Influence of Cynara cardunculus L. Phenolic Compounds on Pseudomonas putida Isolated from the Dairy Industry: Growth and Melanin Bioproduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. C. cardunculus Leaves Extract: Chemical Characterization by LC-HRMS/MS
2.3. Screening of Antimicrobial Activity
2.4. Inhibition of Growth and Melanin Bioproduction
2.5. Melanin Extraction, Purification, and Quantification
2.6. Statistical Analysis
3. Results and Discussion
3.1. Aqueous Extract from C. cardunculus Leaves: Compound Identification by LC-HRMS/MS
3.2. Influence of C. cardunculus Extracts on Bacterial Growth
3.2.1. Antimicrobial Activity of C. cardunculus Extracts Using Solid Method
3.2.2. Antimicrobial Activity of C. cardunculus Extracts Using Liquid Method
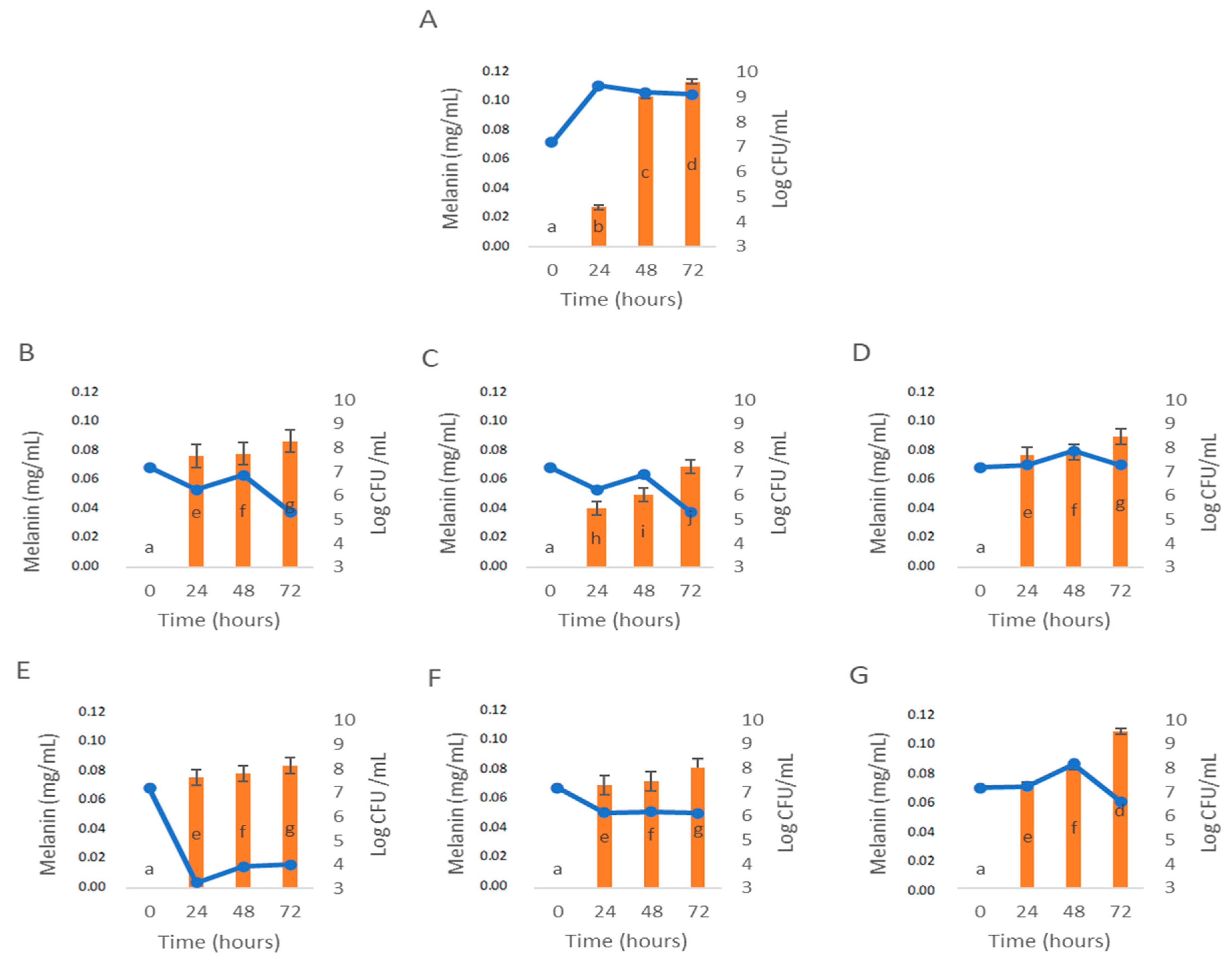
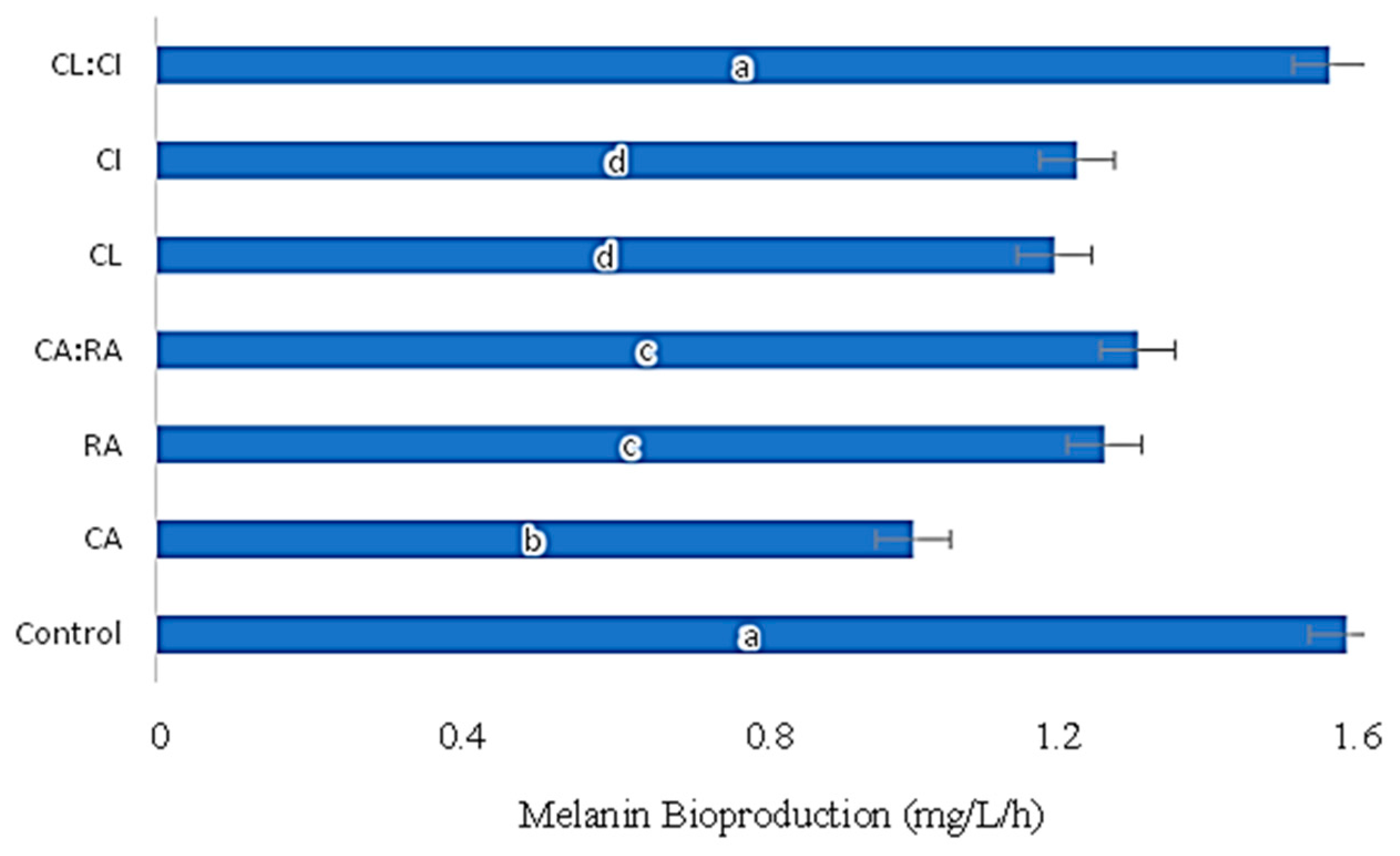
3.3. Influence of C. cardunculus Extracts in Melanin Bioproduction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zayed, A.; Serag, A.; Farag, M.A. Cynara cardunculus L.: Outgoing and potential trends of phytochemical, industrial, nutritive and medicinal merits. J. Funct. Foods 2020, 69, 103937. [Google Scholar] [CrossRef]
- De Falco, B.; Incerti, G.; Amato, M.; Lanzotti, V. Artichoke: Botanical, agronomical, phytochemical, and pharmacological overview. Phytochem. Rev. 2015, 14, 993–1018. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Veríssimo, P.; Esteves, C.; Faro, C.; Pires, E. The vegetable rennet of Cynara cardunculus L. contains two proteinases with chymosin and pepsin-like specificities. Biotechnol. Lett. 1995, 17, 621–626. [Google Scholar] [CrossRef]
- Mandim, F.; Petropoulos, S.A.; Dias, M.I.; Pinela, J.; Kostic, M.; Soković, M.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Barros, L. Seasonal variation in bioactive properties and phenolic composition of cardoon (Cynara cardunculus var. altilis) bracts. Food Chem. 2021, 336, 127744. [Google Scholar] [CrossRef]
- Mileo, A.M.; Di Venere, D.; Linsalata, V.; Fraioli, R.; Miccadei, S. Artichoke polyphenols induce apoptosis and decrease the invasive potential of the human breast cancer cell line MDA-MB231. J. Cell. Physiol. 2012, 227, 3301–3309. [Google Scholar] [CrossRef]
- Falé, P.L.; Ferreira, C.; Rodrigues, A.M.; Serralheiro, M.L. Studies on the molecular mechanism of cholesterol reduction by Fraxinus angustifolia, Peumus boldus, Cynara cardunculus and Pterospartum tridentatum infusions. J. Med. Plants Res. 2014, 8, 9–17. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Dueñas, M.; Pereira, E.; Carvalho, A.M.; Alves, R.C.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical composition of wild and commercial Achillea millefolium L. and bioactivity of the methanolic extract, infusion and decoction. Food Chem. 2013, 141, 4152–4160. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Silva, S.; Henriques, M.; Ferreira, I.C.F.R. Decoction, infusion and hydroalcoholic extract of cultivated thyme: Antioxidant and antibacterial activities, and phenolic characterisation. Food Chem. 2015, 167, 131–137. [Google Scholar] [CrossRef]
- Falé, P.L.; Ferreira, C.; Rodrigues, A.M.; Cleto, P.; Madeira, P.J.A.; Florêncio, M.H.; Frazatilde, F.N.; Serralheiro, M.L.M. Antioxidant and anti-acetylcholinesterase activity of commercially available medicinal infusions after in vitro gastrointestinal digestion. J. Med. Plants Res. 2013, 7, 1370–1378. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, H.; Lo, R. Phenolic Compounds from the Leaf Extract of Artichoke (Cynara scolymus L.) and Their Antimicrobial Activities. J. Agric. Food Chem. 2004, 52, 7272–7278. [Google Scholar] [CrossRef] [PubMed]
- Scavo, A.; Pandino, G.; Restuccia, C.; Parafati, L.; Cirvilleri, G.; Mauromicale, G. Antimicrobial activity of cultivated cardoon (Cynara cardunculus L. var. altilis DC.) leaf extracts against bacterial species of agricultural and food interest. Ind. Crop. Prod. 2019, 129, 206–211. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Barreira, J.C.M.; Alves, M.J.; Barracosa, P.; Ferreira, I.C.F.R. Phenolic profile and bioactivity of cardoon (Cynara cardunculus L.) inflorescence parts: Selecting the best genotype for food applications. Food Chem. 2018, 268, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Mandim, F.; Petropoulos, S.A.; Dias, M.I.; Pinela, J.; Kostić, M.; Soković, M.S.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Barros, L. Phenolic Composition and Biological Properties of Cynara cardunculus L. var. altilis Petioles: Influence of the Maturity Stage. Antioxidants 2021, 10, 1907. [Google Scholar] [CrossRef]
- Carreira, A.; Paloma, L.; Loureiro, V. Pigment producing yeasts involved in the brown surface discoloration of ewes’ cheese. Int. J. Food Microbiol. 1998, 41, 223–230. [Google Scholar] [CrossRef]
- Ferraz, A.R.; Pacheco, R.; Vaz, P.D.; Pintado, C.S.; Ascensão, L.; Serralheiro, M.L. Melanin: Production from Cheese Bacteria, Chemical Characterization, and Biological Activities. Int. J. Environ. Res. Public Health 2021, 18, 10562. [Google Scholar] [CrossRef]
- Arslan, S.; Eyi, A.; Özdemir, F. Spoilage potentials and antimicrobial resistance of Pseudomonas spp. isolated from cheeses. J. Dairy Sci. 2011, 94, 5851–5856. [Google Scholar] [CrossRef]
- Abdulkadir, N.; Usman, N.A.H.M.; Gani, H.M.M.M. Bacterial Pigments and its Significance. MOJ Bioequivalence Bioavailab. 2017, 4, 285–288. [Google Scholar] [CrossRef]
- Guedes, L.; Reis, P.B.P.S.; Machuqueiro, M.; Ressaissi, A.; Pacheco, R.; Serralheiro, M.L. Bioactivities of Centaurium erythraea (Gentianaceae) Decoctions: Antioxidant Activity, Enzyme Inhibition and Docking Studies. Molecules 2019, 24, 3795. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing–EUCAST, Antimicrobial susceptibility testing EUCAST disk diffusion method version 8.0 January. Eur. Soc. Clin. Microbiol. Infect. Deseases 2020, 1–21.
- Falleh, H.; Ksouri, R.; Chaieb, K.; Karray-Bouraoui, N.; Trabelsi, N.; Boulaaba, M.; Abdelly, C. Phenolic composition of Cynara cardunculus L. organs, and their biological activities. Comptes Rendus Biol. 2008, 331, 372–379. [Google Scholar] [CrossRef]
- Hedden, P.; Sponsel, V. A Century of Gibberellin Research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef]
- Toner, P.; Nelson, D.; Rao, J.R.; Ennis, M.; Moore, J.E.; Schock, B. Antimicrobial properties of phytohormone (gibberellins) against phytopathogens and clinical pathogens. Access Microbiol. 2021, 3, 000278. [Google Scholar] [CrossRef]
- Fratianni, F.; Pepe, R.; Nazzaro, F. Polyphenol Composition, Antioxidant, Antimicrobial and Quorum Quenching Activity of the “Carciofo di Montoro” (Cynara cardunculus var. scolymus) Global Artichoke of the Campania Region, Southern Italy. Food Nutr. Sci. 2014, 5, 2053–2062. [Google Scholar] [CrossRef][Green Version]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial Activity and Mechanism of Action of Chlorogenic Acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef]
- Matejczyk, M.; Świsłocka, R.; Golonko, A.; Lewandowski, W.; Hawrylik, E. Cytotoxic, genotoxic and antimicrobial activity of caffeic and rosmarinic acids and their lithium, sodium and potassium salts as potential anticancer compounds. Adv. Med. Sci. 2018, 63, 14–21. [Google Scholar] [CrossRef]
- Osés, S.M.; Pascual-Maté, A.; de la Fuente, D.; de Pablo, A.; Fernández-Muiño, M.A.; Sancho, M.T. Comparison of methods to determine antibacterial activity of honeys against Staphylococcus aureus. NJAS-Wagening. J. Life Sci. 2016, 78, 29–33. [Google Scholar] [CrossRef]
- Hudzicki, J. Kirby-Bauer Disk diffusion susceptibility test protocol author information. Am. Soc. Microbiol. 2012, 15, 1–13. [Google Scholar]
- Flanagan, J.N.; Steck, T.R. The Relationship Between Agar Thickness and Antimicrobial Susceptibility Testing. Indian J. Microbiol. 2017, 57, 503–506. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Li, H.-R.; Habasi, M.; Xie, L.-Z.; Aisa, H.A. Molecules Effect of Chlorogenic Acid on Melanogenesis of B16 Melanoma Cells. Molecules 2014, 19, 12940–12948. [Google Scholar] [CrossRef]
- Oh, H.-N.; Park, D.-H.; Park, J.-Y.; Song, S.-Y.; Lee, S.-H.; Yoon, G.; Moon, H.-S.; Oh, D.-S.; Rhee, S.-H.; Im, E.-O.; et al. Molecules communication tyrosinase inhibition antioxidant effect and cytotoxicity studies of the extracts of Cudrania tricuspidata fruit standardized in chlorogenic acid. Molecules 2019, 24, 3266. [Google Scholar] [CrossRef]
- Al Khatib, M.; Harir, M.; Costa, J.; Baratto, M.C.; Schiavo, I.; Trabalzini, L.; Pollini, S.; Rossolini, G.M.; Basosi, R.; Pogni, R. Spectroscopic characterization of natural melanin from a Streptomyces cyaneofuscatus strain and somparison with melanin enzymatically synthesized by tyrosinase and laccase. Molecules 2018, 23, 1916. [Google Scholar] [CrossRef]
- Plonka, P.M.; Grabacka, M. Melanin synthesis in microorganisms-biotechnological and medical aspects. Acta Biochim. Pol. 2006, 53, 429–443. [Google Scholar] [CrossRef]
- Tran-Ly, A.N.; Reyes, C.; Schwarze, F.W.M.R.; Ribera, J. Microbial production of melanin and its various applications. World J. Microbiol. Biotechnol. 2020, 36, 1–9. [Google Scholar] [CrossRef]
- Claus, H.; Decker, H. Bacterial tyrosinases. Syst. Appl. Microbiol. 2006, 29, 3–14. [Google Scholar] [CrossRef]


| Retention Time (min) | Proposed Compound | Molecular Form (Error-ppm) | [M-H]—m/z | MS/MS Fragment Ions [m/z (Intensity %)] | Intensities |
|---|---|---|---|---|---|
| 1.8 | Isocitric acid | C6H7O7 (−2.5) | 191.0196 | 111.00 (44); 103.04 (3); 87.00 (100); 57.03 (22) | 153,408 |
| 2.0 | Methylmalonic acid | C4H6O4 (−1.4) | 117.0195 | 73.02 (100); 68.95 (12); 56.02 (8) | 59,154 |
| 3.9 | 4-caffeoylquinic acid | C16H18O9 (1.4) | 353.0873 | 191.05 (100) 179.03 (1); 161.02 (2); 109.03 (2); 87.08 (2); 85.02 (8) | 56,606 |
| 4.4 | 5-caffeoylquinic acid | C16H18O9 (0.0) | 353.0878 | 191.05 (100); 179.06 (65); 135.04 (49); 85.02 (8) | 247,994 |
| 5.1 | 3-caffeoylquinic acid = Chlorogenic | C16H18O9 (−0.6) | 353.0880 | 191.05 (100); 85.02 (23) | 303,408 |
| 5.6 | Chlorogenic derivative | C22H32O11 (0.2) | 471.1871 | 353.09 (5); 272.94 (5); 117.03 (4); 101.02 (22); 75.09 (7); 71.04 (44); 59.01 (28) | 28.042 |
| 5.7 | Cynarin | C25H24O12 (0.0) | 515.1195 | 353.08 (67); 191.05 (100); 179.05 (63); 135.04 (74); 85.02 (10) | 118,826 |
| 6.6 | Luteolin-7-O-glucoside | C21H20O11 (1.3) | 447.0928 | 285.03 (100); 256.03 (1); 133.02(11); 107.01 (6) | 108,168 |
| 6.7 | Cynarin isomer | C25H24O12 (2.3) | 515.1183 | 353.08 (89); 191.05 (30); 179.03 (93); 173.04 (100); 135.04 (78); 161.02 (18) | 61,892 |
| 7.0 | Luteolin-7-O-(6″ malonylglucoside) | C24H22O14 (0.5) | 533.0934 | 489.1 (100); 285.03 (42); 256.03 (5); 151.00 (8) | 109,720 |
| 7.2 | Gibberellin A8 | C16H24O7 (0.1) | 363.1449 | 261.11 (1); 243.10 (1); 199.11 (2); 101.02 (100) | 117,618 |
| 7.6 | Coumaric acid-glycosidic | C16H20O6 (1.0) | 307.1184 | 200.12 (1); 161.10 (1); 146.96 (1); 44.99 (100) | 24,658 |
| 8.3 | Gibberellin A28 dehydrogenated | C20H24O8 (1.4) | 391.1393 | 273.0192 (1); 2453.1028 (1); 218.98 (1) 107.05 (1); 44.99 (100) | 195,196 |
| Strains | Origin | Zone of Inhibition, mm | |||||
|---|---|---|---|---|---|---|---|
| CL | CI | CA | RA | C− | C+ | ||
| Pseudomonas fluorescens ESACB 2 | Sheep’s raw milk | NI | NI | 8.15 ± 0.09 a | 8.85 ± 0.09 a | 9.08 ± 0.06 a | NI |
| Alcaligenes faecalis ESACB 7 | Cow’s raw milk | NI | NI | 8.48 ± 0.07 a | 8.95 ± 0.04 b | 8.28 ± 0.03 a | NI |
| Pseudomonas putida ESACB 27 | Goat’s cheese rind | NI | NI | 6.48 ± 0.06 b | 6.58 ± 0.08 c | 8.27 ± 0.04 a | NI |
| Pseudomonas putida ESACB 29 | Water | NI | NI | 6.73 ± 0.05 b | 7.28 ± 0.03 a | 7.14 ± 0.04 a | NI |
| Pseudomonas fluorescens ESACB 67 | Goat’s raw milk | NI | NI | 7.65 ± 0.05 a | 7.28 ± 0.03 b | 7.69 ± 0.04 a | NI |
| Pseudomonas fluorescens ESACB 137 | Washing car | NI | NI | 8.04±0.11a | 8.03 ± 0.10 a | 8.28 ± 0.03 a | NI |
| Pseudomonas putida ESACB 184 | Sheep’s raw milk | NI | NI | 7.36 ± 0.04 b | 7.28 ± 0.03 b | 9.08 ± 0.06 a | NI |
| Pseudomonas putida ESACB 191 | Goat’s cheese rind | NI | NI | 8.18 ± 0.10 a | 7.27 ± 0.08 b | 8.28 ± 0.10 a | NI |
| Pseudomonas aeruginosa ESACB 217 | Water | NI | NI | 6.44 ± 0.04 a | 6.67 ± 0.06 a | 6.34 ± 0.21 a | NI |
| Pseudomonas aeruginosa ESA 1 | Cow’s raw milk | NI | NI | 8.28 ± 0.07 a | 8.08 ± 0.09 a | 8.38 ± 0.07 a | NI |
| Pseudomonas aeruginosa ESA 2 | Cow’s raw milk | NI | NI | 8.73 ± 0.09 b | 8.93 ± 0.03 b | NI | NI |
| Pseudomonas aeruginosa ATCC 27853 | - | NI | NI | 8.58 ± 0.06 a | 8.79 ± 0.09 a | 8.55 ± 0.05 a | NI |
| Pseudomonas fluorescens ATCC 13525 | - | NI | NI | 7.96 ± 0.12 a | 8.03 ± 0.09 a | 8.08 ± 0.06 a | NI |
| Staphylococcus aureus ATCC 25923 | - | NI | NI | NI | 8.12 ± 0.09 a | 8.23 ± 0.65 a | NI |
| Listeria monocytogenes NCTC 11994 | - | NI | NI | 7.73 ± 0.24 a | 6.93 ± 0.65 a | 8.08 ± 0.06 a | NI |
| Compounds Tested | Log CFU/mL Reduction | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| Chlorogenic acid (CA) | 3.22 ± 0.04 a | 2.32 ± 0.05 a | 3.80 ± 0.05 a |
| Rosmarinic acid (RA) | 2.25 ± 0.14 b | 1.32 ± 0.13 b | 1.95 ± 0.13 b |
| Chlorogenic acid:Rosmarinic acid (CA:RA) 50:50 | 2.18 ± 0.07 b | 1.31 ± 0.0 b | 1.83 ± 0.07 b |
| C. cardunculus leaves extract (CL) | 6.20 ± 0.45 c | 5.25 ± 0.04 c | 5.10 ± 0.14 c |
| C. cardunculus inflorescence extract (CI) | 3.31 ± 0.13 d | 3.00 ± 0.14 d | 2.98 ± 0.15 d |
| C. cardunculus leaves extract: inflorescence extract (CL:CI) 50:50 | 2.22 ± 0.15 b | 1.01 ± 0.07 b | 2.49 ± 0.00 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraz, A.R.; Pintado, C.M.B.S.; Serralheiro, M.L. Influence of Cynara cardunculus L. Phenolic Compounds on Pseudomonas putida Isolated from the Dairy Industry: Growth and Melanin Bioproduction. Appl. Sci. 2022, 12, 3629. https://doi.org/10.3390/app12073629
Ferraz AR, Pintado CMBS, Serralheiro ML. Influence of Cynara cardunculus L. Phenolic Compounds on Pseudomonas putida Isolated from the Dairy Industry: Growth and Melanin Bioproduction. Applied Sciences. 2022; 12(7):3629. https://doi.org/10.3390/app12073629
Chicago/Turabian StyleFerraz, Ana Rita, Cristina M. B. S. Pintado, and Maria Luísa Serralheiro. 2022. "Influence of Cynara cardunculus L. Phenolic Compounds on Pseudomonas putida Isolated from the Dairy Industry: Growth and Melanin Bioproduction" Applied Sciences 12, no. 7: 3629. https://doi.org/10.3390/app12073629
APA StyleFerraz, A. R., Pintado, C. M. B. S., & Serralheiro, M. L. (2022). Influence of Cynara cardunculus L. Phenolic Compounds on Pseudomonas putida Isolated from the Dairy Industry: Growth and Melanin Bioproduction. Applied Sciences, 12(7), 3629. https://doi.org/10.3390/app12073629







