Anti-Hyperuricemic Effect of Ethyl Acetate Sub-Fractions from Chrysanthemum morifolium Ramat. Dried Flowers on Potassium Oxonate-Induced Hyperuricemic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Reagents
2.3. Plant Extraction and Solvent-Solvent Partition
2.4. Xanthine Oxidase Inhibitory Activity
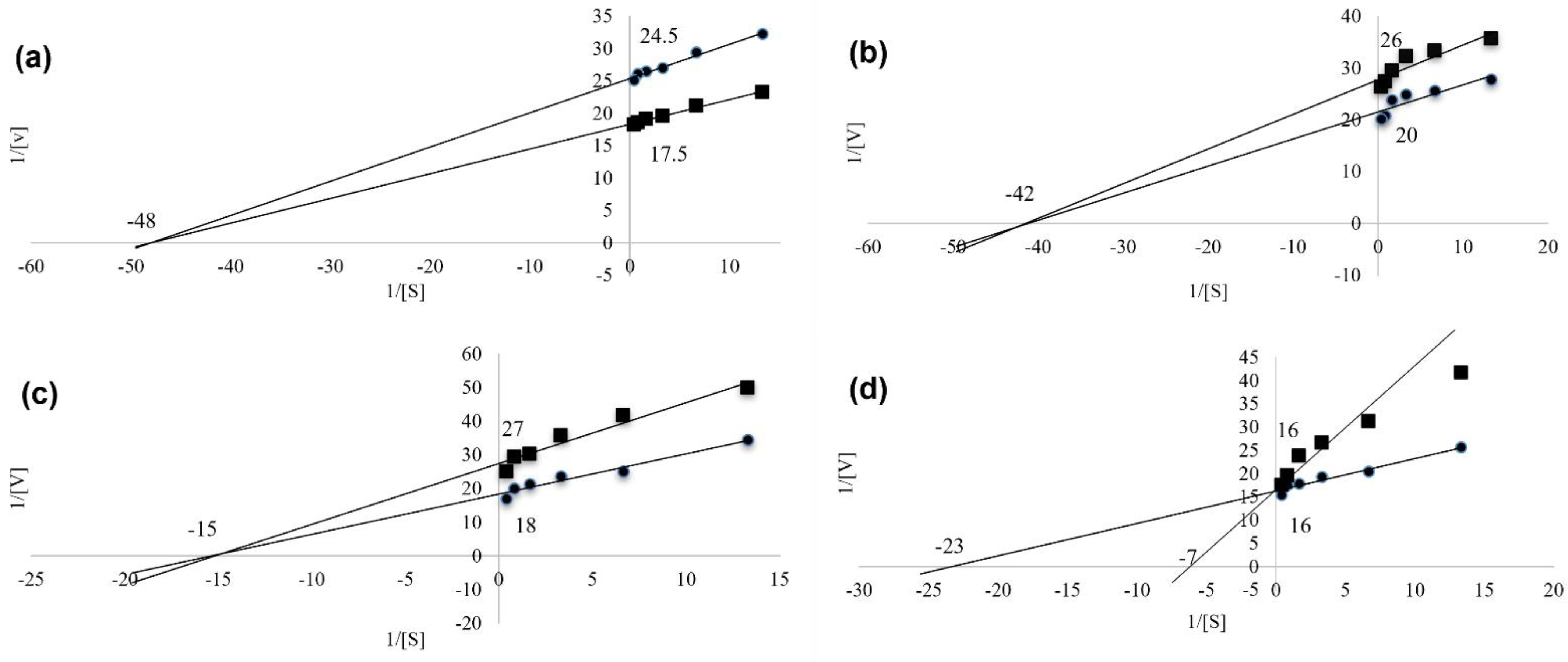
2.5. Xanthine Oxidase Enzyme Kinetic
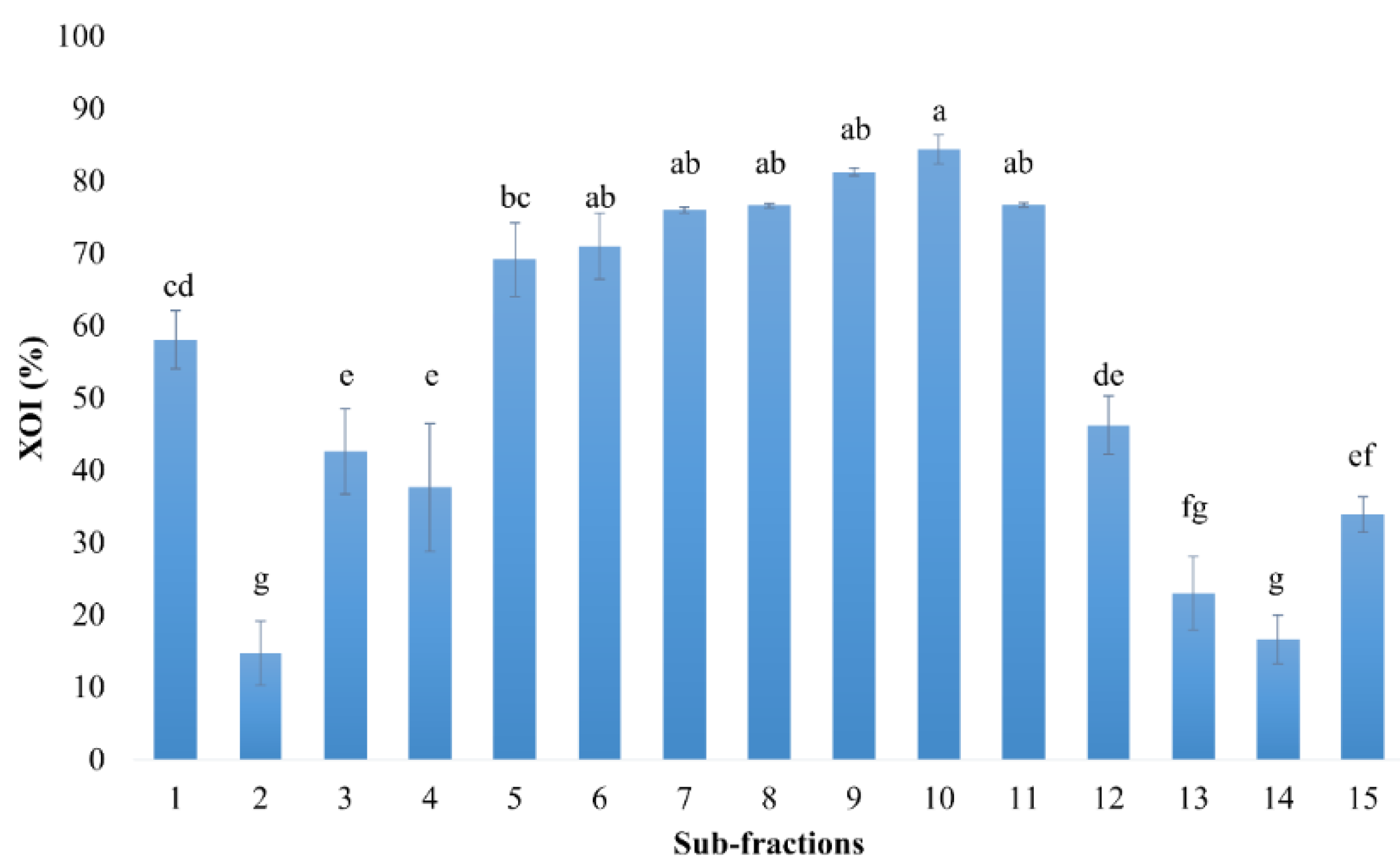
2.6. Bioassay-Guided Fractionation Using Column Chromatography
2.7. Trypan Blue Exclusion Assay
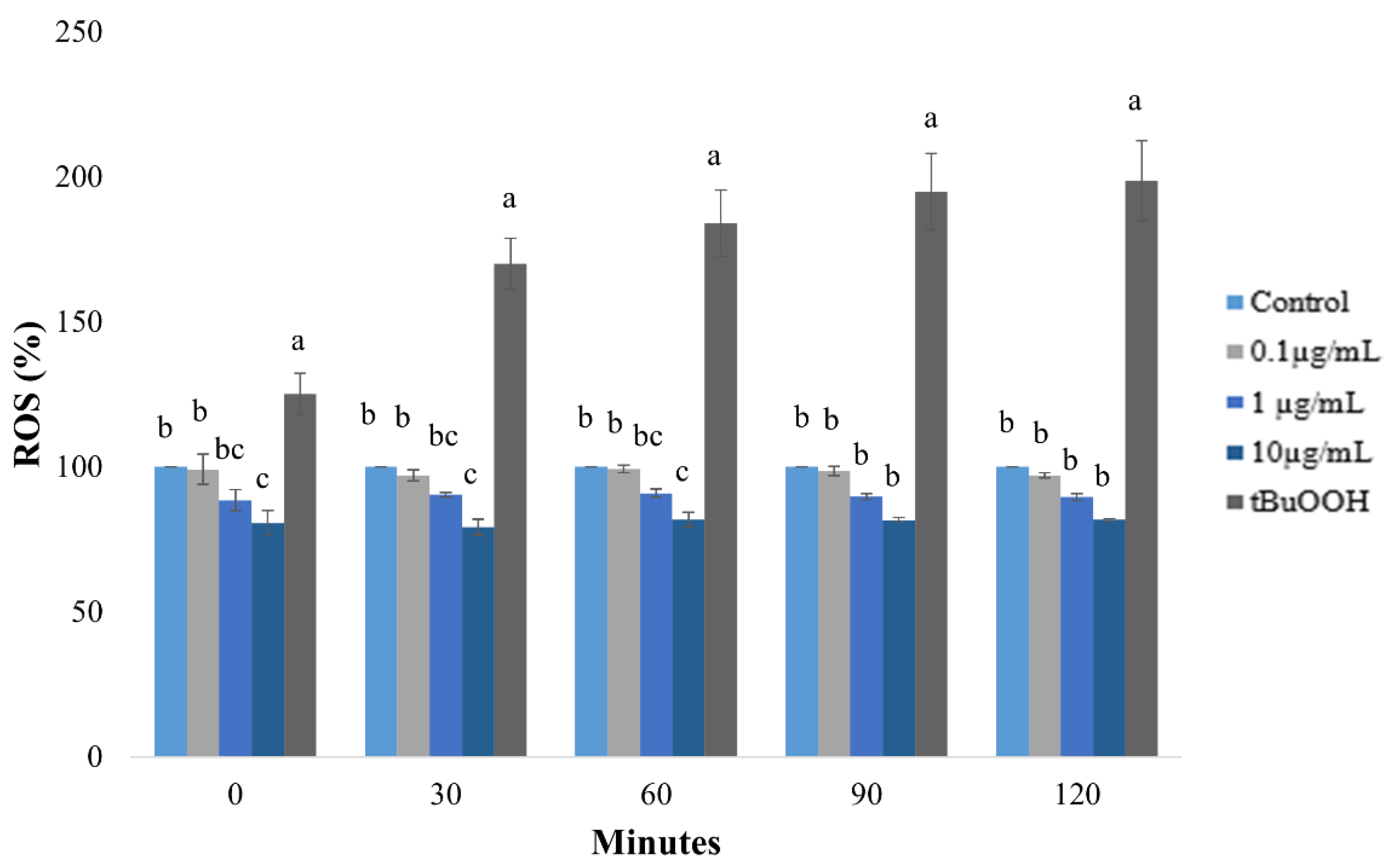
2.8. Reactive Oxygen Species (ROS) Direct Assay
2.9. Anti-Hyperuricemic Effects of C. morifolium in Potassium Oxonate-Induced Rats
2.10. qPCR Analysis of XO Gene Expression
2.11. Putative Identification of XO Inhibitors Using HPLC-Q-TOF-MS/MS
2.12. Statistical Analysis
3. Results
3.1. XO Inhibitory Activity of C. morifolium Fractions
3.2. Cellular Assays
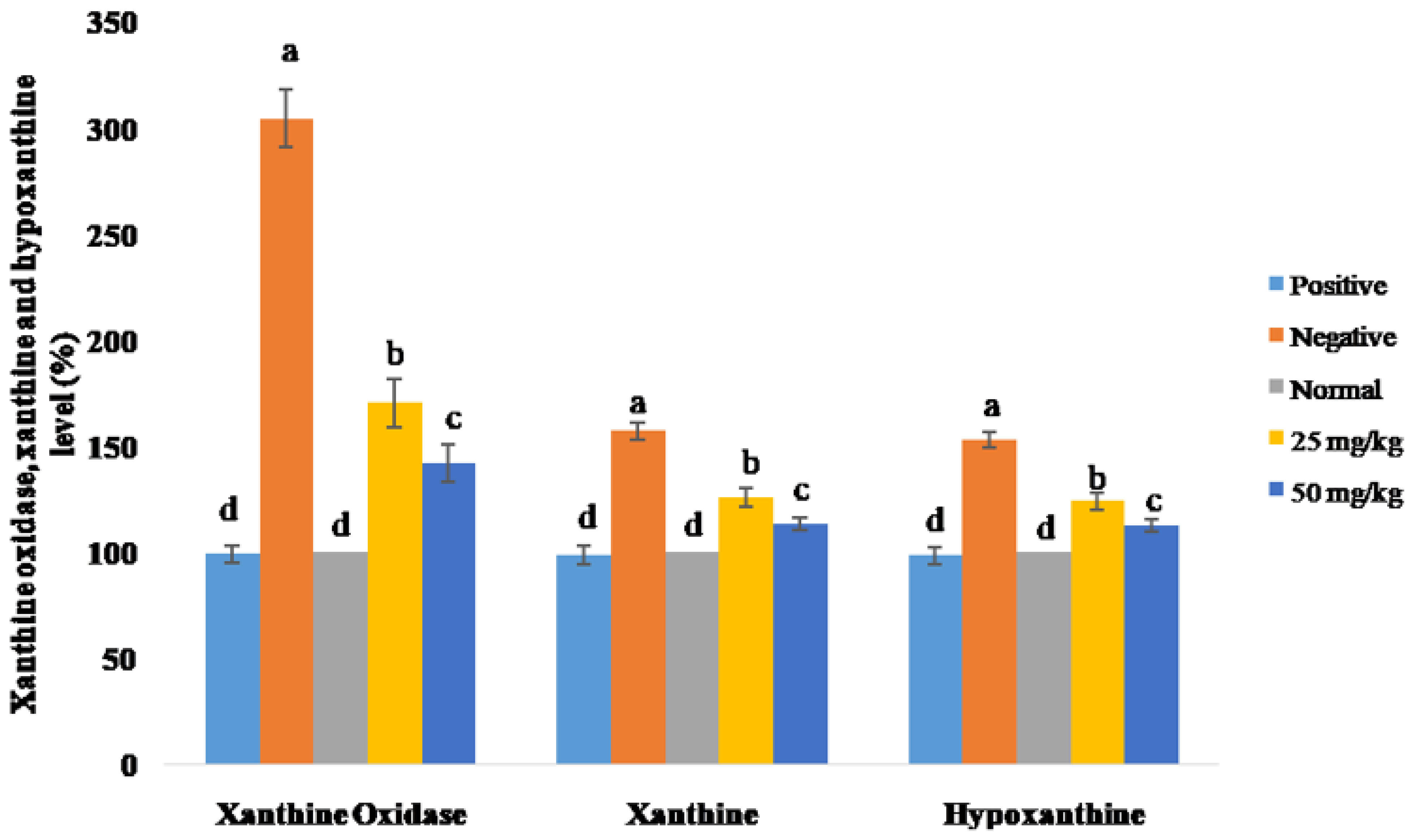
3.3. Anti-Hyperuricemic Effects of C. morifolium in Potassium Oxonate-Induced Rats
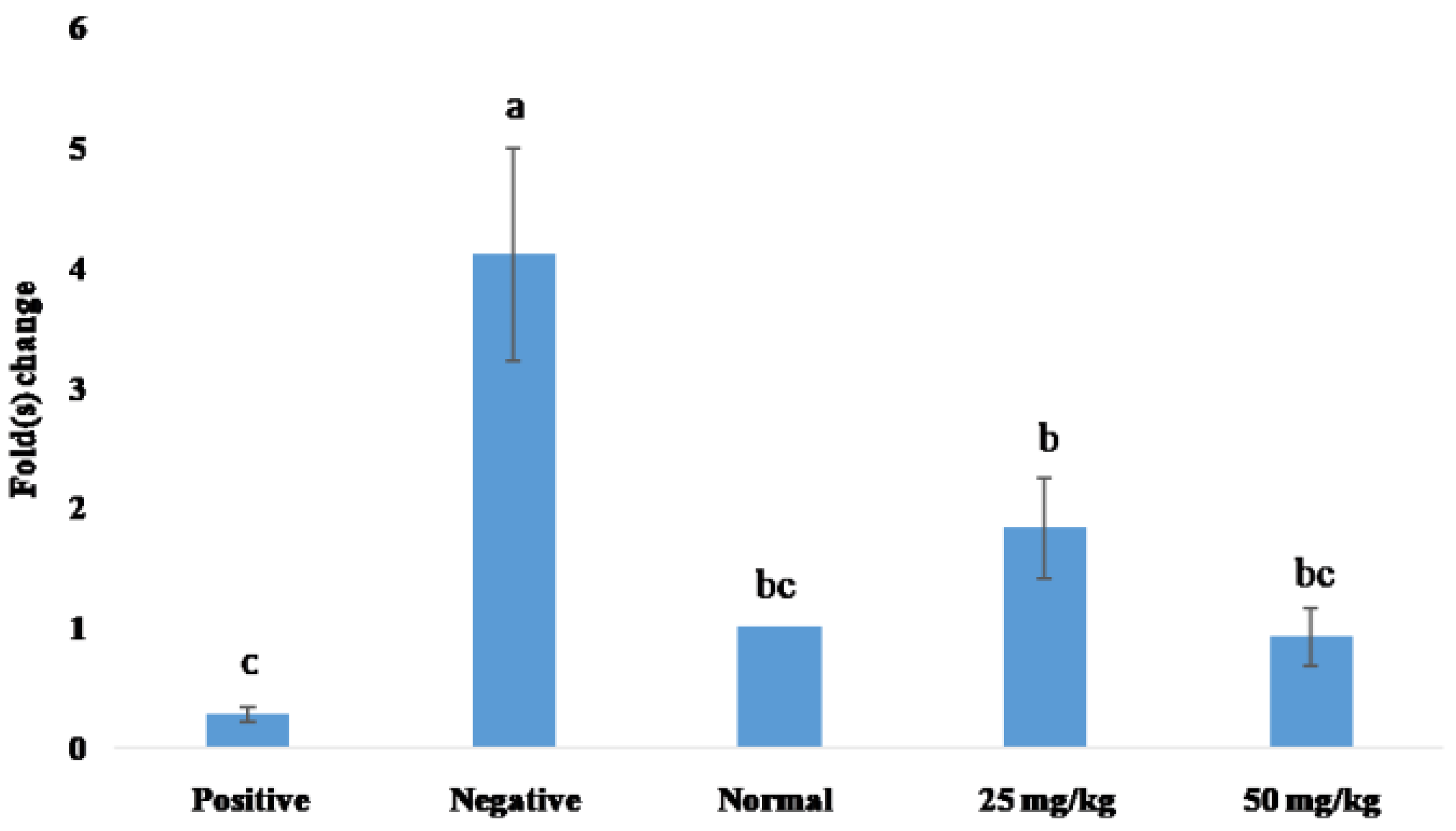
3.4. qPCR Analysis of XO Gene Expression
3.5. Putative Identification of XO Inhibitors Using HPLC-Q-TOF-MS/MS
4. Discussion
4.1. XO Inhibitory Activity of C. morifolium Fractions
4.2. Cellular Assays
4.3. Anti-Hyperuricemic Effects of C. morifolium in Potassium Oxonate-Induced Rats
4.4. qPCR Analysis of XO Gene Expression
4.5. Putative Identification of XO Inhibitors Using HPLC-Q-TOF-MS/MS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Honda, S.; Kawamoto, S.; Tanaka, H.; Kishida, H.; Kitagawa, M.; Nakai, Y.; Abe, K.; Hirata, D. Administered chrysanthemum flower oil attenuates hyperuricemia: Mechanism of action as revealed by DNA microarray analysis. Biosci. Biotechnol. Biochem. 2014, 78, 655–661. [Google Scholar] [CrossRef]
- Villegas, R.; Xiang, Y.B.; Elasy, T.; Xu, W.H.; Cai, H.; Cai, Q.; Linton, M.; Fazio, S.; Zheng, W.; Shu, X.O. Purine-rich foods, protein intake, and the prevalence of hyperuricemia: The Shanghai Men’s Health Study. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Park, J.E.; Yeom, Z.; Park, K.T.; Han, E.H.; Yu, H.J.; Kang, H.S.; Lim, Y.H. Hypouricemic effect of ethanol extract of Aster glehni leaves in potassium oxonate-induced hyperuricemic rats. Clin. Nutr. Res. 2018, 7, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Yang, Q.; Zhao, K.; Zhao, M. Identification of the free phenolic profile of Adlay bran by UPLC-QTOF-MS/MS and inhibitory mechanisms of phenolic acids against xanthine oxidase. Food Chem. 2018, 253, 108–118. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China; Chinese Medical Science and Technology Press: Beijing, China, 2010; Volume 1, p. 292. [Google Scholar]
- Hirano, M.; Takeda, S.; Hitoe, S.; Shimoda, H. Luteolin-rich chrysanthemum flower extract suppresses baseline serum uric acid in Japanese subjects with mild hyperuricemia. Integr. Mol. Med. 2017, 4, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Loh, K.E.; Chin, Y.S.; Ismail, I.S.; Tan, H.Y. Rapid characterization of xanthine oxidase inhibitors from the flowers of Chrysanthemum morifolium Ramat using metabolomics approach. Phytochem. Anal. 2021, 33, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.L.Y.; Kuksin, D.; Laverty, D.J.; Saldi, S.; Qiu, J. Morphological observation and analysis using automated image cytometry for the comparison of trypan blue and fluorescence-based viability detection method. Cytotechnology 2015, 67, 461–473. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.A.; Lim, S.Y.; Law, C.S.; Yue, C.S.; Poh, T.V.; Saad, W.Z.; Ismail, S.; Yusoff, K.M.; Loke, C.F. Antioxidative and photocytotoxic effects of standardized Clinacanthus nutans and Strobilanthes crispus extracts toward HepG2 liver cells. Pharmacogn. Mag. 2019, 15, 613–620. [Google Scholar]
- Kurien, B.T.; Everds, N.E.; Scofield, R.H. Experiment animal urine collection: A review. Lab. Anim. 2004, 38, 333–361. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.M. Identification of the phenolic components of chrysanthemum flower (Chrysanthemum morifolium Ramat). Food Chem. 2010, 120, 319–326. [Google Scholar] [CrossRef]
- Cecilia, M.K.; Abrha, A.H.; Hlengilizwe, N.; Hintsa, A.T.; Umer, M.; Nixwell, M.F.; Ali, S.W.; Afzal, M.I.; Martorell, M.; Salehi, B.; et al. Untargeted profiling of field cultivated bush tea (Athrixia phylicoides DC.) based on metabolite analysis. Cell. Mol. Biol. 2020, 66, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, J.; Dong, G.; Zhang, X.; Liu, Y.; Sun, W.; Liu, A. Flavonoids and caffeoylquinic acids in Chrysanthemum morifolium Ramat flowers: A potentially rich source of bioactive compounds. Food Chem. 2021, 344, 128733. [Google Scholar] [CrossRef]
- Chang, K.L.; Tey, M.K.; Chow, W.M.; Loh, K.E.; Intan, S.I. In-vitro antioxidant, xanthine oxidase inhibitory activity and antimicrobial properties of Chrysanthemum morifolium. In Proceedings of the 14th Symposium of Malaysian Society of Applied Biology: Bridging between Knowledge and Innovation, Melaka, Malaysia, 29–31 May 2016; pp. 161–166. [Google Scholar]
- Galanakis, C.M.; Goulas, V.; Tsakona, S.; Manganaris, G.A.; Gekas, V. A knowledge base for the recovery of natural phenols with different solvents. Int. J. Food Prop. 2013, 16, 382–396. [Google Scholar] [CrossRef] [Green Version]
- Kennouche, S.; Bicha, S.; Bentamene, A.; Crèche, J.; Benayache, F.; Benayache, S. In vitro antioxidant activity, phenolic and flavonoid contents of different polarity extracts from Chrysanthemum segetum L. growing in Algeria. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1522–1525. [Google Scholar]
- Gomez, A.R.; Rocha-Santos, T.A.P. Bioassays, enzyme assays. In Encyclopedia of Analytical Science, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 271–277. [Google Scholar]
- Spanou, C.; Veskoukis, A.S.; Kerasioti, T.; Kontou, M.; Angelis, A.; Aligiannis, N.; Skaltsounis, A.L.; Kouretas, D. Flavonoid glycosides isolated from unique legume plant extracts as novel inhibitors of xanthine oxidase. PLoS ONE 2012, 7, e32214. [Google Scholar] [CrossRef] [Green Version]
- Mohan, C.; Long, K.D.; Mutneja, M. An Introduction to Inhibitors and Their Biological Applications, 1st ed.; EMD Millipore Corporation: Billerica, MA, USA, 2013; pp. 3–41. [Google Scholar]
- Liu, L.; Zhang, L.; Ren, L. Advances in structures required of polyphenols for xanthine oxidase inhibition. Food Front. 2020, 1, 152–167. [Google Scholar] [CrossRef]
- Nile, S.H.; Keum, Y.S.; Nile, A.S.; Kwon, Y.D.; Kim, D.H. Potential cow milk xanthine oxidase inhibitory and antioxidant activity of selected phenolic acid derivatives. J. Biochem. Mol. Toxicol. 2018, 32, e22005. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.D.; Pi, L.Q.; Lee, W.S. Effect of Chrysanthemum zawadskii extract on dermal papilla cell proliferation and hair growth. Ann. Dermatol. 2020, 32, 395–401. [Google Scholar] [CrossRef]
- Marongiu, B.; Piras, A.; Porcedda, S.; Tuveri, E.; Laconi, S.; Deidda, D.; Maxia, A. Chemical and biological comparisons on supercritical extracts of Tanacetum cinerariifolium (Trevir) Sch. Bip. with three related species of chrysanthemums of Sardinia (Italy). Nat. Prod. Res. 2009, 23, 190–199. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Candra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, T.; Igarashi, K. Cultivar variation in flavonoid components and radical scavenging activity of polyphenol fractions among edible chrysanthemum flowers. J. Jpn. Soc. Food Sci. Technol. 2009, 56, 600–604. [Google Scholar] [CrossRef] [Green Version]
- Aziz, N.; Jamil, R.T. Biochemistry, xanthine oxidase. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Puig, J.G.; Mateos, F.A.; Jiménez, M.L.; Ramos, T.H. Renal excretion of hypoxanthine and xanthine in primary gout. Am. J. Med. 1988, 85, 533–537. [Google Scholar] [CrossRef]
- Lyngdoh, T.; Marques-Vidal, P.; Paccaud, F.; Preisig, M.; Waeber, G.; Bochud, M.; Vollenweider, P. Elevated serum uric acid is associated with high circulating inflammatory cytokines in the population based colaus study. PLoS ONE 2011, 6, e19901. [Google Scholar] [CrossRef] [Green Version]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine oxidoreductase-derived reactive species: Physiological and pathological effects. Oxid. Med. Cell Longev. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [Green Version]
- McNally, J.S.; Saxena, A.; Cai, H.; Dikalov, S.; Harrison, D.G. Regulation of xanthine oxidoreductase protein expression by hydrogen peroxide and calcium. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1623–1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu Bakar, F.I.; Abu Bakar, M.F.; Rahmat, A.; Abdullah, N.; Sabran, S.F.; Endrini, S. Anti-gout potential of Malaysian medicinal plants. Front. Pharmacol. 2018, 9, 261. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Zhao, X.L.; Liu, A.W.; Nian, H.; Zhang, S.H. Apigenin inhibits hepatoma cell growth through alteration of gene expression patterns. Phytomedicine 2011, 18, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Chen, C.S.; Chen, C.T.; Liang, Y.C.; Lin, J.G. Molecular modeling of flavonoids that inhibits xanthine oxidase. Biochem. Biophys. Res. Commun. 2002, 294, 167–172. [Google Scholar] [CrossRef]
- Sarg, T.; Ateya, A.M.; Abdel-Ghani, A.; Badr, W.; Shams, G. Phytochemical and pharmacological studies of Dalbergia sissoo growing in Egypt. Pharm. Biol. 1999, 37, 54–62. [Google Scholar] [CrossRef]
- Kozachok, S.; Pecio, L.; Orhan, I.E.; Deniz, F.S.S.; Marchyshyn, S.; Oleszek, W. Reinvestigation of Herniaria glabra L. saponins and their biological activity. Phytochemistry 2020, 169, 112162. [Google Scholar] [CrossRef]
- Schramm, S.; Köhler, N.; Rozhon, W. Pyrrolizidine alkaloids: Biosynthesis, biological activities and occurrence in crop plants. Molecules 2019, 24, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hainer, B.L.; Matheson, E.; Wilkes, R.T. Diagnosis, treatment, and prevention of gout. Am. Fam. Physician 2014, 90, 831–836. [Google Scholar] [PubMed]
- Burns, C.M.; Wortmann, R.L. Latest evidence on gout management: What the clinician needs to know. Ther. Adv. Chronic Dis. 2012, 3, 271–286. [Google Scholar] [CrossRef] [Green Version]
- Husain, N.; Kumar, A. Comparative study of phytochemical constituents in flower of Wedelia trilobata, Achyranthes aspera and Chrysanthemum from Durg District of Chhattisgarh, India. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 150–156. [Google Scholar]
- Fachriyah, E.; Ghifari, M.A.; Anam, K. Isolation, identification, and xanthine oxidase inhibition activity of alkaloid compound from Peperomia pellucid. IOP Conf. Ser. Mater. Sci. Eng. 2018, 349, 012017. [Google Scholar] [CrossRef]


| Samples | Xanthine Oxidase Inhibition (%) |
|---|---|
| PE | 28.09 ± 2.09 c |
| RS | 33.86 ± 3.25 c |
| EtOAc | 51.77 ± 0.98 b |
| Crude | 11.55 ± 1.21 d |
| Allopurinol | 99.79 ± 0.21 a |
| Treatments | Serum Uric Acid Content (%) | Urine Uric Acid Content (%) |
|---|---|---|
| Positive control | 108.30 ± 5.05 c | 98.97 ± 5.17 c |
| Negative control | 217.79 ± 10.28 a | 249.67 ± 9.93 a |
| Normal control | 100.00 ± 0.00 c | 100.00 ± 0.00 c |
| 25 mg/kg EtOAc F10 | 134.38 ± 7.91 b | 157.13 ± 5.71 b |
| 50 mg/kg EtOAc F10 | 96.74 ± 4.81 c | 143.97 ± 5.80 b |
| Peak | tR (min) | Chemical Formula | Mass | Adduct | Parent Ions (m/z) | Fragments (m/z) | Identification | References |
|---|---|---|---|---|---|---|---|---|
| 1 | 13.172 | C18H28N2O2 | 304.2135 | [M+Na] | 327.2028 | 133.0860, 177.1124 | 4′-Hydroxybupivacaine | MassHunter library |
| 2 | 13.566 | C17H30N4O5 | 370.2218 | [M+H] | 371.2291 | 133.0862 | - | - |
| 3 | 14.544 | C30H46O6 | 502.3005 | [M+H] | 503.3087 | 221.1386, 459.2797 | Medicagenic acid | HMDB0034551 |
| 4 | 14.840 | C25H24O14 | 546.3268 | [M+H] | 547.3342 | - | - | - |
| 5 | 15.929 | C31H55N6O2 | 543.4393 | [M+Na] | 566.4286 | 283.7175 | - | - |
| 6 | 16.403 | C34H30O15 | 678.5065 | [M+H] | 679.5138 | 354.2610 | 3,4,5-Tricaffeoylquinic acid | [11] |
| 7 | 20.947 | C15H22O | 218.1682 | [M+H] | 219.1756 | 201.1644 | Zerumbone | HMDB0036667 |
| 8 | 22.531 | C15H22O | 218.1680 | [M+H] | 219.1754 | 201.1644 | Vulgarone B | HMDB0035657 |
| 9 | 23.094 | C15H22O | 218.1684 | [M+H] | 219.1758 | 201.1644 | Vulgarone A | FDB015670 |
| 10 | 24.161 | C18H26O3 | 290.1895 | [M+H] | 291.1969 | 273.1855 | 4-oxo-9Z, 11Z, 13E, 15E-octadecatetraenoic acid | MassHunter library |
| 11 | 25.207 | C14H31NO2 | 245.2366 | [M+H] | 246.2440 | - | - | - |
| 12 | 28.133 | C16H12O5 | 284.2024 | [M+H] | 285.2055 | 211.1118 | Prunetin | HMDB0034127 |
| 13 | 29.319 | C24H30O6 | 414.2055 | [M+H] | 415.2129 | 119.0856 | Magnoshinin | [12] |
| 14 | 13.142 | C15H18O8 | 326.1952 | [M+H] | 327.2025 | 309.1906, 133.0859 | trans-p-Coumaroyl beta-D-glucopyranoside | HMDB0302089 |
| 15 | 14.247 | C17H23N4O9P | 458.2738 | [M+H] | 459.2812 | 441.2697 | Flavin mononucleotide hydroquinone | HMDB0001142 |
| 16 | 14.527 | C6H4O2 | 108.0581 | [M+H] | 109.0654 | - | 1,2-benzoquinone | HMDB0012133 |
| 17 | 15.225 | C23H22O11 | 474.3185 | [M+H] | 475.3260 | 271.1588 | Apigenin 7- O-6”-acetylglucoside | [11] |
| 18 | 15.891 | C26H28O14 | 565.4190 | [M+H] | 566.4264 | 548.4134 | 6-C-glucosyl-8-C-arabinosylapigenin | [11] |
| 19 | 17.373 | C21H20O10 | 432.1066 | [M+H] | 433.1139 | 271.0605 | Apigenin 7-O- glucoside | MassHunter library |
| 20 | 19.596 | C17H27NO6 | 341.1847 | [M+H] | 342.1919 | 337.1626 | Acetylintermedine | MassHunter library |
| 21 | 20.642 | C22H22O10 | 446.1223 | [M+H] | 447.1297 | 285.0759 | Prunetin 4′-O- galactoside | MassHunter library |
| 22 | 23.272 | C8H10N4O2 | 193.1589 | [M+H] | 194.1662 | - | - | - |
| 23 | 25.702 | C11H6O3 | 186.1204 | [M+H] | 187.1278 | 115.0551, 131.0851 | Psoralen | HMDB0034272 |
| 24 | 27.557 | C9H15N3O11P2 | 403.2004 | [M+H] | 404.2078 | 105.0703, 387.1805 | Cytidine diphosphate (CDP) | HMDB0001546 |
| 25 | 29.395 | C16H27NO11 | 409.2500 | [M+Na] | 432.2390 | 415.2114 | Linustatin | HMDB0303673 |
| 26 | 30.776 | C16H35NO2 | 273.2682 | [M+H] | 274.2754 | - | - | - |
| 27 | 11.043 | C5H9NO | 99.0692 | [M+H] | 100.0765 | - | 2-Hydroxy-2-methylbutanenitrile | HMDB0060309 |
| 28 | 11.636 | C12H8O4 | 216.0988 | [M+H] | 217.1061 | 173.0767, 201.0716 | 5-methoxypsoralen | HMDB0030637 |
| 29 | 13.134 | C18H16O7 | 344.2292 | [M+H] | 345.2320 | 328.2056 | Eupatilin | [13] |
| 30 | 13.935 | C26H38O4 | 414.2476 | [M+H] | 415.2550 | 371.2273, 397.2434 | Lupulone | HMDB0030140 |
| 31 | 15.853 | C30H51N8O4 | 587.4037 | [M+H] | 588.4111 | 318.1141 | - | - |
| 32 | 28.295 | C16H33NO3 | 287.2464 | [M+H] | 288.2537 | 242.2475 | Prosopinine | MassHunter Library |
| 33 | 13.280 | C16H28O8 | 348.1778 | [M+H] | 349.1851 | 333.1541 | Schizonepetoside E | MassHunter library |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, T.L.; Loh, K.E.; Tan, S.-A.; Tan, H.Y.; Yue, C.S.; Wee, S.P.; Tey, Z.T. Anti-Hyperuricemic Effect of Ethyl Acetate Sub-Fractions from Chrysanthemum morifolium Ramat. Dried Flowers on Potassium Oxonate-Induced Hyperuricemic Rats. Appl. Sci. 2022, 12, 3487. https://doi.org/10.3390/app12073487
Ng TL, Loh KE, Tan S-A, Tan HY, Yue CS, Wee SP, Tey ZT. Anti-Hyperuricemic Effect of Ethyl Acetate Sub-Fractions from Chrysanthemum morifolium Ramat. Dried Flowers on Potassium Oxonate-Induced Hyperuricemic Rats. Applied Sciences. 2022; 12(7):3487. https://doi.org/10.3390/app12073487
Chicago/Turabian StyleNg, Teng Lit, Khye Er Loh, Sheri-Ann Tan, Hui Yin Tan, Chen Son Yue, Sze Ping Wee, and Zi Tong Tey. 2022. "Anti-Hyperuricemic Effect of Ethyl Acetate Sub-Fractions from Chrysanthemum morifolium Ramat. Dried Flowers on Potassium Oxonate-Induced Hyperuricemic Rats" Applied Sciences 12, no. 7: 3487. https://doi.org/10.3390/app12073487
APA StyleNg, T. L., Loh, K. E., Tan, S.-A., Tan, H. Y., Yue, C. S., Wee, S. P., & Tey, Z. T. (2022). Anti-Hyperuricemic Effect of Ethyl Acetate Sub-Fractions from Chrysanthemum morifolium Ramat. Dried Flowers on Potassium Oxonate-Induced Hyperuricemic Rats. Applied Sciences, 12(7), 3487. https://doi.org/10.3390/app12073487