Alkaline Extraction of Organomineral Potassium Humate from Coal Mining Waste
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
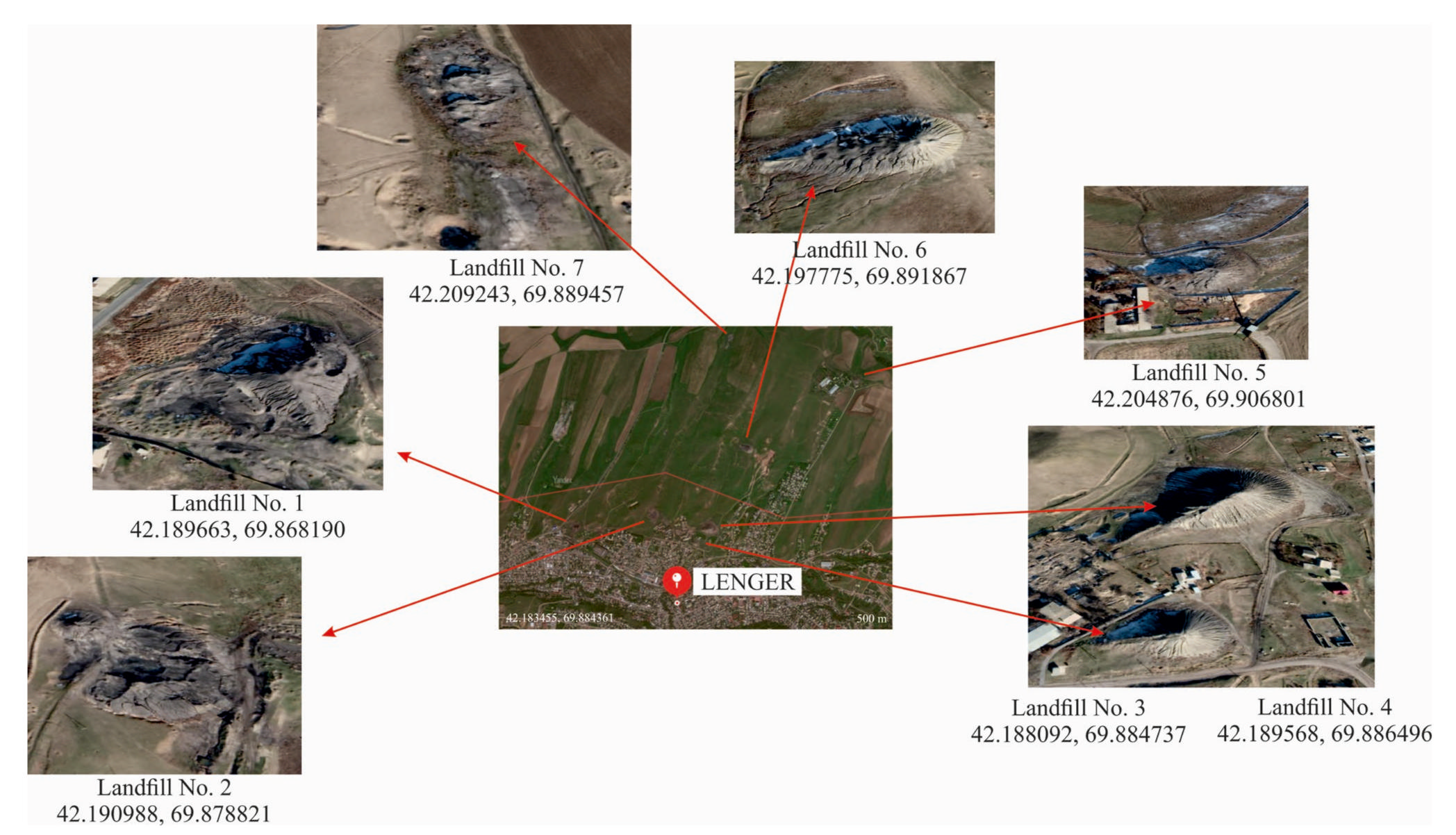
3.1. Characteristics of Coal Mining Waste
3.2. Kinetic Study of the Alkaline Extraction Process
- at 60 °C, tgφ1 = k1 = 0.0005458;
- at 80 °C, tgφ2 = k2 = 0.0003379;
- at 100 °C, tgφ3 = k3 = 0.0001375.
3.3. Characteristics of the Obtained Potassium Humate
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, T.; Wang, M.; Ferng, Y.; Huang, P. Catalytic synthesis of humic substances by natural clays, silts, and soils. Soil Sci. 1983, 135, 350–360. Available online: https://journals.lww.com/soilsci/Abstract/1983/06000/Catalytic_Synthesis_of_Humic_Substances_By_Natural.3.aspx (accessed on 3 February 2022). [CrossRef]
- Klavins, M.; Purmalis, O. Properties and structure of raised bog peat humic acids. J. Mol. Struct. 2013, 1050, 103–113. [Google Scholar] [CrossRef]
- Skripkina, T.; Belokozenko, M.; Shatskaya, S.; Tikhova, V.; Lomovskiy, I. Concentrating rare earth elements in brown coal humic acids by mechanochemical treatment. RSC Adv. 2021, 11, 36016–36022. [Google Scholar] [CrossRef]
- Platonova, D.; Adeeva, L. Use of Humic Sorbent from Sapropel for Extraction of Palladium Ions from Chloride Solutions. Open Eng. 2018, 8, 176–181. [Google Scholar] [CrossRef]
- Rupiasih, N.; Vidyasagar, P. A Review: Compositions, Structures, Properties and Applications of Humic Substances. J. Adv. Sci. Technol. 2005, 8, 16–25. Available online: https://www.researchgate.net/publication/236347209 (accessed on 5 February 2022).
- Jarukas, L.; Ivanauskas, L.; Kasparaviciene, G.; Baranauskaite, J.; Marksa, M.; Bernatoniene, J. Determination of Organic Compounds, Fulvic Acid, Humic Acid, and Humin in Peat and Sapropel Alkaline Extracts. Molecules 2021, 26, 2995. [Google Scholar] [CrossRef] [PubMed]
- Kitapova, R.R.; Ziganshin, A.U. Biologic activity of humic substances from peat and sapropel. Kazan Med. J. 2015, 96, 84–89. [Google Scholar] [CrossRef]
- van Rensburg, C.J. The Antiinflammatory Properties of Humic Substances: A Mini Review. Phytother. Res. 2015, 29, 791–795. [Google Scholar] [CrossRef]
- Stefanova, M.; Gonsalvesh, L.; Marinov, S.; Czech, J.; Carleer, R.; Yperman, J. Reductive pyrolysis of Miocene-aged lignite humic acids, Bulgaria. Fuel 2016, 165, 324–330. [Google Scholar] [CrossRef]
- Novotny, E.; deAzevedo, E.; Bonagamba, T.; Cunha, T.; Madari, B.; Benites, V. Studies of the Compositions of Humic Acids from Amazonian Dark Earth Soils. Environ. Sci. Technol. 2007, 41, 400–405. [Google Scholar] [CrossRef]
- Rahmi, E.; Suwardi, S.; Sumawinata, B. Characterization of Humic Substance Extracted from Andisols, Spodosols, Peat, and Lignite. SAINS Tanah-J. Soil Sci. Agroclimatol. 2018, 15, 35–45. [Google Scholar] [CrossRef][Green Version]
- Klavins, M.; Grandovska, S.; Obuka, V.; Ievinsh, G. Comparative Study of Biostimulant Properties of Industrially and Experimentally Produced Humic Substances. Agronomy 2021, 11, 1250. [Google Scholar] [CrossRef]
- Arif, M.; Alagawany, M.; Abd El-Hack, M.E.; Saeed, M.; Arain, M.A.; Elnesr, S.S. Humic acid as a feed additive in poultry diets: A review. Iran. J. Vet. Res. 2019, 20, 167–172. [Google Scholar] [PubMed]
- Alekseeva, O. Istorija Lengerskih shaht. Tolebijskij Jekspress 2012, 25, 25. [Google Scholar]
- Visscher, C.; Hankel, J.; Nies, A.; Keller, B.; Galvez, E.; Strowig, T.; Keller, C.; Breves, G. Performance, Fermentation Characteristics and Composition of the Microbiome in the Digest of Piglets Kept on a Feed with Humic Acid-Rich Peat. Front. Vet.-Sci. 2019, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Niu, Z.; Zhang, C.; Zhang, X.; Li, X. Extraction of Humic Acid from Lignite by KOH-Hydrothermal Method. Appl. Sci. 2019, 9, 1356. [Google Scholar] [CrossRef]
- Saito, B.; Seckler, M.M. Alkaline extraction of humic substances from peat applied to organic-mineral fertilizer production. Braz. J. Chem. Eng. 2014, 31, 675–682. [Google Scholar] [CrossRef]
- Technical Committee for Standardization. National Standard of the Russian Federation: Humic Substances from Brown Coals, Lignites and Oxidized Coals. Test Methods. GOST R 54221-2010. Moscow. 2012. Available online: https://docs.cntd.ru/document/1200084825 (accessed on 27 December 2021).
- Bowen, B.H.; Irwin, M.W. Coal Characteristics; Indiana Center for Coal Technology Research: West Lafayette IN, USA, 2008. [Google Scholar]
- Mirzakhodzhaeva, Z. Inhaling Is Prohibited [In Russian: Vdoh Zapreshhjon]. TITUS Citizen Journalism Agency. 2013. Available online: http://titus.kz/?type=shym&previd=34323 (accessed on 21 February 2022).
- Pritula, I. Environmental Scandal in the City of Lenger South Kazakhstan [In Russian: Jekologicheskij Skandal v Gorode Lengere JuKO]. OTYRAY News Portal. 2012. Available online: https://otyrar.kz/2012/10/ekologicheskij-skandal-v-lengere-yuzhnogo-kazaxstana/ (accessed on 21 February 2022).
- Coal Mine Dust Exposures and Associated Health Outcomes; Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health: Cincinnati, OH, USA, 2011.
- Ul-Hamid, A. A Beginners’ Guide to Scanning Electron Microscope; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Tarasevich, B.N. IR spectra of the main classes of organic compounds. In Reference Materials; Lomonosov Moscow State University: Moscow, Russia, 2012. [Google Scholar]
- Technical Committee for Standardization. National Standard of the Russian Federation: Production and Preparation Waste of Coals. Classification. GOST 57011-2016. Moscow. 2017. Available online: https://docs.cntd.ru/document/1200137234 (accessed on 15 February 2022).
- Helal, A.A.; Murad, G. Characterization of different humic materials by various analytical techniques. Arab. J. Chem. 2011, 4, 51–54. [Google Scholar] [CrossRef]
- Gostishcheva, M.V.; Belousov, M.V.; Yusubov, M.S.; Ismatova, R.R.; Dmitruk, S.E. The Comparative Characteristic of Infra-Red Spectra of Humic Acids from Peats of a Different Origin of Tomsk Area. Pharm. Chem. J. 2009, 43, 418–421. [Google Scholar] [CrossRef]
- Machado, W.; Franchini, J.C.; de Fátima Guimarães, M.; Filho, J.T. Spectroscopic characterization of humic and fulvic acids in soil aggregates, Brazil. Heliyon 2020, 6, e04078. [Google Scholar] [CrossRef]
- Volkov, D.S.; Rogova, O.B.; Proskurnin, M.A. Temperature Dependences of IR Spectra of Humic Substances of Brown Coal. Agronomy 2021, 11, 1822. [Google Scholar] [CrossRef]
- Madejova, J.; Komadel, P. Baseline Studies of the Clay Minerals Society Source Clays: Infrared Methods. Clays Clay Miner. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Frost, R.L.; Vassallo, A.M. The Dehydroxylation of the Kaolinite Clay Minerals using Infrared Emission Spectroscopy. Clays Clay Miner. 1996, 44, 635–651. [Google Scholar] [CrossRef]
- Mahdi, A.H.A.; Badawy, S.A.; Abdel Latef, A.A.H.; El Hosary, A.A.A.; Abd El Razek, U.A.; Taha, R.S. Integrated Effects of Potassium Humate and Planting Density on Growth, Physiological Traits and Yield of Vicia faba L. Grown in Newly Reclaimed Soil. Agronomy 2021, 11, 461. [Google Scholar] [CrossRef]
- Multi-Active Biostimulant HUMICRAFT® Liquid. HuminTech. Available online: https://www.humintech.com/agriculture/products/humicraft-liquid (accessed on 21 February 2022).
- Imbufe, A.U.; Patti, A.F.; Burrow, D.; Surapaneni, A.; Jackson, W.R.; Milner, A.D. Effects of potassium humate on aggregate stability of two soils from Victoria, Australia. Geoderma 2005, 125, 321–330. [Google Scholar] [CrossRef]










| Compounds | Composition, % | Standard Deviation, s, n = 8 |
|---|---|---|
| C | 48.19 | 1.84 |
| H | 5.30 | 1.21 |
| N | 2.12 | 0.43 |
| Al2O3 | 5.14 | 2.20 |
| SiO2 | 9.86 | 2.13 |
| K2O | 0.91 | 0.56 |
| CaO | 0.29 | 0.73 |
| Fe2O3 | 5.73 | 1.05 |
| Element | Weight, % | Oxides | In Terms of Oxides, % |
|---|---|---|---|
| C | 43.00 | - | - |
| O | 39.43 | - | - |
| Al | 3.79 | Al2O3 | 7.16 |
| Si | 6.42 | SiO2 | 13.73 |
| S | 1.03 | SO3 | 2.57 |
| K | 0.40 | K2O | 0.48 |
| Ca | 0.43 | CaO | 0.60 |
| Fe | 5.49 | Fe2O3 | 7.85 |
| No. | Peak | Intensity | Corr. Intensity | Base (H) | Base (L) | Area | Corr. Area |
|---|---|---|---|---|---|---|---|
| 1 | 555.50 | 87.320 | 3.204 | 570.93 | 540.07 | 1.610 | 0.269 |
| 2 | 597.93 | 86.390 | 3.257 | 613.36 | 574.79 | 2.111 | 0.317 |
| 3 | 644.22 | 83.190 | 0.746 | 648.08 | 617.22 | 2.034 | 0.092 |
| 4 | 694.37 | 80.421 | 2.647 | 721.38 | 655.80 | 5.771 | 0.488 |
| 5 | 759.10 | 81.668 | 0.230 | 759.38 | 725.23 | 2.942 | 0.033 |
| 6 | 779.24 | 81.211 | 0.717 | 786.96 | 763.81 | 2.056 | 0.043 |
| 7 | 794.67 | 81.701 | 0.746 | 860.25 | 786.96 | 5.389 | −0.173 |
| 8 | 910.40 | 82.571 | 4.018 | 948.98 | 860.25 | 6.375 | 0.782 |
| 9 | 1010.70 | 77.444 | 0.798 | 1014.56 | 952.84 | 5.143 | 0.099 |
| 10 | 1029.99 | 76.948 | 1.394 | 1153.43 | 1018.41 | 12.500 | 0.452 |
| 11 | 1238.30 | 86.867 | 0.109 | 1269.16 | 1234.44 | 2.106 | 0.020 |
| 12 | 1361.74 | 88.043 | 0.198 | 1489.05 | 1357.89 | 6.502 | 0.241 |
| 13 | 1504.48 | 90.824 | 0.075 | 1508.33 | 1492.90 | 0.630 | 0.000 |
| 14 | 1589.34 | 87.409 | 3.778 | 1674.21 | 1516.05 | 7.906 | 1.583 |
| 15 | 1697.36 | 90.939 | 1.460 | 1816.94 | 1678.07 | 3.745 | 0.380 |
| 16 | 2854.65 | 93.339 | 0.902 | 2881.65 | 2827.64 | 1,472 | 0.080 |
| 17 | 2924.09 | 92.271 | 1.826 | 2989.66 | 2881.65 | 3.198 | 0.364 |
| Time, min | pH | C, mol/L | α, % | Product Yield, gr |
|---|---|---|---|---|
| 60 °C | ||||
| 60 | 9.69 | 0.000000000204 | 37.11 | 40.76 |
| 70 | 9.61 | 0.000000000245 | 49.73 | 45.51 |
| 80 | 9.54 | 0.000000000288 | 56.08 | 49.18 |
| 90 | 9.46 | 0.000000000346 | 61.12 | 53.75 |
| 100 | 9.31 | 0.000000000489 | 64.76 | 56.57 |
| 80 °C | ||||
| 60 | 8.57 | 0.000000002691 | 48.85 | 52.19 |
| 70 | 8.49 | 0.000000003235 | 57.16 | 57.28 |
| 80 | 8.33 | 0.000000004677 | 70.70 | 65.21 |
| 90 | 8.25 | 0.000000005623 | 87.03 | 73.84 |
| 100 | 8.11 | 0.000000007762 | 85.98 | 71.66 |
| 100 °C | ||||
| 60 | 8.03 | 0.000000009332 | 62.96 | 62.71 |
| 70 | 7.96 | 0.000000010964 | 66.84 | 68.16 |
| 80 | 7.92 | 0.000000012022 | 78.09 | 66.94 |
| 90 | 7.91 | 0.000000012302 | 89.01 | 65.36 |
| 100 | 7.90 | 0.000000012589 | 88.12 | 62.23 |
| α (Unit Fraction) | ||
|---|---|---|
| 60 °C | ||
| 0.3711 | 0.321241 | 60 |
| 0.4973 | 0.303349 | 70 |
| 0.5608 | 0.291102 | 80 |
| 0.6112 | 0.279459 | 90 |
| 0.6476 | 0.269809 | 100 |
| 80 °C | ||
| 0.4885 | 0.304858 | 60 |
| 0.5716 | 0.288761 | 70 |
| 0.7070 | 0.251387 | 80 |
| 0.8703 | 0.174138 | 90 |
| 0.8598 | 0.180902 | 100 |
| 100 °C | ||
| 0.6296 | 0.274721 | 60 |
| 0.6684 | 0.263764 | 70 |
| 0.7809 | 0.222527 | 80 |
| 0.8901 | 0.160306 | 90 |
| 0.8812 | 0.166712 | 100 |
| Compounds | Composition, % | Standard Deviation, s, n = 8 |
|---|---|---|
| C | 47.29 | 1.34 |
| H | 5.83 | 1.15 |
| N | 2.28 | 0.81 |
| CaO, % | 0.42 | 1.29 |
| K2O, % | 19.21 | 2.60 |
| Al2O3, % | 0.63 | 1.11 |
| SiO2, % | 0.97 | 1.56 |
| Fe2O3, % | 0.36 | 0.94 |
| Element | Weight, % | Oxides | In Terms of Oxides, % |
|---|---|---|---|
| C | 42.30 | - | - |
| O | 41.88 | - | - |
| Al | 0.64 | Al2O3 | 1.20 |
| Si | 0.85 | SiO2 | 1.81 |
| S | 0.33 | SO3 | 2.57 |
| K | 14.11 | K2O | 17.01 |
| Na | 0.17 | Na2O | 0.22 |
| No. | Peak | Intensity | Corr. Intensity | Base (H) | Base (L) | Area | Corr. Area |
|---|---|---|---|---|---|---|---|
| 1 | 601.79 | 72.808 | 0.759 | 609.51 | 597.93 | 1.576 | 0.033 |
| 2 | 648.08 | 63.858 | 1.094 | 651.94 | 613.36 | 6.041 | 0.039 |
| 3 | 667.37 | 61.698 | 0.910 | 675.09 | 675.80 | 3.933 | 0.062 |
| 4 | 682.80 | 61.226 | 1.322 | 871.82 | 675.09 | 31.869 | 1.708 |
| 5 | 883.40 | 79.655 | 0.946 | 914.26 | 871.82 | 3.960 | 0.052 |
| 6 | 937.40 | 79.228 | 4.267 | 1014.56 | 914.26 | 7.291 | 0.718 |
| 7 | 1072.42 | 81.501 | 9.272 | 1103.28 | 1018.41 | 5.402 | 1.765 |
| 8 | 1153.43 | 85.666 | 4.698 | 1195.87 | 1118.71 | 4.407 | 0.994 |
| 9 | 1643.35 | 82.088 | 13.154 | 1739.79 | 1566.20 | 8.961 | 5.333 |
| 10 | 3240.41 | 74.757 | 0.327 | 3244.27 | 2916.37 | 25.535 | 0.744 |
| 11 | 3263.56 | 74.475 | 0.114 | 3271.27 | 3248.13 | 2.945 | 0.009 |
| 12 | 3332.99 | 74.218 | 0.053 | 3336.85 | 3302.13 | 4.473 | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarbek, U.; Abdurazova, P.; Nazarbekova, S.; Assylbekova, D.; Kambatyrov, M.; Raiymbekov, Y. Alkaline Extraction of Organomineral Potassium Humate from Coal Mining Waste. Appl. Sci. 2022, 12, 3658. https://doi.org/10.3390/app12073658
Nazarbek U, Abdurazova P, Nazarbekova S, Assylbekova D, Kambatyrov M, Raiymbekov Y. Alkaline Extraction of Organomineral Potassium Humate from Coal Mining Waste. Applied Sciences. 2022; 12(7):3658. https://doi.org/10.3390/app12073658
Chicago/Turabian StyleNazarbek, Ulzhalgas, Perizat Abdurazova, Saule Nazarbekova, Dina Assylbekova, Maksat Kambatyrov, and Yerkebulan Raiymbekov. 2022. "Alkaline Extraction of Organomineral Potassium Humate from Coal Mining Waste" Applied Sciences 12, no. 7: 3658. https://doi.org/10.3390/app12073658
APA StyleNazarbek, U., Abdurazova, P., Nazarbekova, S., Assylbekova, D., Kambatyrov, M., & Raiymbekov, Y. (2022). Alkaline Extraction of Organomineral Potassium Humate from Coal Mining Waste. Applied Sciences, 12(7), 3658. https://doi.org/10.3390/app12073658






