The Role of an Industrial Alkaline Wastewater in the Alkali Activation of Biomass Fly Ash
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Alkali-Activated Material Preparation
2.3. Alkali-Activated Material Characterisation
2.3.1. Fresh Paste Characterisation
- 1.
- Consistency and setting time
- 2.
- Dissolution assays
- 3.
- Calorimetric characterisation
2.3.2. Hardened State Characterisation
3. Results and Discussion
3.1. Characterisation of the Solid Precursors and the Industrial Wastewater
3.2. Fresh Paste Characterisation
3.2.1. Consistency and Setting Time
3.2.2. Dissolution Tests
3.2.3. Calorimetric Characterisation
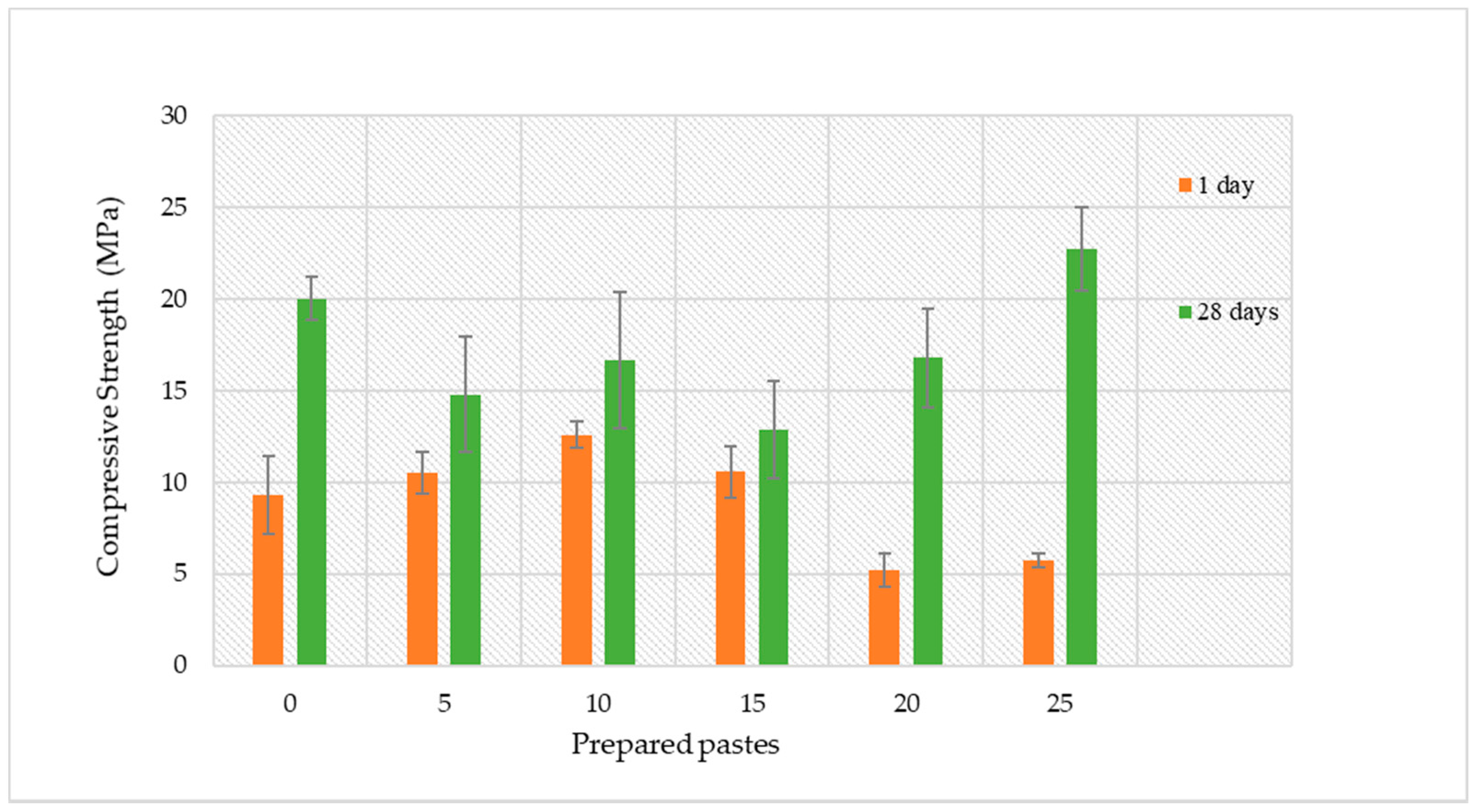
3.3. Hardened State Characterisation: Compressive Strength
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adesanya, E.; Perumal, P.; Luukkonen, T.; Yliniemi, J.; Ohenoja, K.; Kinnunen, P.; Illikainen, M. Opportunities to improve sustainability of alkali-activated materials: A review of side-stream based activators. J. Clean. Prod. 2021, 286, 125558. [Google Scholar] [CrossRef]
- Mesgari, S.; Akbarnezhad, A.; Xiao, J.Z. Recycled geopolymer aggregates as coarse aggregates for Portland cement concrete and geopolymer concrete: Effects on mechanical properties. Constr. Build. Mater. 2020, 236, 117571. [Google Scholar] [CrossRef]
- Imtiaz, L.; Ur Rehman, S.K.; Memon, S.A.; Khan, M.K.; Javed, M.F. A review of recent developments and advances in eco-friendly geopolymer concrete. Appl. Sci. 2020, 10, 7838. [Google Scholar] [CrossRef]
- Tailby, J.; MacKenzie, K.J.D. Structure and mechanical properties of aluminosilicate geopolymer composites with Portland cement and its constituent minerals. Cem. Concr. Res. 2010, 40, 787–794. [Google Scholar] [CrossRef]
- Obonyo, E.; Kamseu, E.; Melo, U.C.; Leonelli, C. Advancing the use of secondary inputs in geopolymer binders for sustainable cementitious composites: A review. Sustainability 2011, 3, 410. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Geopolymer foam concrete: An emerging material for sustainable construction. Constr. Build. Mater. 2014, 56, 113–127. [Google Scholar] [CrossRef]
- Panizza, M.; Natali, M.; Garbin, E.; Tamburini, S.; Secco, M. Assessment of geopolymers with Construction and Demolition Waste (CDW) aggregates as a building material. Constr. Build. Mater. 2018, 181, 119–133. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Liew, Y.M.; Heah, C.Y.; Mohd Mustafa, A.B.; Kamarudin, H. Structure and properties of clay-based geopolymer cements: A review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- Zhang, P.; Zheng, Y.; Wang, K.; Zhang, J. A review on properties of fresh and hardened geopolymer mortar. Compos. Part B Eng. 2018, 152, 79–95. [Google Scholar] [CrossRef]
- Ulugöl, H.; Kul, A.; Yıldırım, G.; Şahmaran, M.; Aldemir, A.; Figueira, D.; Ashour, A. Mechanical and microstructural characterization of geopolymers from assorted construction and demolition waste-based masonry and glass. J. Clean. Prod. 2021, 280, 124358. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Kaze, C.R.; Lecomte-Nana, G.L.; Adesina, A.; Nemaleu, J.G.D.; Kamseu, E.; Chinje Melo, U. Influence of mineralogy and activator type on the rheology behaviour and setting time of laterite based geopolymer paste. Cem. Concr. Compos. 2022, 126, 104345. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; Cui, M.; Wang, J.; Lyu, X. Preparation of eco-friendly one-part geopolymers from gold mine tailings by alkaline hydrothermal activation. J. Clean. Prod. 2021, 298, 126806. [Google Scholar] [CrossRef]
- McLellan, B.C.; Williams, R.P.; Lay, J.; Van Riessen, A.; Corder, G.D. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef] [Green Version]
- Mendes, B.C.; Pedroti, L.G.; Vieira, C.M.F.; Marvila, M.; Azevedo, A.R.G.; Franco de Carvalho, J.M.; Ribeiro, J.C.L. Application of eco-friendly alternative activators in alkali-activated materials: A review. J. Build. Eng. 2021, 35, 102010. [Google Scholar] [CrossRef]
- Saeli, M.; Tobaldi, D.M.; Seabra, M.P.; Labrincha, J.A. Mix design and mechanical performance of geopolymeric binders and mortars using biomass fly ash and alkaline effluent from paper-pulp industry. J. Clean. Prod. 2019, 208, 1188–1197. [Google Scholar] [CrossRef]
- Alnahhal, M.F.; Kim, T.; Hajimohammadi, A. Waste-derived activators for alkali-activated materials: A review. Cem. Concr. Compos. 2021, 118, 103980. [Google Scholar] [CrossRef]
- Emdadi, Z.; Asim, N.; Amin, M.H.; Yarmo, M.A.; Maleki, A.; Azizi, M.; Sopian, K. Development of green geopolymer using agricultural and industrial waste materials with high water absorbency. Appl. Sci. 2017, 7, 514. [Google Scholar] [CrossRef] [Green Version]
- El-Naggar, M.R.; El-Dessouky, M.I. Re-use of waste glass in improving properties of metakaolin-based geopolymers: Mechanical and microstructure examinations. Constr. Build. Mater. 2017, 132, 543–555. [Google Scholar] [CrossRef]
- Vinai, R.; Soutsos, M. Production of sodium silicate powder from waste glass cullet for alkali activation of alternative binders. Cem. Concr. Res. 2019, 116, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Ogundiran, M.B.; Nugteren, H.W.; Witkamp, G.J. Geopolymerisation of fly ashes with waste aluminium anodising etching solutions. J. Environ. Manag. 2016, 181, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Jiménez, A.; Cristelo, N.; Miranda, T.; Palomo, Á. Sustainable alkali activated materials: Precursor and activator derived from industrial wastes. J. Clean. Prod. 2017, 162, 1200–1209. [Google Scholar] [CrossRef]
- Van Riessen, A.; Jamieson, E.; Kealley, C.S.; Hart, R.D.; Williams, R.P. Bayer-geopolymers: An exploration of synergy between the alumina and geopolymer industries. Cem. Concr. Compos. 2013, 41, 29–33. [Google Scholar] [CrossRef]
- Novais, R.M.; Caetano, A.P.F.; Seabra, M.P.; Labrincha, J.A.; Pullar, R.C. Extremely fast and efficient methylene blue adsorption using eco-friendly cork and paper waste-based activated carbon adsorbents. J. Clean. Prod. 2018, 197, 1137–1147. [Google Scholar] [CrossRef]
- Novais, R.M.; Carvalheiras, J.; Senff, L.; Labrincha, J.A. Upcycling unexplored dregs and biomass fly ash from the paper and pulp industry in the production of eco-friendly geopolymer mortars: A preliminary assessment. Constr. Build. Mater. 2018, 184, 464–472. [Google Scholar] [CrossRef]
- Novais, R.M.; Carvalheiras, J.; Senff, L.; Seabra, M.P.; Pullar, R.C.; Labrincha, J.A. In-depth investigation of the long-term strength and leaching behaviour of inorganic polymer mortars containing green liquor dregs. J. Clean. Prod. 2019, 220, 630–641. [Google Scholar] [CrossRef]
- Novais, R.M.; Senff, L.; Carvalheiras, J.; Labrincha, J.A. Bi-layered porous/cork-containing waste-based inorganic polymer composites: Innovative material towards green buildings. Appl. Sci. 2020, 10, 2995. [Google Scholar] [CrossRef]
- Novais, R.M.; Ascensão, G.; Buruberri, L.H.; Senff, L.; Labrincha, J.A. Influence of blowing agent on the fresh- and hardened-state properties of lightweight geopolymers. Mater. Des. 2016, 108, 551–559. [Google Scholar] [CrossRef]
- Buruberri, L.H.; Tobaldi, D.M.; Caetano, A.; Seabra, M.P.; Labrincha, J.A. Evaluation of reactive Si and Al amounts in various geopolymer precursors by a simple method. J. Build. Eng. 2019, 22, 48–55. [Google Scholar] [CrossRef]
- Firdous, R.; Stephan, D. Effect of silica modulus on the geopolymerization activity of natural pozzolans. Constr. Build. Mater. 2019, 219, 31–43. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, K.; Wang, X.; Hua, S. Effects of mix design parameters on heat of geopolymerization, set time, and compressive strength of high calcium fly ash geopolymer. Constr. Build. Mater. 2019, 228, 116763. [Google Scholar] [CrossRef]
- Nedeljković, M.; Li, Z.; Ye, G. Setting, strength, and autogenous shrinkage of alkali-activated fly ash and slag pastes: Effect of slag content. Materials 2018, 11, 2121. [Google Scholar] [CrossRef] [Green Version]
- Teo, W.; Shirai, K.; Lim, J.H.; Jack, L.B.; Nikbakht, E. Experimental Investigation on Ambient-Cured One-Part Alkali-Activated Binders Using Combined High-Calcium Fly Ash (HCFA) and Ground Granulated Blast Furnace Slag (GGBS). Materials 2022, 15, 1612. [Google Scholar] [CrossRef]
- Saeli, M.; Senff, L.; Tobaldi, D.M.; Carvalheiras, J.; Seabra, M.P.; Labrincha, J.A. Unexplored alternative use of calcareous sludge from the paper-pulp industry in green geopolymer construction materials. Constr. Build. Mater. 2020, 246, 118457. [Google Scholar] [CrossRef]
- Shang, J.; Dai, J.G.; Zhao, T.J.; Guo, S.Y.; Zhang, P.; Mu, B. Alternation of traditional cement mortars using fly ash-based geopolymer mortars modified by slag. J. Clean. Prod. 2018, 203, 746–756. [Google Scholar] [CrossRef]
- Quiatchon, P.R.J.; Dollente, I.J.R.; Abulencia, A.B.; De Guzman Libre, R.G.; Villoria, M.B.D.; Guades, E.J.; Promentilla, M.A.B.; Ongpeng, J.M.C. Investigation on the compressive strength and time of setting of low-calcium fly ash geopolymer paste using response surface methodology. Polymers 2021, 13, 3461. [Google Scholar] [CrossRef]
- Phair, J.W.; Van Deventer, J.S.J. Effect of silicate activator pH on the leaching and material characteristics of waste-based inorganic polymers. Miner. Eng. 2001, 14, 289–304. [Google Scholar] [CrossRef]
- Vogt, O.; Ukrainczyk, N.; Ballschmiede, C.; Koenders, E. Reactivity and microstructure of metakaolin based geopolymers: Effect of fly Ash and liquid/solid contents. Materials 2019, 12, 3485. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Zhang, Z.; Zhu, H.; Chen, Y. Geopolymerization process of alkali-metakaolinite characterized by isothermal calorimetry. Thermochim. Acta 2009, 493, 49–54. [Google Scholar] [CrossRef]
- Ravikumar, D.; Neithalath, N. Reaction kinetics in sodium silicate powder and liquid activated slag binders evaluated using isothermal calorimetry. Thermochim. Acta 2012, 546, 32–43. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH-activated ground fly ash geopolymer cured at ambient temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Saeli, M.; Senff, L.; Tobaldi, D.M.; La Scalia, G.; Seabra, M.P.; Labrincha, J.A. Innovative recycling of lime slaker grits from paper-pulp industry reused as aggregate in ambient cured biomass fly ash-based geopolymers for sustainable construction material. Sustainability 2019, 11, 3481. [Google Scholar] [CrossRef] [Green Version]
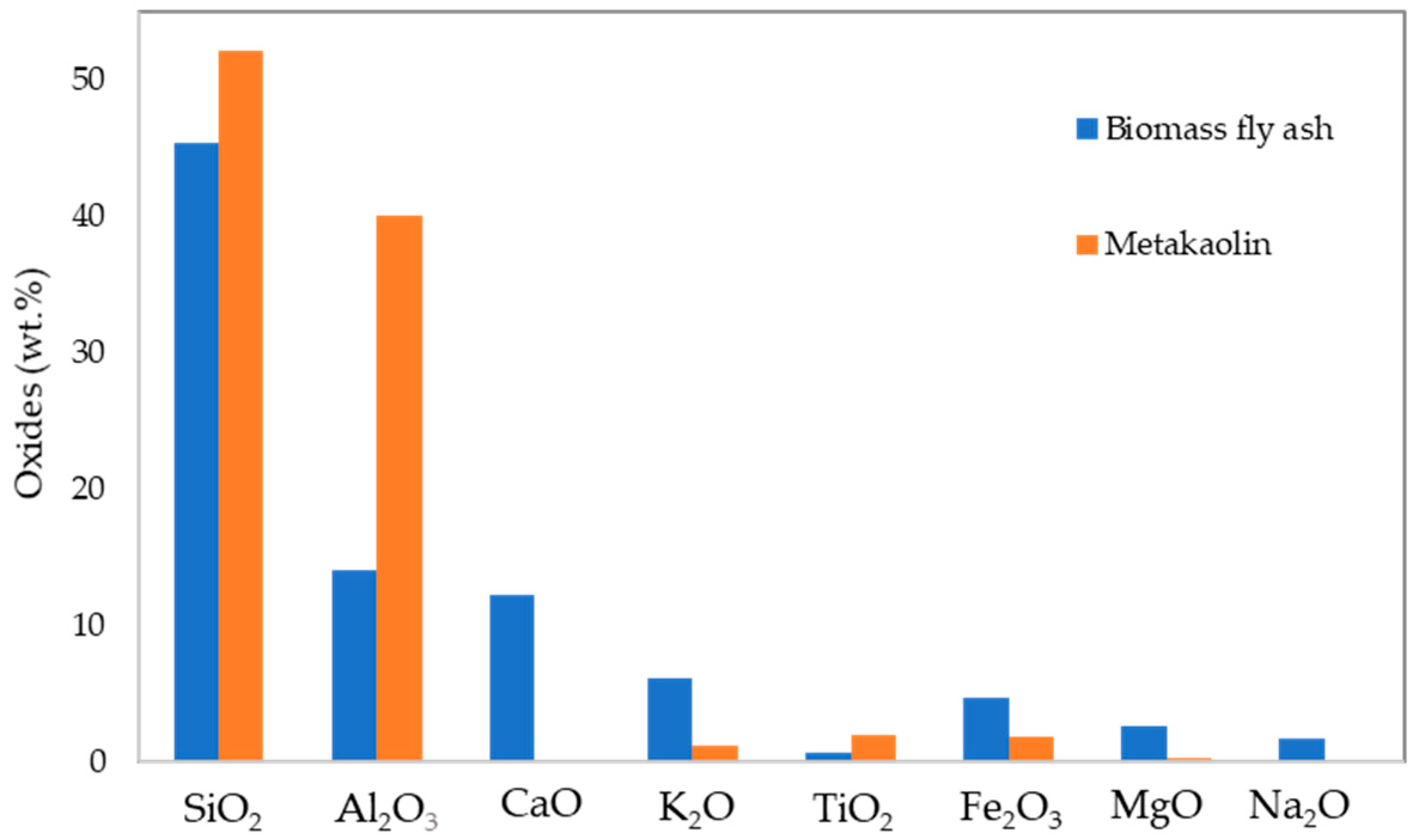
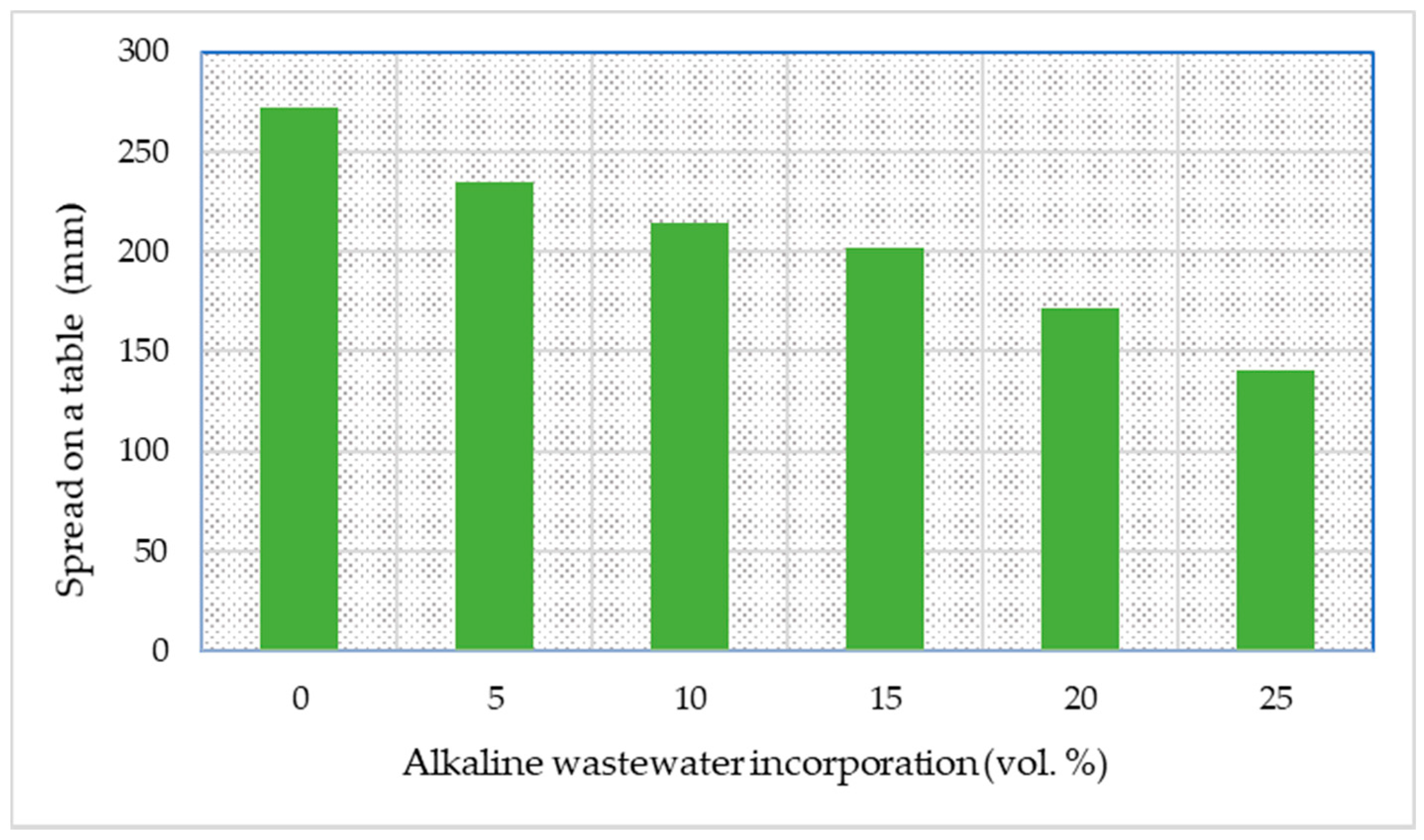
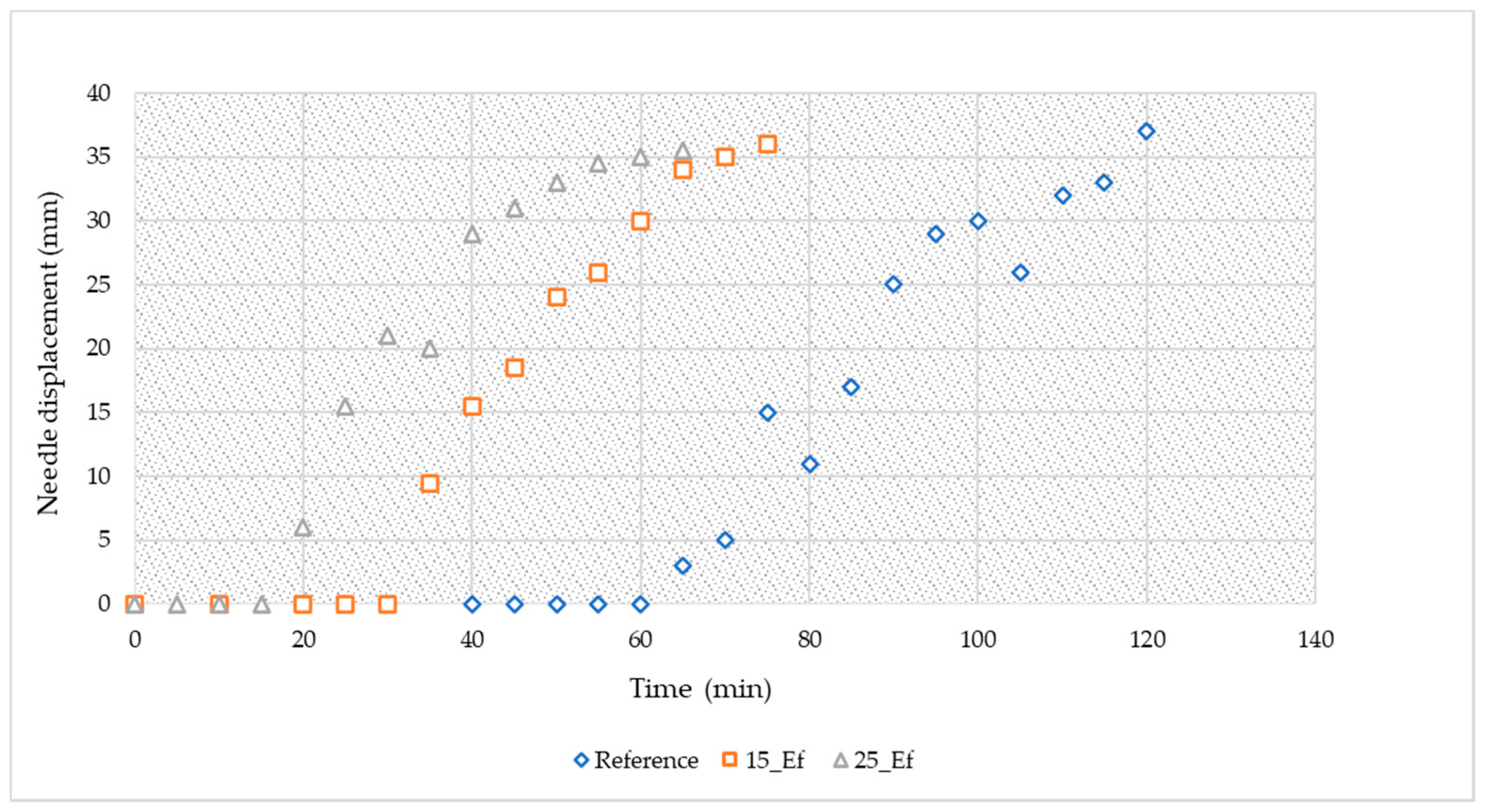
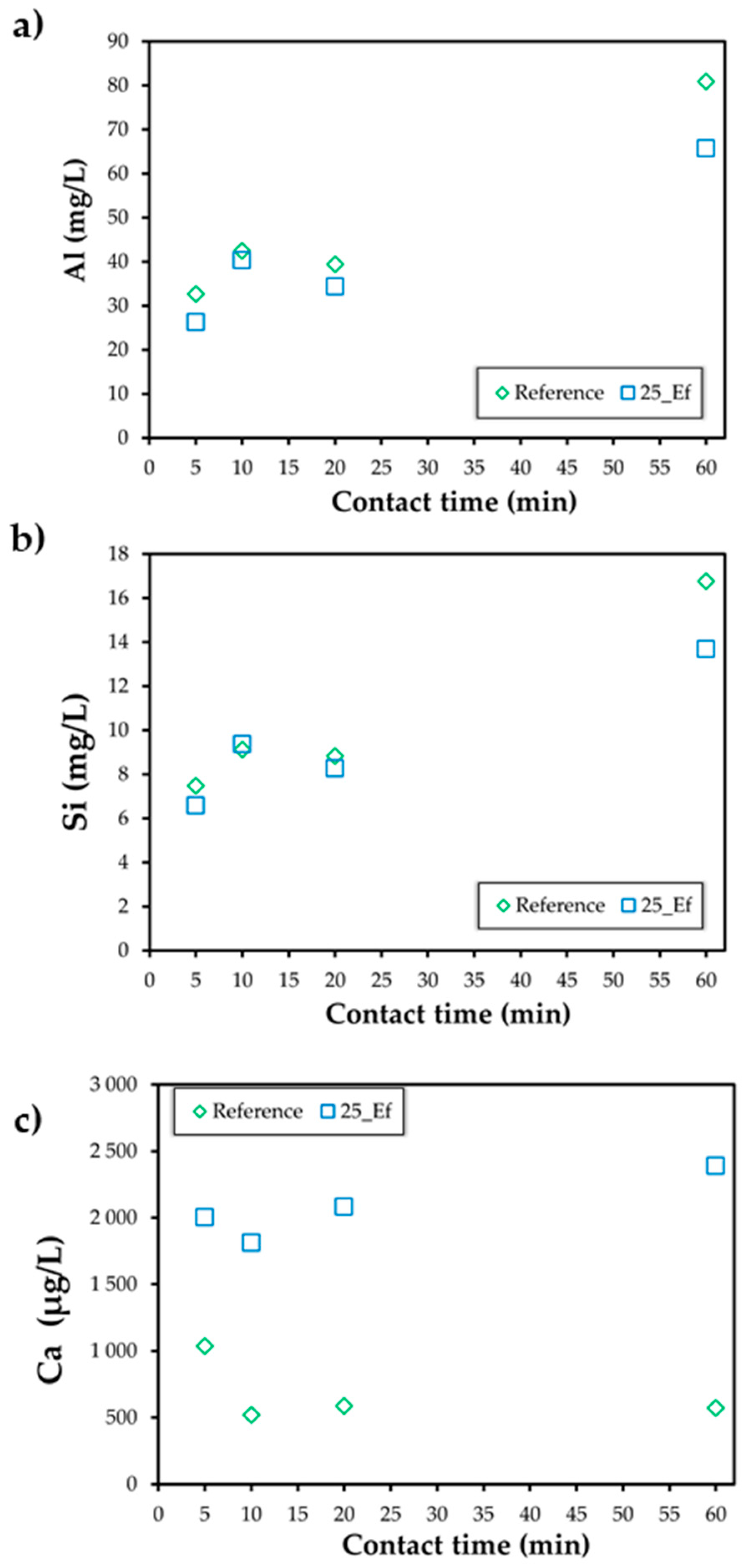

| Sample ID | Metakaolin (g) | Fly Ash (g) | Sodium Silicate (g) | Sodium Hydroxide Solution (g) | Industrial Effluent (mL) | Effluent (vol.%) |
|---|---|---|---|---|---|---|
| Reference (0_Ef) | 30.00 | 70.00 | 75.00 | 25.00 | - | 0 |
| 5_Ef | 23.75 | 0.97 | 5 | |||
| 10_Ef | 22.50 | 1.94 | 10 | |||
| 15_Ef | 21.25 | 2.92 | 15 | |||
| 20_Ef | 20.00 | 3.89 | 20 | |||
| 25_Ef | 18.75 | 4.86 | 25 |
| Element | Na | K | Cl |
|---|---|---|---|
| Concentration (mg/L) | 1130 | 11.3 | 653 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novo, C.C.; Senff, L.; Seabra, M.P.; Novais, R.M.; Labrincha, J.A. The Role of an Industrial Alkaline Wastewater in the Alkali Activation of Biomass Fly Ash. Appl. Sci. 2022, 12, 3612. https://doi.org/10.3390/app12073612
Novo CC, Senff L, Seabra MP, Novais RM, Labrincha JA. The Role of an Industrial Alkaline Wastewater in the Alkali Activation of Biomass Fly Ash. Applied Sciences. 2022; 12(7):3612. https://doi.org/10.3390/app12073612
Chicago/Turabian StyleNovo, Catarina C., Luciano Senff, Maria P. Seabra, Rui M. Novais, and João A. Labrincha. 2022. "The Role of an Industrial Alkaline Wastewater in the Alkali Activation of Biomass Fly Ash" Applied Sciences 12, no. 7: 3612. https://doi.org/10.3390/app12073612






