochloride) chloride | Antibacterial (AB) | AB activity against Bacillus subtilis, Pseudomonas aeruginosa, and Staphylococcus aureus. AF activity against Candida albicans. | [75] |
| Antifungal (AF) | |||
| Chitosan/Tigecycline composite (1–5) | Antibacterial (AB) | The AB activity of chitosan/tigecycline composite depends on the levels of Tigecycline. Increasing the concentration of Tigecycline increase the AB activity against Staphylococcus aureus. | [76] |
| Chitosan-depolymerization products | Antibacterial (AB) | AB activity against both Gram (+) and Gram (−) bacteria. AF compared to untreated chitosan. High antioxidant activity | [77] |
| Antifungal (AF) | |||
| Antioxidant | |||
| Tetramethyl urea thiosemicarbazone carboxymethyl chitosan nanofibers | Antibacterial (AB) | AB activity against Escherichia coli and Staphylococcus aureus. Strong AC activity against 4T1 breast cancer cells. | [78] |
| Anticancer (AC) | |||
| Heterocyclic (1,2,4-oxadiazoline) chitosan derivatives and their derived nanoparticles (NPs) | Antibacterial (AB) | AB activity against Escherichia coli and Staphylococcus aureus. Low toxicity against human embryonic kidney 293 cells. | [79] |
| Cytotoxicity | |||
| Betaine chitosan derivatives and nanoparticles (NPs) based on them | Antibacterial (AB) | AB activity against Escherichia coli and Staphylococcus aureus low toxicity against human embryonic kidney 293 cells. | [80] |
| Cytotoxicity | |||
| 1-phenyl-3-(thiophene-2-yl)-1H-pyrazole-4-carbaldehyde/ 1-phenyl-3-(furan-2-yl)-1H-pyrazole-4-carbaldehyde/1-phenyl-3-(pyridine-3-yl)-1H-pyrazole-4-carbaldehyde | Antibacterial (AB) | AB activity against Escherichia coli, Klebsiella pneumonia, Staphylococcus, and Streptococcus mutans. AF activity against Aspergillus fumigatus and Candida albicans. non-toxic to normal retinal cells. | [81] |
| Antifungal (AF) | |||
| Cytotoxicity | |||
| Coupling of chitosan Schiff base derivatives with 2-chloroquinoline-3-carbaldehyde, quinazoline-6-carbaldehyde, and oxazole-4-carbaldehyde | Antibacterial (AB) | AB activity against Staphylococcus and Streptococcus mutans. AF activity against Aspergillus fumigates and Candida albicans. non-toxic to mouse fibroblast cells. | [82] |
| Antifungal (AF) | |||
| Cytotoxicity | |||
| Chitosan/poly(vinyl alcohol)/guar gum blends | Antibacterial (AB) | AB against Bacillus subtilis, Escherichia coli, Pasteurella multocida and Staphylococcus aureus. | [83] |
| Chitosan-2-imidazolecarboxaldehyde Chitosan-2-thiophenecarboxaldehyde | Antibacterial (AB) | AB activity against Escherichia coli. | [84] |
| Chitosan derivatives inspired heterocyclic anhydride (Chitosan derivative 6, 7 and 8) | Antibacterial (AB) | AB activity against eight different pathogens highest enzymatic inhibitory activity. highest toxicity against the Vero cell line. | [85] |
| Enzyme inhibitory | |||
| Cytotoxicity | |||
| 2,6-diamino chitosan | Antibacterial (AB) | AB activity against both Gram-positive and Gram-negative bacteria. | [86] |
| Chitosan oligosaccharide derivative | Antibacterial (AB) | AB activity against Aeromonas spp., Pseudomonas spp., and Shewanella putrefaciens | [87] |
| N,O-acyl chitosan (NOAC) derivatives (1–18) | Antifungal (AF) | The AF activity of NOAC derivatives is higher than that of unmodified chitosan | [88] |
| N-heterocyclic chitosan derivatives | Antifungal (AF) | N-[(5-methylfuran-2-yl)methyl] chitosan AF against Pyricularia grisea and N-(benzo[d][1,3]dioxol-5-ylmethyl) chitosan and N-(methyl-4H-chromen-4-one) chitosan AF against Fusarium oxysporum and Pythium debaryanum. significant growth inhibition and antifeedant activity against the larvae of Spodoptera littoralis. | [89] |
| Insecticidal | |||
| 4-(5-chloro-2-hydroxybenzylideneamino)-pyridine/4-(5-bromo-2-hydroxybenzylideneamino)-pyridine | Antifungal (AF) | AF activities against Cladosporium cucumerinum, Colletotrichum lagenarium, Monilinia fructicola, and Fusarium oxysporum | [90] |
| Phenylaldehyde thiosemicarbazone chitosan o-hydroxyphenylaldehyde thiosemicarbazone chitosan/p-methoxyphenylaldehyde thiosemicarbazone chitosan | Antifungal (AF) | AF activity against four phytopathogenic fungi. | [91] |
| 1,3,4-thiadiazole/2-methyl-1,3,4-thiadiazole/ 2-phenyl-1,3,4-thiadiazole | Antifungal (AF) | AF activity against Colletotrichum lagenarium, Monilinia fructicola, and Monilinia fructicola than chitosan and other derivatives. | [92] |
| Diethyl dithiocarbamate chitosan | Antifungal (AF) | AF activity against Gloeosporium theae sinensis followed by Alternaria porri and Stemphylium solani. | [93] |
| Pyrimethanil grafted chitosan derivatives (1–3) | Antifungal (AF) | AF activity against Rhizoctonia solani and Gibberella zeae | [94] |
| 1,2,3-triazole (TCTS, CTCTS and BTCTS) | Antifungal (AF) | The AF activity against Colletotrichum lagenarium, Fusarium oxysporum f.sp. niveum and Fusarium oxysporum f.sp. cucumebrium | [95] |
| Tricyclohexylphosphonium acetyl chitosan chloride/Triphenylphosphonium acetyl chitosan chloride | Antifungal (AF) | AF activity against Colletotrichum lagenarium, Fusarium oxysporum, and Watermelon fusarium. | [96] |
| Cationic chitosan derivatives/quaternary ammonium and phosphonium salts | Antifungal (AF) | enhanced AF activity than chitosan. | [97] |
| Polyaminoethyl chitosan Schiff base derivatives Diethoxyphosphoryl polyaminoethyl chitosan Schiff base derivatives | Antifungal (AF) | AF activity against Botrytis cinerea, Fusarium solani and Phytophthora capsici. weak cytotoxicity against HepG2 cells. | [98] |
| Cytotoxicity | |||
| Chitosan derivative bearing 1,2,3-triazole and pyridine/cationic chitosan derivative possessing 1,2,3-triazolium and pyridinium | Antifungal (AF) | AF activity against Colletotrichum lagenarium. | [99] |
| Coumarin-functionalized chitosan derivatives (1–4) | Antifungal (AF) | AF activity against Alternaria solani, Fusarium moniliforme, and Fusarium oxysporum f.sp. vasinfectum. | [100] |
| Chitosan derivatives with triple quaternary ammonium groups using 3-aminopyridine and 3-amino-4-methylpyridine. | Antifungal (AF) | AF activity against Phytophthora capsica and Rhizoctonia solani. | [101] |
| Quaternary ammonium salt chitosan derivatives | Antifungal (AF) | AF activity against Botrytis cinerea and Gibberella zeae. Higher antioxidant activity than chitosan. good biocompatibility on HaCaT cells. | [102] |
| Antioxidant | |||
| Cytotoxicity | |||
| Novel urea-functionalized chitosan derivatives | Antifungal (AF) | Superior AF activity compared with chitosan. Five chitosan derivatives displayed higher antioxidant activities than chitosan. Weak cytotoxicity against L929 cells. | [103] |
| Antioxidant | |||
| Cytotoxicity | |||
| Chitosan derivatives bearing Schiff bases and quaternary ammonium salts. | Antifungal (AF) | AF activity against Botrytis cinerea, Fusarium oxysporum f.sp. cucumerium, and F. oxysporum f.sp. niveum. Greater antioxidant activity as compared with chitosan. | [104] |
| Antioxidant | |||
| Cationic chitosan derivative bearing triphenylphosphonium salt | Antifungal (AF) | Stronger antifungal activity against phytopathogens. | [105] |
| Chitosan derivatives bearing urea groups | Antifungal (AF) | The derivatives displayed higher AF activity than pristine chitosan. The derivatives possess enhanced radical (DPPH, hydroxyl, and superoxide) scavenging activity than pristine chitosan. 2,6-(3-(benzylureido)-pyridyl)acetyl chitosan chloride showed low toxicity to L929 cells. | [106] |
| Antioxidant | |||
| Cytotoxicity | |||
| Various carboxymethyl chitosan conjugates | Antifungal (AF) | Excellent AF activity against Colletotrichum lagenarium and Phomopsis asparagi. Enhanced DPPH and superoxide-radical scavenging activity. Low toxicity to L929 cells. | [107] |
| Antioxidant | |||
| Cytotoxicity | |||
| N,N,N-trimethyl-O-(ureidopyridinium)acetyl chitosan derivatives | Antifungal (AF) | AF activity against Botrytis cinerea and Phomopsis asparagus. Higher radical (DPPH, hydroxyl, and superoxide) scavenging activity than chitosan. | [108] |
| Antioxidant |
| Novel Chitosan Derivative Type | Biological Application | Bio-Functionality | Reference |
|---|---|---|---|
| Novel films based on sulfonamide-chitosan derivatives | Antioxidant | Stronger antioxidant properties than chitosan and other film derivatives. Highest swelling ratio. | [109] |
| Swelling Ratio | |||
| Amino-pyridine functionalized chitosan quaternary ammonium derivatives | Antioxidant | Stronger radical (DPPH and hydroxyl) scavenging activity than chitosan. | [110] |
| Double quaternized chitosan derivatives | Antioxidant | Stronger radical (DPPH, hydroxyl, and superoxide) scavenging activity. | [111] |
| Chitosan derivatives with 1,2,3-Triazolium | Antioxidant | Higher radical (DPPH, hydroxyl, and superoxide) scavenging activity than chitosan. | [112] |
| Pyridylurea-functionalized chitosan derivatives | Antioxidant | Enhanced antioxidant activity than N-pyridylurea chitosan derivatives. Low cytotoxicity to L929 cells. | [113] |
| Cytotoxicity | |||
| Carboxymethyl chitosan derivatives with thiourea salts | Antioxidant | Higher antioxidant activities. Viability of L929 cells not affected. | [114] |
| Cytotoxicity | |||
| N, O-selenized N-(2-carboxyethyl) chitosan 1, 2 and 3 | Antioxidant | Strong antioxidant properties Caco-2 cell viability unaffected | [115] |
| Cytotoxicity | |||
| Water-soluble chitosan with 4-fluorescein-carboxaldehyde/N-methyl-carbazole-3-aldehyde | Bio-imaging | Used to detect of Fe3+ in water and living MCF-7 cells. | [116] |
| Chitosan with phenolic hydroxyl groups | Cytotoxicity | Low toxicity to L929 cells. | [117] |
| Oligo-chitosan N,O-carboxymethylchitosan N-carboxymethyl-chitosan | Cytotoxicity | Excellent cytocompatibility and cell viability. | [118] |
| Water soluble phosphonium chitosan derivatives | Cytotoxicity | Low toxicity to L929 cell lines (mouse fibroblasts). | [119] |
| Norcantharidin-conjugated chitosan conjugate 1 Norcantharidin-conjugated chitosan conjugate 2 | Cytotoxicity | Conjugate 1 was more cytotoxic to the human gastric cancer cell line (MGC80-3) than conjugate 2. | [120] |
| Raloxifene–chitosan conjugate (Run 3 and 6) | Cytotoxicity | Both low toxicity to MCF-7 cells. | [121] |
| Thiolated methylated N-(4-N,N-dimethylaminobenzyl) chitosan | Cytotoxicity | Did not affect the viability of human embryonic kidney 293 cells. | [122] |
| Aminophosphonate-containing chitosan polymer derivatives | Cytotoxicity | Few moderately toxic to human liver cancer cell line (HepG2) while highly toxic to MCF-7 (breast cancer cells). | [123] |
| N-mPEG-N-octyl-O-sulfate chitosan derivatives | Drug delivery | The highest paclitaxel (3.94 mg/mL) was found in N-mPEG-N-octyl-O-sulfate chitosan. | [124] |
| Novel films based on sulfonamide-chitosan derivatives | Antioxidant | Stronger antioxidant properties than chitosan and other film derivatives. Highest swelling ratio. | [109] |
| Swelling Ratio | |||
| Amino-pyridine functionalized chitosan quaternary ammonium derivatives | Antioxidant | Stronger radical (DPPH and hydroxyl) scavenging activity than chitosan. | [110] |
| Double quaternized chitosan derivatives | Antioxidant | Stronger radical (DPPH, hydroxyl, and superoxide) scavenging activity. | [111] |
| Chitosan derivatives with 1,2,3-Triazolium | Antioxidant | Higher radical (DPPH, hydroxyl, and superoxide) scavenging activity than chitosan. | [112] |
| Pyridylurea-functionalized chitosan derivatives | Antioxidant | Enhanced antioxidant activity than N-pyridylurea chitosan derivatives. Low cytotoxicity to L929 cells. | [113] |
| Cytotoxicity |
| Novel ChDs | Cancer Type | Anticancer Application | References |
|---|---|---|---|
| Chitosan–thymine conjugate | Liver cancer | Antiproliferative activity towards HepG2 liver carcinoma cells, no cytotoxic activity on normal mouse fibroblast cells, confirming selective targeting of cancer cells | [59] |
| Polypyrrole–chitosan | Ehrlich ascites carcinoma, breast cancer | Active against Ehrlich ascites carcinoma (EAC) cells and MCF-7 breast cancer cells | [129,130] |
| Sulfated chitosan | Breast cancer | Blocks cell cycle and FGF-2 mediated phosphorylation ERK | [131] |
| Sulfated benzaldehyde chitosan | Breast cancer | Induces apoptosis and blocks FGF-2 mediated phosphorylation ERK | [131] |
| Carboxymethyl chitosan | Liver cancer | Antiangiogenic activity decreasing VEGF and stimulating immune activity | [132] |
| Quaternized amino chitooligosaccharides | Cervical cancer, colon cancer | Induces necrosis | [133] |
| Sulfated chitooligosaccharides: | Cervical cancer, colon cancer | Induces necrosis | [133] |
| Chitohexose | Cervical cancer, colon cancer | Downregulates cyclin D1 and bcl-xl mRNA induction of apoptosis | [134] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivanesan, I.; Hasan, N.; Kashif Ali, S.; Shin, J.; Gopal, J.; Muthu, M.; Oh, J.-W. Novel Chitosan Derivatives and Their Multifaceted Biological Applications. Appl. Sci. 2022, 12, 3267. https://doi.org/10.3390/app12073267
Sivanesan I, Hasan N, Kashif Ali S, Shin J, Gopal J, Muthu M, Oh J-W. Novel Chitosan Derivatives and Their Multifaceted Biological Applications. Applied Sciences. 2022; 12(7):3267. https://doi.org/10.3390/app12073267
Chicago/Turabian StyleSivanesan, Iyyakkannu, Nazim Hasan, Syed Kashif Ali, Juhyun Shin, Judy Gopal, Manikandan Muthu, and Jae-Wook Oh. 2022. "Novel Chitosan Derivatives and Their Multifaceted Biological Applications" Applied Sciences 12, no. 7: 3267. https://doi.org/10.3390/app12073267
APA StyleSivanesan, I., Hasan, N., Kashif Ali, S., Shin, J., Gopal, J., Muthu, M., & Oh, J.-W. (2022). Novel Chitosan Derivatives and Their Multifaceted Biological Applications. Applied Sciences, 12(7), 3267. https://doi.org/10.3390/app12073267








