Transition Metal Coordination Compounds as Novel Materials for Dye-Sensitized Solar Cells
Abstract
:1. Introduction
2. Characterization and Principle of Operation of Dye-Sensitized Solar Cells
2.1. Compounds Used as Dyes
- Absorb electromagnetic radiation in the visible light range to the greatest possible extent;
- Have energy levels matching the energy levels of the redox mediator and the conduction band edge of TiO2, so as to ensure efficient electron transport;
- Be chemically accessible and (photo)chemically stable.
2.2. Compounds Used as Redox Mediators
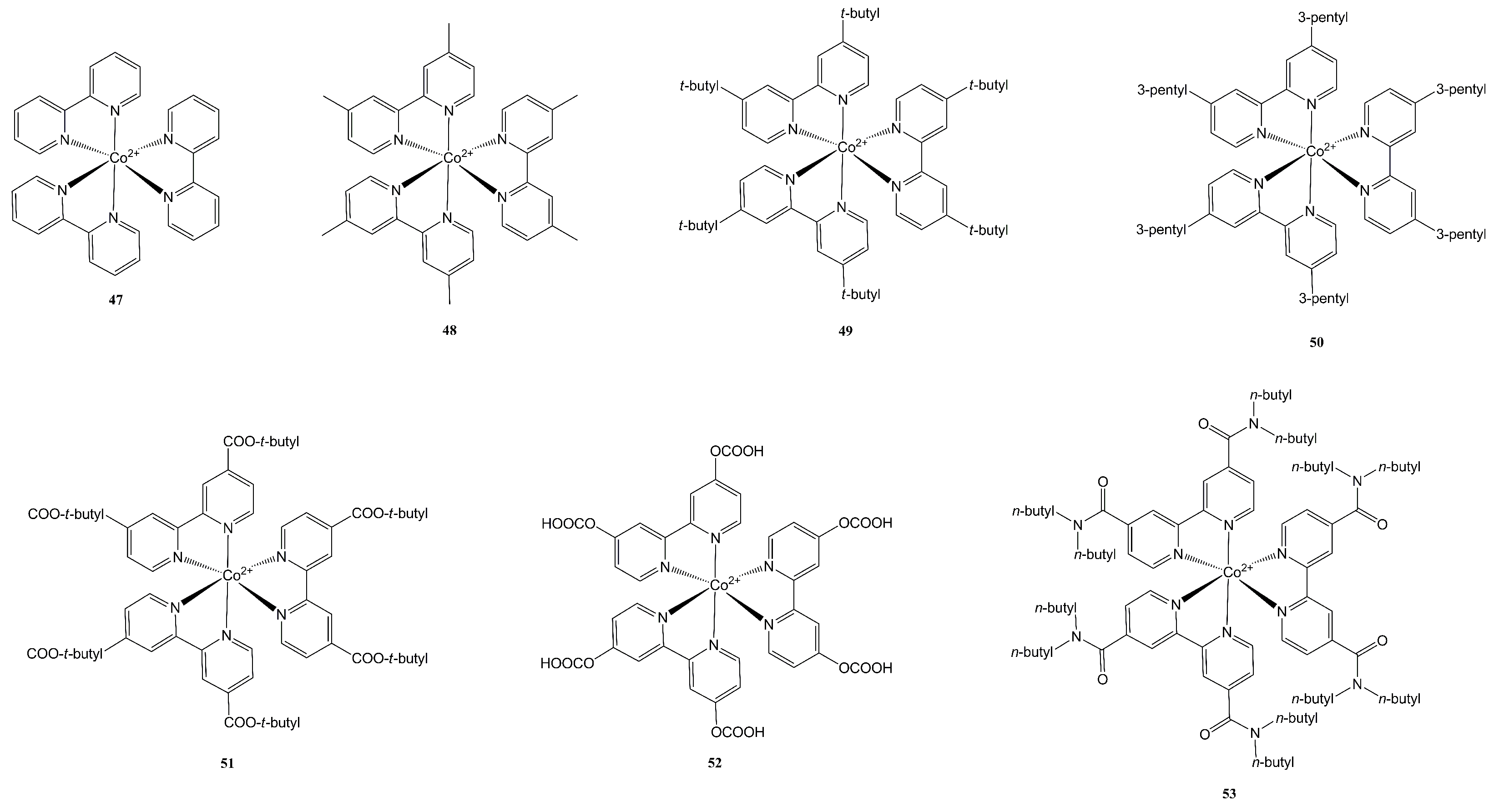
- Co2+/3+ tris(bipyridine) complexes;
- Co2+/3+ bis(terpyridine) complexes;
- Co2+/3+ (tris)phenanthroline complexes;
- Co2+/3+ bis(benzimidazole-2-yl)pyridine complexes;
- Co2+/3+ pyrazole-based complexes;
- Co2+/3+ complexes with pentadentate ligands;
- Co2+/3+ complexes with non-pyridyl ligands.
2.3. Alternative Electrodes
2.4. Application of Coordination Compounds in DSSCs
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stergiopoulos, T.; Falaras, P. Minimizing Energy Losses in Dye-Sensitized Solar Cells Using Coordination Compounds as Alternative Redox Mediators Coupled with Appropriate Organic Dyes. Adv. Energy Mater. 2012, 2, 616–627. [Google Scholar] [CrossRef]
- Schlichthörl, G.; Park, N.; Frank, A.J. Evaluation of the charge-collection efficiency of dye-sensitized nanocrystalline TiO2 solar cells. J. Phys. Chem. 1999, 103, 782–791. [Google Scholar] [CrossRef]
- Teo, L.P.; Buraidah, M.H.; Arof, A.K. Development on Solid Polymer Electrolytes for Electrochemical Devices. Molecules 2021, 26, 6499. [Google Scholar] [CrossRef]
- Castillo-Robles, J.A.; Rocha-Rangel, E.; Ramírez-de León, J.A.; Caballero-Rico, F.C.; Armendáriz-Mireles, E.N. Advances on Dye-Sensitized Solar Cells (DSSCs) Nanostructures and Natural Colorants: A Review. J. Compos. Sci. 2021, 5, 288. [Google Scholar] [CrossRef]
- Grifoni, F.; Bonomo, M.; Naim, W.; Barbero, N.; Alnasser, T.; Dzeba, I.; Giordano, M.; Tsaturyan, A.; Urbani, M.; Torres, T.; et al. Toward Sustainable, Colorless, and Transparent Photovoltaics: State of the Art and Perspectives for the Development of Selective Near-Infrared Dye-Sensitized Solar Cells. Adv. Energy Mater. 2021, 11, 2101598. [Google Scholar] [CrossRef]
- Saeed, M.A.; Yoo, K.; Kang, H.C.; Shim, J.W.; Lee, J.J. Recent developments in dye-sensitized photovoltaic cells under ambient illumination. Dyes Pigments 2021, 194, 109626. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Yahya, M.; Bouziani, A.; Ocak, C.; Seferoğlu, Z.; Sillanpää, M. Organic/Metal-organic photosensitizers for dye-sensitized solar cells (DSSC): Recent developments, new trends, and future perceptions. Dyes Pigments 2021, 192, 109227. [Google Scholar] [CrossRef]
- Inomata, T.; Hatano, M.; Kawai, Y.; Matsunaga, A.; Kitagawa, T.; Wasada-Tsutsui, Y.; Ozawa, T.; Masuda, H. Synthesis and Physico-Chemical Properties of Homoleptic Copper(I) Complexes with Asymmetric Ligands as a DSSC Dye. Molecules 2021, 26, 6835. [Google Scholar] [CrossRef]
- Sauve, G.; Cass, M.E.; Coia, G.; Doig, S.J.; Lauermann, I.; Pomykal, K.E.; Lewis, N.S. Dye sensitization of nanocrystalline titanium dioxide with osmium and ruthenium polypyridyl complexes. J. Phys. Chem. 2000, 104, 6821–6836. [Google Scholar] [CrossRef] [Green Version]
- Wong, H.L.; Mak, C.S.; Chan, W.K.; Djurišić, A.B. Efficient photovoltaic cells with wide photosensitization range fabricated from rhenium benzathiazole complexes. Appl. Phys. Lett. 2007, 90, 081107. [Google Scholar] [CrossRef] [Green Version]
- Islam, A.; Sugihara, H.; Hara, K.; Singh, L.P.; Katoh, R.; Yanagida, M.; Takahashi, Y.; Murata, S.; Arakawa, H. New platinum(II) polypyridyl photosensitizers for TiO2 solar cells. New J. Chem. 2000, 24, 343–345. [Google Scholar] [CrossRef]
- Boschloo, G.; Hagfeldt, A. Characteristics of the iodide/triiodide redox mediator in dye-sensitized solar cells. Accounts Chem. Res. 2009, 42, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Benesperi, I.; Singh, R.; Freitag, M. Copper coordination complexes for energy-relevant applications. Energies 2020, 13, 2198. [Google Scholar] [CrossRef]
- Wu, K.; Liu, S.; Wang, Z.; Qi, X.; Chen, R.; Wu, M. The modified W2C@C composites derived from the polyoxotungstate-based organic complexes assisted by pyrrole for efficient counter electrode in dye-sensitized solar cells. Surf. Interfaces 2022, 28, 101628. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X.; Tang, S.; Wang, K.; Tian, Y.; Zhong, C. Novel metal complexes for D-(A-π-A) 2 motif dye sensitizer: Synthesis and photovoltaic application. Appl. Organomet. Chem. 2021, 35, e6220. [Google Scholar] [CrossRef]
- Conradie, M.M.; Langner, E.H.; Conradie, J. DFT data to relate calculated LUMO energy with experimental reduction potentials of Cu(II)-β-diketonato complexes. Data Brief 2021, 38, 107331. [Google Scholar] [CrossRef]
- Baker, K.; Sikkema, R.; Liang, W.; Zhitomirsky, I. Multifunctional Properties of Commercial Bile Salts for Advanced Materials Engineering. Adv. Eng. Mater. 2021, 23, 2001261. [Google Scholar] [CrossRef]
- Stojanović, M.; Flores-Diaz, N.; Ren, Y.; Vlachopoulos, N.; Pfeifer, L.; Shen, Z.; Liu, Y.; Zakeeruddin, S.M.; Milić, J.V.; Hagfeldt, A. The Rise of Dye-Sensitized Solar Cells: From Molecular Photovoltaics to Emerging Solid-State Photovoltaic Technologies. Helv. Chim. Acta 2021, 104, e2000230. [Google Scholar] [CrossRef]
- Conesa-Egea, J.; Nogal, N.; Martínez, J.I.; Fernández-Moreira, V.; Rodríguez-Mendoza, U.R.; González-Platas, J.; Gómez-García, C.J.; Delgado, S.; Zamora, F.; Amo-Ochoa, P. Smart composite films of nanometric thickness based on copper–iodine coordination polymers. Toward sensors. Chem. Sci. 2018, 9, 8000–8010. [Google Scholar] [CrossRef] [Green Version]
- López-Molina, J.; Hernández-Rodríguez, C.; Guerrero-Lemus, R.; Cantelar, E.; Lifante, G.; Muñoz, M.; Amo-Ochoa, P. Cu(i)–I coordination polymers as the possible substitutes of lanthanides as downshifters for increasing the conversion efficiency of solar cells. Dalton Trans. 2020, 49, 4315–4322. [Google Scholar] [CrossRef] [PubMed]
- Higashino, T.; Iiyama, H.; Nimura, S.; Kurumisawa, Y.; Imahori, H. Effect of Ligand Structures of Copper Redox Shuttles on Photovoltaic Performance of Dye-Sensitized Solar Cells. Inorg. Chem. 2019, 59, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, N.; Hagfeldt, A.; Benesperi, I.; Freitag, M.; Hashmi, G.; Jia, G.; Wahyuono, R.A.; Plentz, J.; Dietzek, B. New approaches in component design for dye-sensitized solar cells. Sustain. Energy Fuels 2021, 5, 367–383. [Google Scholar] [CrossRef]
- Khalil, M.M.; El-Sayed, A.H.; Masaoud, M.; Hamad, M.A. Dielectric properties and potential applications of alizarin yellow GG-Cu(II) complex film blended with polyvinyl alcohol. J. Mater. Res. Technol. 2021, 11, 1799–1805. [Google Scholar] [CrossRef]
- Bai, Y.; Yu, Q.; Cai, N.; Wang, Y.; Zhang, M.; Wang, P. High-efficiency organic dye-sensitized mesoscopic solar cells with a copper redox shuttle. Chem. Commun. 2011, 47, 4376–4378. [Google Scholar] [CrossRef] [PubMed]
- Rahmalia, W.; Septiani, S.; Naselia, U.; Usman, T.; Silalahi, I.; Mouloungui, Z. Performance improvements of bixin and metal-bixin complexes sensitized solar cells by 1-methyl-3-propylimidazolium iodide in electrolyte system. Indones. J. Chem. 2021, 21, 669–678. [Google Scholar] [CrossRef]
- Li, Y.; Wang, G.; Feng, X.; Jia, Q.; Li, Y.; Liu, J.; Cao, J.; Liu, J. Double-layer novel zinc porphyrin based on axial coordination self-assembly for dye-sensitized solar cells. J. Mol. Struct. 2021, 1242, 130819. [Google Scholar] [CrossRef]
- Lindh, L.; Chábera, P.; Rosemann, N.W.; Uhlig, J.; Wärnmark, K.; Yartsev, A.; Sundström, V.; Persson, P. Photophysics and photochemistry of iron carbene complexes for solar energy conversion and photocatalysis. Catalysts 2020, 10, 315. [Google Scholar] [CrossRef] [Green Version]
- Xiang, N.; Huang, X.; Feng, X.; Liu, Y.; Zhao, B.; Deng, L.; Shen, P.; Fei, J.; Tan, S. The structural modification of thiophene-linked porphyrin sensitizers for dye-sensitized solar cells. Dyes Pigments 2011, 88, 75–83. [Google Scholar] [CrossRef]
- Yang, K.; Yang, X.; Zhang, L.; An, J.; Wang, H.; Deng, Z. Copper redox mediators with alkoxy groups suppressing recombination for dye-sensitized solar cells. Electrochim. Acta 2021, 368, 137564. [Google Scholar] [CrossRef]
- Kashif, M.K.; Benesperi, I.; Milhuisen, R.A.; Meyer, S.; Hellerstedt, J.; Zee, D.; Duffy, N.W.; Halstead, B.; Fuhrer, M.S.; Cashion, J.; et al. Polypyridyl Iron Complex as a Hole-Transporting Material for Formamidinium Lead Bromide Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 1855–1859. [Google Scholar] [CrossRef]
- Lin, L.; Lian, C.; Jones, T.W.; Bennett, R.D.; Mihaylov, B.; Yang, T.C.J.; Wang, J.T.W.; Chi, B.; Duffy, N.W.; Li, J.; et al. Tunable transition metal complexes as hole transport materials for stable perovskite solar cells. Chem. Commun. 2021, 57, 2093–2096. [Google Scholar] [CrossRef] [PubMed]
- Fränkel, R.; Kernbach, U.; Bakola-Christianopoulou, M.; Plaia, U.; Suter, M.; Ponikwar, W.; Nöth, H.; Moinet, C.; Fehlhammer, W.P. Homoleptic carbene complexes: Part VIII. Hexacarbene complexes. J. Organomet. Chem. 2001, 617, 530–545. [Google Scholar] [CrossRef]
- Luo, Y.N.; Kong, M.; Lu, Y.H.; Gao, Y.W.; Ren, H.; Yu, X.Y. A new (3, 6)-connected silver(I) coordination polymer with photovoltaic performance. Inorg. Chem. Commun. 2020, 120, 108079. [Google Scholar] [CrossRef]
- Luo, Y.; Jiang, H.; Yu, L.; Liu, Z.; Yu, X. Synthesis, crystal structure, and photoelectrical property of a new (3, 6) connected 3D Zn(II) coordination polymer. Inorg. Chem. Commun. 2017, 86, 304–307. [Google Scholar] [CrossRef]
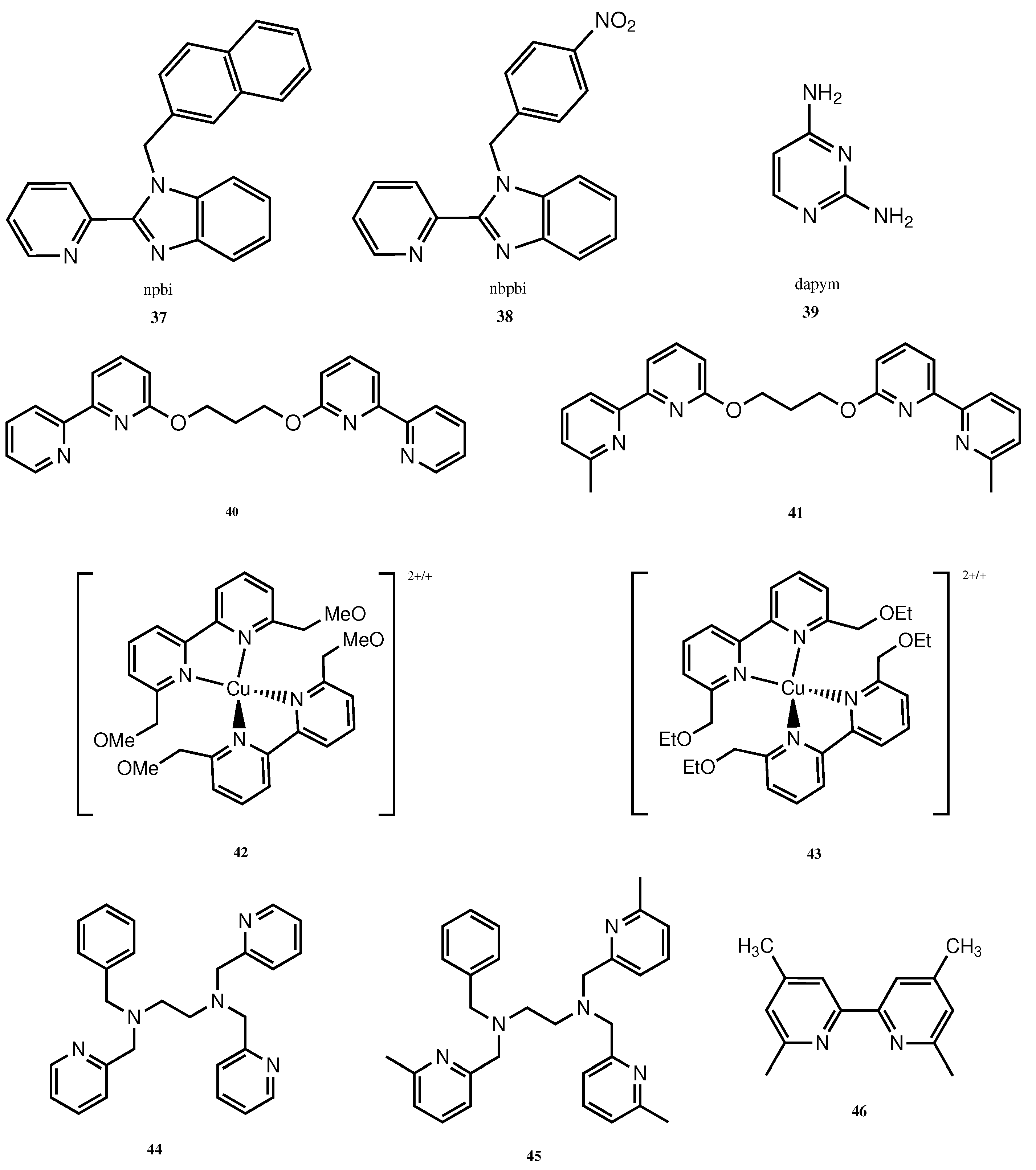
- Selvaraj, B.; Shanmugam, G.; Kamaraj, S.; Gunasekeran, A.; Sambandam, A. Effect of 1-Substituted 2-(Pyridin-2-yl)-1H-Benzo[d] imidazole Ligand-Coordinated Copper and Cobalt Complex Redox Electrolytes on Performance of Ru(II) Dye-Based Dye-Sensitized Solar Cells. Inorg. Chem. 2021, 60, 1937–1947. [Google Scholar] [CrossRef]
- Deng, Z.; Yang, X.; Yang, K.; Zhang, L.; Wang, H.; Wang, X.; Sun, L. Helical Copper Redox Mediator with Low Electron Recombination for Dye-Sensitized Solar Cells. ACS Sustain. Chem. Eng. 2021, 9, 5252–5259. [Google Scholar] [CrossRef]
- Rui, H.; Shen, J.; Yu, Z.; Li, L.; Han, H.; Sun, L. Stable Dye-Sensitized Solar Cells Based on Copper(II/I) Redox Mediators Bearing a Pentadentate Ligand. Angew. Chem. 2021, 133, 16292–16299. [Google Scholar] [CrossRef]
- Colombo, A.; Dragonetti, C.; Roberto, D.; Fagnani, F. Copper Complexes as Alternative Redox Mediators in Dye-Sensitized Solar Cells. Molecules 2021, 26, 194. [Google Scholar] [CrossRef]
- Bella, F.; Galliano, S.; Gerbaldi, C.; Viscardi, G. Cobalt-based electrolytes for dye-sensitized solar cells: Recent advances towards stable devices. Energies 2016, 9, 384. [Google Scholar] [CrossRef] [Green Version]
- Giribabu, L.; Bolligarla, R.; Panigrahi, M. Recent Advances of Cobalt(II/III) Redox Couples for Dye-Sensitized Solar Cell Applications. Chem. Rec. 2015, 15, 760–788. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Huang, W.; Kong, L.; Guo, S.; Cheng, Y. Ditungsten Carbide Nanoparticles Homogeneously Embedded in Carbon Nanofibers for Efficient Hydrogen Production. Chem. Eng. J. 2021, 420, 130480. [Google Scholar] [CrossRef]
- Fan, J.; Dou, Y.; Jiang, R.; Du, K.; Deng, B.; Wang, D. Electro-synthesis of tungsten carbide containing catalysts in molten salt for efficiently electrolytic hydrogen generation assisted by urea oxidation. Int. J. Hydrog. Energy 2021, 46, 14932–14943. [Google Scholar] [CrossRef]
- Yang, A.N.; Lin, J.T.; Li, C.T. Electroactive and Sustainable Cu-MOF/PEDOT Composite Electrocatalysts for Multiple Redox Mediators and for High-Performance Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 8435–8444. [Google Scholar] [CrossRef] [PubMed]
- Marchini, E.; Caramori, S.; Bignozzi, C.A.; Carli, S. On the Use of PEDOT as a Catalytic Counter Electrode Material in Dye-Sensitized Solar Cells. Appl. Sci. 2021, 11, 3795. [Google Scholar] [CrossRef]
- Yun, S.; Hagfeldt, A.; Ma, T. Pt-free counter electrode for dye-sensitized solar cells with high efficiency. Adv. Mater. 2014, 26, 6210–6237. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Patyal, M.; Gupta, N. A review on the use of carbon matrix incorporated with macrocyclic metal complexes as counter electrodes for platinum free dye sensitized solar cells. J. Coord. Chem. 2021, 74, 543–562. [Google Scholar] [CrossRef]
- Hattori, S.; Wada, Y.; Yanagida, S.; Fukuzumi, S. Blue copper model complexes with distorted tetragonal geometry acting as effective electron-transfer mediators in dye-sensitized solar cells. J. Am. Chem. Soc. 2005, 127, 9648–9654. [Google Scholar] [CrossRef]
- Freitag, M.; Giordano, F.; Yang, W.; Pazoki, M.; Hao, Y.; Zietz, B.; Gratzel, M.; Hagfeldt, A.; Boschloo, G. Copper phenanthroline as a fast and high-performance redox mediator for dye-sensitized solar cells. J. Phys. Chem. C 2016, 120, 9595–9603. [Google Scholar] [CrossRef]
- Pradhan, S.C.; Hagfeldt, A.; Soman, S. Resurgence of DSCs with copper electrolyte: A detailed investigation of interfacial charge dynamics with cobalt and iodine based electrolytes. J. Mater. Chem. A 2018, 6, 22204–22214. [Google Scholar] [CrossRef]
- Zhang, J.; Vlachopoulos, N.; Jouini, M.; Johansson, M.B.; Zhang, X.; Nazeeruddin, M.K.; Boschloo, G.; Johansson, E.M.; Hagfeldt, A. Efficient solid-state dye sensitized solar cells: The influence of dye molecular structures for the in-situ photoelectrochemically polymerized PEDOT as hole transporting material. Nano Energy 2016, 19, 455–470. [Google Scholar] [CrossRef]
- Day, J.; Senthilarasu, S.; Mallick, T.K. Improving spectral modification for applications in solar cells: A review. Renew. Energy 2019, 132, 186–205. [Google Scholar] [CrossRef]
- López, J.; Platas, J.G.; Rodríguez-Mendoza, U.R.; Martínez, J.I.; Delgado, S.; Lifante-Pedrola, G.; Cantelar, E.; Guerrero-Lemus, R.; Hernández-Rodríguez, C.; Amo-Ochoa, P. Cu(I)–I-2,4-diaminopyrimidine Coordination Polymers with Optoelectronic Properties as a Proof of Concept for Solar Cells. Inorg. Chem. 2020, 60, 1208–1219. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.X.; Gao, M.; Wen, T.; Kang, Y.; Chen, S. Synthesis of halide-modulated cuprous(I) coordination polymers with mechanochromic and photocatalytic properties. Inorg. Chem. 2017, 56, 6507–6511. [Google Scholar] [CrossRef] [PubMed]

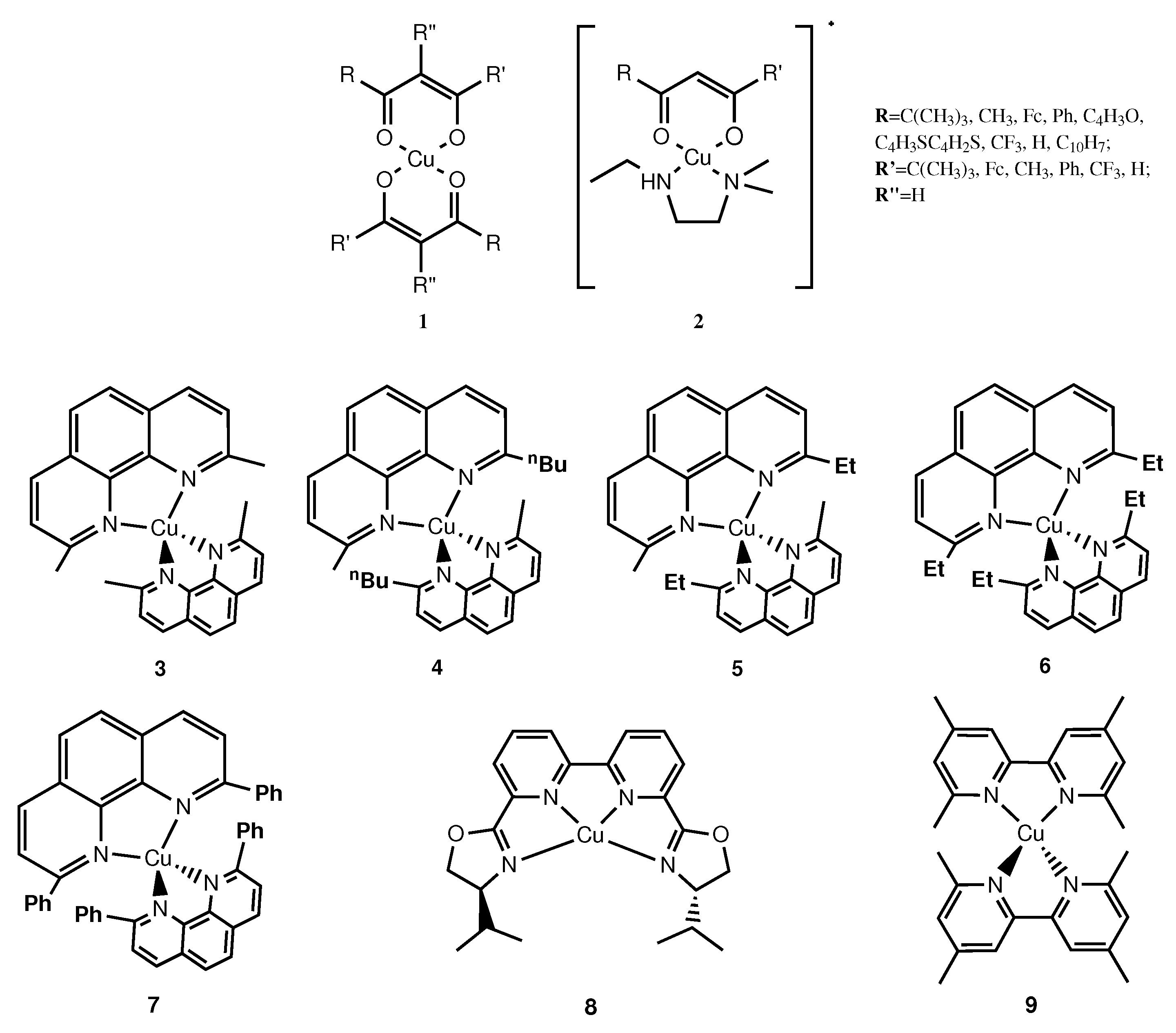
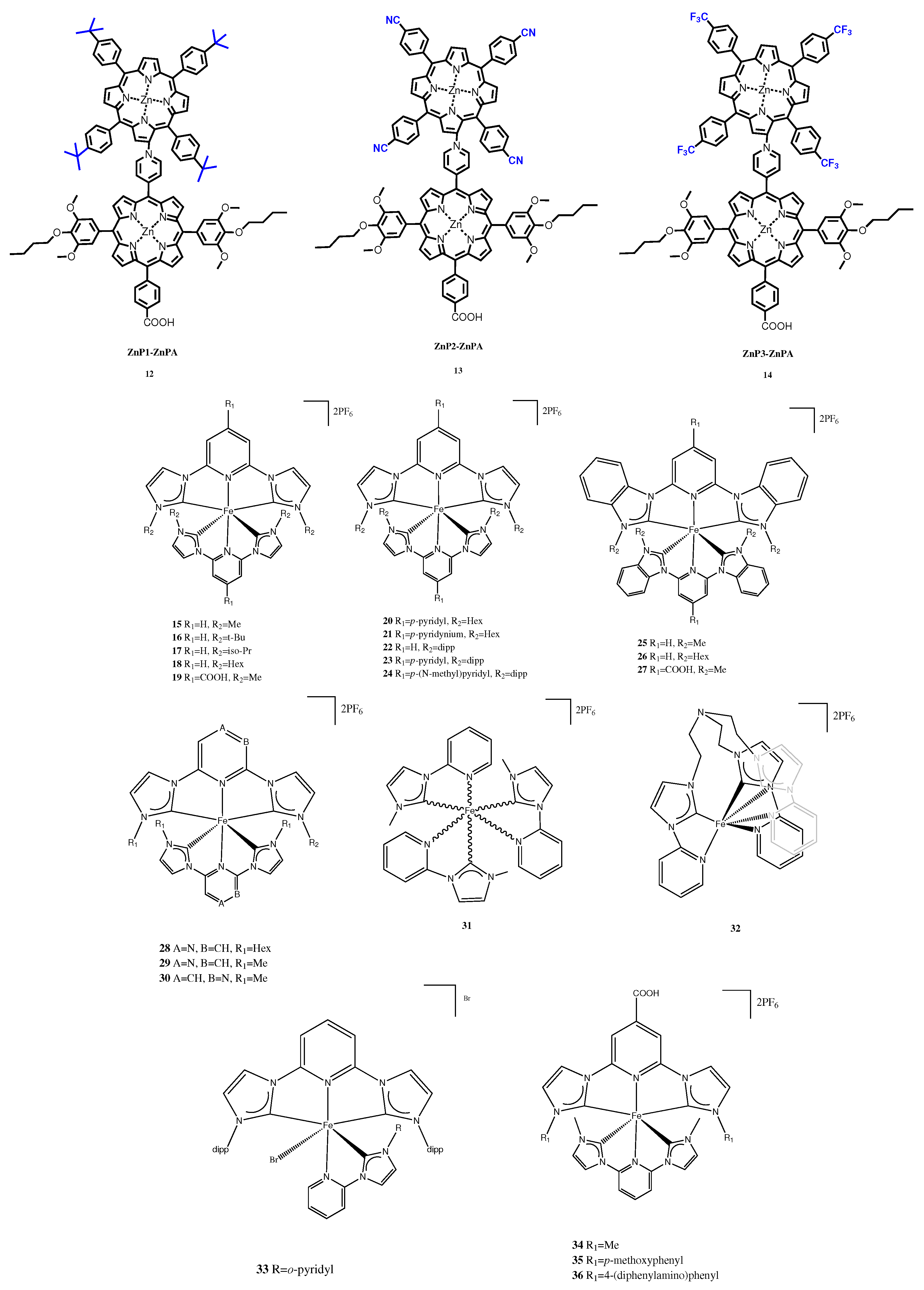
| Electrolyte | Dye | JSC (mA·cm−2) | VOC (V) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| 3 | LEG4 | 10.00 | 1.03 | 60.9 | 6.29 |
| 4 | LEG4 | 10.06 | 0.84 | 66.4 | 5.90 |
| 5 | LEG4 | 7.52 | 0.82 | 52.6 | 3.25 |
| 6 | LEG4 | 6.77 | 0.81 | 46.4 | 2.56 |
| 7 | LEG4 | 4.18 | 0.85 | 62.4 | 2.21 |
| 8 | Y123 | 9.75 | 0.92 | 69 | 6.20 |
| 9 | D203 | 6.12 | 0.92 | 82 | 4.60 |
| 9 | D205Si | 6.40 | 0.95 | 78 | 4.75 |
| 9 | WS-72 | 13.8 | 1.07 | 79 | 11.7 |
| Light | JSC (mA·cm−2) | VOC (V) | FF (%) | PCE (%) |
|---|---|---|---|---|
| White | 0.021 | 0.37 | 0.47 | 3.96 × 10−3 |
| Yellow | 0.020 | 0.39 | 0.44 | 3.44 × 10−3 |
| Green | 0.051 | 0.40 | 0.43 | 8.67 × 10−3 |
| Blue | 0.022 | 0.40 | 0.44 | 3.8 × 10−3 |
| Dye | JSC (mA·cm−2) | VOC (V) | FF (%) | PCE (%) |
|---|---|---|---|---|
| 12 | 5.63 ± 0.30 | 0.76 ± 0.01 | 0.66 ± 0.1 | 2.83 ± 0.12 |
| 13 | 6.59 ± 0.28 | 0.74 ± 0.02 | 0.65 ± 0.1 | 3.17 ± 0.12 |
| 14 | 8.02 ± 0.21 | 0.71 ± 0.02 | 0.60 ± 0.2 | 3.44 ± 0.11 |
| 19 | 3.30 | 0.44 | 0.63 | 0.92 |
| 27 | 0.12 | 0.37 | 0.71 | 0.03 |
| 34 | 0.33 | 0.40 | 0.73 | 0.10 |
| 35 | 0.36 | 0.44 | 0.73 | 0.11 |
| 36 | 0.36 | 0.39 | 0.71 | 0.10 |
| 42 | 14.50 | 1.02 | 70.50 | 10.50 |
| 43 | 14.40 | 1.03 | 69.30 | 10.30 |
| Counter Electrode | JSC (mA·cm−2) | VOC (V) | FF (%) | PCE (%) |
|---|---|---|---|---|
| Pt a | 16.08 ± 0.13 | 748 ± 2 | 0.64 ± 0.01 | 7.67 ± 0.04 |
| Cu-MOF a | 0.88 ± 0.88 | 618 ± 166 | 0.10 ± 0.03 | 0.04 ± 0.04 |
| PEDOT a | 15.49 ± 0.48 | 769 ± 7 | 0.64 ± 0.01 | 7.58 ± 0.25 |
| Cu-MOF/PEDOT a | 16.36 ± 0.27 | 777 ± 3 | 0.65 ± 0.02 | 8.26 ± 0.06 |
| Cu-MOF/PEDOT b | 17.48 ± 0.92 | 786 ± 9 | 0.69 ± 0.01 | 9.45 ± 0.35 |
| Counter Electrode | Redox Mediator | Dye | PCE (%) |
|---|---|---|---|
| PEDOT-TsO | I3−/I− | N719 | 4.60 |
| PEDOT-PSS | I3−/I− | N719 | 7.60 |
| PEDOT-ClO4 | T−/T2 | TH305 | 6.00 |
| PEDOT-TFSI | I3−/I− | C106 | 9.80 |
| PEDOT-Cl | I3−/I− | N719 | 8.42 |
| PEDOT-FAP | [Co(phen)3]3+/2+ | Y123 | 10.30 |
| PEDOT-SDS | [Cu(tmby)2]1+/2+ | Y123/XY1b | 13.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlus, K.; Jarosz, T. Transition Metal Coordination Compounds as Novel Materials for Dye-Sensitized Solar Cells. Appl. Sci. 2022, 12, 3442. https://doi.org/10.3390/app12073442
Pawlus K, Jarosz T. Transition Metal Coordination Compounds as Novel Materials for Dye-Sensitized Solar Cells. Applied Sciences. 2022; 12(7):3442. https://doi.org/10.3390/app12073442
Chicago/Turabian StylePawlus, Klaudia, and Tomasz Jarosz. 2022. "Transition Metal Coordination Compounds as Novel Materials for Dye-Sensitized Solar Cells" Applied Sciences 12, no. 7: 3442. https://doi.org/10.3390/app12073442






