Abstract
Postoperative tissue regeneration can be negatively affected by bisphosphonate administration, especially in patients with oncologic diseases. A serious complication of bisphosphonate therapy is the medication-related osteonecrosis of the jaw (MRONJ), which can be observed mainly after dental surgery. MRONJ is a progressive destruction of the bone that requires patients to stay in hospital for extended periods of time. For this reason, primary wound closure is particularly important in surgical procedures. In the case of wound dehiscence, there is a very high risk for MRONJ. In recent years, non-invasive physical plasma (NIPP) has become known for improving wound healing on the one hand, but also for its promising efficacy in cancer therapy on the other hand. We report on a 63-year-old patient with a history of multiple myeloma and receiving zoledronate, who developed wound dehiscence after tooth extraction. NIPP treatment resulted in complete epithelialization of the entire wound dehiscence. In conclusion, the use of NIPP in patients receiving antiresorptive drugs seems to support tissue regeneration and thus could be an important tool for the prevention of MRONJ.
1. Introduction
Cellular processes of postoperative tissue regeneration are negatively affected by antiresorptive drugs, especially in patients with breast cancer, prostate cancer, and multiple myeloma [1,2]. A serious complication associated with the application of bisphosphonates, monoclonal antibodies, or angiogenesis inhibitors is medication-related osteonecrosis of the jaw (MRONJ). MRONJ is frequently observed after dental surgery and due to denture pressure points [3,4].
MRONJ is the progressive destruction of alveolar bone that may remain asymptomatic for years, but may also be associated with pain, ulceration, suppuration, and exposed or sequestered bone [4,5,6]. Once MRONJ has developed, complex surgical procedures are frequently required, necessitating prolonged hospitalisation of the patients. Usually, these complicated interventions are a burden on the health care system as well as a psychological burden for patients [7,8].
Therefore, in patients treated with these drugs, increased safety precautions must be taken when planning oral surgical interventions. These include perioperative antibiosis and primary wound closure using a muco-periosteal flap [9]. However, since the bone metabolism is affected by bisphosphonates and monoclonal antibodies for a long period of time, the stability of wounds after surgical dental procedures is not guaranteed. Early dehiscence of the wound increases the risk of MRONJ many times over, especially since inflammation and bacterial infection due to bacteria of the oral flora and insufficient oral hygiene can already be a trigger for MRONJ [4,10].
In recent years, non-invasive physical plasma (NIPP) has received increased attention with regard to novel therapeutic procedures. The most important effects of NIPP treatment are the highly effective antimicrobial efficacy on a variety of pathogens and the promotion of wound healing. Additionally, NIPP has also been shown to be very effective in devitalizing neoplastic tissue [11,12,13,14,15]. NIPP corresponds to a highly reactive gas that affects miscellaneous cells and tissues, particularly through reactive oxygen and nitrogen species [16]. Different types of NIPP devices are known in the field of medical applications: dielectric barrier discharges (DBD), plasma jets, as well as hybrid devices that combine both technologies. With DBD devices, NIPP is generated directly at the site of action, with the treated object serving as a counter-electrode. Plasma jets form NIPP inside the device between the cathode and the anode and use a carrier gas to transport NIPP to the place of action [16,17]. Thus far, the utilization of NIPP in the field of oral and craniomaxillofacial surgery and therapy is not a routine procedure. However, assumingly, NIPP has positive effects on tissue regeneration, due to antimicrobial factors, anti-inflammatory effects and additionally, promotes wound healing.
This report presents the case of a wound dehiscence associated with zoledronate–therapy for oncological reasons and the benefit of non-invasive treatment with an intraoral certified NIPP device. Additionally, the effect of NIPP on factors associated with wound healing were to be studied using XTT, rtPCR, and scratch assay. Since a healing wound is characterised by a high level of proliferating cells, typically associated proliferation proteins Ki67 and Proliferating Cell Nuclear Antigen (PCNA) [18] should be investigated.
2. Case Report
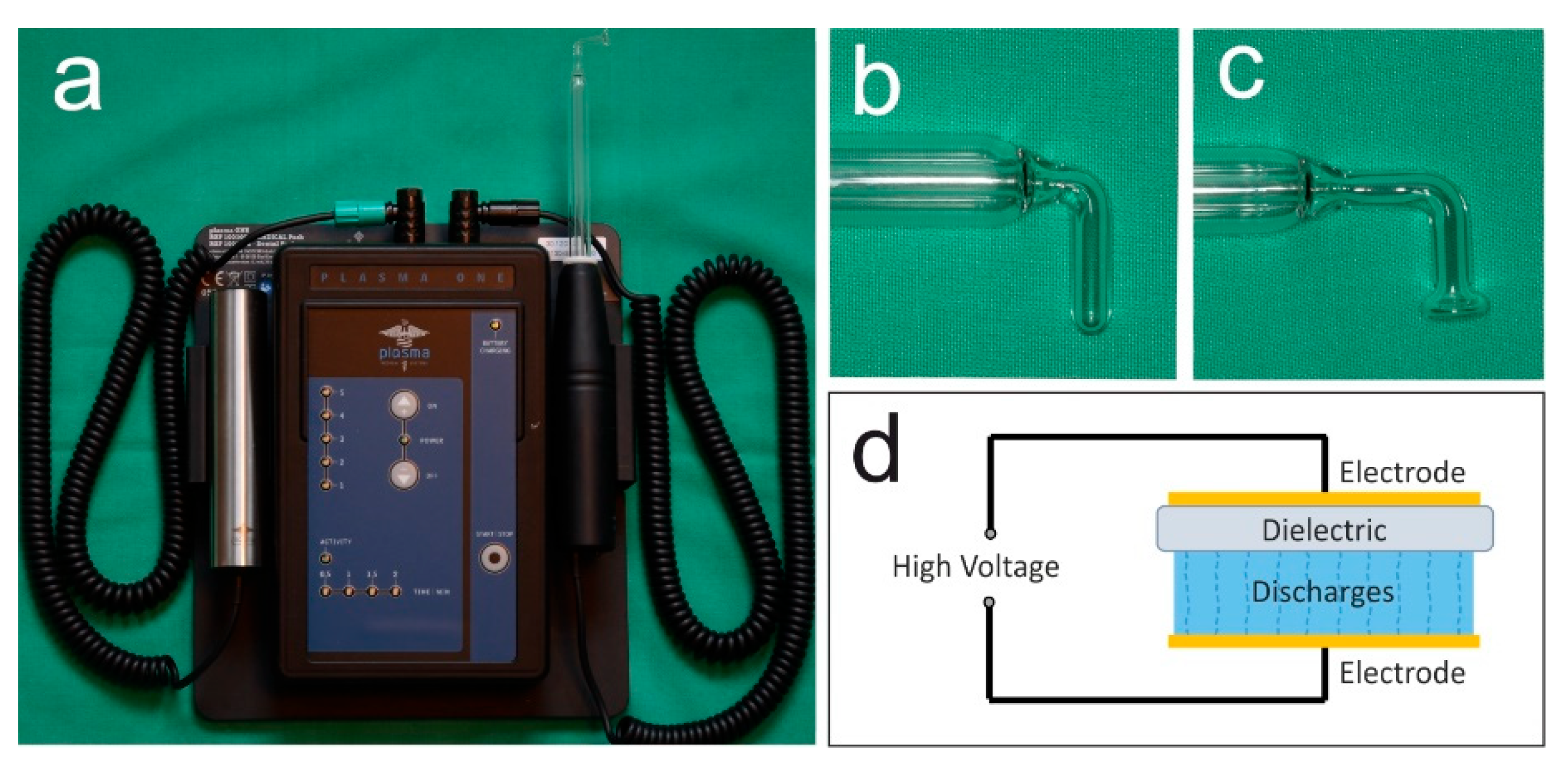
The present case demonstrates the use of NIPP for wound healing disorders after tooth extraction following muco-periosteal flap in one patient receiving antiresorptive medication due to multiple myeloma. The plasma ONE Dental (plasma Medical Systems GmbH, Nievern, Germany), a DBD certified for intraoral use, was used with probe PS08 and PS12 at the highest intensity level (5) for 60 s per cm2 (Figure 1a–d). The device operates on 12 V DC and modulates a high voltage of 18 kV, which generates an electric field at the tip of the probe. Thereby, the plasma is created. According to the company’s information, the predominant reactive species generated by the plasma are NO, NO2, and O3. Detailed information about the device is shown in Table 1.
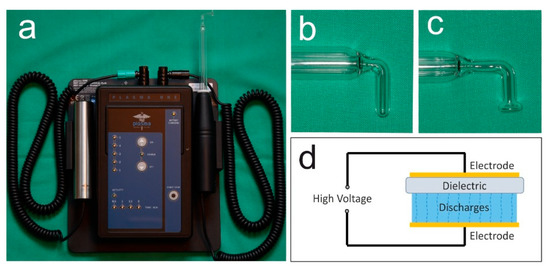
Figure 1.
Ambient-air NIPP was generated by a dielectric barrier discharge (a) plasma ONE Dental (plasma Medical Systems GmbH, Nievern, Germany); (b) instrument probe PS08; (c) instrument probe PS12; (d) principle of dielectric barrier discharge.

Table 1.
Plasma ONE Dental. Technical Data (intensity level 5, DBD mode).
The treatments were performed in accordance with the Declaration of Helsinki. The patient gave written informed consent to participate.
A 63-year-old man presented to our clinic 2 weeks after all wisdom teeth and the first lower left molar had been removed under general anaesthesia (Figure 2a). Primary wound closure had been achieved for every wound area with a mucoperiosteal flap, which was necessary because the patient had been taking 4 mg of zoledronate intravenously every 3 months due to multiple myeloma diagnosed 2 years prior. Two weeks before the tooth removal, the drug had been discontinued by the oncologist for one dose. The bisphosphonate was resumed at the end of our therapy. In addition, the patient received clindamycin 600 mg three times daily for 8 days perioperatively due to an existing penicillin allergy. The patient’s medical history included hypertension and a heart attack suffered 4 years prior.

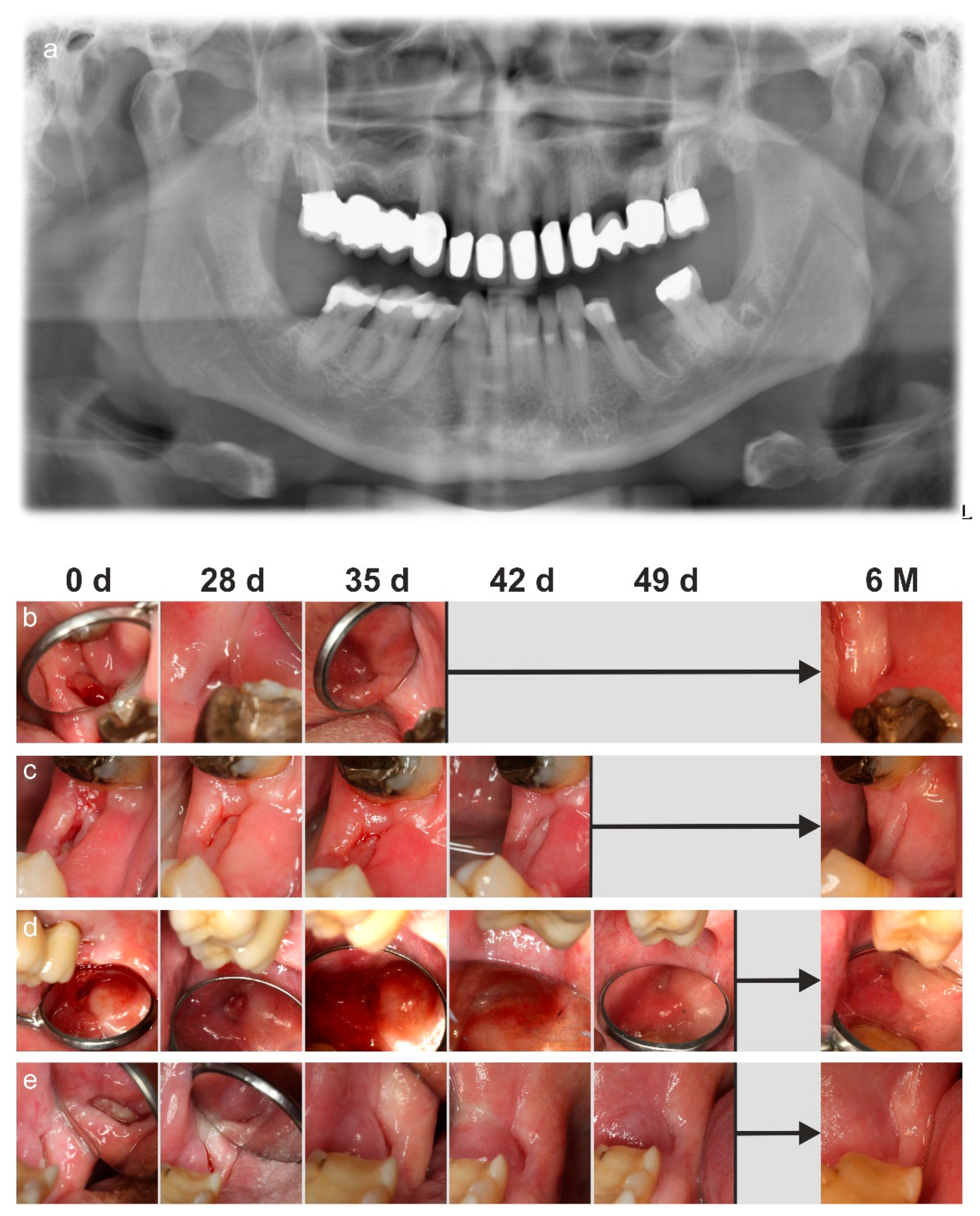
Figure 2.
A 63-year-old patient receiving zoledronate for oncological reasons developed wound dehiscence 2 weeks after tooth extractions. (a) Panoramic X-ray at first presentation showing previous extraction of all wisdom teeth and left lower first molar; (b) wound dehiscence of the area of the former left lower wisdom tooth at first presentation with complete epithelization after 35 days and follow-up after 6 months; (c) wound dehiscence of the area of the former first lower left molar at first presentation with complete epithelization after 42 days and follow-up after 6 months; (d) wound dehiscence of the area of the former right upper wisdom tooth at first presentation with complete epithelization after 49 days and follow-up after 6 month; (e) wound dehiscence of the area of the former right lower wisdom tooth at first presentation with complete epithelization after 49 days and follow-up after 6 months.
On first presentation, all wounds except the site of the former upper left wisdom tooth presented as dehiscent with exposed bone (Figure 2b–e). The patient did not report any pain or sensory disturbances and denied having had radiotherapy in the head and neck area in the past. Since reoperation is not possible until about 6 to 12 weeks after dehiscence with stable wound conditions, the patient agreed to treat the wounds with NIPP in the intermediate period.
In the following period, the patient presented himself weekly at our clinic. At each appointment, the wounds were irrigated with chlorhexidine (Kreussler Pharma, Wiesbaden, Germany) and photographic documentation was taken. Additionally, a bulbous probe (Aesculap AG, Tuttlingen, Germany) was used to carefully check the progress of epithelialization so as not to oversee small areas of exposed bone. The region was only considered to be healed when no more bone was palpable. The wounds were then treated with NIPP. Twenty-eight days after the initial presentation, the wound of the lower wisdom tooth was completely epithelialized. Quantification of the wound surface (ImageJ, Bethesda, MD, USA) indicated that the healed and epithelialized area represented only 68% of the original defect size (Figure 2b). Overall, this wound showed the most increased healing tendency. After 35 days, the structure of the gums was almost regenerated and the area of the former wound represented 48% as compared to the first image (Figure 2b). A passive fistula had developed in the wound region of the former left lower first molar during the healing process, and an exposed bone could be probed at the base of the fistula (Figure 2c). The passive fistula developed during healing due to the fact that the soft tissue healed faster than deeper areas in the former alveolus. The fistula did not form an exudate, but there was bleeding on probing which indicated an underlying infection. With the use of the PS08 probe of the NIPP device (Figure 1b), the fistula had been closed by NIPP application so that after 42 days the wound was epithelialized (Figure 2c). The wound size decreased to 23% (Figure 2c). The area of the former upper right wisdom tooth showed an increase in granulation tissue with a delay of four weeks, representing a wound area of 63% as compared to the initial size (Figure 2d). However, the subsequent epithelialisation of the wound took place rapidly so that the treatment was completed after 49 days (size of the former defect: 43%) (Figure 2d). The healing of the right lower wisdom tooth showed a clear increase in the epithelium due to the application of NIPP. Within 4 weeks the bone was covered by a thin layer of tissue, which gradually became more stable, so that the NIPP treatment was completed after 7 weeks (Figure 2e). The defect contracted to 36% of the original size down to 23%. Throughout the therapy, the patient reported neither pain nor sensory disturbances in the treated areas. At a follow-up 6 months after therapy, all wounds were still found to remain epithelialized (Figure 2).
3. Materials and Methods
3.1. Cell Culture
Commercially available Human Primary Gingival Keratinocytes (HPGK) (ATCC, Manassas, VA, USA) were propagated in a humidified atmosphere at 5% CO2 and 37 °C in Keratinocyte Growth Medium 2 (Promocell, Heidelberg, Germany) supplemented with 1% penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific, Waltham, MA, USA). For in vitro cell culture experiments, cells were seeded at 50.000 viable cells/mL into 3.5 cm2 cell culture Petri dishes (VWR, Radnor, PA, USA).
3.2. NIPP Treatment
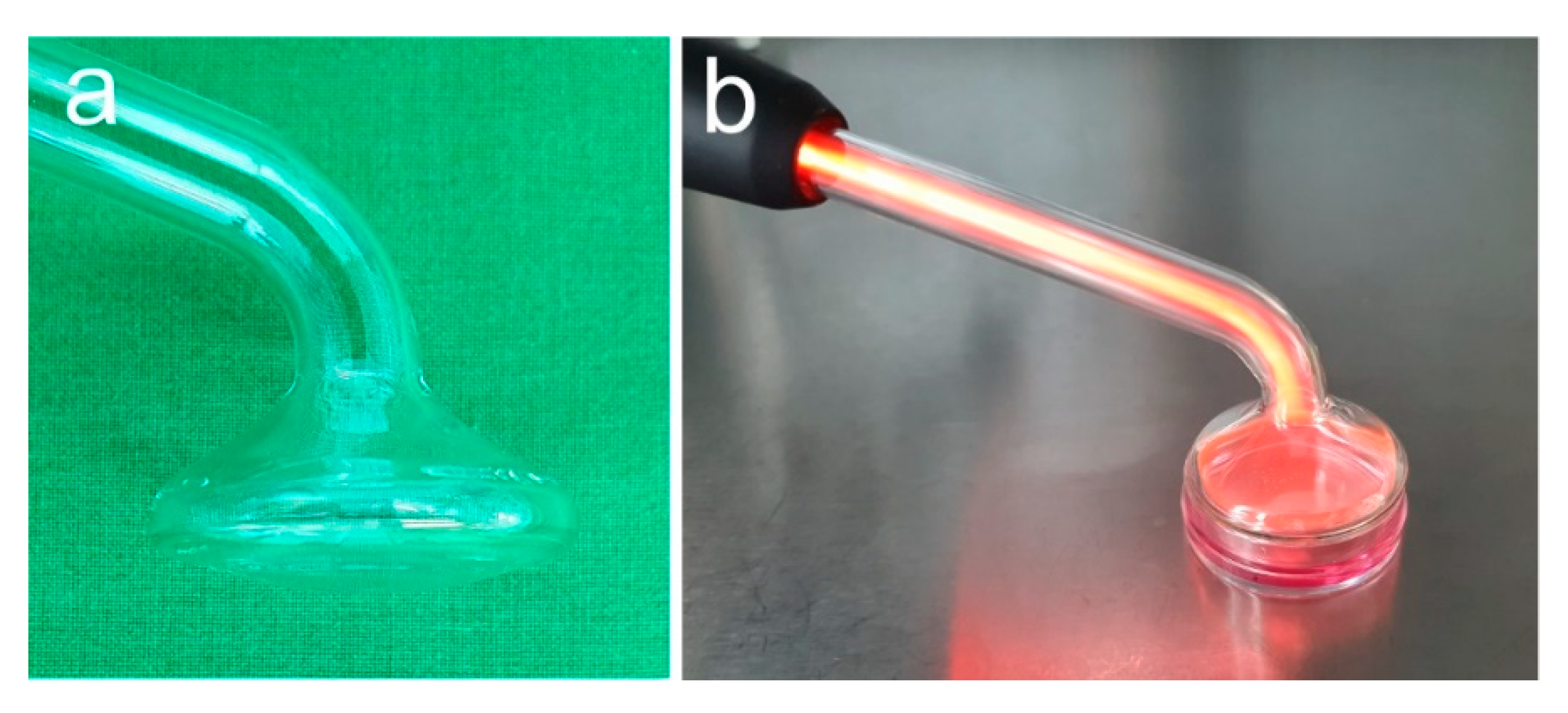
NIPP treatment was performed as shown in Figure 3 using instrument probe PS30 (Figure 3a,b). Cells were treated for 60 s and analysed at 1 day. Untreated cells served as negative control.
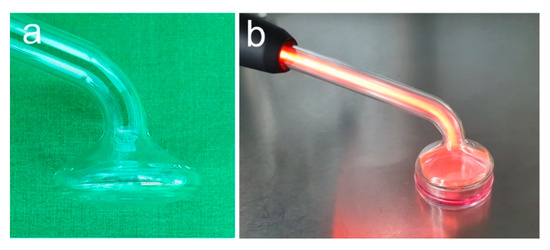
Figure 3.
In vitro NIPP treatment. (a) Instrument probe PS30; (b) treatment of medium covered cells.
3.3. Temperature and pH Measurements
The temperature of the cell culture medium was determined before and after NIPP treatment for the indicated duration at the surface with an FLIR One infrared camera (Teledyne Flir, Wilsonville, OR, USA). In parallel, this method was used to determine the temperature of the probe during the NIPP treatment. Additionally, the temperature of the liquid below the surface was measured and the pH was determined with a benchtop pH 50 VioLab (XS instruments, Carpi, Italy).
3.4. XTT Assay
For analysing metabolic activity of the cells, Cell proliferation Kit XTT (AppliChem, Darmstadt, Germany) was used. After NIPP treatment, cells were incubated for 20 h. Subsequently, XTT reagent solution was added to the culturing medium for 4 h, according to the manufacturer’s instructions. Analysis was performed with a microplate reader at an absorbance of 475 nm (Epoch™ Microplate Spectrophotometer, BioTek Instruments, Winooski, VT, USA).
3.5. Analysis of mRNA Expression
RNA was isolated using the RNeasy protect Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Concentration of the isolated RNA was determined using the Nano Drop ND-2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). For cDNA synthesis, 500 ng of RNA was reverse transcribed using the iScript™ Select cDNA Synthesis Kit (Bio-Rad Laboratories, Munich, Germany) according to the manufacturer’s instructions. Real-time PCR was used to detect the expression of the target mRNA, using 1 µL of cDNA, 2.5 μL of specific primers (Ki67, PCNA, GAPDH; QuantiTect Primer Assay, Qiagen), 12.5 μL of SYBR Green QPCR Master Mix (Bio-Rad) and 9 μL of deionised water in a 25 µL reagent mixture. Here, the following protocol was used: initial denaturation and thermal activation of polymerase (95 °C, 5 min), 40 cycles of denaturation (95 °C, 10 s), and a combined annealing/elongation (60 °C, 30 s). Quantification of mRNA was performed using the comparative threshold cycle method.
3.6. Scratch Assay
Scratch assay was performed as previously described [19]: A standardized sterile instrument was used to generate a 3 mm wide cell-free area within the cell monolayer. The confluence of the cell free area was measured automatically every 30 min for 48 h using JuLI™ Br and JuLI™Br PC software (NanoEnTek, Seoul, Korea).
3.7. Statistical Analysis
All in vitro experiments were performed at least three times with independent replicates (n) and the means and standard error of the mean (SEM) were calculated. For statistics, GraphPad Prism Software (GraphPad Software, San Diego, CA, USA) was used with Mann–Whitney U test at the significance level of p < 0.05.
4. Results
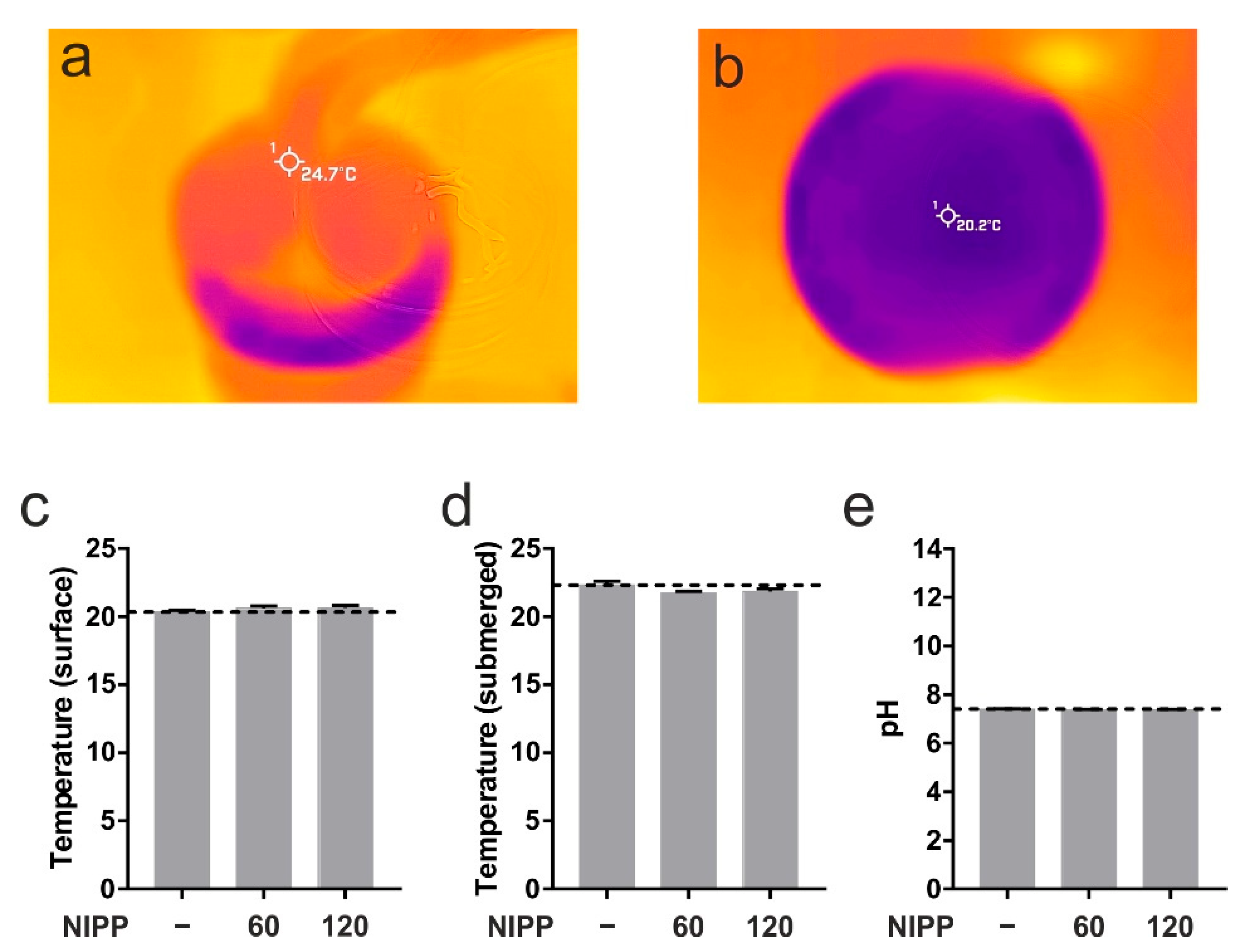
First, we wanted to prove that NIPP has no negative effects on cells and tissues. At room temperature the PS30 probe runs with a certain surface temperature of 24.7 °C (Figure 4a). The measurement of the surface temperature and the core temperature of the cell culture medium showed no change due to the application of NIPP (Figure 4b–d). Neither was there any change in the pH value (Figure 4e). For this reason, damage to the treated cells and tissues could be ruled out.
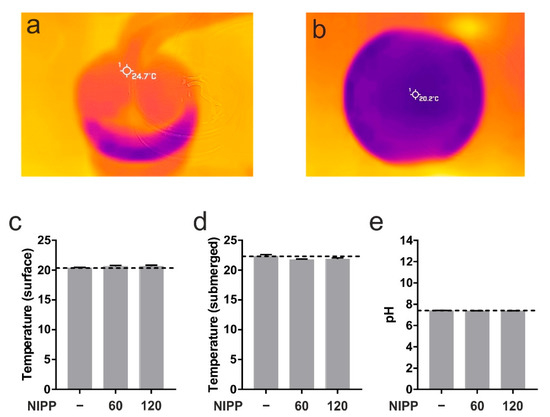
Figure 4.
Basic characterisation of NIPP. (a) Superficial temperature of NIPP probe PS30 at DBD mode; (b,c) superficial temperature of cell culture medium prior to NIPP treatment (-) up to 120 s of treatment, respectively; (d) submerged temperature of cell culture medium during 120 s of NIPP treatment; (e) pH of cell culture medium during 120 s of NIPP treatment.
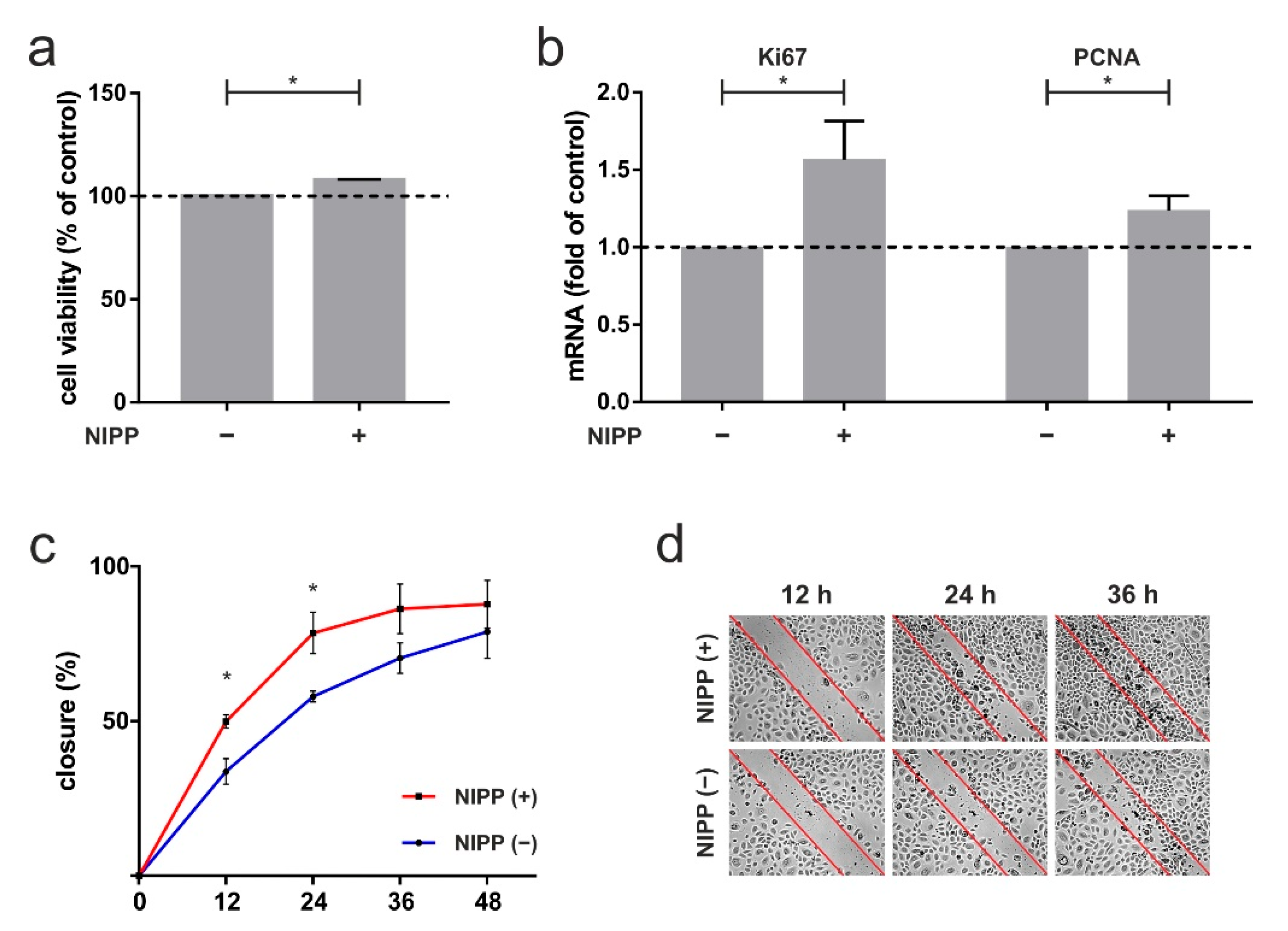
To quantify the in vivo effect of NIPP described in the case report, human gingival keratinocytes were used for the following experiments. First, we investigated the influence of NIPP on HPGK’s metabolic activity. NIPP treatment of 60 s caused a significant increase in cell viability of 8% after 24 h (p = 0.0022; Figure 5a). Next, we focused on crucial markers of proliferation: NIPP treatment resulted in a significant 1.6-fold increase in Ki67 mRNA levels (p = 0.0476) and a 1.2-fold increase in PCNA mRNA levels (p = 0.0476; Figure 5b). Finally, we investigated effects of NIPP on in vitro wound healing using an established scratch-assay. As shown in Figure 5c, NIPP lead to a significant faster closure of an artificial scratch, as compared to untreated cells (12 h: 0.0159; 24 h: p = 0.0317; Figure 5c,d).
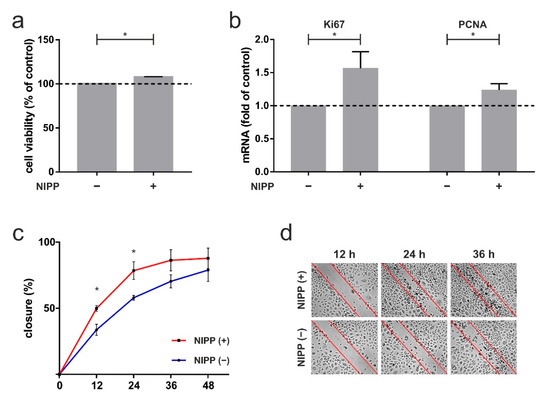
Figure 5.
The 60 s NIPP treatment of primary human gingival keratinocytes (HPGK) at one day. (a) Influence of NIPP on cell viability, shown by XTT assay, n = 6; (b) impact of NIPP on mRNA level of proliferation markers Ki67 and PCNA, n = 6; (c) in vitro wound healing assay, displayed by scratch assay, n = 5; (d) representative images are visualized. * statistical significance (p < 0.05).
5. Discussion
Over the last two decades, drug therapies have been frequently associated with osteonecrosis of the jaw. Thus, these diseases are no longer solely called bisphosphonate associated osteonecrosis, but MRONJ. Bisphosphonates administered for oncologic reasons are associated with the development of osteonecrosis. The risk of developing MRONJ in oncologic patients is 1.3–1.8% [20]. Especially patients treated for multiple myeloma have a rather high incidence for MRONJ [21]. Thus, in these patients, it is mandatory to ensure primary wound healing when surgery is performed. In cases of wound dehiscence, it is very important that the wound is covered with a mucoperiosteal flap with sufficiently stable tissue [22]. We report about a conservative treatment option of a wound dehiscence using NIPP with successful healing of the affected tissue in a patient under bisphosphonate therapy for multiple myeloma.
Zoledronate is an intravenous bisphosphonate used to treat multiple myeloma, metastatic breast and prostate cancer, Paget’s disease, and osteoporosis [23]. It has a high affinity for mineralised bone, more pronounced than other bisphosphonates, and accumulates particularly at sites of high bone turnover [24,25]. The effect of bisphosphonates is characterised by reducing bone turnover and increasing osteoblast function [26]. However, zoledronate has been shown to impair wound healing after dental surgery, such as tooth extraction [27]. In our case, the wound healing took about 5–9 weeks in total. Additionally, zoledronate is known to negatively affect wound healing by causing mucosal lesions, inflammatory infiltrates and non-vital bone after tooth extraction [28,29].
The application of NIPP has been described as a promising method for delayed wound healing and in cancer therapy [14,30], and a proliferation-promoting effect has been shown in many in vitro, in vivo and even clinical studies [13,31,32,33,34,35,36,37,38]. The exact mode of action of NIPP is not known; however, a number of reactive oxygen and nitrogen species have an effect on the cells and tissues involved. In the in vitro part of our study, we were able to demonstrate a proliferation-promoting effect of NIPP on keratinocytes, in terms of upregulation of proliferation markers Ki67 and PCNA mRNA. Additionally, higher metabolic activity of the cells by XTT and improved cell migration by scratch assay has been shown. However, the NIPP effect was not as pronounced in keratinocytes as in cementoblasts or periodontal ligament cells [13,39]. A reason might be that keratinocytes are slow-proliferating cells [40]. It is possible that a study after longer incubation times (after 2 or 3 d) would have shown even stronger effects. Nevertheless, a significant positive effect of NIPP was evident, which may explain the successful clinical response observed in the case report. However, since mesenchymal stem cells in particular provide key factors that accelerate healing during wound healing [41,42], it may be that NIPP influences these cells during tissue stimulation. On the other hand, NIPP is also able to stimulate certain cytokines and chemokines and MMPs [13,32], which are also components of the mesenchymal stem cell secretome and modulate immune processes as well as cell migration and proliferation [43,44,45]. However, the cellular processes involved in NIPP-induced tissue regeneration and wound healing are still poorly understood. Additionally, as it is also known that microtrauma can influence the secretome of the cells, a detailed investigation of the influence on tissue healing is necessary [46]. Nevertheless, a proliferation promoting effect of NIPP on mesenchymal stem cells in vitro has been described by other authors [47].
Since the wound healing effect of NIPP has been demonstrated in vitro, in vivo, and in randomised clinical trials by other authors [30,48,49], we treated the patient with this technology. The NIPP application was intended to minimize the risk of developing MRONJ. Various devices are currently used in surgical practice. NIPP is generated in different ways, either by using noble gases such as argon or helium, or by using the ambient air, as shown in our case [13,14,31]. In the present study, only a 60 s treatment was performed once a week. Although the device is a certified medical device, there is little evidence-based information on the exact application therapy, except for an application time between 30 s and 120 s. In previous in vitro studies, we found that NIPP has a proliferation-promoting effect when applied for 60 s [13,32,39]. A significant increase in wound healing in patients with diabetic foot ulcers by eight applications of NIPP has been shown by Stratmann et al. In this study, treatment with an argon-based NIPP device is performed for 30 s per cm2 [30]. Other authors applied a treatment-time of 2 min for 14 patients with chronically infected wounds using argon generated NIPP [37]. Just a few clinical studies have shown that intraoral wound healing can be improved by NIPP: Kusakci-Seher et al. have proven that a single application of NIPP significantly improves gingival wound healing after gingivectomy [50] and Pekbağrıyanık et al. have shown that gingival epithelization after surgery can be significantly accelerated by a single application of NIPP [51]. In these studies, application times of 60 s and 120 s, respectively, have been described. Further systematic studies with NIPP treatments of varying duration are needed to reveal the effects of NIPP on intraoral wound healing.
Other approaches to prevent MRONJ also exist. For example, it has been shown that dental pulp stem cell conditioned medium or platelet rich fibrin could also be used to prevent MRONJ [52,53]. However, the NIPP-based treatment modality would be a very cost-effective and easy-to-use method for MRONJ prevention that could also be applied in dental practices.
As wound healing problems can only indicate MRONJ at least 8 weeks after surgery under current or previous treatment with antiresorptive drugs with exposed bone and without any history of radiation therapy [4], we wanted to prevent the development of MRONJ by starting NIPP therapy 2 weeks after surgery. In our case, necrotic bone that regularly accompanied MRONJ, was not evident clinically or on radiograph. It has also been described in the literature that the duration of taking antiresorptive drugs seems to have an important influence on the occurrence of MRONJ [54,55]: Patients receiving antiresorptive drugs for longer than 8 months have a significantly higher incidence of MRONJ than patients receiving these drugs for a shorter period of time [54]. This might suggest that NIPP treatment was only possible because the side effects of zoledronate were not yet very pronounced. However, as the patient we presented had been taking zoledronate for a period of more than 8 months and all primary surgically closed wounds had become dehiscent, we considered the risk of developing MRONJ to be high. However, it may be possible that extensive MRONJ cannot be treated with NIPP. Although a proliferation-promoting effect of NIPP has also been shown in hard tissue cells [32,56], further studies are needed to decipher the exact effect of NIPP in MRONJ.
A limitation of our case report, as with all observational studies, is the possibility of bias and the risk of overinterpretation because it is impossible to control for all variability in a retrospective design. For ethical reasons, we decided not to use a split mouth design. Therefore, no statement can be made about what the healing would have been without NIPP. A clinical trial is needed to objectively investigate the benefits of NIPP treatment. Moreover, effects on wound healing were also not objectively quantified, but based on photographic comparisons, which of course depend on photographic settings such as the camera angle. Additionally, it must be considered that the success of healing was due to good oral hygiene and regular mouth rinsing, which has been described for the treatment of MRONJ [57]. Only a clinical study can provide data. It must also be considered that the drug holiday ordered by the oncologist had an impact on the wound healing process. Nevertheless, there is strong evidence that a drug holiday actually has no effect, which has been demonstrated in clinical studies [58,59]. However, we did not want to interfere with the oncologist’s therapy protocol and did not modify the therapy. Additionally, a single case report has limited power. A randomized controlled trial would provide the best possible evidence and is already being planned. Another limitation of our study is, that in our in vitro experiments we only examined the proliferation-associated genes Ki67 and PCNA. Since other effects of CAP on antimicrobial and remodelling effects are known, further studies of cytokines, chemokines, and matrix metalloproteinases at the mRNA and protein level should be carried out to better understand the effect of NIPP on HPGK. Additionally, since different reactive species have been described to be responsible for biological effects [60] a chemical analysis should be carried out to further decipher the effect of NIPP. Optical emission spectroscopy should also be performed during these further investigations.
However, to the best of our knowledge, this is the first report of NIPP treatment in a patient under bisphosphonate therapy who developed wound dehiscence after oral surgery. Since any following surgical intervention may also provoke the development the outcome and treatment of MRONJ [61,62], our case shows an interesting therapeutic approach in preventing MRONJ.
We acknowledge that we cannot prove that the observed healing was solely due to NIPP treatment by describing a single case, but we clearly observed an improvement in the patient’s quality of life, so that the planned second surgery was no longer necessary.
6. Conclusions
The use of NIPP for wound healing disorders after tooth extractions in patients receiving antiresorptive drugs may support tissue regeneration and avoid the need for additional surgery. Since the incidence of MRONJ in patients with bone metastases after treatment with antiresorptive drugs is 7.3% [56], the use of NIPP is a simple and cost-effective method which may help to minimise the risk of MRONJ-development. However, a clinical trial is required to conclusively prove the effect of NIPP in patients on antiresorptive drugs.
Author Contributions
Conceptualization, B.E., A.M., M.B.S., N.H. and F.-J.K.; methodology, B.E., M.N., N.H. and F.-J.K. software, B.E.; validation, B.E. and N.H.; formal analysis, B.E. and M.B.S.; investigation, B.E. and M.N.; data curation, B.E.; writing—original draft preparation, B.E.; writing—review and editing, A.M., M.B.S., N.H. and F.-J.K.; visualization, B.E.; supervision, N.H. and F.-J.K.; project administration, B.E. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. Ethical review and approval were waived for this study due to its representation of a case report and the use of commercially available cells.
Informed Consent Statement
Informed consent was obtained from the subject involved in the case report.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Landesberg, R.; Cozin, M.; Cremers, S.; Woo, V.; Kousteni, S.; Sinha, S.; Garrett-Sinha, L.; Raghavan, S. Inhibition of Oral Mucosal Cell Wound Healing by Bisphosphonates. J. Oral Maxillofac. Surg. 2008, 66, 839–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, A.; Munz, A.; Reinert, S.; Hoefert, S. Gingival Fibroblasts and Medication-Related Osteonecrosis of the Jaw: Results by Real-Time and Wound Healing in Vitro Assays. J. Cranio-Maxillofac. Surg. 2019, 47, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Eguia, A.; Bagán-Debón, L.; Cardona, F. Review and Update on Drugs Related to the Development of Osteonecrosis of the Jaw. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e71–e83. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons American Association of Oral and Maxillofacial Surgeons Position Paper on Medication-Related Osteonecrosis of the Jaw—2014 Update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.R.; Ruggiero, S.L. Higher Bone Matrix Density Exists in Only a Subset of Patients with Bisphosphonate-Related Osteonecrosis of the Jaw. J. Oral Maxillofac Surg. 2009, 67, 1373–1377. [Google Scholar] [CrossRef]
- Sharma, D.; Ivanovski, S.; Slevin, M.; Hamlet, S.; Pop, T.S.; Brinzaniuc, K.; Petcu, E.B.; Miroiu, R.I. Bisphosphonate-Related Osteonecrosis of Jaw (BRONJ): Diagnostic Criteria and Possible Pathogenic Mechanisms of an Unexpected Anti-Angiogenic Side Effect. Vasc. Cell 2013, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- McKay, R.; Haider, B.; Duh, M.S.; Valderrama, A.; Nakabayashi, M.; Fiorillo, M.; Ristovska, L.; Wen, L.; Kantoff, P. Impact of Symptomatic Skeletal Events on Health-Care Resource Utilization and Quality of Life among Patients with Castration-Resistant Prostate Cancer and Bone Metastases. Prostate Cancer Prostatic Dis. 2017, 20, 276–282. [Google Scholar] [CrossRef]
- Hoefeler, H.; Duran, I.; Hechmati, G.; Garzon Rodriguez, C.; Lüftner, D.; Ashcroft, J.; Bahl, A.; Atchison, C.; Wei, R.; Thomas, E.; et al. Health Resource Utilization Associated with Skeletal-Related Events in Patients with Bone Metastases: Results from a Multinational Retrospective—Prospective Observational Study—A Cohort from 4 European Countries. J. Bone Oncol. 2014, 3, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Spanou, A.; Nelson, K.; Ermer, M.A.; Steybe, D.; Poxleitner, P.; Voss, P.J. Primary Wound Closure and Perioperative Antibiotic Therapy for Prevention of Bisphosphonate-Related Osteonecrosis of the Jaw after Tooth Extraction. Quintessence Int. 2020, 51, 220–228. [Google Scholar] [CrossRef]
- Mawardi, H.; Giro, G.; Kajiya, M.; Ohta, K.; Almazrooa, S.; Alshwaimi, E.; Woo, S.-B.; Nishimura, I.; Kawai, T. A Role of Oral Bacteria in Bisphosphonate-Induced Osteonecrosis of the Jaw. J. Dent. Res. 2011, 90, 1339–1345. [Google Scholar] [CrossRef]
- Kluge, S.; Bekeschus, S.; Bender, C.; Benkhai, H.; Sckell, A.; Below, H.; Stope, M.B.; Kramer, A. Investigating the Mutagenicity of a Cold Argon-Plasma Jet in an HET-MN Model. PLoS ONE 2016, 11, e0160667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dijksteel, G.S.; Ulrich, M.M.W.; Vlig, M.; Sobota, A.; Middelkoop, E.; Boekema, B.K.H.L. Safety and Bactericidal Efficacy of Cold Atmospheric Plasma Generated by a Flexible Surface Dielectric Barrier Discharge Device against Pseudomonas Aeruginosa in Vitro and in Vivo. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Kleineidam, B.; Nokhbehsaim, M.; Deschner, J.; Wahl, G. Effect of Cold Plasma on Periodontal Wound Healing-an in Vitro Study. Clin. Oral Investig. 2019, 23, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, J.M.; Strakeljahn, S.; Nitsch, A.; Bekeschus, S.; Hinz, P.; Mustea, A.; Ekkernkamp, A.; Tzvetkov, M.V.; Haralambiev, L.; Stope, M.B. An Innovative Therapeutic Option for the Treatment of Skeletal Sarcomas: Elimination of Osteo- and Ewing’s Sarcoma Cells Using Physical Gas Plasma. Int. J. Mol. Sci. 2020, 21, 4460. [Google Scholar] [CrossRef] [PubMed]
- Duchesne, C.; Banzet, S.; Lataillade, J.-J.; Rousseau, A.; Frescaline, N. Cold Atmospheric Plasma Modulates Endothelial Nitric Oxide Synthase Signalling and Enhances Burn Wound Neovascularisation. J. Pathol. 2019, 249, 368–380. [Google Scholar] [CrossRef]
- Weltmann, K.-D.; Kindel, E.; Brandenburg, R.; Meyer, C.; Bussiahn, R.; Wilke, C.; von Woedtke, T. Atmospheric Pressure Plasma Jet for Medical Therapy: Plasma Parameters and Risk Estimation. Contrib. Plasma Phys. 2009, 49, 631–640. [Google Scholar] [CrossRef]
- Gan, L.; Zhang, S.; Poorun, D.; Liu, D.; Lu, X.; He, M.; Duan, X.; Chen, H. Medical Applications of Nonthermal Atmospheric Pressure Plasma in Dermatology. J. Der Dtsch. Dermatol. Ges. 2018, 16, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Bologna-Molina, R.; Mosqueda-Taylor, A.; Molina-Frechero, N.; Mori-Estevez, A.D.; Sánchez-Acuña, G. Comparison of the Value of PCNA and Ki-67 as Markers of Cell Proliferation in Ameloblastic Tumor. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e174–e179. [Google Scholar] [CrossRef]
- Memmert, S.; Nokhbehsaim, M.; Damanaki, A.; Nogueira, A.V.B.; Papadopoulou, A.K.; Piperi, C.; Basdra, E.K.; Rath-Deschner, B.; Götz, W.; Cirelli, J.A.; et al. Role of Cathepsin S In Periodontal Wound Healing–an in Vitro Study on Human PDL Cells. BMC Oral Health 2018, 18, 60. [Google Scholar] [CrossRef]
- Chalem, M.; Medina, A.; Sarmiento, A.K.; Gonzalez, D.; Olarte, C.; Pinilla, E.; Paz, J.; Casas, N.; Vega, M.P.; Diaz, E. Therapeutic Approach and Management Algorithms in Medication-Related Osteonecrosis of the Jaw (MONJ): Recommendations of a Multidisciplinary Group of Experts. Arch. Osteoporos. 2020, 15, 101. [Google Scholar] [CrossRef]
- Van Poznak, C.H.; Unger, J.M.; Darke, A.K.; Moinpour, C.; Bagramian, R.A.; Schubert, M.M.; Hansen, L.K.; Floyd, J.D.; Dakhil, S.R.; Lew, D.L.; et al. Association of Osteonecrosis of the Jaw with Zoledronic Acid Treatment for Bone Metastases in Patients with Cancer. JAMA Oncol. 2021, 7, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-H.; Won, Y.-J.; Kim, M.-K. Surgical Treatment of Stage 2 Medication-Related Osteonecrosis of the Jaw Compared to Osteomyelitis. Cranio 2018, 36, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Green, J.R.; Lyles, K.W.; Reid, D.M.; Trechsel, U.; Hosking, D.J.; Black, D.M.; Cummings, S.R.; Russell, R.G.G.; Eriksen, E.F. Zoledronate. Bone 2020, 137, 115390. [Google Scholar] [CrossRef]
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Cooper, C.; Rizzoli, R.; Reginster, J.-Y. Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF) European Guidance for the Diagnosis and Management of Osteoporosis in Postmenopausal Women. Osteoporos. Int. 2013, 24, 23–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nancollas, G.H.; Tang, R.; Phipps, R.J.; Henneman, Z.; Gulde, S.; Wu, W.; Mangood, A.; Russell, R.G.G.; Ebetino, F.H. Novel Insights into Actions of Bisphosphonates on Bone: Differences in Interactions with Hydroxyapatite. Bone 2006, 38, 617–627. [Google Scholar] [CrossRef]
- Recker, R.R.; Delmas, P.D.; Halse, J.; Reid, I.R.; Boonen, S.; García-Hernandez, P.A.; Supronik, J.; Lewiecki, E.M.; Ochoa, L.; Miller, P.; et al. Effects of Intravenous Zoledronic Acid Once Yearly on Bone Remodeling and Bone Structure. J. Bone Miner. Res. 2008, 23, 6–16. [Google Scholar] [CrossRef]
- Soundia, A.; Hadaya, D.; Esfandi, N.; Gkouveris, I.; Christensen, R.; Dry, S.M.; Bezouglaia, O.; Pirih, F.; Nikitakis, N.; Aghaloo, T.; et al. Zoledronate Impairs Socket Healing after Extraction of Teeth with Experimental Periodontitis. J. Dent. Res. 2018, 97, 312–320. [Google Scholar] [CrossRef]
- Kozutsumi, R.; Kuroshima, S.; Kaneko, H.; Sasaki, M.; Ishisaki, A.; Sawase, T. Zoledronic Acid Deteriorates Soft and Hard Tissue Healing of Murine Tooth Extraction Sockets in a Dose-Dependent Manner. Calcif. Tissue Int. 2021, 110, 104–116. [Google Scholar] [CrossRef]
- Ratzkowski, B.; Koth, V.S.; Azambuja, A.A.; Salum, F.G.; de Figueiredo, M.A.Z.; Cherubini, K. Effect of Tyrosine Kinase Inhibitor Sunitinib on Tissue Repair at Tooth Extraction Sites. Oral Dis. 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- Stratmann, B.; Costea, T.-C.; Nolte, C.; Hiller, J.; Schmidt, J.; Reindel, J.; Masur, K.; Motz, W.; Timm, J.; Kerner, W.; et al. Effect of Cold Atmospheric Plasma Therapy vs Standard Therapy Placebo on Wound Healing in Patients with Diabetic Foot Ulcers: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2010411. [Google Scholar] [CrossRef]
- Lou, B.-S.; Hsieh, J.-H.; Chen, C.-M.; Hou, C.-W.; Wu, H.-Y.; Chou, P.-Y.; Lai, C.-H.; Lee, J.-W. Helium/Argon-Generated Cold Atmospheric Plasma Facilitates Cutaneous Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 683. [Google Scholar] [CrossRef]
- Eggers, B.; Marciniak, J.; Memmert, S.; Kramer, F.J.; Deschner, J.; Nokhbehsaim, M. The Beneficial Effect of Cold Atmospheric Plasma on Parameters of Molecules and Cell Function Involved in Wound Healing in Human Osteoblast-like Cells in Vitro. Odontology 2020, 108, 607–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arndt, S.; Unger, P.; Berneburg, M.; Bosserhoff, A.-K.; Karrer, S. Cold Atmospheric Plasma (CAP) Activates Angiogenesis-Related Molecules in Skin Keratinocytes, Fibroblasts and Endothelial Cells and Improves Wound Angiogenesis in an Autocrine and Paracrine Mode. J. Dermatol. Sci. 2018, 89, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Hasse, S.; Duong Tran, T.; Hahn, O.; Kindler, S.; Metelmann, H.-R.; von Woedtke, T.; Masur, K. Induction of Proliferation of Basal Epidermal Keratinocytes by Cold Atmospheric-Pressure Plasma. Clin. Exp. Dermatol. 2016, 41, 202–209. [Google Scholar] [CrossRef]
- Jung, J.M.; Yoon, H.K.; Jung, C.J.; Jo, S.Y.; Hwang, S.G.; Lee, H.J.; Lee, W.J.; Chang, S.E.; Won, C.H. Cold Plasma Treatment Promotes Full-Thickness Healing of Skin Wounds in Murine Models. Int. J. Low. Extrem. Wounds 2021, 15347346211002144. [Google Scholar] [CrossRef]
- Schmidt, A.; Bekeschus, S.; Wende, K.; Vollmar, B.; von Woedtke, T. A Cold Plasma Jet Accelerates Wound Healing in a Murine Model of Full-Thickness Skin Wounds. Exp. Dermatol. 2017, 26, 156–162. [Google Scholar] [CrossRef]
- Isbary, G.; Heinlin, J.; Shimizu, T.; Zimmermann, J.L.; Morfill, G.; Schmidt, H.-U.; Monetti, R.; Steffes, B.; Bunk, W.; Li, Y.; et al. Successful and Safe Use of 2 Min Cold Atmospheric Argon Plasma in Chronic Wounds: Results of a Randomized Controlled Trial. Br. J. Dermatol. 2012, 167, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.; Momeni, M.; Jahandideh, A.; Ghoranneviss, M.; Soudmand, S.; Yousefi, P.; Khandan, S.; Amini, M. Tendon Repair by Plasma Jet Treatment. J. Diabetes Metab. Disord. 2021, 20, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Eggers, B.; Marciniak, J.; Deschner, J.; Stope, M.B.; Mustea, A.; Kramer, F.-J.; Nokhbehsaim, M. Cold Atmospheric Plasma Promotes Regeneration-Associated Cell Functions of Murine Cementoblasts In Vitro. Int. J. Mol. Sci. 2021, 22, 5280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, D.; Cheng, K.; Zhou, Z.; Liu, S.; Chen, L.; Hu, Y.; Mao, C.; Liu, S. Spontaneous Evolution of Human Skin Fibroblasts into Wound-Healing Keratinocyte-like Cells. Theranostics 2019, 9, 5200–5213. [Google Scholar] [CrossRef]
- Riedl, J.; Popp, C.; Eide, C.; Ebens, C.; Tolar, J. Mesenchymal Stromal Cells in Wound Healing Applications: Role of the Secretome, Targeted Delivery and Impact on Recessive Dystrophic Epidermolysis Bullosa Treatment. Cytotherapy 2021, 23, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Boccaccio, A.; Saini, R.; Van Pham, P.; Tatullo, M. Dental-Derived Stem Cells and Their Secretome and Interactions with Bioscaffolds/Biomaterials in Regenerative Medicine: From the In Vitro Research to Translational Applications. Stem Cells Int. 2017, 2017, 6975251. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Gonçalves, R.M. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-Conditioning. Front. Immunol 2018, 9, 2837. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.G.; Sanders, A.J.; Ruge, F.; Harding, K.G. Influence of Interleukin-8 (IL-8) and IL-8 Receptors on the Migration of Human Keratinocytes, the Role of PLC-γ and Potential Clinical Implications. Exp. Ther. Med. 2012, 3, 231–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Bressan, E.; Ferroni, L.; Gardin, C.; Bellin, G.; Sbricoli, L.; Sivolella, S.; Brunello, G.; Schwartz-Arad, D.; Mijiritsky, E.; Penarrocha, M.; et al. Metal Nanoparticles Released from Dental Implant Surfaces: Potential Contribution to Chronic Inflammation and Peri-Implant Bone Loss. Materials 2019, 12, 2036. [Google Scholar] [CrossRef] [Green Version]
- Ermakov, A.M.; Ermakova, O.N.; Afanasyeva, V.A.; Popov, A.L. Dose-Dependent Effects of Cold Atmospheric Argon Plasma on the Mesenchymal Stem and Osteosarcoma Cells In Vitro. Int. J. Mol. Sci. 2021, 22, 6797. [Google Scholar] [CrossRef]
- Bekeschus, S.; Kramer, A.; Schmidt, A. Gas Plasma-Augmented Wound Healing in Animal Models and Veterinary Medicine. Molecules 2021, 26, 5682. [Google Scholar] [CrossRef]
- Chatraie, M.; Torkaman, G.; Khani, M.; Salehi, H.; Shokri, B. In Vivo Study of Non-Invasive Effects of Non-Thermal Plasma in Pressure Ulcer Treatment. Sci. Rep. 2018, 8, 5621. [Google Scholar] [CrossRef]
- Kusakci-Seker, B.; Demirayak-Akdemir, M. The Effect of Non-Thermal Atmospheric Pressure Plasma Application on Wound Healing after Gingivectomy. Int. Wound J. 2020, 17, 1376–1383. [Google Scholar] [CrossRef]
- Pekbağrıyanık, T.; Dadas, F.K.; Enhoş, Ş. Effects of Non-Thermal Atmospheric Pressure Plasma on Palatal Wound Healing of Free Gingival Grafts: A Randomized Controlled Clinical Trial. Clin. Oral Investig. 2021, 25, 6269–6278. [Google Scholar] [CrossRef] [PubMed]
- Kushiro, H.; Takahashi, H.; Tanaka, A. Effects of the Prevention of Medication-Related Osteonecrosis of the Jaw by Local Administration of a Dental Pulp Stem Cell-Conditioned Medium to the Rat Tooth Extraction Socket. Odontology 2021, 109, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Gianfreda, F.; Raffone, C.; Antonacci, D.; Pistilli, V.; Bollero, P. The Role of Platelet-Rich Fibrin (PRF) in the Prevention of Medication-Related Osteonecrosis of the Jaw (MRONJ). Biomed. Res. Int. 2021, 2021, 4948139. [Google Scholar] [CrossRef]
- Hasegawa, T.; Hayashida, S.; Kondo, E.; Takeda, Y.; Miyamoto, H.; Kawaoka, Y.; Ueda, N.; Iwata, E.; Nakahara, H.; Kobayashi, M.; et al. Medication-Related Osteonecrosis of the Jaw after Tooth Extraction in Cancer Patients: A Multicenter Retrospective Study. Osteoporos. Int. 2019, 30, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Nicolatou-Galitis, O.; Papadopoulou, E.; Vardas, E.; Kouri, M.; Galiti, D.; Galitis, E.; Alexiou, K.-E.; Tsiklakis, K.; Ardavanis, A.; Razis, E.; et al. Alveolar Bone Histological Necrosis Observed Prior to Extractions in Patients, Who Received Bone-Targeting Agents. Oral Dis. 2020, 26, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Eggers, B.; Marciniak, J.; Memmert, S.; Wagner, G.; Deschner, J.; Kramer, F.-J.; Nokhbehsaim, M. Influences of Cold Atmospheric Plasma on Apoptosis Related Molecules in Osteoblast-like Cells in Vitro. Head Face Med. 2021, 17, 37. [Google Scholar] [CrossRef]
- Nicolatou-Galitis, O.; Schiødt, M.; Mendes, R.A.; Ripamonti, C.; Hope, S.; Drudge-Coates, L.; Niepel, D.; Van den Wyngaert, T. Medication-Related Osteonecrosis of the Jaw: Definition and Best Practice for Prevention, Diagnosis, and Treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 117–135. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, T.; Kawakita, A.; Ueda, N.; Funahara, R.; Tachibana, A.; Kobayashi, M.; Kondou, E.; Takeda, D.; Kojima, Y.; Sato, S.; et al. A Multicenter Retrospective Study of the Risk Factors Associated with Medication-Related Osteonecrosis of the Jaw after Tooth Extraction in Patients Receiving Oral Bisphosphonate Therapy: Can Primary Wound Closure and a Drug Holiday Really Prevent MRONJ? Osteoporos Int. 2017, 28, 2465–2473. [Google Scholar] [CrossRef]
- Ottesen, C.; Schiodt, M.; Jensen, S.S.; Kofod, T.; Gotfredsen, K. Tooth Extractions in Patients with Cancer Receiving High-Dose Antiresorptive Medication: A Randomized Clinical Feasibility Trial of Drug Holiday versus Drug Continuation. Oral Surg Oral Med. Oral Pathol. Oral Radiol. 2022, 133, 165–173. [Google Scholar] [CrossRef]
- Sardella, E.; Veronico, V.; Gristina, R.; Grossi, L.; Cosmai, S.; Striccoli, M.; Buttiglione, M.; Fracassi, F.; Favia, P. Plasma Treated Water Solutions in Cancer Treatments: The Contrasting Role of RNS. Antioxidants 2021, 10, 605. [Google Scholar] [CrossRef]
- Aljohani, S.; Fliefel, R.; Ihbe, J.; Kühnisch, J.; Ehrenfeld, M.; Otto, S. What Is the Effect of Anti-Resorptive Drugs (ARDs) on the Development of Medication-Related Osteonecrosis of the Jaw (MRONJ) in Osteoporosis Patients: A Systematic Review. J. Cranio-Maxillofac. Surg. 2017, 45, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, A.M.; Lemound, J.; Lindhorst, D.; Rana, M.; Gellrich, N.-C. Surgical Management of Bisphosphonate-Related Osteonecrosis of the Jaw in Oncologic Patients: A Challenging Problem. Anticancer Res. 2011, 31, 2313–2318. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).