Liquid Biopsy and Dielectrophoretic Analysis—Complementary Methods in Skin Cancer Monitoring
Abstract
:1. Introduction
2. Liquid Biopsy and Its Utility in Skin Cancer Monitoring
3. Circulating Tumor Cells—Relevance to Skin Cancers
3.1. From Origin to Metastasis—CTCs as Key Players of the Process
3.2. Advances in CTCs Detection
3.3. From CTCs to Other Important Components Obtained via Liquid Biopsy
4. The Role of Dielectrophoresis in the Characterization of Skin Tumor Cells
4.1. Theoretical Background
4.2. Clinical Applications
5. Conclusions and Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCC | basal cell carcinoma |
| CTCs | circulating tumor cells |
| ctDNA | circulating tumor deoxyribonucleic acid |
| DEP | dielectrophoresis |
| miRNA | micro ribonucleic acid |
| MM | malignant melanoma |
| NGS | next-generation sequencing |
| NMSC | non-melanoma skin cancers |
| PCR | polymerase chain reaction |
| SCC | squamous cell carcinoma |
References
- Leiter, U.; Keim, U.; Garbe, C. Epidemiology of Skin Cancer: Update 2019. Adv. Exp. Med. Biol. 2020, 1268, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Vita Mare, L.; Porumb-Andrese, E.; Monu, A.M.; Adam, M.G.; Gheuca Solovastru, L.; Vata, D. Update on Epidemiology of Non-Melanocytic Skin Tumors. Rev. Chim. 2019, 70, 3050–3052. [Google Scholar] [CrossRef]
- Katalinic, A.; Kunze, U.; Schäfer, T. Epidemiology of cutaneous melanoma and non-melanoma skin cancer in Schleswig-Holstein, Germany: Incidence, clinical subtypes, tumour stages and localization (epidemiology of skin cancer). Br. J. Dermatol. 2003, 149, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Samarasinghe, V.; Madan, V. Nonmelanoma skin Cancer. J. Cutan. Aesthet. Surg. 2012, 5, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Han, J.; Li, W.Q.; Li, T.; Qureshi, A.A. Basal-cell carcinoma incidence and associated risk factors in U.S. women and men. Am. J. Epidemiol. 2013, 178, 890–897. [Google Scholar] [CrossRef] [Green Version]
- Dika, E.; Scarfì, F.; Ferracin, M.; Broseghini, E.; Marcelli, E.; Bortolani, B.; Campione, E.; Riefolo, M.; Ricci, C.; Lambertini, M. Basal Cell Carcinoma: A Comprehensive Review. Int. J. Mol. Sci. 2020, 21, 5572. [Google Scholar] [CrossRef]
- Tillman, E.; Parekh, P.K.; Grimwood, R.E., Jr. Locally destructive metastatic basal cell carcinoma. Cutis 2019, 103, E23–E25. [Google Scholar]
- Teng, Y.; Yu, Y.; Li, S.; Huang, Y.; Xu, D.; Tao, X.; Fan, Y. Ultraviolet Radiation and Basal Cell Carcinoma: An Environmental Perspective. Front. Public Health 2021, 9, 666528. [Google Scholar] [CrossRef]
- Abi Karam, M.; Kourie, H.R.; Jalkh, N.; Mehawej, C.; Kesrouani, C.; Haddad, F.G.; Feghaly, I.; Chouery, E.; Tomb, R. Molecular profiling of basal cell carcinomas in young patients. BMC Med. Genomics 2021, 14, 187. [Google Scholar] [CrossRef]
- Parekh, V.; Seykora, J.T. Cutaneous Squamous Cell Carcinoma. Clin. Lab. Med. 2017, 37, 503–525. [Google Scholar] [CrossRef] [PubMed]
- Stang, A.; Khil, L.; Kajüter, H.; Pandeya, N.; Schmults, C.D.; Ruiz, E.S.; Karia, P.S.; Green, A.C. Incidence and mortality for cutaneous squamous cell carcinoma: Comparison across three continents. J. Eur. Acad. Dermatol. Venereol. 2019, 33 (Suppl. 8), 6–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobl, M.; Grinnell, M.; Phillips, A.; Abels, J.; Wysong, A. The Correlation Between Immunohistochemistry Findings and Metastasis in Squamous Cell Carcinoma: A Review. Dermatol. Surg. 2021, 47, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Green, A.C.; Olsen, C.M. Cutaneous squamous cell carcinoma: An epidemiological review. Br. J. Dermatol. 2017, 177, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Porumb-Andrese, E.; Scutariu, M.M.; Luchian, I.; Schreiner, T.G.; Mârtu, I.; Porumb, V.; Popa, C.G.; Sandu, D.; Ursu, R.G. Molecular Profile of Skin Cancer. Appl. Sci. 2021, 11, 9142. [Google Scholar] [CrossRef]
- Hasanovic, A.; Mus-Veteau, I. Targeting the Multidrug Transporter Ptch1 Potentiates Chemotherapy Efficiency. Cells 2018, 7, 107. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.D.; Haensel, D.; Gaddam, S.; Patel, T.; Atwood, S.X.; Sarin, K.Y.; Whitson, R.J.; McKellar, S.; Shankar, G.; Aasi, S.; et al. AP-1 and TGFß cooperativity drives non-canonical Hedgehog signaling in resistant basal cell carcinoma. Nat. Commun. 2020, 11, 5079. [Google Scholar] [CrossRef]
- Petrilli, R.; Eloy, J.O.; Saggioro, F.P.; Chesca, D.L.; de Souza, M.C.; Dias, M.V.S.; DaSilva, L.L.P.; Lee, R.J.; Lopez, R.F.V. Skin cancer treatment effectiveness is improved by iontophoresis of EGFR-targeted liposomes containing 5-FU compared with subcutaneous injection. J. Control. Release 2018, 283, 151–162. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef]
- Sandru, A.; Voinea, S.; Panaitescu, E.; Blidaru, A. Survival rates of patients with metastatic malignant melanoma. J. Med. Life 2014, 7, 572–576. [Google Scholar]
- Sample, A.; He, Y.Y. Mechanisms and prevention of UV-induced melanoma. Photodermatol. Photoimmunol. Photomed. 2018, 34, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.E.; Esfandiari, A.; Ho, Y.H.; Wang, N.; Mahdi, A.K.; Aptullahoglu, E.; Lovat, P.; Lunec, J. Targeting negative regulation of p53 by MDM2 and WIP1 as a therapeutic strategy in cutaneous melanoma. Br. J. Cancer 2018, 118, 495–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porumb-Andrese, E.; Ursu, R.G.; Ivanov, I.; Caruntu, I.-D.; Porumb, V.; Ferariu, D.; Damian, C.; Ciobanu, D.; Terinte, C.; Iancu, L.S. The BRAF V600E Mutation Detection by quasa Sensitive Real-Time PCR Assay in Northeast Romania Melanoma Patients. Appl. Sci. 2021, 11, 9511. [Google Scholar] [CrossRef]
- Kilic, A.; Kilic, A.; Kivanc, A.E.; Sisik, A. Biopsy Techniques for Skin Disease and Skin Cancer: A New Approach. J. Cutan. Aesthet. Surg. 2020, 13, 251–254. [Google Scholar] [CrossRef]
- Nischal, U.; Nischal, K.C.; Khopkar, U. Techniques of skin biopsy and practical considerations. J. Cutan. Aesthet. Surg. 2008, 1, 107–111. [Google Scholar] [CrossRef]
- Fattore, L.; Ruggiero, C.F.; Liguoro, D.; Castaldo, V.; Catizone, A.; Ciliberto, G.; Mancini, R. The Promise of Liquid Biopsy to Predict Response to Immunotherapy in Metastatic Melanoma. Front. Oncol. 2021, 11, 645069. [Google Scholar] [CrossRef]
- Kamińska, P.; Buszka, K.; Zabel, M.; Nowicki, M.; Alix-Panabières, C.; Budna-Tukan, J. Liquid Biopsy in Melanoma: Significance in Diagnostics, Prediction and Treatment Monitoring. Int. J. Mol. Sci. 2021, 22, 9714. [Google Scholar] [CrossRef]
- Poulet, G.; Massias, J.; Taly, V. Liquid Biopsy: General Concepts. Acta Cytol. 2019, 63, 449–455. [Google Scholar] [CrossRef]
- Lin, C.; Liu, X.; Zheng, B.; Ke, R.; Tzeng, C.M. Liquid Biopsy, ctDNA Diagnosis through NGS. Life 2021, 11, 890. [Google Scholar] [CrossRef]
- Stevenson, P.; Rodins, K. Improving diagnostic accuracy of skin biopsies. Aust. J. Gen. Pract. 2018, 47, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Lorente, D.; Olmos, D.; Mateo, J.; Dolling, D.; Bianchini, D.; Seed, G.; Flohr, P.; Crespo, M.; Figueiredo, I.; Miranda, S.; et al. Circulating tumour cell increase as a biomarker of disease progression in metastatic castration-resistant prostate cancer patients with low baseline CTC counts. Ann. Oncol. 2018, 29, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Hepp, P.; Andergassen, U.; Jäger, B.; Trapp, E.; Alunni-Fabbroni, M.; Friedl, T.W.; Hecker, N.; Lorenz, R.; Fasching, P.; Schneeweiss, A.; et al. Association of CA27.29 and Circulating Tumor Cells Before and at Different Times After Adjuvant Chemotherapy in Patients with Early-stage Breast Cancer—The SUCCESS Trial. Anticancer Res. 2016, 36, 4771–4776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, C.R.; Seagle, B.L.; Friedl, T.W.P.; Rack, B.; Lato, K.; Fink, V.; Cristofanilli, M.; Donnelly, E.D.; Janni, W.; Shahabi, S.; et al. Association of Circulating Tumor Cell Status With Benefit of Radiotherapy and Survival in Early-Stage Breast Cancer. JAMA Oncol 2018, 4, e180163. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Bode, A.M.; Dong, Z. Circulating tumor cells: Moving biological insights into detection. Theranostics 2017, 7, 2606–2619. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, V.; Verzoni, E.; Ratta, R.; Vismara, M.; Silvestri, M.; Montone, R.; Miodini, P.; Reduzzi, C.; Claps, M.; Sepe, P.; et al. Analysis of Single Circulating Tumor Cells in Renal Cell Carcinoma Reveals Phenotypic Heterogeneity and Genomic Alterations Related to Progression. Int. J. Mol. Sci. 2020, 21, 1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micalizzi, D.S.; Maheswaran, S.; Haber, D.A. A conduit to metastasis: Circulating tumor cell biology. Genes Dev. 2017, 31, 1827–1840. [Google Scholar] [CrossRef]
- Keller, L.; Pantel, K. Unravelling tumour heterogeneity by single-cell profiling of circulating tumour Cells. Nat. Rev. Cancer 2019, 19, 553–567. [Google Scholar] [CrossRef] [Green Version]
- Kiniwa, Y.; Nakamura, K.; Mikoshiba, A.; Ashida, A.; Akiyama, Y.; Morimoto, A.; Okuyama, R. Usefulness of monitoring circulating tumor cells as a therapeutic biomarker in melanoma with BRAF mutation. BMC Cancer 2021, 21, 287. [Google Scholar] [CrossRef]
- Hong, X.; Sullivan, R.J.; Kalinich, M.; Kwan, T.T.; Giobbie-Hurder, A.; Pan, S.; LiCausi, J.A.; Milner, J.D.; Nieman, L.T.; Wittner, B.S.; et al. Molecular signatures of circulating melanoma cells for monitoring early response to immune checkpoint therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 2467–2472. [Google Scholar] [CrossRef] [Green Version]
- Lucci, A.; Hall, C.S.; Patel, S.P.; Narendran, B.; Bauldry, J.B.; Royal, R.E.; Karhade, M.; Upshaw, J.R.; Wargo, J.A.; Glitza, I.C.; et al. Circulating Tumor Cells and Early Relapse in Node-positive Melanoma. Clin. Cancer Res. 2020, 26, 1886–1895. [Google Scholar] [CrossRef]
- De Giorgi, V.; Pinzani, P.; Salvianti, F.; Panelos, J.; Paglierani, M.; Janowska, A.; Grazzini, M.; Wechsler, J.; Orlando, C.; Santucci, M.; et al. Application of a filtration- and isolation-by-size technique for the detection of circulating tumor cells in cutaneous melanoma. J. Investig. Dermatol. 2010, 130, 2440–2447. [Google Scholar] [CrossRef] [PubMed]
- Aya-Bonilla, C.A.; Morici, M.; Hong, X.; McEvoy, A.C.; Sullivan, R.J.; Freeman, J.; Calapre, L.; Khattak, M.A.; Meniawy, T.; Millward, M.; et al. Detection and prognostic role of heterogeneous populations of melanoma circulating tumour Cells. Br. J. Cancer 2020, 122, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Swennenhuis, J.F.; van Dalum, G.; Zeune, L.L.; Terstappen, L.W. Improving the CellSearch® system. Expert Rev. Mol. Diagn. 2016, 16, 1291–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolazzo, C.; Gradilone, A.; Loreni, F.; Raimondi, C.; Gazzaniga, P. EpCAMlow Circulating Tumor Cells: Gold in the Waste. Dis. Markers 2019, 2019, 1718920. [Google Scholar] [CrossRef] [Green Version]
- De Wit, S.; van Dalum, G.; Lenferink, A.T.; Tibbe, A.G.; Hiltermann, T.J.; Groen, H.J.; van Rijn, C.J.; Terstappen, L.W. The detection of EpCAM(+) and EpCAM(-) circulating tumor Cells. Sci. Rep. 2015, 5, 12270. [Google Scholar] [CrossRef] [Green Version]
- Kulasinghe, A.; Kenny, L.; Perry, C.; Thiery, J.P.; Jovanovic, L.; Vela, I.; Nelson, C.; Punyadeera, C. Impact of label-free technologies in head and neck cancer circulating tumour cells. Oncotarget 2016, 7, 71223–71234. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Mao, X.; Imrali, A.; Syed, F.; Mutsvangwa, K.; Berney, D.; Cathcart, P.; Hines, J.; Shamash, J.; Lu, Y.J. Optimization and Evaluation of a Novel Size Based Circulating Tumor Cell Isolation System. PLoS ONE 2015, 10, e0138032. [Google Scholar] [CrossRef] [Green Version]
- Tang, F. Fabrication of perforated polyethylene microfiltration membranes for circulating tumor cells separation by thermal nanoimprInt. method. Appl. Phys. A Mater. Sci. Process. 2019, 125, 55. [Google Scholar] [CrossRef]
- Galanzha, E.I.; Menyaev, Y.A.; Yadem, A.C.; Sarimollaoglu, M.; Juratli, M.A.; Nedosekin, D.A.; Foster, S.R.; Jamshidi-Parsian, A.; Siegel, E.R.; Makhoul, I.; et al. In vivo liquid biopsy using Cytophone platform for photoacoustic detection of circulating tumor cells in patients with melanoma. Sci. Transl. Med. 2019, 11, eaat5857. [Google Scholar] [CrossRef]
- Lemaire, C.A.; Liu, S.Z.; Wilkerson, C.L.; Ramani, V.C.; Barzanian, N.A.; Huang, K.W.; Che, J.; Chiu, M.W.; Vuppalapaty, M.; Dimmick, A.M.; et al. Fast and Label-Free Isolation of Circulating Tumor Cells from Blood: From a Research Microfluidic Platform to an Automated Fluidic Instrument, VTX-1 Liquid Biopsy System. SLAS Technol. 2018, 23, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Wurdinger, T.; In’t Veld, S.G.J.G.; Best, M.G. Platelet RNA as Pan-Tumor Biomarker for Cancer Detection. Cancer Res. 2020, 80, 1371–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ambrosi, S.; Nilsson, R.J.; Wurdinger, T. Platelets and tumor-associated RNA transfer. Blood 2021, 137, 3181–3191. [Google Scholar] [CrossRef] [PubMed]
- Heinhuis, K.M.; In’t Veld, S.G.J.G.; Dwarshuis, G.; van den Broek, D.; Sol, N.; Best, M.G.; Coevorden, F.V.; Haas, R.L.; Beijnen, J.H.; van Houdt, W.J.; et al. RNA-Sequencing of Tumor-Educated Platelets, a Novel Biomarker for Blood-Based Sarcoma Diagnostics. Cancers 2020, 12, 1372. [Google Scholar] [CrossRef] [PubMed]
- Meldolesi, J. Exosomes and Ectosomes in Intercellular Communication. Curr. Biol. 2018, 28, R435–R444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kok, V.C.; Yu, C.C. Cancer-Derived Exosomes: Their Role in Cancer Biology and Biomarker Development. Int. J. Nanomed. 2020, 15, 8019–8036. [Google Scholar] [CrossRef]
- Song, Z.; Mao, J.; Barrero, R.A.; Wang, P.; Zhang, F.; Wang, T. Development of a CD63 Aptamer for Efficient Cancer Immunochemistry and Immunoaffinity-Based Exosome Isolation. Molecules 2020, 25, 5585. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Lei, Y.; Wang, J.; Zhu, L.; Wu, Y.; Zhang, H.; Wu, L.; Zhang, P.; Yang, C. Microfluidic-Based Exosome Analysis for Liquid Biopsy. Small Methods 2021, 5, e2001131. [Google Scholar] [CrossRef]
- Bardi, G.T.; Smith, M.A.; Hood, J.L. Melanoma exosomes promote mixed M1 and M2 macrophage polarization. Cytokine 2018, 105, 63–72. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, W.; McGinley, E.C.; Klinke, D.J., 2nd. Melanoma exosomes deliver a complex biological payload that upregulates PTPN11 to suppress T lymphocyte function. Pigment. Cell Melanoma Res. 2017, 30, 203–218. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Kalimuthu, S.; Gangadaran, P.; Oh, J.M.; Lee, H.W.; Baek, S.H.; Jeong, S.Y.; Lee, S.W.; Lee, J.; Ahn, B.C. Exosomes Derived From Natural Killer Cells Exert Therapeutic Effect in Melanoma. Theranostics 2017, 7, 2732–2745. [Google Scholar] [CrossRef]
- Pessoa, L.S.; Heringer, M.; Ferrer, V.P. ctDNA as a cancer biomarker: A broad overview. Crit. Rev. Oncol. Hematol. 2020, 155, 103109. [Google Scholar] [CrossRef] [PubMed]
- Franczak, C.; Filhine-Tresarrieu, P.; Gilson, P.; Merlin, J.L.; Au, L.; Harlé, A. Technical considerations for circulating tumor DNA detection in oncology. Expert Rev. Mol. Diagn. 2019, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Butler, K.S.; Young, M.Y.; Li, Z.; Elespuru, R.K.; Wood, S.C. Performance characteristics of the AmpliSeq Cancer Hotspot panel v2 in combination with the Ion Torrent Next Generation Sequencing Personal Genome Machine. Regul. Toxicol. Pharmacol. 2016, 74, 178–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phallen, J.; Sausen, M.; Adleff, V.; Leal, A.; Hruban, C.; White, J.; Anagnostou, V.; Fiksel, J.; Cristiano, S.; Papp, E.; et al. Direct detection of early-stage cancers using circulating tumor DNA. Sci. Transl. Med. 2017, 9, eaan2415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manier, S.; Park, J.; Capelletti, M.; Bustoros, M.; Freeman, S.S.; Ha, G.; Rhoades, J.; Liu, C.J.; Huynh, D.; Reed, S.C.; et al. Whole-exome sequencing of cell-free DNA and circulating tumor cells in multiple myeloma. Nat. Commun. 2018, 9, 1691. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Fujita, M. Whole genome sequencing analysis for cancer genomics and precision medicine. Cancer Sci. 2018, 109, 513–522. [Google Scholar] [CrossRef] [Green Version]
- Griewank, K.G.; Schilling, B. Next-Generation Sequencing to Guide Treatment of Advanced Melanoma. Am. J. Clin. Dermatol. 2017, 18, 303–310. [Google Scholar] [CrossRef]
- Sacco, A.; Forgione, L.; Carotenuto, M.; Luca, A.; Ascierto, P.A.; Botti, G.; Normanno, N. Circulating Tumor DNA Testing Opens New Perspectives in Melanoma Management. Cancers 2020, 12, 2914. [Google Scholar] [CrossRef]
- Gerloff, D.; Sunderkötter, C.; Wohlrab, J. Importance of microRNAs in Skin Oncogenesis and Their Suitability as Agents and Targets for Topical Therapy. Skin Pharmacol. Physiol. 2020, 33, 270–279. [Google Scholar] [CrossRef]
- Latchana, N.; Ganju, A.; Howard, J.H.; Carson, W.E., 3rd. MicroRNA dysregulation in melanoma. Surg. Oncol. 2016, 25, 184–189. [Google Scholar] [CrossRef]
- Al-Eryani, L.; Jenkins, S.F.; States, V.A.; Pan, J.; Malone, J.C.; Rai, S.N.; Galandiuk, S.; Giri, A.K.; States, J.C. miRNA expression profiles of premalignant and malignant arsenic-induced skin lesions. PLoS ONE 2018, 13, e0202579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neagu, M.; Constantin, C.; Cretoiu, S.M.; Zurac, S. miRNAs in the Diagnosis and Prognosis of Skin Cancer. Front. Cell Dev. Biol. 2020, 8, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turcan, I.; Caras, I.; Schreiner, T.G.; Tucureanu, C.; Salageanu, A.; Vasile, V.; Avram, M.; Tincu, B.; Olariu, M.A. Dielectrophoretic and Electrical Impedance Differentiation of Cancerous Cells Based on Biophysical Phenotype. Biosensors 2021, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.J.; Hsieh, C.H.; Chiu, T.K.; Zhu, Y.X.; Wang, H.M.; Hung, F.C.; Chou, W.P.; Wu, M.H. An Optically Induced Dielectrophoresis (ODEP)-Based Microfluidic System for the Isolation of High-Purity CD45neg/EpCAMneg Cells from the Blood Samples of Cancer Patients-Demonstration and Initial Exploration of the Clinical Significance of These Cells. Micromachines 2018, 9, 563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudagunti, F.D.; Velmanickam, L.; Nawarathna, D.; Lima, I.T., Jr. Nucleotide Identification in DNA Using Dielectrophoresis Spectroscopy. Micromachines 2019, 11, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Z.; Deng, Y.; Kim, D.; Chen, Z.; Xiao, Y.; Fan, R. An Integrated Dielectrophoresis-Trapping and Nanowell Transfer Approach to Enable Double-Sub-Poisson Single-Cell RNA Sequencing. ACS Nano 2020, 14, 7412–7424. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, L.; Ye, Y.; Li, Y.; Luan, X.; Liu, J.; Cheng, J.; Zhao, Y.; Li, M.; Huang, C. Microsphere mediated exosome isolation and ultra-sensitive detection on a dielectrophoresis integrated microfluidic device. Analyst 2021, 146, 5962–5972. [Google Scholar] [CrossRef]
- Pohl, H.A. The Motion and Precipitation of Suspensoids in Divergent Electric Fields. J. Appl. Phys. 1951, 22, 869–871. [Google Scholar] [CrossRef]
- Okayama, H.; Tomita, M.; Suzuki, M.; Yasukawa, T. Rapid Formation of Arrayed Cells on an Electrode with Microwells by a Scanning Electrode Based on Positive Dielectrophoresis. Anal. Sci. 2019, 35, 701–704. [Google Scholar] [CrossRef] [Green Version]
- Nakano, M.; Ding, Z.; Matsuda, K.; Xu, J.; Inaba, M.; Suehiro, J. Simple microfluidic device for detecting the negative dielectrophoresis of DNA labeled microbeads. Biomicrofluidics 2019, 13, 064109. [Google Scholar] [CrossRef]
- Matyushov, D.V. Electrostatic solvation and mobility in uniform and non-uniform electric fields: From simple ions to proteins. Biomicrofluidics 2019, 13, 064106. [Google Scholar] [CrossRef] [PubMed]
- Gimsa, J. Active, Reactive, and Apparent Power in Dielectrophoresis: Force Corrections from the Capacitive Charging Work on Suspensions Described by Maxwell-Wagner’s Mixing Equation. Micromachines 2021, 12, 738. [Google Scholar] [CrossRef] [PubMed]
- Turcan, I.; Olariu, M.A. Dielectrophoretic Manipulation of Cancer Cells and Their Electrical Characterization. ACS Comb. Sci. 2020, 22, 554–578. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.Y.; Lo, Y.J.; Lei, U. Measurement of the Imaginary Part of the Clausius-Mossotti Factor of Particle/Cell via Dual Frequency Electrorotation. Micromachines 2020, 11, 329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gascoyne, P.R.; Shim, S. Isolation of circulating tumor cells by dielectrophoresis. Cancers 2014, 6, 545–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.L.; Lan, K.C.; Jang, L.S. Electrical Characteristics Analysis of Various Cancer Cells Using a Microfluidic Device Based on Single-Cell Impedance Measurement. Sens. Actuators B 2012, 173, 927–934. [Google Scholar] [CrossRef]
- Wei, M.; Zhang, Y.; Li, G.; Ni, Y.; Wang, S.; Zhang, F.; Zhang, R.; Yang, N.; Shao, S.; Wang, P. A cell viability assessment approach based on electrical wound-healing impedance characteristics. Biosens. Bioelectron. 2019, 124–125, 25–32. [Google Scholar] [CrossRef]
- Ponti, G.; Manfredini, M.; Greco, S.; Pellacani, G.; Depenni, R.; Tomasi, A.; Maccaferri, M.; Cascinu, S. BRAF, NRAS and C-KIT Advanced Melanoma: Clinico-pathological Features, Targeted-Therapy Strategies and Survival. Anticancer Res. 2017, 37, 7043–7048. [Google Scholar]
- Kulkarni, A.; Al-Hraishawi, H.; Simhadri, S.; Hirshfield, K.M.; Chen, S.; Pine, S.; Jeyamohan, C.; Sokol, L.; Ali, S.; Teo, M.L.; et al. BRAF Fusion as a Novel Mechanism of Acquired Resistance to Vemurafenib in BRAFV600E Mutant Melanoma. Clin. Cancer Res. 2017, 23, 5631–5638. [Google Scholar] [CrossRef] [Green Version]
- Loureiro, J.; Abrantes, M.; Oliveira, P.; Saraiva, L. P53 in skin cancer: From a master player to a privileged target for prevention and therapy. Biochim. Biophys. Acta (BBA) Bioenerg. 2020, 1874, 188438. [Google Scholar] [CrossRef]
- Di Nardo, L.; Pellegrini, C.; Di Stefani, A.; Del Regno, L.; Sollena, P.; Piccerillo, A.; Longo, C.; Garbe, C.; Fargnoli, M.C.; Peris, K. Molecular genetics of cutaneous squamous cell carcinoma: Perspective for treatment strategies. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Durand, N.; Simsir, M.; Signetti, L.; Labbal, F.; Ballotti, R.; Mus-Veteau, I. Methiothepin Increases Chemotherapy Efficacy against Resistant Melanoma Cells. Molecules 2021, 26, 1867. [Google Scholar] [CrossRef] [PubMed]
- Kowacz, M.; Pollack, G.H. Cells in New Light: Ion Concentration, Voltage, and Pressure Gradients across a Hydrogel Membrane. ACS Omega 2020, 5, 21024–21031. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.; Shim, J.; Dutta, P. Effect of Joule heating on isoelectric focusing of proteins in a microchannel. Biomicrofluidics 2014, 8, 064125. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Ota, C.; Fujino, N.; Tando, Y.; Suzuki, S.; Yamada, M.; Kondo, T.; Okada, Y.; Kubo, H. Improving the viability of tissue-resident stem cells using an organ-preservation solution. FEBS Open Bio 2019, 9, 2093–2104. [Google Scholar] [CrossRef]
- Miccio, L.; Cimmino, F.; Kurelac, I.; Villone, M.M.; Bianco, V.; Memmolo, P.; Merola, F.; Mugnano, M.; Capasso, M.; Iolascon, A.; et al. Perspectives on liquid biopsy forlabel-free detection of “circulating tumor cells”through intelligent lab-on-chips. View 2020, 1, 20200034. [Google Scholar] [CrossRef]
- Russo, G.I.; Musso, N.; Romano, A.; Caruso, G.; Petralia, S.; Lanzanò, L.; Broggi, G.; Camarda, M. The Role of Dielectrophoresis for Cancer Diagnosis and Prognosis. Cancers 2022, 14, 198. [Google Scholar] [CrossRef]
- Li, M.; Anand, R.K. High-Throughput Selective Capture of Single Circulating Tumor Cells by Dielectrophoresis at a Wireless Electrode Array. J. Am. Chem Soc. 2017, 139, 8950–8959. [Google Scholar] [CrossRef] [Green Version]
- Clawson, G.A.; Kimchi, E.; Patrick, S.D.; Xin, P.; Harouaka, R.; Zheng, S.; Berg, A.; Schell, T.; Staveley-O’Carroll, K.F.; Neves, R.I.; et al. Circulating tumor cells in melanoma patients. PLoS ONE 2012, 7, e41052. [Google Scholar] [CrossRef] [Green Version]
- Fukumoto, T.; Nishiumi, S.; Fujiwara, S.; Yoshida, M.; Nishigori, C. Novel serum metabolomics-based approach by gas chromatography/triple quadrupole mass spectrometry for detection of human skin cancers: Candidate bioMarkers. J. Dermatol. 2017, 44, 1268–1275. [Google Scholar] [CrossRef]
- Surman, M.; Kędracka-Krok, S.; Hoja-Łukowicz, D.; Jankowska, U.; Drożdż, A.; Stępień, A.; Przybyło, M. Mass Spectrometry-Based Proteomic Characterization of Cutaneous Melanoma Ectosomes Reveals the Presence of Cancer-Related Molecules. Int. J. Mol. Sci. 2020, 21, 2934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosmopoulou, M.; Giannopoulou, A.F.; Iliou, A.; Benaki, D.; Panagiotakis, A.; Velentzas, A.D.; Konstantakou, E.G.; Papassideri, I.S.; Mikros, E.; Stravopodis, D.J.; et al. Human Melanoma-Cell Metabolic Profiling: Identification of Novel Biomarkers Indicating Metastasis. Int. J. Mol. Sci. 2020, 21, 2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, J.; Jirovsky, D.; Lee, S.Y.; Zimmermann, H.; Müller, C.E. Capillary electrophoresis-based nanoscale assays for monitoring ecto-5′-nucleotidase activity and inhibition in preparations of recombinant enzyme and melanoma cell membranes. Anal. Biochem. 2008, 373, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Po, J.W.; Ma, Y.; Balakrishna, B.; Brungs, D.; Azimi, F.; de Souza, P.; Becker, T.M. Immunomagnetic isolation of circulating melanoma cells and detection of PD-L1 status. PLoS ONE 2019, 14, e0211866. [Google Scholar] [CrossRef]
- Ozkumur, E.; Shah, A.M.; Ciciliano, J.C.; Emmink, B.L.; Miyamoto, D.T.; Brachtel, E.; Yu, M.; Chen, P.I.; Morgan, B.; Trautwein, J.; et al. Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci. Transl. Med. 2013, 5, 179ra47. [Google Scholar] [CrossRef] [Green Version]
- Strizzi, L.; Margaryan, N.V.; Gilgur, A.; Hardy, K.M.; Normanno, N.; Salomon, D.S.; Hendrix, M.J. The significance of a Cripto-1 positive subpopulation of human melanoma cells exhibiting stem cell-like characteristics. Cell Cycle 2013, 12, 1450–1456. [Google Scholar] [CrossRef] [Green Version]
- Silveira, L., Jr.; Silveira, F.L.; Bodanese, B.; Zângaro, R.A.; Pacheco, M.T. Discriminating model for diagnosis of basal cell carcinoma and melanoma in vitro based on the Raman spectra of selected biochemicals. J. Biomed. Opt. 2012, 17, 077003. [Google Scholar] [CrossRef]
- Galanzha, E.I.; Shashkov, E.V.; Spring, P.M.; Suen, J.Y.; Zharov, V.P. In vivo, noninvasive, label-free detection and eradication of circulating metastatic melanoma cells using two-color photoacoustic flow cytometry with a diode laser. Cancer Res. 2009, 69, 7926–7934. [Google Scholar] [CrossRef] [Green Version]
- Mischel, M.; Rougé, F.; Lamprecht, I.; Aubert, C.; Prota, G. Dielectrophoresis of malignant human melanocytes. Arch. Dermatol. Res. 1983, 275, 141–143. [Google Scholar] [CrossRef]
- Altomare, L.; Borgatti, M.; Medoro, G.; Manaresi, N.; Tartagni, M.; Guerrieri, R.; Gambari, R. Levitation and movement of human tumor cells using a printed circuit board device based on software-controlled dielectrophoresis. Biotechnol. Bioeng. 2003, 82, 474–479. [Google Scholar] [CrossRef]
- Sabuncu, A.C.; Liu, J.A.; Beebe, S.J.; Beskok, A. Dielectrophoretic separation of mouse melanoma clones. Biomicrofluidics 2010, 4, 021101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyata, S.; Ojima, Y. Discrimination of normal skin fibroblast and malignant melanocytes using dielectrophoretic force and fluid-induced shear force. In Proceedings of the 2014 World Automation Congress (WAC), Waikoloa, HI, USA, 3–7 August 2014; pp. 109–113. [Google Scholar] [CrossRef]
- Mi, Y.; Li, P.; Liu, Q.; Xu, J.; Yang, Q.; Tang, J. Multi-Parametric Study of the Viability of in Vitro Skin Cancer Cells Exposed to Nanosecond Pulsed Electric Fields Combined with Multi-Walled Carbon Nanotubes. Technol. Cancer Res. Treat. 2019, 18, 1533033819876918. [Google Scholar] [CrossRef]
- Kelp, G.; Li, J.; Lu, J.; DiNapoli, N.; Delgado, R.; Liu, C.; Fan, D.; Dutta-Gupta, S.; Shvets, G. Infrared spectroscopy of live cells from a flowing solution using electrically-biased plasmonic metasurfaces. Lab Chip 2020, 20, 2136–2153. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.P. Fifty years of dielectrophoretic cell separation technology. Biomicrofluidics 2016, 10, 032801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benhal, P.; Quashie, D.; Kim, Y.; Ali, J. Insulator Based Dielectrophoresis: Micro, Nano, and Molecular Scale Biological Applications. Sensors 2020, 20, 5095. [Google Scholar] [CrossRef] [PubMed]
- Douglas, T.A.; Alinezhadbalalami, N.; Balani, N.; Schmelz, E.M.; Davalos, R.V. Separation of Macrophages and Fibroblasts Using Contactless Dielectrophoresis and a Novel ImageJ. Macro. Bioelectricity 2019, 1, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Cen, E.G.; Dalton, C.; Li, Y.; Adamia, S.; Pilarski, L.M.; Kaler, K.V. A combined dielectrophoresis, traveling wave dielectrophoresis and electrorotation microchip for the manipulation and characterization of human malignant cells. J. Microbiol. Methods 2004, 58, 387–401. [Google Scholar] [CrossRef]
- Chou, W.P.; Wang, H.M.; Chang, J.H.; Chiu, T.K.; Hsieh, C.H.; Liao, C.J.; Wu, M.H. The utilization of optically-induced-dielectrophoresis (ODEP)-based virtual cell filters in a microfluidic system for continuous isolation and purification of circulating tumour cells (CTCs) based on their size characteristics. Sens. Actuators B. 2017, 241, 245–254. [Google Scholar] [CrossRef]
- Chiu, T.K.; Chao, A.C.; Chou, W.P.; Liao, C.J.; Wang, H.M.; Chang, J.H.; Chen, P.H.; Wu, M.H. Optically-induced-dielectrophoresis (ODEP)-based cell manipulation in a microfluidic system for high-purity isolation of integral circulating tumor cell (CTC) clusters based on their size characteristics. Sens. Actuators B. 2018, 258, 1161–1173. [Google Scholar] [CrossRef]
- Shamloo, A.; Yazdani, A.; Saghafifar, F. Investigation of a two-step device implementing magnetophoresis and dielectrophoresis for separation of circulating tumor cells from blood cells. Eng. Life Sci. 2020, 20, 296–304. [Google Scholar] [CrossRef]
- Luo, T.; Fan, L.; Zeng, Y.; Liu, Y.; Chen, S.; Tan, Q.; Lam, R.H.W.; Sun, D. A simplified sheathless cell separation approach using combined gravitational-sedimentation-based prefocusing and dielectrophoretic separation. Lab Chip 2018, 18, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.V.; Jen, C.P. Impedance detection integrated with dielectrophoresis enrichment platform for lung circulating tumor cells in a microfluidic channel. Biosens. Bioelectron. 2018, 121, 10–18. [Google Scholar] [CrossRef] [PubMed]

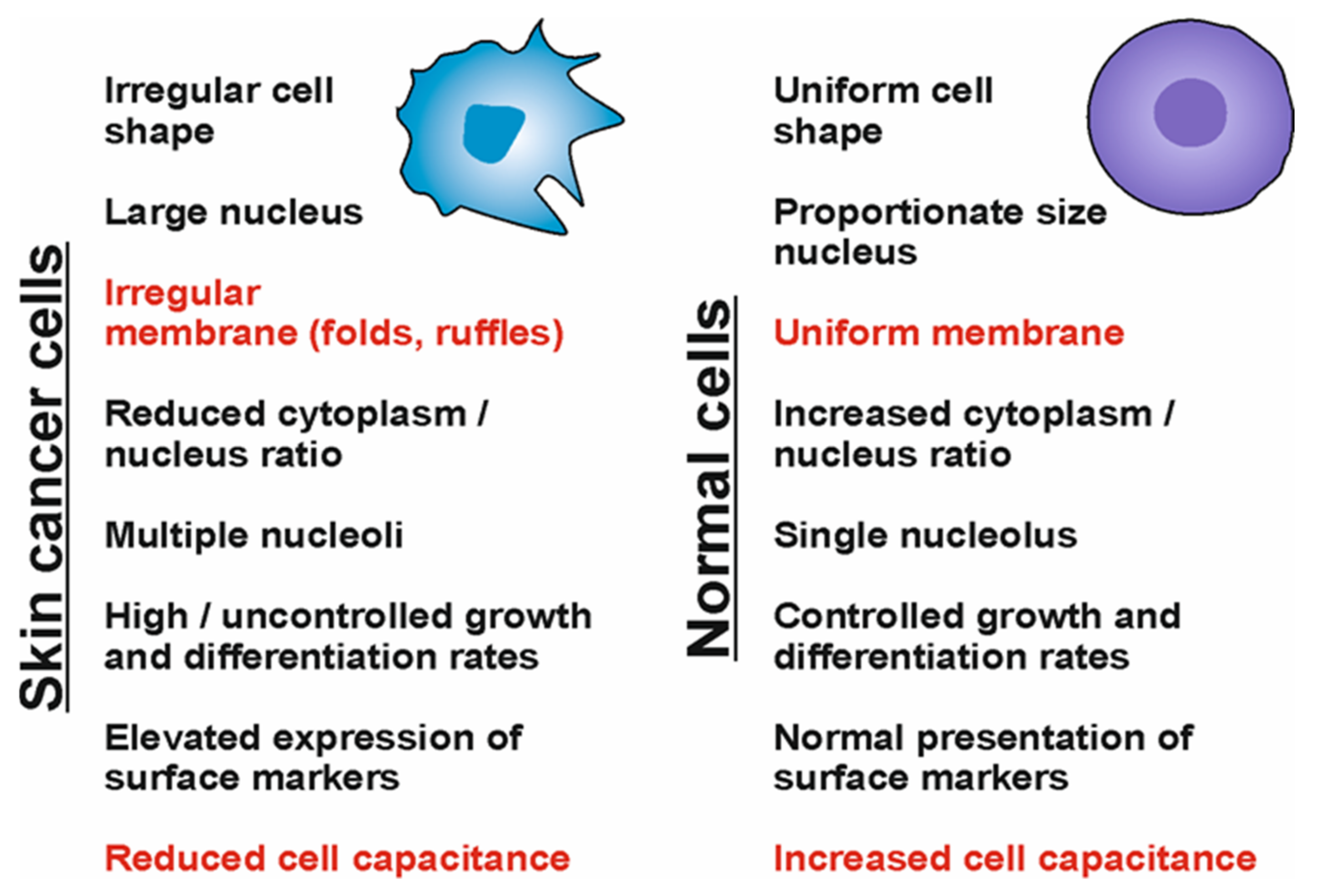
| Liquid Biopsy | Tissue (Conventional) Biopsy |
|---|---|
| Advantages of liquid biopsy over conventional biopsy | |
| Easy to obtain (blood sample) | Most often easy to obtain (localization dependent) |
| Non- or minimally invasive | Invasive |
| Virtually no clinical complications | Low risk of complications (pain, bleeding, infection) |
| Possibility to highlight intra- and inter-tumor heterogeneity, and multiple tumor sites | Localized analysis (no intra- or inter-tumor heterogeneity |
| Applicable to serial monitoring | Not applicable to serial monitoring |
| Unmodified/fresh DNA study | Possibility of DNA study (possible technique-related DNA alterations) |
| Limitations of liquid biopsy compared to conventional biopsy | |
| Limited use in clinical practice | Currently gold standard approach |
| Utility under investigation | |
| Limited histopathological analysis to CTCs | Histopathological analysis and staging |
| CTCs | TEPs | Exosomes | ctDNA | miRNAs | |
|---|---|---|---|---|---|
| Advantages | Morphological characterization; Functional analysis; Possibility of cellular RNA analysis; Interest/correlation with tumor diagnosis, prediction, prognosis | Abundant; Relatively simple isolation technique; Possibility of tumor RNA analysis | Potential correlation with tumor drug-resistance and metastasis; Possibility of tumor DNA, RNA analysis | Good representation of tumor heterogeneity; Relatively simple isolation technique; Useful in tumor monitoring and prognosis | Released by different structures (TEPs, exosomes); Stable in peripheral blood and other fluids |
| Limitations | Extreme rarity; Fragility; Heterogeneity | No standardized approach; Time consuming | No effective enrichment method; Heterogeneity | Rarity; Fragility; Contamination with normal cell DNA; No functional analysis | High variability; Unspecific to cancer type |
| Method | Pros | Cons | References |
|---|---|---|---|
| DEP | Non-invasive detection in liquid biopsy; Both label-free and biomarker-based discrimination; Single-cell isolation possibilities; Cells’ evolution monitoring in real-time based on electrical properties | Low selectivity; Limited reproducibility | [73,96,97,98] |
| Density gradient centrifugation | Label-free; Simultaneous isolation of more than two types of cells | Low specificity; Physical stress—destructive for the CTCs | [99] |
| Gas chromatography | Highly reproducible; High peak capacity molecules analysis | Use of high temperature (destructive for the sample); Limited to volatile compounds | [100] |
| Mass spectrometry | Accurate determination of mass; High throughput | Destructive for the sample; Less reproducible | [101] |
| Liquid chromatography | Adequate for a broad range of molecules; High peak capacity molecules analysis | Limited throughput; Potential longer separation time | [102] |
| Capillary electrophoresis | Measurements possible on small samples; Automated method (offers the possibility to process larger batches) | Sensitivity and resolution limits; Increased immunofixation rate | [103] |
| Immunomagnetic separation | High detection sensitivity (high purity) and effectiveness; High-throughput | Limitation related to surface markers; Antibody-mediated method | [104,105] |
| Fluorescence-activated cell sorting (FACS) | High-throughput; Simultaneous isolation of more than two types of cells | High costs in processing and pre-processing steps; Skilled staff required | [106] |
| Raman spectroscopy | High specificity; Little sample preparation required | Sensitive and highly optimized instrumentation; Use of high temperature (Destructive for the sample) | [107] |
| Photoacoustic flow cytometry | Label-free; Quantitative flow cytometry imaging | Limitation related to sample size and device parameters | [108] |
| Skin Tumor Type | Main Findings | Reference |
|---|---|---|
| Human malign melanocytes | Cells’ behavior in DEP is markedly dependent upon pigmentation (melanin content), age, and drug treatment (chlorpromazine) | [109] |
| Melanoma Colo38 cells | Separation of melanoma Colo 38 cells from other cancer cells using levitation principle in software-controlled DEP | [110] |
| Mouse melanoma B16F10 cells | DEP has the ability to differentiate between two malignant cells of the same origin, based on their melanin content | [111] |
| Human skin fibroblast and human malignant melanocytes | Distinguishing healthy and cancer cells using the microfluidic chamber DEP device | [112] |
| A375 human skin cancer cells | Assessment of the effects of various parameters of nanosecond pulsed electric fields combined with multi-walled carbon nanotubes on skin cancer cells viability | [113] |
| A431 human skin cancer cells | Separation of A431 cells from liquid samples based on their DEP parameters | [114] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schreiner, T.G.; Turcan, I.; Olariu, M.A.; Ciobanu, R.C.; Adam, M. Liquid Biopsy and Dielectrophoretic Analysis—Complementary Methods in Skin Cancer Monitoring. Appl. Sci. 2022, 12, 3366. https://doi.org/10.3390/app12073366
Schreiner TG, Turcan I, Olariu MA, Ciobanu RC, Adam M. Liquid Biopsy and Dielectrophoretic Analysis—Complementary Methods in Skin Cancer Monitoring. Applied Sciences. 2022; 12(7):3366. https://doi.org/10.3390/app12073366
Chicago/Turabian StyleSchreiner, Thomas Gabriel, Ina Turcan, Marius Andrei Olariu, Romeo Cristian Ciobanu, and Maricel Adam. 2022. "Liquid Biopsy and Dielectrophoretic Analysis—Complementary Methods in Skin Cancer Monitoring" Applied Sciences 12, no. 7: 3366. https://doi.org/10.3390/app12073366
APA StyleSchreiner, T. G., Turcan, I., Olariu, M. A., Ciobanu, R. C., & Adam, M. (2022). Liquid Biopsy and Dielectrophoretic Analysis—Complementary Methods in Skin Cancer Monitoring. Applied Sciences, 12(7), 3366. https://doi.org/10.3390/app12073366






