1. Introduction
The core aim of precision oncology is to identify targetable alterations through the molecular profiling of tumors. Recent developments in sequencing technologies and bioinformatics analysis [
1], combined with major advances in molecular biology and cancer immunology research [
2], have made precision oncology feasible. To make sense of the acquired data, genetic variants must be classified according to gene function, oncogenicity, druggability, and evidence level, resulting in personalized therapy recommendations that target the specific molecular alterations responsible for the phenotypic expression of cancer [
3]. This complex diagnostic process is challenging, time-consuming, and requires the collaboration of a set of experts from different scientific fields such as oncology, pathology, bioinformatics, and genetics.
To meet this challenge, Molecular Tumor Boards (MTB) have been established. In general, an MTB process includes patient enrolment, sample assessment, bioinformatic analysis, clinical interpretation of the molecular data, and its discussion during the MTB meeting by a multidisciplinary team of experts. After the personalized therapy recommendation has been prepared, it is presented by the molecular oncologist assigned to the particular case, who must merge the patient’s prior therapy and diagnosis, the new molecular diagnosis, and its clinical interpretation into one MTB report [
4]. The clinical interpretation of the molecular data is commonly the bottleneck of the MTB process, as the physician must research each tumor-driven event in the context of oncogenicity, drug–gene interaction, the current literature, and relevant clinical trials from external knowledge bases [
5]. Due to this complex and time-consuming process, the number of patients who can benefit from personalized therapy is limited. Process-optimization with software support for physicians can help mitigate this constraint.
Service-Oriented Architecture (SOA) is a framework for integrating business processes and supporting IT infrastructure as secure, standardized components (services) that can be reused and combined to address changing business priorities [
6]. SOA is an enterprise-wide IT architecture that promotes loose coupling, reuse, and interoperability between systems, which makes it especially suitable for optimizing business processes. While companies have long adopted SOA as a strategy [
7], healthcare organizations have only discovered its benefits in recent years [
8,
9]. The Object Management Group (OMG) [
10] is a standards consortium in the computer industry, with expertise in the development of interoperability standards. OMG’s Business Process Modeling and Notation (BPMN) is the preferred standard for business modeling in companies [
11], but some in healthcare are still hesitant to use it [
12]. It is a formal graphical and computable language, “designed to be understandable by both business professionals and IT specialists. The explicit design for non-technical users makes it a promising candidate for healthcare process modeling, where medical staff needs to understand and discuss the process models” [
13].
To support the use of SOA in clinical practice, OMG and Health Level Seven International [
14], which provides standards for health data interoperability, collaborated in the Healthcare Services Specification Project, creating solutions for use in clinical processes.
Here, we describe in detail the current MTB process at the University Cancer Center Hamburg (UCCH) using BPMN as well as a proposed simplified process with software support for the MTB physician.
2. Materials and Methods
2.1. BPMN Process Creation
The BPMN diagrams were created with the Camunda Modeler version 4.11.1 Platform. The Camunda Platform is a lightweight, open-source platform for Business Process Management. We used BPMN on the Camunda Platform version 7.16.0. A Java Runtime Environment (JRE) or a java development kit (JDK) version 6.0 or greater must be installed to run the software.
2.2. BPMN Elements
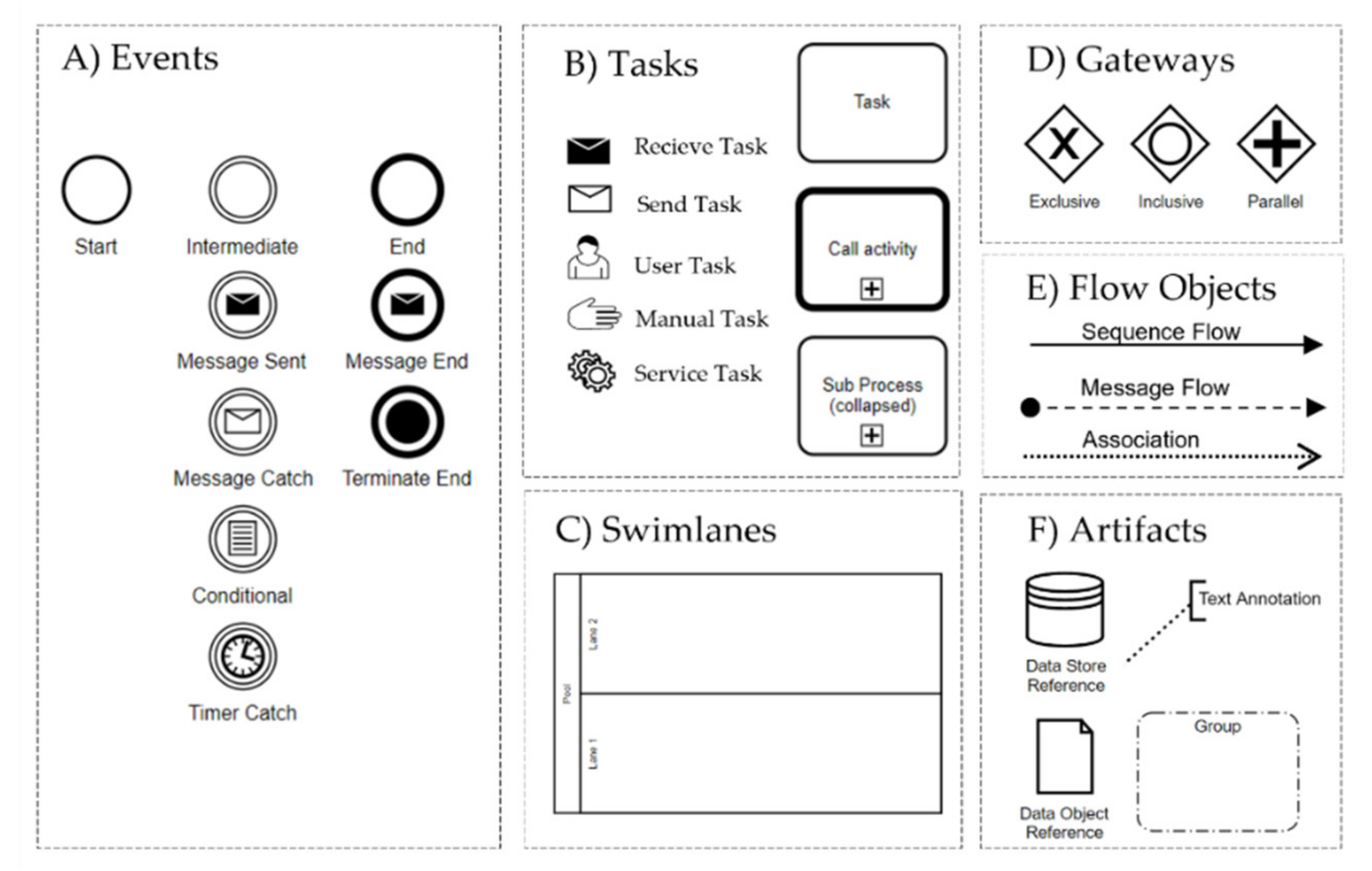
The graphical elements of BPMN are divided into Flow Objects, Connecting Objects, Pools and Swimlanes, and Artifacts (
Figure 1).
- (A)
Events: Events occur during a process and are labeled according to their position in the business process such as the start, intermediate, and end events.
- (B)
Tasks: A task is an activity which has to be completed in a business process. Tasks with thick boundaries are referred to an activity in an associated process.
- (C)
Swimlanes: A pool represents the main process participants, typically different organizations. A lane is a subdivision of a pool that spans the entire length of the pool and represents a participant in a workflow such as a user, a user role, or a system.
- (D)
Gateways: A gateway is a decision point (split/fork) or a point where different control flows converge (join/merge).
- (E)
Flow Objects: Sequence flows connect activities, gateways, and events. They represent the sequence in which activities are executed. A message flow indicates that two lanes or pools in a business process diagram or two elements from it exchange messages.
- (F)
Artifacts: Data Objects are used to represent electronic objects; a Group is a tool to visually summarize elements of a business process.
A list of all modeling elements with detailed explanations can be found in Section 7.2 of the OMG BPMN Specification [
11].
2.3. Data Collection
The work of MTB physicians, from the diagnostics request of the referrer to the personalized therapy recommendation, was recorded through the method of “participatory open observation” [
15]. It was prepared for via an observation plan, and all details were accurately recorded during the observation. In addition, separate standardized interviews were conducted with the physicians of the MTB, the molecular pathologists, the nurse at reception of the UCCH, and employees of the clinical cancer registry. Furthermore, to get a complete picture, we participated in several molecular tumor boards.
3. Results
The BPMN representation of the workflow of an MTB at the UCCH revealed a high workload for the MTB physician regarding administration, documentation, and interpretation (
Figure 2A). The developed simplified process, in which custom IT solutions have been provided, shows a significant reduction in workload (
Figure 2B). The following describes in detail the MTB process alongside its corresponding optimization. Since workload reduction for the physician was the driving force behind these optimizations, we show the BPMN process from the perspective of the MTB physician. All processes that are not part of the MTB physician’s work are shown as abbreviated sub-processes, which are expanded to the whole process in detail in
Supplementary Materials Figures S1 and S2.
The process of an MTB at the UCCH begins with the request for a personalized therapy recommendation for a patient diagnosed with cancer. The attending physician contacts either the UCCH reception or the MTB physician directly as they are often already in possession of their contact details. In this case, the MTB physician subsequently passes the information concerning the request to the UCCH reception for the patient data to be entered into the clinic’s admission system. This creates a patient case in Soarian, the UKE’s hospital information system. Without a software solution in place to track MTB patients, the MTB physician must write down all such cases by hand in a notebook. As the molecular pathology department is not linked to Soarian, the MTB physician must submit the diagnostic requests by fax. In addition, the physician arranges for the biospecimen transport to the molecular pathology lab and then sends a fax as a notification of the sample transfer to the department. All these steps are performed manually without the help of any administration software. In the suggested simplified process, the entering of patient data into the clinic’s admission system by the nurse at reception creates the patient case not only in Soarian but also in the MTB Software, which then displays all patients in the pipeline. The physician can now request a molecular diagnostic via a structured selection mask and/or request an MTB for a patient. The MTB software will then send necessary requests for sample shipment and molecular diagnostics automatically, resulting in a significant administrative workload reduction for the physician.
After the molecular pathology department receives the molecular diagnostic request and the biospecimen, Next-Generation Sequencing can be performed. The molecular pathologist then generates a pathology report containing identified molecular alterations. In the current process, the physician needs to manually check if the pathology report has been completed and uploaded to the Soarian Health Archive as there is no notification. Then, the physician begins to generate the MTB report, which is written as free text directly in Soarian and is written by the MTB physician alone. The MTB report contains the sections Pre-Diagnosis and Pre-Therapy, Current Diagnosis, Scientific Background, and Procedure. Pre-Diagnosis and Pre-Therapy are transferred by hand into the report from the doctor’s letter. For the Current Diagnosis, the identified variations are transferred manually from the pathology report. Subsequently, each variation is checked for its therapeutic relevance using various public genetic, pathway, and literature databases. These results are then noted in the Scientific Background section. Finally, a therapy recommendation based on findings from drug databases is documented by the MTB physician in the Procedure section. In the optimized process, a first interpretation of the Next-Generation Sequencing data, including the generation of a diagnostic report, will be performed by the MIRACUM Pipeline [
16]. After the input of the raw sequencing files in FASTQ format and the patient’s gender from the MTB Software, the pipeline automatically generates an interactive PDF report containing sequencing quality assessments, the identified and annotated variants, and highlighted hotspot mutations.
The recording of Pre-Diagnosis and Pre-Therapy as well as Current Diagnosis into a structured documentation form in the MTB software is completed by a designated documentation specialist.
The data from the MIRACUM report is then annotated with further external genetic and drug databases using a variant interpretation software developed in house, which presents relevant clinical data together with the sequencing results and matches them with additional information from public knowledge databases. With help from this interpretation software, the physician can then create the sections Scientific Background and Procedure, the second part of the MTB report.
After completion, the second part is transferred into the MTB software, where it is merged with the first part containing the Pre-Diagnosis and Pre-Therapy section, resulting in the final MTB report. After review by a senior physician, the MTB report is ready for presentation at the MTB meeting, where the findings and therapy recommendations are discussed by an interdisciplinary team of physicians. After all have agreed on one or more personalized therapy recommendations, this is noted in the Procedure section and digitally signed by all participants.
In the current process, the signed report will now be sent to the referring physician or, in the case of internal patients, can be looked up in Soarian. In the optimized process, the signed MTB report is transferred to Soarian by the MTB Software and, in the case of external patients, sent automatically to the treating physician. Finally, the data from the MTB report is transferred to the Giessener Tumor Documentation Software (GTDS) [
17] at the Clinical Cancer Registry (KKR). In the current process, the MTB physician manually enters Pre-Therapy and Pre-Diagnosis, Current Diagnosis, and Procedure into the GTDS. In the optimized process, this can be performed automatically since the structured form of documentation in the MTB software enables an automated transfer into the GTDS.
4. Discussion
Here, we created an optimized version of the process of an entire MTB at the UCCH based on the shortcomings of the current process using the graphical representation standard BPMN. Our core aim was to increase the efficiency of the MTB physician by introducing improved software support and automation.
The performed workflow analysis revealed a high degree of inefficiency and disorganization for the MTB physician. The entire process of preparing the MTB report as well as the subsequent tumor documentation are currently performed entirely by the physician alone. Variants of the current diagnosis are typed manually into both the MTB report and the GTDS, increasing the likelihood of transfer errors. Without an administration software in place, MTB patients need to be tracked manually, tying up valuable resources of the MTB physician. The envisaged automation of variant calling, data integration, and quality control using the MIRACUM Pipeline, and the variant annotation by the KC will increase efficiency and are essential to scaling up the process of personalized treatment recommendations in oncology. Since the molecular diagnosis is automatically transferred to the MTB report and GTDS in the optimized process, genetic variations no longer need to be typed in manually. Furthermore, the MTB software tracks all patients who are to receive a personalized therapy recommendation. Therefore, the optimized MTB process will improve patient safety, the reproducibility of the process, and provide a more accurate interpretation of the molecular data. In addition, it will lead to a significant increase in efficiency for the MTB physician, thus optimizing costs for the healthcare system.
The process of workflow visualization was complex and challenging. We therefore chose the SOA using BPMN as different studies showed a reduction of costs, IT infrastructure integration, and better business alignment with IT upon the implementation of SOA in the industry [
18]. Although SOA has been slow in making its way into healthcare, it is now widely and successfully used in clinical decision support [
12]. For personalized medicine, however, there is no published data on the benefits of SOA. Additionally, the BPMN standard for business process modelling that we used is still only rarely used in healthcare, which can be attributed to difficulties in its user-centric design [
12], as every pool of a lane in BPMN needs to include a user (see Methods section). Having many different users within a process can make it challenging to represent especially larger processes. We solved this problem by splitting the overall process into different sub-processes, which were displayed in separate pools. This demonstrates the suitability of BPMN to represent a complex healthcare process.
The accuracy of the chosen representation enabled us to identify the weaknesses of the current process and to target them for optimization. The BPMN process provided a consistent visual representation of all stakeholders throughout the project cycle, facilitating communication between IT staff and physicians, thus supporting interdisciplinary coordination.
There were several challenges which the use of BPMN helped to overcome. For one, the technical challenges of the project included the use of several different networks and IT systems. As patient data had to be documented with the highest data protection standards while having to access external research databases, different IT systems had to be used in different networks. Moreover, interoperability and the optimal embedding of IT solutions into the process was of utmost importance. Furthermore, due to the scope constraints of the project, the novel structured documentation needed to be performed outside the hospital information system, creating the need for a separate documentation software (MTB-Software).
Another major advantage of process visualization using BPMN is the quick and effective adaptability of the process to changing conditions, in line with the fast advances in the field of personalized oncology. Additionally, since MTB processes vary greatly across different hospitals in Germany, the standardization of these processes will become an increasingly important task in the future. Consortia such as the German Network for Personalized Medicine (DNPM) or the C4 Project (Connecting Comprehensive Cancer Centers) are striving for a uniform quality standard for MTBs. The fixed set of rules of the BPMN language introduces uniform quality standards so that an MTB process is conducted in the same way for every clinic, regardless of the person performing them.
5. Conclusions
In summary, for the first time, we implemented a BPMN model for the optimization of an MTB process, demonstrating its suitability for the visualization of complex processes in healthcare. Using BPMN for the MTB process facilitates collaboration, promotes rapid adaptability, standardization, and quality assurance. Its use could also provide great benefits to other areas of personalized medicine.
Supplementary Materials
The following supporting information can be downloaded at:
https://www.mdpi.com/article/10.3390/app12073485/s1, Figure S1: The current process of a Molecular Tumor Board at the University Cancer Center Hamburg. The tasks associated with the physician’s work are shown in blue; Figure S2: The optimized process of a Molecular Tumor Board at the University Cancer Center Hamburg. The tasks associated with the physician’s work are shown in blue.
Author Contributions
Conceptualization, F.U. and K.L.; methodology, K.L.; validation, S.N., F.U., J.-L.V., M.-C.P.; formal analysis, K.L.; investigation, K.L.; resources, F.U.; data curation, K.L.; writing—original draft preparation, K.L.; writing—review and editing, S.N., F.U., J.-L.V. and M.-C.P.; visualization, K.L.; supervision, F.U.; project administration, F.U.; funding acquisition, F.U. All authors have read and agreed to the published version of the manuscript.
Funding
Katharina Lauk is funded by the German Network of Personalized Medicine (DNPM).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kamps, R.; Brandão, R.; Bosch, B.; Paulussen, A.; Xanthoulea, S.; Blok, M.; Romano, A. Next-Generation Sequencing in Oncology: Genetic Diagnosis, Risk Prediction and Cancer Classification. Int. J. Mol. Sci. 2017, 18, 308. [Google Scholar] [CrossRef] [PubMed]
- Suda, K. Recent Advances in Cancer Immunotherapy. Biomolecules 2021, 11, 335. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharm. 2018, 834, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Missios, P.; Beha, J.; Bitzer, M.; Malek, N.P. The molecular tumor board. Chirurg 2021, 92, 1011–1015. [Google Scholar] [CrossRef]
- Good, B.M.; Ainscough, B.J.; McMichael, J.F.; Su, A.I.; Griffith, O.L. Organizing knowledge to enable personalization of medicine in cancer. Genome Biol. 2014, 15, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bieberstein, N.; Bose, S.; Walker, L.; Lynch, A. Impact of Service-Oriented Architecture on enterprise systems, organizational structures, and individuals. IBM Syst. J. 2005, 44, 691–708. [Google Scholar] [CrossRef]
- Seth, A.; Singla, A.R.; Aggarwal, H. Service Oriented Architecture Adoption Trends: A Critical Survey; Springer: Berlin/Heidelberg, Germany, 2012; pp. 164–175. [Google Scholar]
- Dobrescu, R.; Purcarea, V. Impact of Information Technology on the Quality of Health Services; Springer: Berlin/Heidelberg, Germany, 2012; pp. 307–319. [Google Scholar]
- Koumaditis, K.; Themistocleous, M.; Da Cunha, P.R. SOA implementation critical success factors in healthcare. J. Enterp. Inf. Manag. 2013, 26, 343–362. [Google Scholar] [CrossRef]
- Mission & Vision. Available online: https://www.omg.org/about/index.htm (accessed on 24 February 2022).
- Bussines Process Model and Notation (BPMN). Available online: http://www.omg.org/spec/BPMN/2.0 (accessed on 24 February 2022).
- Loya, S.R.; Kawamoto, K.; Chatwin, C.; Huser, V. Service Oriented Architecture for Clinical Decision Support: A Systematic Review and Future Directions. J. Med. Syst. 2014, 38, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, R.; Rogge-Solti, A. BPMN for healthcare processes. In Proceedings of the 3rd Central-European Workshop on Services and their Composition (ZEUS 2011), Karlsruhe, Germany, 21–22 February 2011. [Google Scholar]
- About HL7. Available online: https://www.hl7.org/about/ (accessed on 24 February 2022).
- Ciesielska, M.; Boström, K.W.; Öhlander, M. Observation Methods; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 33–52. [Google Scholar]
- MIRACUM-Pipe. Available online: https://github.com/AG-Boerries/MIRACUM-Pipe/ (accessed on 24 February 2022).
- Altmann, U.; Dudeck, J. The Giessen Tumor Documentation System (GTDS)—Review and perspectives. Methods Inf. Med. 2006, 45, 108–115. [Google Scholar] [PubMed]
- Mueller, B.; Viering, G.; Legner, C.; Riempp, G. Understanding the Economic Potential of Service-Oriented Architecture. J. Manag. Inf. Syst. 2010, 26, 145–180. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).