Abstract
Purpose: To investigate the applicability of strip meniscometry tube (SMT) in the measurement of tear lactoferrin in non-obese diabetic mice (NOD). Methods: SMT (SMTube, Echo Electricity Co., Ltd., Tokyo, Japan) and fluorescein staining tests were performed on 7–14 week NOD- male mice (n = 4) and age and sex matched wild–type (Balbc, WT) mice (n = 5). Tears collected during SMT underwent lactoferrin concentration measurement by ELISA. Results: The mean SMT value was significantly lower in NOD mice compared to wild-type mice (p = 0.01). The mean corneal fluorescein staining score in the NOD mice was significantly lower compared with the wild-type mice (p = 0.03). The mean tear lactoferrin level also showed a significantly lower concentration in NOD mice (p = 0.02). Conclusions: SMT has been shown to be an effective tool in measuring tear volume in humans, cats, dogs, and mice. SMT may also serve as a useful tool for tear lactoferrin assessment in NOD and WT mice in experimental settings.
1. Introduction
Sjögren’s syndrome (SS) is a multifactorial autoimmune disorder, mainly affecting salivary and lacrimal glands, which is influenced by genetic as well as environmental factors that are not completely understood as yet. Although dry eyes and dry mouth characterize the disease, the expression of clinical spectrum is diverse, extending from a solitary organ-specific autoimmune exocrinopathy to a systemic disorder affecting several organs. While Sjögren’s syndrome is one of the most common autoimmune diseases, it has no specific and non-invasive diagnostic tests. Diagnosis is conducted by biopsy of the exocrine glands and blood tests for specific antibodies [1]. Since ethical issues strongly hamper the collection and study of tissue or tear samples in humans in order to investigate pathogenetic mechanisms, animal models of SS or dry eye disease help us to analyze many pathogenetic relations from which conclusions can be drawn for similar human diseases. NOD mice have been reported to have similar ocular surface disease and dry eye features to human ocular surface disease, including decreased tear volume, the loss of corneal barrier integrity (increased staining scores), reduced tear mucins, and increased lacrimal gland inflammatory infiltrates [2], which led us to choose NOD mice to examine tear lactoferrin changes. Indeed, tear lactoferrin has been reported to be a good biomarker of dry eye disease in SS. The severity of ocular surface damage due to SS-related dry eye largely depends on the tear secretory function of the lacrimal gland and that the function of the lacrimal gland can be evaluated by determination of the level of tear lactoferrin [3].
The strip meniscometry tube (SMT) has been defined as a non-invasive tool for measuring tear quantity in human tear meniscus [4]. It has been shown to have a strong correlation with the Schirmer test, tear film break-up time (BUT), and ocular surface vital staining scores [5]. This new method of testing has not only been applied to the diagnosis of dry eye disease [6,7] but also for the assessment of the efficacy of punctal occlusion [8,9,10]. Recently, it has been shown to be a very useful tool in evaluating epiphora and related symptoms in humans [11].The applicability of SMT in tear volume measurements has also been shown in cats, dogs, mice, and rabbits [12,13].
This new method has not been employed for the evaluation of tear protein levels in humans and in mice until now.
In this study, we examined the usefulness of SMT in tear lactoferrin concentration measurement in NOD and WT mice.
2. Materials and Methods
2.1. Animals
Seven eyes of four 7–14 week NOD male mice with ICR-JCL background and 8 eyes of 5 age and sex matched Balb/c strain wild–type (WT) mice were examined. NOD and WT mice were purchased from Japan Clea (Osaka, Japan). NOD mice had not yet developed type 1 diabetes at the time of the conduct of the current study. All studies were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
2.2. Strip Meniscometry Tube Measurements in Mice
SMT was gently immersed into the inferior TM of the mice with touch to the eyelid and ocular surface for 5 s. Three SMT tear collections were performed on a single eye on the same day with an interval of one hour between the tests. An electronic metronome was used for the strict measurement of the test’s duration. The SMT measurement and all the following tests were performed by the same examiner.
2.3. Tear Lactoferrin Assessment by ELISA
SMTs were gently inserted into the lower conjunctival fornix for 5 s. The collections yielded 1–3 μL of tears/eye. After the collection of tears, the wetted parts of the SMTs were cut with scissors and placed within a 1.5 mL Eppendorf tube to be stored in a −80 °C freezer until ELISA measurement. For ELISA, samples were allowed to defrost slowly on ice. 0.5 M NaCl, and 0.5% Tween80 in PBS solution was used to extract the collected tears from the SMTs. A 10 times dilution was performed using this extracting solution based on the mm of wet reading on the SMTs, which yielded between 10 and 30 μL of diluted tears for the assays. Diluted tears were transferred into new Eppendorf tubes, which were shaken up and down a few times to allow mixing. The Eppendorf tubes were then placed in a refrigerator at 4 °C for 3 h. After centrifuging samples at 3500 rpm for 10 min, the supernatant was collected and used for tear lactoferrin assessment by ELISA following kit instructions (Lactoferrin mouse ELISA kit, BioVision, Milpitas, CA, USA).
2.4. Corneal Fluorescein Vital Staining
Sodium fluorescein (FS) 0.5% dye was used for vital staining examinations. Using the cobalt blue light filter of a handheld slit lamp (an SL-15, Kowa, Tokyo, Japan), the FS staining was scored. 2 µL FS was instilled in the eyes using a micropipette. Vital staining scores of FS were evaluated by a grading system of 0–3 points for superior, central, and inferior corneal areas (min: 0 point, max: 9 points).
2.5. Statistical Analysis
The Mann–Whitney U test was used for statistical analysis, and a p value less than 5% was considered statistically significant.
3. Results
3.1. Comparison of SMT Values between the NOD and Wild-Type Mice
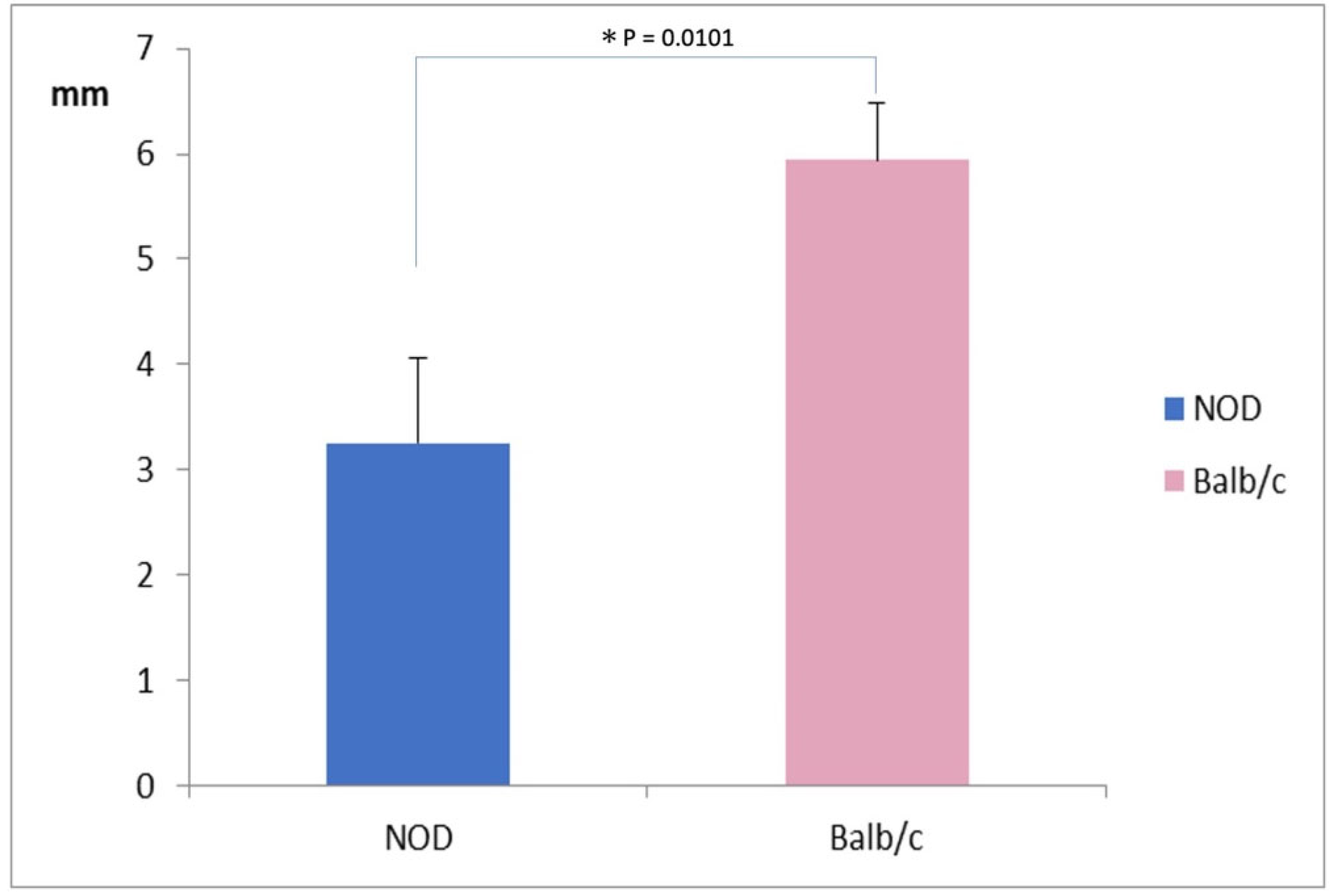
The mean SMT value was significantly lower in NOD mice (3.25 ± 0.75 mm) compared to WT mice (6.0 ± 0.50 mm) (p = 0.0101) (Figure 1).
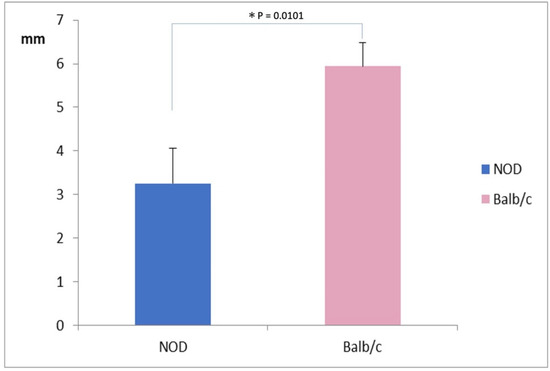
Figure 1.
Comparison of Strip Meniscometry Tube values between the NOD and the Balb/c mice. Note the significantly lower SMT value in NOD mice compared to the WT mouse (p = 0.00101).* represents p < 0.05.
3.2. Comparison of Tear Lactoferrin Concentration in the Tears
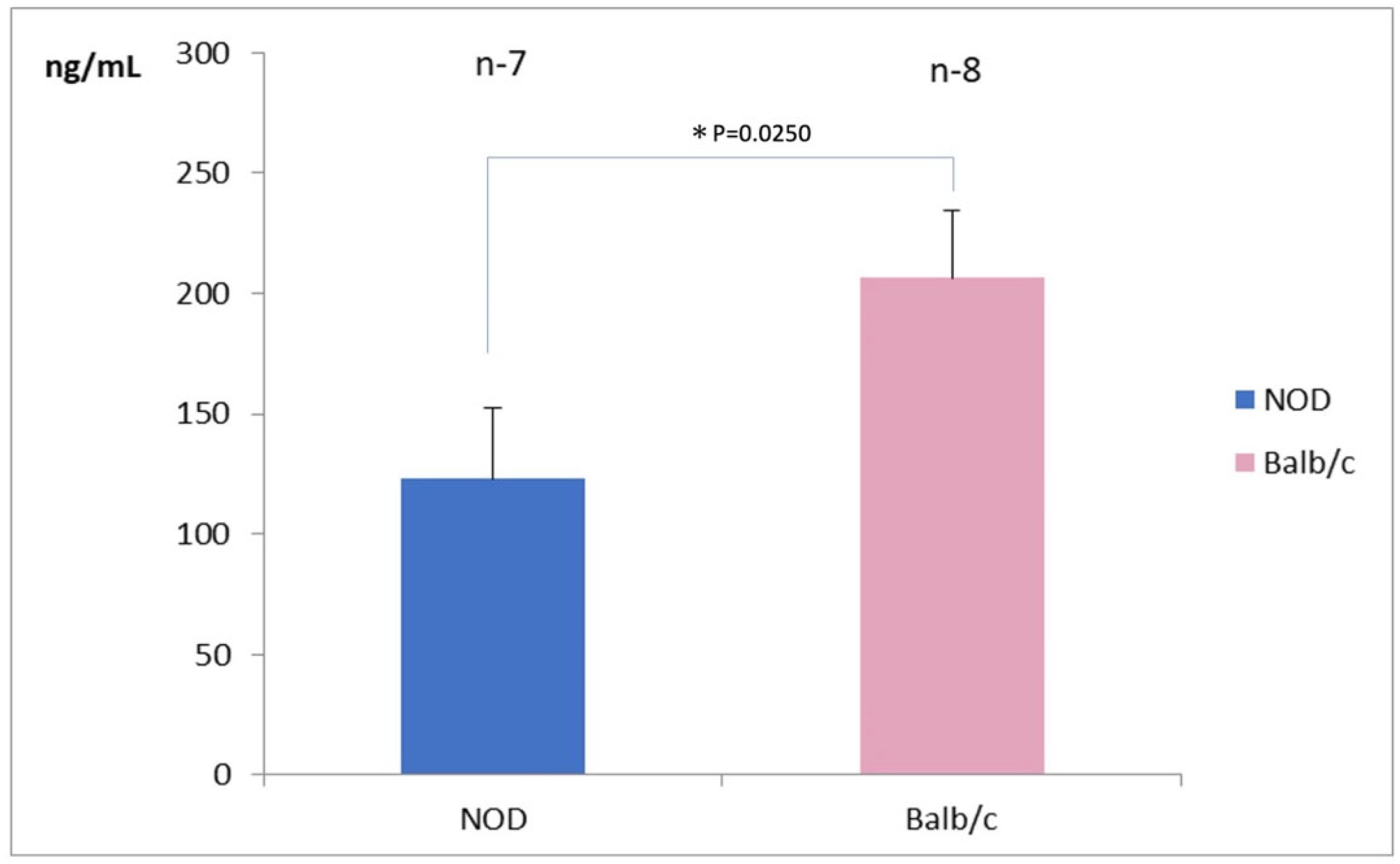
Seven eyes in the NOD mice and 8 eyes in the WT mice were available for tear lactoferrin measurements. The mean tear lactoferrin concentration in the NOD mice was 125 ± 25 ng/mL in the NOD mice and 200 ± 25 ng/mL in the WT mice, respectively; the difference between the two groups was statistically significant (p = 0.025) (Figure 2).
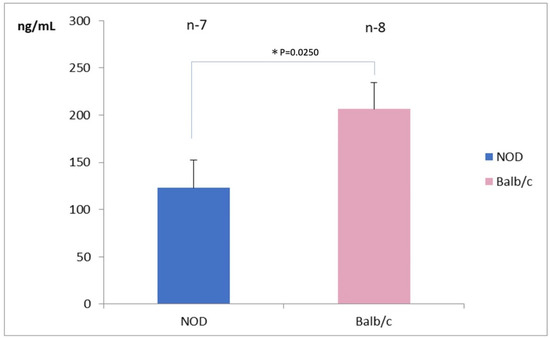
Figure 2.
Comparison of tear lactoferrin concentrations between NOD and the Balb/c mice. Note the significantly lower tear lactoferrin concentrations in the NOD mice compared to the WT mouse (p = 0.025). * represents p < 0.05.
3.3. Comparison of Corneal Vital Staining Scores
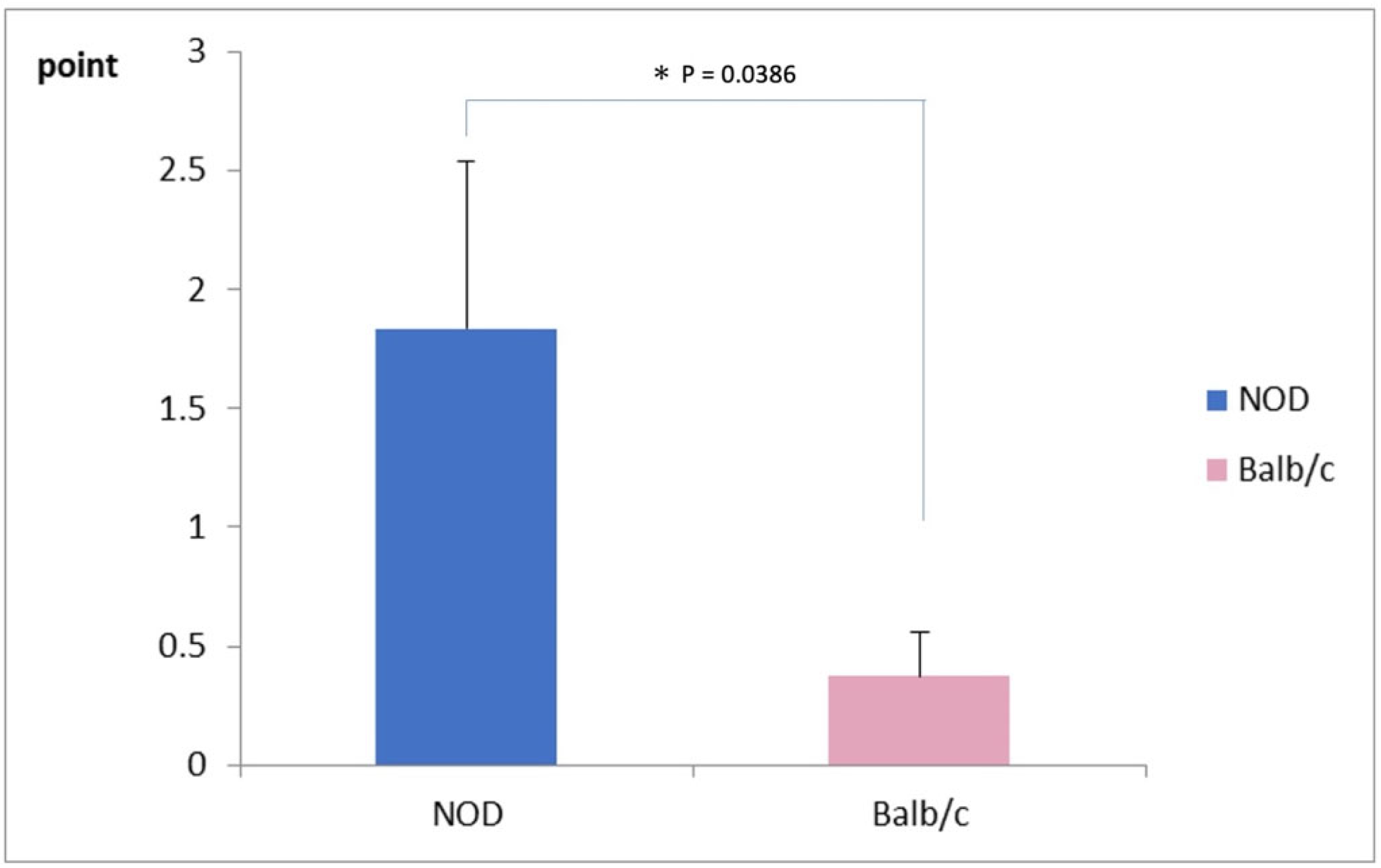
The mean corneal fluorescein staining score was significantly worse in NOD mice (1.75 ± 0.75 points) compared to the WT mice, as shown in Figure 3 (0.25 ± 0.25 points) (p = 0.03).
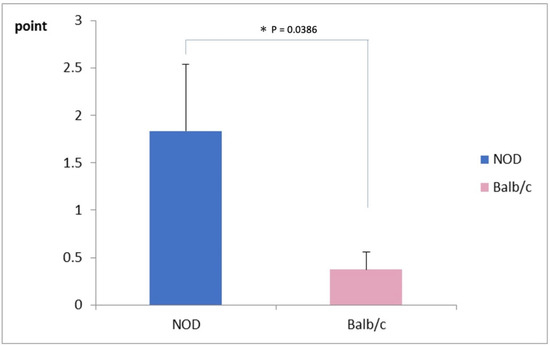
Figure 3.
Comparison of corneal vital staining scores between NOD and the Balb/c mice. Note the significantly higher staining scores in the NOD mice compared to the WT mouse (p = 0.03). * represents p < 0.05.
4. Discussion
Experimental settings using dry eye mouse models are necessary to elucidate the pathophysiology of dry eye disease and to help with the development of new treatment modalities and agents. Strip meniscometry has been shown to be a reliable method of measuring the tear meniscus volume and to have a strong correlation with tear function tests and vital staining scores in humans [4,5,6,8]. Previously, we have shown that the SMT test was quite efficient in measuring tear volumes in wild type and an environmental stress dry eye mouse model [13], where this testing was observed to be well correlated with other tear function parameters. This previous report emphasized the stability of the material while holding, the overall convenience of the technique, and the short measurement time as advantages of this new methodology [13]. In this investigation, we attempted to study if SMT could be used for the assessment of tear proteins. We have decided to concentrate on tear lactoferrin this time, which is known to be reduced in tears in dry eye disease and Sjogren syndrome [14,15]. Moreover, a previous clinical investigation conducted by us demonstrated that lactoferrin was an efficient treatment modality in improving tear stability and ocular surface epithelium in dry eye patients with Sjögren’s syndrome [16]. We employed NOD mice in this study because this model has been shown to harbor the clinical dry eye and ocular surface characteristics as observed in Sjogren’s syndrome [2,17]. The results of the current investigation showed that SMT could detect the tear volume differences between the WT and NOD mice, which was significantly lower in the latter. The NOD mice also had a significantly higher extent of ocular surface epithelial damage when compared to the wild-type mice. To our surprise, we found that tears collected with SMT harbored sufficient lactoferrin detectable by ELISA testing, also suggesting that SMT testing could reveal lactoferrin concentration differences between the SS model mouse and the wild-type mouse.
While our observations should be retested in a large number of NOD mice and other models of mice with dry eye disease, the initial findings of the current study pave the way to a novel method of tear lactoferrin assessment in murine experimental models. Our findings also justify the SMT testing that is used for the evaluation of other tear proteins or inflammatory markers including cytokines and chemokines. Future testing should also establish sensitivity and specificity of this method in assessing tear lactoferrin concentrations in human subjects and murine models with dry eyes. The new SMT has yet to be tested in human tear lactoferrin concentration assessments, which we believe should be much easier to perform because the human eyes are bigger and SMT testing can be readily carried out by dipping the tip of the tube into the tear meniscus without touching the lids or the ocular surface. This eliminates the possibility of reflex tearing, which would have an effect on tear protein concentrations. Therefore, one possible shortcoming of the new SMT methodology is that because the mouse eyes are small, the tips of the strips inevitably touch the lids and the conjunctiva, which might induce reflex tearing and alter the concentration of tears. Still, being able to detect tear protein concentration with small amounts of tears collected over 5 s is an important step forward in animal experiments of dry eye disease.
5. Conclusions
SMT allows an efficient measurement of tear meniscus volume in NOD mice similarly to the experience in the environmental dry eye mouse model. The new method was also feasible in detecting tear lactoferrin concentrations in conjunction with conventional ELISA testing.
Author Contributions
Conceptualization, M.D. and T.K.; methodology, T.N., M.D. and K.T. software, T.N.; validation, M.D., T.K. and K.T.; formal analysis, M.D.; investigation, K.T.; resources, T.N. and K.T.; data curation, M.D. and T.N.; writing, —original draft preparation, M.D.; writing—review and editing, T.K., T.N. and K.T.; visualization, M.D.; supervision, K.T.; project administration, M.D.; funding acquisition, T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Echo Electricity Co., Ltd.
Institutional Review Board Statement
The Animal Experimentation Ethics Committee of the Keio University School of Medicine approved the current research procedures (08067-7, 25 October 2017).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors would like to thank all staff members in the animal facility from our institution who helped during all the procedures included in this series.
Conflicts of Interest
Tsubota Laboratory, Inc. (Tokyo, Japan) holds the co-patent rights for the strip meniscometry tube (JP5138967B2). The other authors declare no conflict of interest.
References
- Nair, J.J.; Singh, T.P. Sjogren’s syndrome: Review of the aetiology, Pathophysiology & Potential therapeutic interventions. J. Clin. Exp. Dent. 2017, 9, e584–e589. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Masli, S.; Dartt, D.A. Mouse Models of Sjogren’s Syndrome with Ocular Surface Disease. Int. J. Mol. Sci. 2020, 21, 9112. [Google Scholar] [CrossRef] [PubMed]
- Ponzini, E.; Scotti, L.; Grandori, R.; Tavazzi, S.; Zambon, A. Lactoferrin Concentration in Human Tears and Ocular Diseases: A Meta-Analysis. Investig. Ophthalmol. Vis. Sci. 2020, 61, 9. [Google Scholar] [CrossRef] [PubMed]
- Dogru, M.; Ishida, K.; Matsumoto, Y.; Goto, E.; Ishioka, M.; Kojima, T.; Goto, T.; Saeki, M.; Tsubota, K. Strip meniscometry: A new and simple method of tear meniscus evaluation. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, O.M.; Dogru, M.; Ward, S.K.; Matsumoto, Y.; Wakamatsu, T.H.; Ishida, K.; Tsuyama, A.; Kojima, T.; Shimazaki, J.; Tsubota, K. The efficacy, sensitivity, and specificity of strip meniscometry in conjunction with tear function tests in the assessment of tear meniscus. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2194–2198. [Google Scholar] [CrossRef] [PubMed]
- Shinzawa, M.; Dogru, M.; Miyasaka, K.; Shimazaki, J.; Sekiryu, T. Application of CASIA SS-1000 Optical Coherence Tomography Tear Meniscus Imaging in Testing the Efficacy of New Strip Meniscometry in Dry Eye Diagnosis. Eye Contact Lens 2018, 44 (Suppl. 1), S44–S49. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Kim, J.Y.; Chin, H.S.; Seo, K.Y.; Kim, T.I.; Jung, J.W. Assessment of the Tear Meniscus by Strip Meniscometry and Keratograph in Patients with Dry Eye Disease According to the Presence of Meibomian Gland Dysfunction. Cornea 2017, 36, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, O.M.; Dogru, M.; Kojima, T.; Matsumoto, Y.; Wakamatsu, T.H.; Tsubota, K.; Fujishima, H. OCT assessment of tear meniscus after punctal occlusion in dry eye disease. Optom. Vis. Sci. 2012, 89, E770–E776. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Matsumoto, Y.; Ibrahim, O.M.; Wakamatsu, T.H.; Dogru, M.; Tsubota, K. Evaluation of a thermosensitive atelocollagen punctal plug treatment for dry eye disease. Am. J. Ophthalmol. 2014, 157, 311–317 e311. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Takeuchi, M.; Kato, N. The combination of strip meniscometry and dry eye-related quality-of-life score is useful for dry eye screening during health checkup: Cross-sectional study. Medicine 2018, 97, e12969. [Google Scholar] [CrossRef] [PubMed]
- Osawa, I.; Esaka, Y.; Kojima, T.; Simsek, C.; Kudo, H.; Dogru, M. Feasibility of Strip Meniscometry for Tear Volume Evaluation in Lacrimal Passage Obstruction. Diagnostics 2020, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Rajaei, S.M.; Ansari Mood, M.; Asadi, F.; Rajabian, M.R.; Aghajanpour, L. Strip meniscometry in dogs, cats, and rabbits. Vet. Ophthalmol. 2018, 21, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Shinzawa, M.; Dogru, M.; Miyasaka, K.; Kojima, T.; Tsubota, K. The Application of Strip Meniscometry to the Evaluation of Tear Volume in Mice. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2088–2091. [Google Scholar] [CrossRef] [PubMed]
- Yolton, D.P.; Mende, S.; Harper, A.; Softing, A. Association of dry eye signs and symptoms with tear lactoferrin concentration. J. Am. Optom. Assoc. 1991, 62, 217–223. [Google Scholar] [PubMed]
- Danjo, Y.; Lee, M.; Horimoto, K.; Hamano, T. Ocular surface damage and tear lactoferrin in dry eye syndrome. Acta Ophthalmol. 1994, 72, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Dogru, M.; Matsumoto, Y.; Yamamoto, Y.; Goto, E.; Saiki, M.; Shimazaki, J.; Takebayashi, T.; Tsubota, K. Lactoferrin in Sjogren’s syndrome. Ophthalmology 2007, 114, 2366–2367. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.P.; Yamachika, S.; Bounous, D.I.; Brayer, J.; Jonsson, R.; Holmdahl, R.; Peck, A.B.; Humphreys-Beher, M.G. A novel NOD-derived murine model of primary Sjogren’s syndrome. Arthritis Rheum. 1998, 41, 150–156. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).