Experimental and Simulation Research on the Process of Nitrogen Migration and Transformation in the Fluctuation Zone of Groundwater Level
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.1.1. Experimental Materials
2.1.2. Experimental Device
2.1.3. Experimental Method
2.2. Numerical Model
2.2.1. Mathematical Model
- Water Movement Model.
- Solute transport model in the Vadose zone.
- Nitrogen migration and transformation model.
2.2.2. Initial Conditions and Boundary Conditions
- Initial conditions.
- Boundary conditions.
2.2.3. Model Parameters
2.2.4. Calibration and Evaluation of the Model
3. Results and Discussion
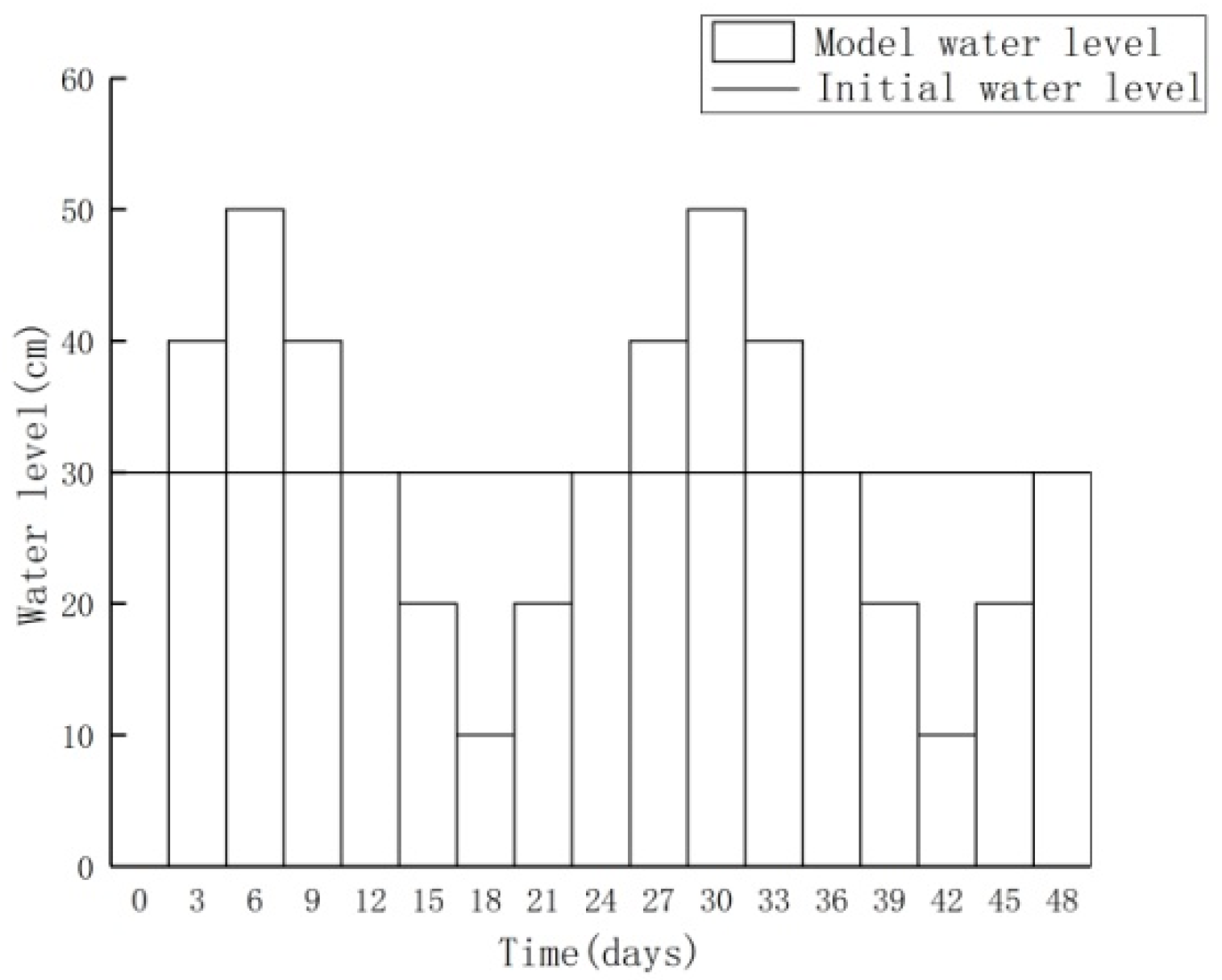
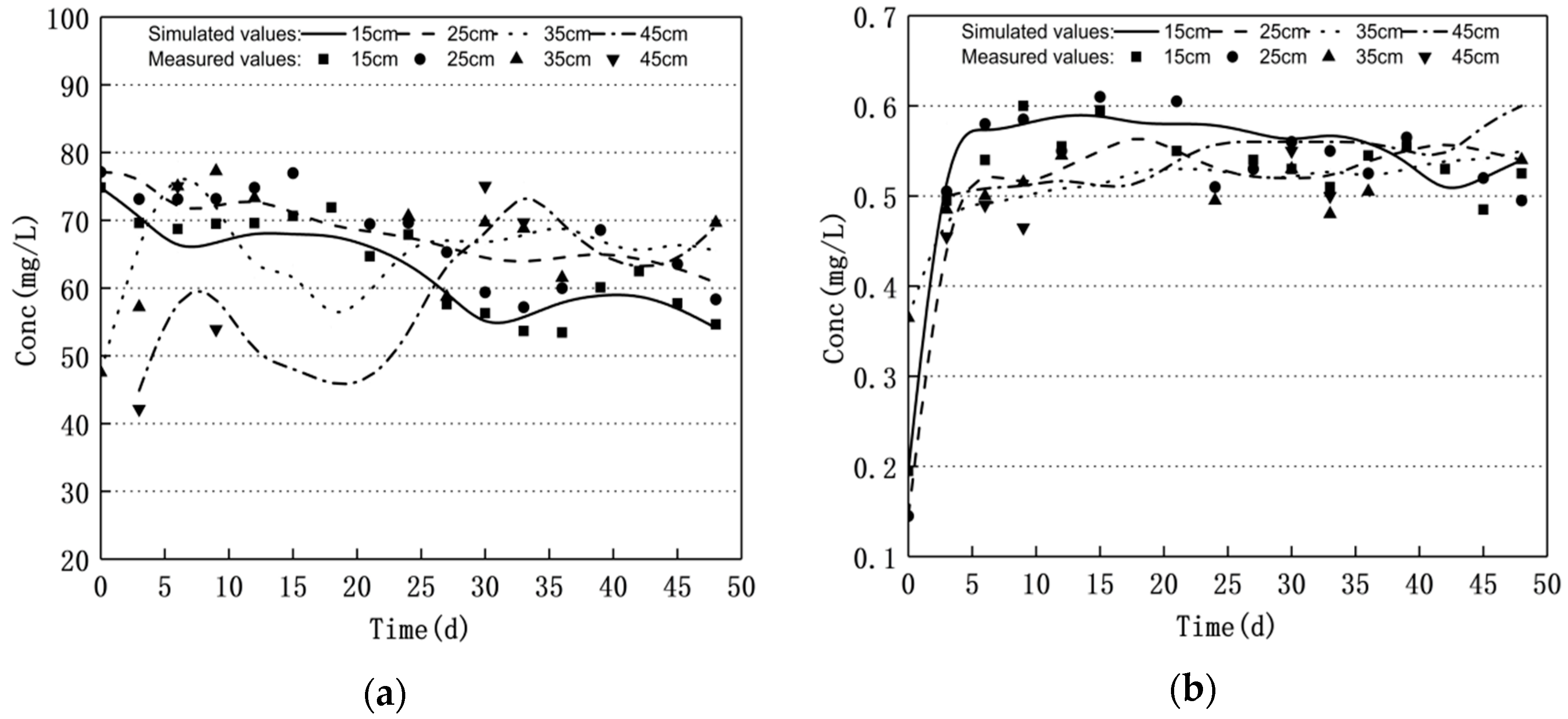
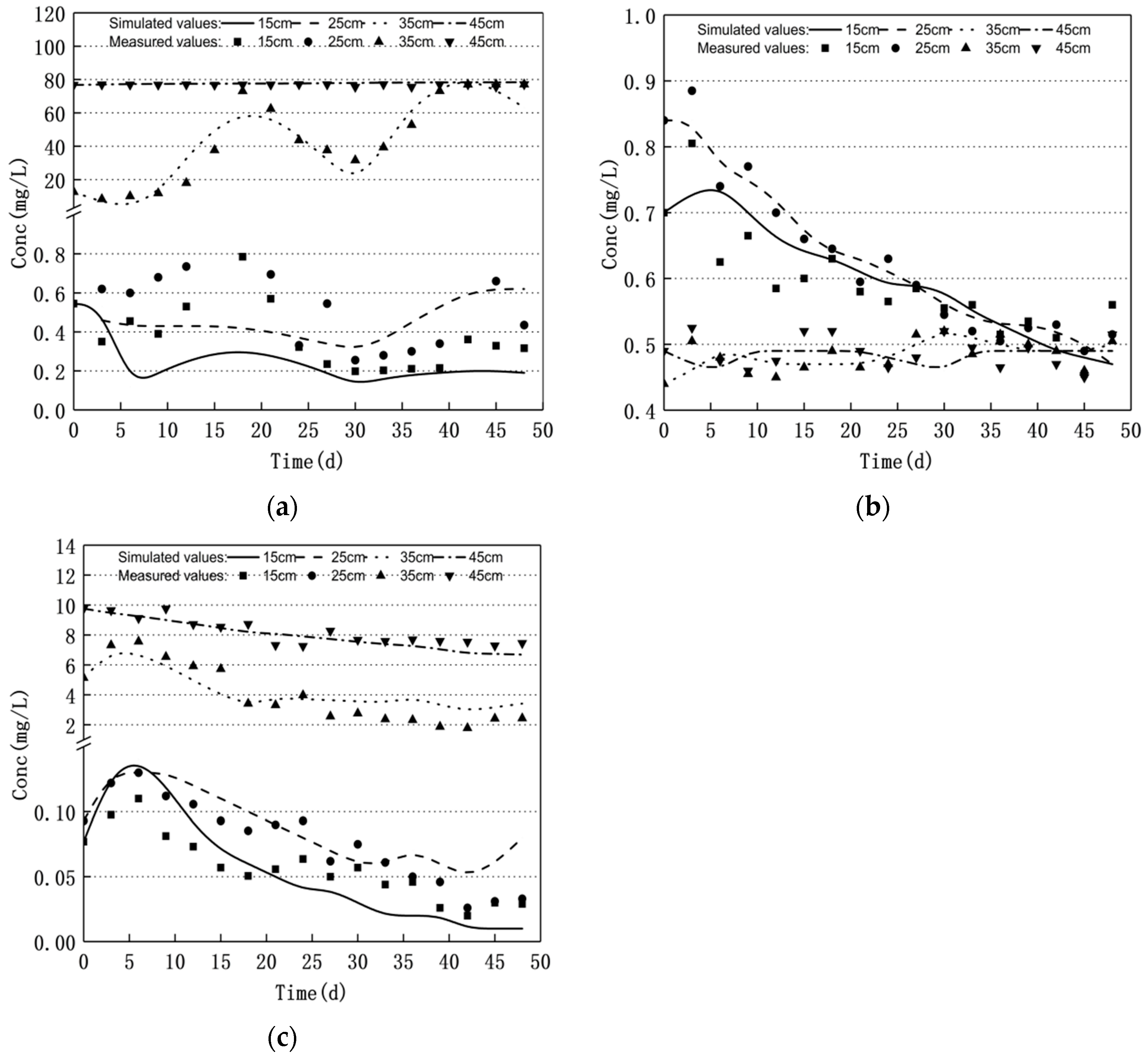
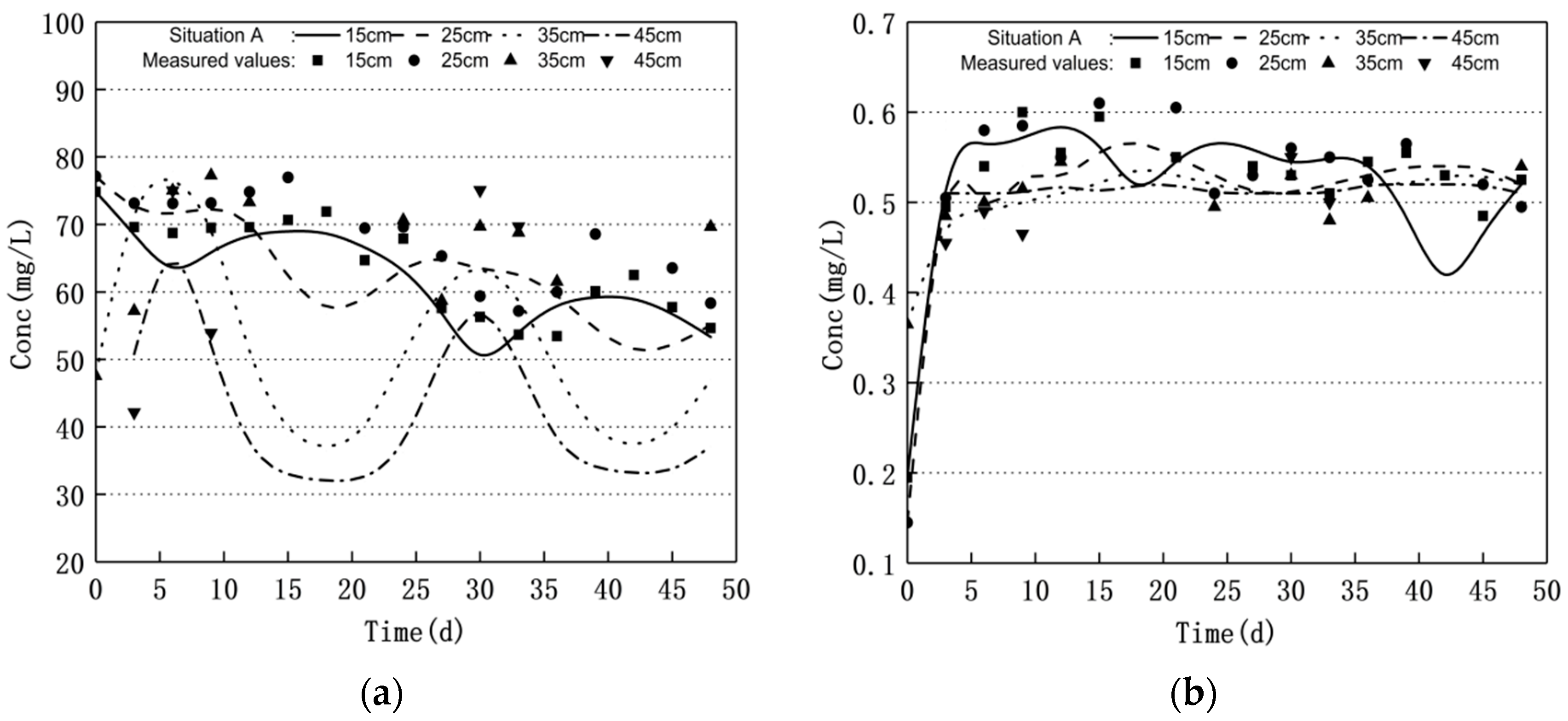
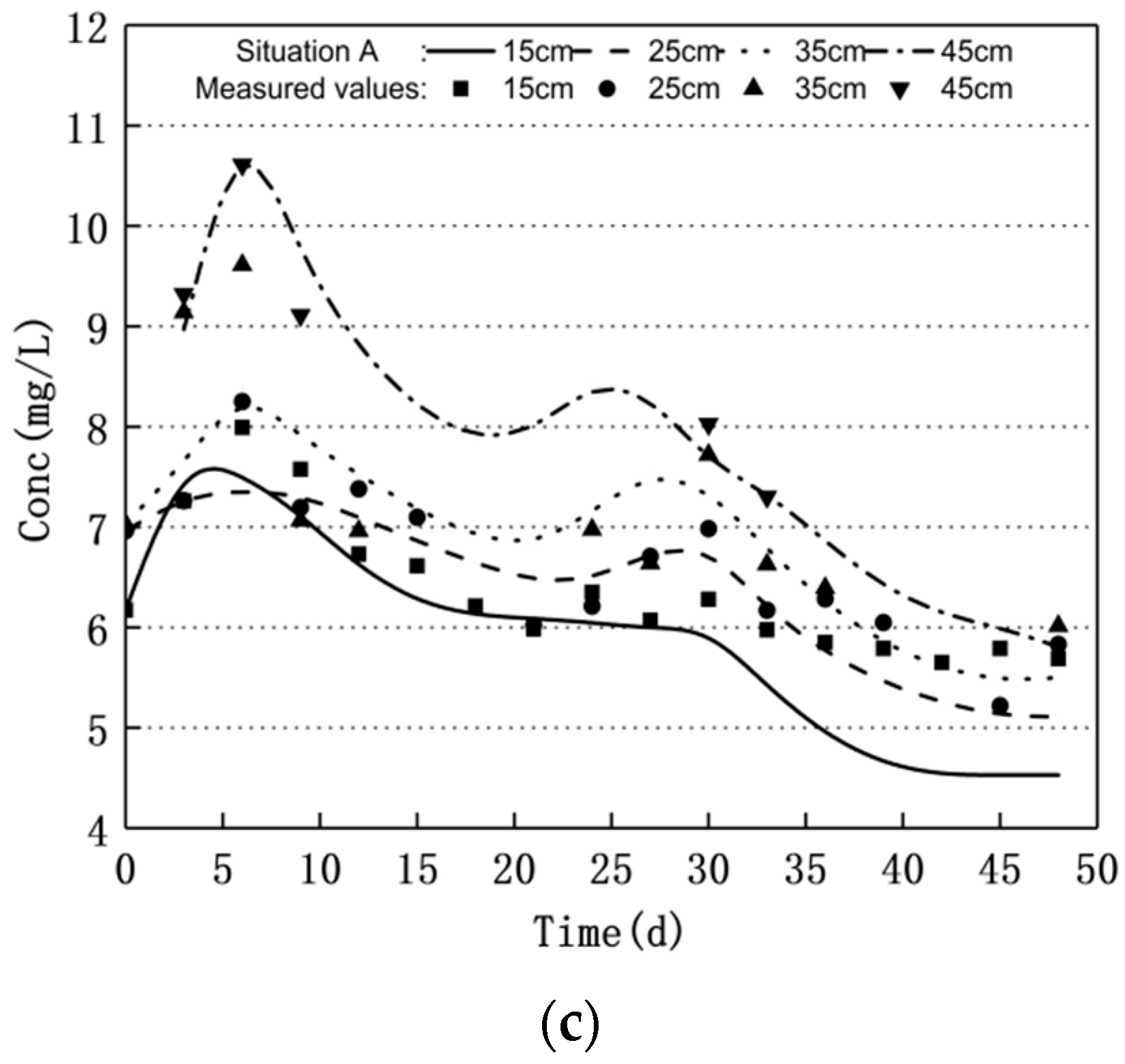
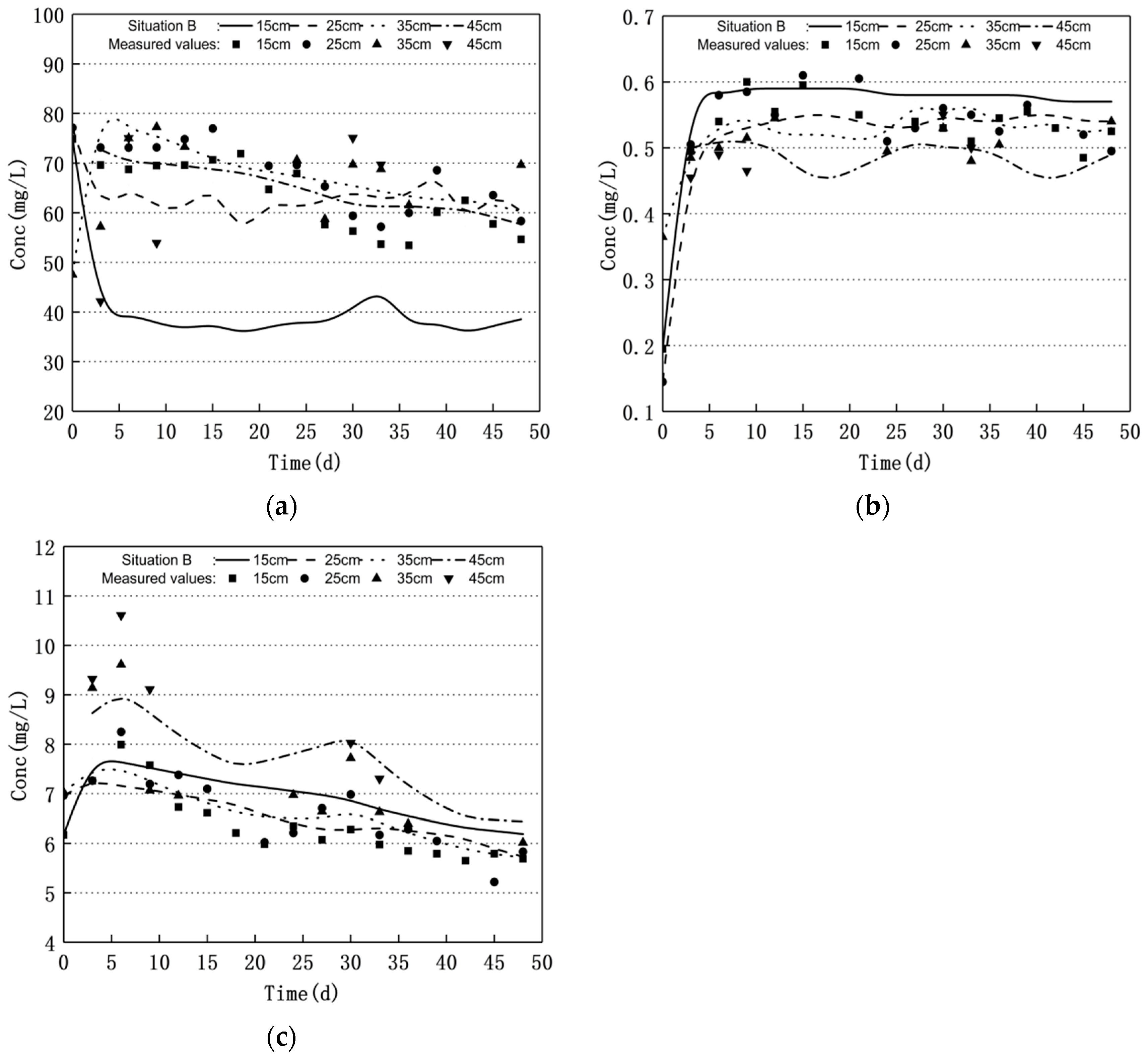
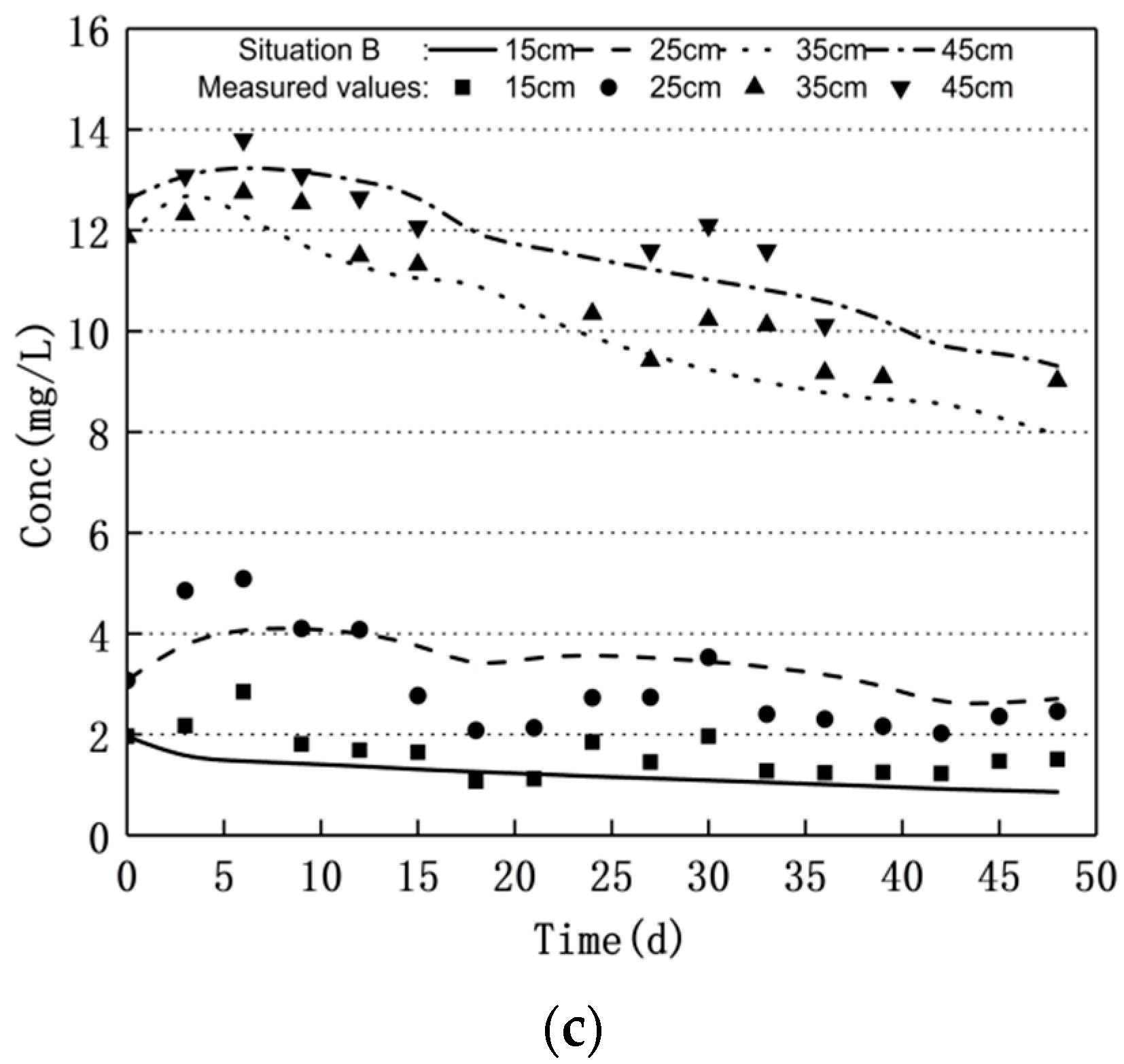
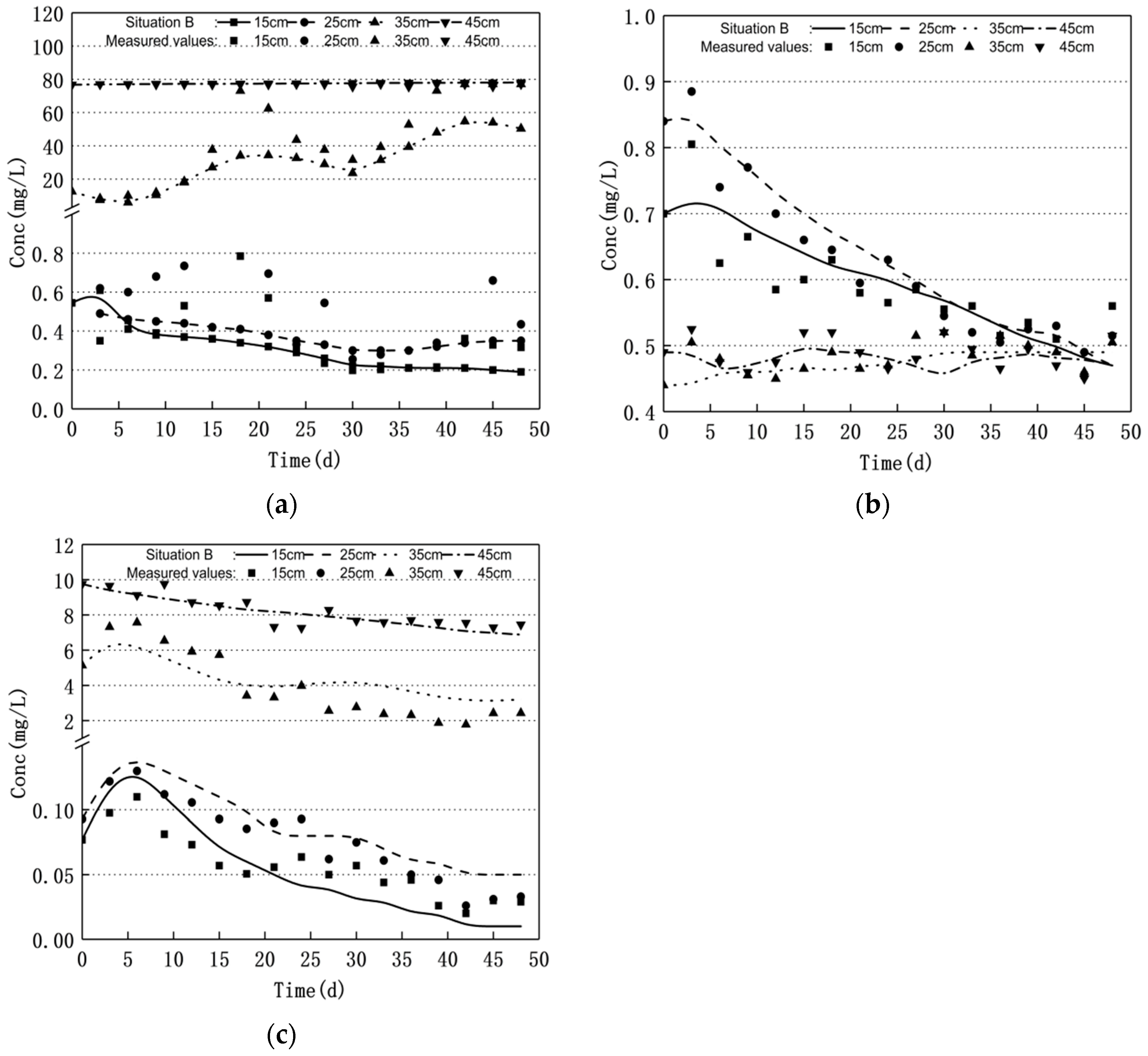
3.1. Nitrogen Changes and Model Validation
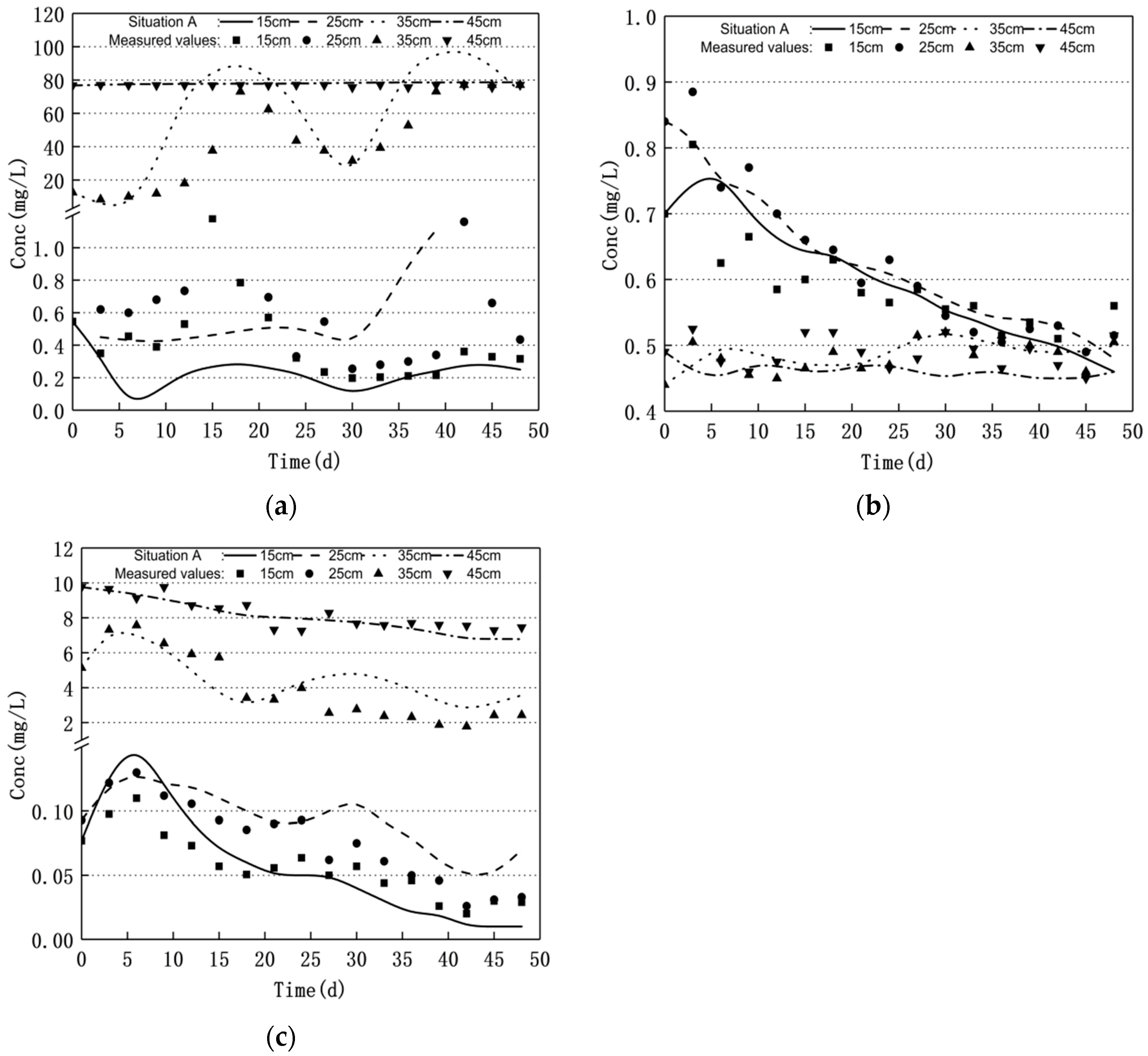
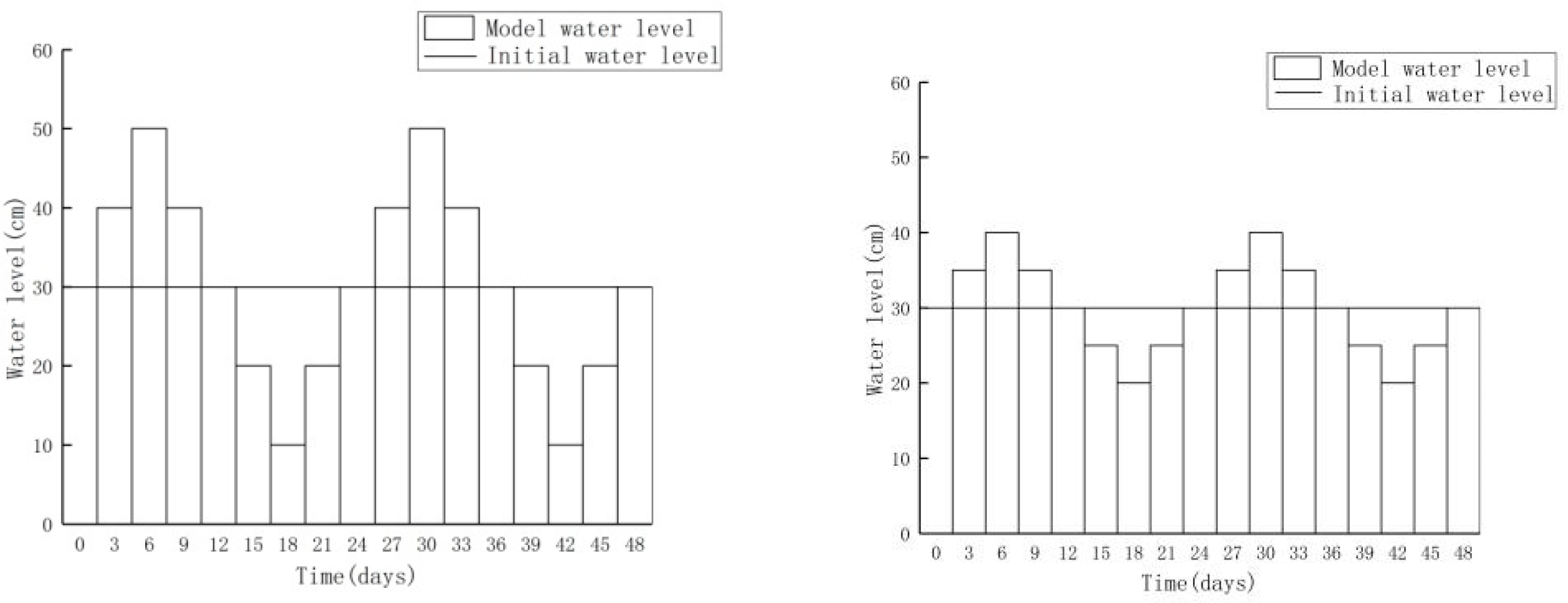
3.2. Scenario Simulation
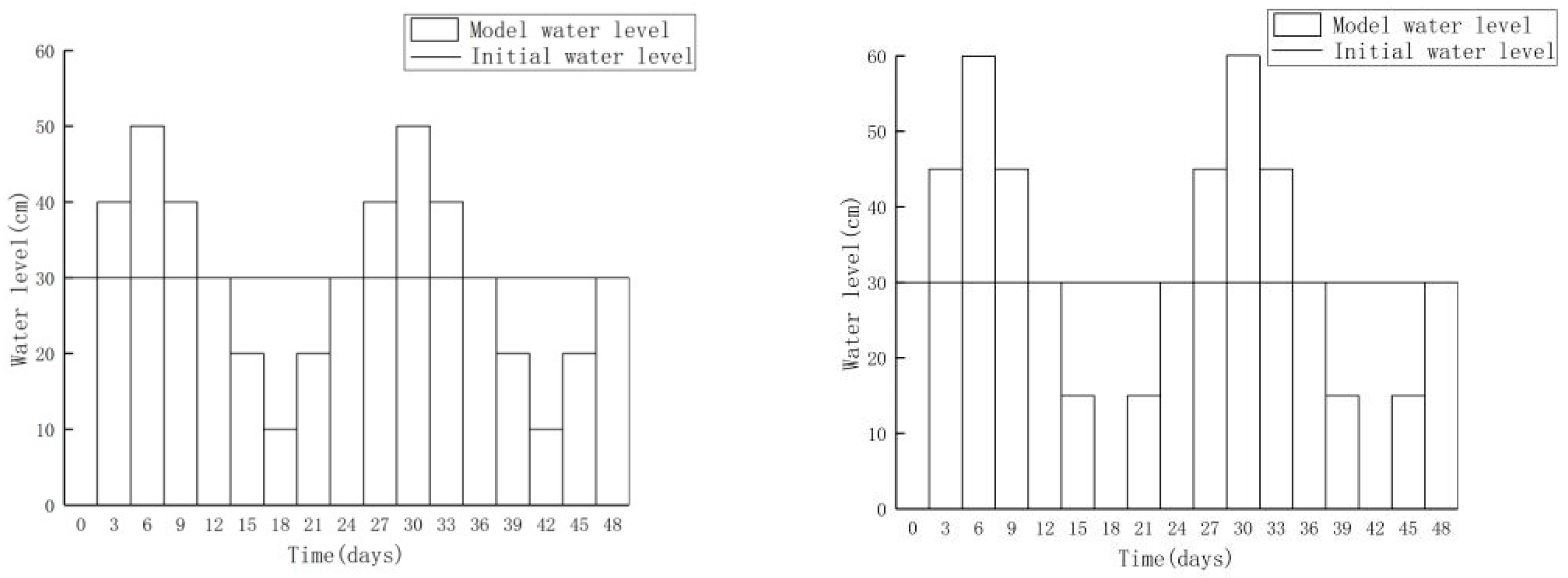
3.2.1. Increasing Water Level Fluctuation
3.2.2. Scenario of Reduced Water Level Fluctuation
4. Conclusions
- Groundwater level fluctuations can significantly affect the nitrogen transport and transformation patterns in soil–groundwater. The nitrate nitrogen concentration increased and the ammonium nitrogen mass concentration decreased when the water level decreased. Moreover, the nitrate nitrogen mass concentration decreased and the ammonium nitrogen mass concentration increased when the water level increased; the nitrite nitrogen did not change significantly.
- In this study, indoor soil column experiments were combined with the Hydrus-1D software simulation prediction to simulate the theoretical values of the tri-nitrogen transformation process in indoor soil columns. Although simple, the software does not take into account the existence of non-homogeneous changes in the soil column, is not accurate enough, there are certain limitations, and the influence of biological processes on the transformation of pollutants has not been taken into account. Furthermore, there is still a certain gap with the actual results.
- As an important factor of the hydrological mechanism, the water level plays a vital role in the groundwater system, and the effect of the level and intensity of fluctuation of the groundwater level on the migration and transformation of nitrogen cannot be ignored. The change in water level will affect the water content in the soil, the state of water movement, the physical and chemical properties of soil and the state of the microorganisms, which in turn, will affect the migration and transformation of nitrogen in the soil.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matiatos, I. Nitrate source identification in groundwater of multiple land-use areas by combining isotopes and multivariate statistical analysis: A case study of Asopos basin (Central Greece). Sci. Total Environ. 2016, 541, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Shan, J.; Yuan, Y.; Gao, Y.; Wu, R.; Yang, J. Comprehensive traceability of nitrogen pollution in the Shenfu section of Hunhe River Basin. Acta Sci. Circumstantiae 2018, 38, 2560–2567. [Google Scholar]
- van der Schans, M.L.; Harter, T.; Leijnse, A.; Mathews, M.C.; Meyer, R.D. Characterizing sources of nitrate leaching from an irrigated dairy farm in Merced County, California. J. Contam. Hydrol. 2009, 110, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, T.S.; Liptzin, D.; Dzurella, K.; Fryjoff-Hung, A.; Hollander, A.; Jensen, V.; King, A.; Kourakos, G.; McNally, A.; Pettygrove, G.S.; et al. Agriculture’s Contribution to Nitrate Contamination of Californian Groundwater (1945–2005). J. Environ. Qual. 2014, 43, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Meghdadi, A.; Javar, N. Quantification of spatial and seasonal variations in the proportional contribution of nitrate sources using a multi-isotope approach and Bayesian isotope mixing model. Environ. Pollut. 2018, 235, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Liu, S. Comprehensive evaluation of soybean meal replacement based on RIAM model. Feed. Res. 2020, 43, 126–130. [Google Scholar]
- Xiaoliang, J.; Xie, R.; Yun, H.; Lu, J. Quantitative identification of nitrate pollution sources and uncertainty analysis based on dual isotope ap-proach in an agricultural watershed. Environ. Pollut. 2017, 229, 586–594. [Google Scholar]
- Burow, K.R.; Nolan, B.T.; Rupert, M.G.; Dubrovsky, N.M. Nitrate in groundwater of the United States, 1991–2003. Environ. Sci. Technol. 2010, 44, 4988–4997. [Google Scholar] [CrossRef]
- Gu, B.; Ge, Y.; Chang, S.X.; Luo, W.; Chang, J. Nitrate in groundwater of China: Sources and driving forces. Glob. Environ. Chang. 2013, 23, 1112–1121. [Google Scholar] [CrossRef]
- Stuart, M.; Gooddy, D.; Bloomfield, J.; Williams, A. A review of the impact of climate change on future nitrate concentrations in groundwater of the UK. Sci. Total Environ. 2011, 409, 2859–2873. [Google Scholar] [CrossRef]
- Cheong, J.-Y.; Hamm, S.-Y.; Lee, J.-H.; Lee, K.-S.; Woo, N. Groundwater nitrate contamination and risk assessment in an agricultural area, South Korea. Environ. Earth Sci. 2011, 66, 1127–1136. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, C.; Du, L.; Liu, B.; An, Z. Investigation of nitrate content in groundwater of seven provinces (cities) around the Bohai Sea. Acta Agri-Environ. Sci. 2007, 2, 779–783. [Google Scholar]
- Zhao, Y.; Shao, M. Experimental study on nitrate-nitrogen migration in farmland under different fertilization conditions. J. Agric. Eng. 2002, 18, 37–40. [Google Scholar]
- Filippis, G.D.; Ercoli, L.; Rossetto, R. A Spatially Distributed, Physically-Based Modeling Approach for Estimating Agricultural Nitrate Leaching to Groundwater. Hydrology 2021, 8, 8. [Google Scholar] [CrossRef]
- Wild, L.M.; Mayer, B.; Einsiedl, F. Decadal Delays in Groundwater Recovery from Nitrate Contamination Caused by Low O2Reduction Rates. Water Resour. Res. 2018, 54, 9996. [Google Scholar] [CrossRef]
- European Commission. Establishing a Framework for the Community Action in the Field of Water Policy. In Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000; European Commission: Brussels, Belgium, 2000. [Google Scholar]
- Liu, M. Migration and Transformation of Petroleum Hydrocarbons in the Envelope Zone with Numerical Simulation; Jilin University: Jilin, China, 2014. [Google Scholar]
- Guo, H.M.; Wang, Y.X.; Li, Y.M. Analysis of arsenic enrichment factors in groundwater in the arsenic poisoning area of Shan Yin water. Environ. Sci. 2003, 4, 60–67. [Google Scholar]
- Sorensen, J.; Butcher, A.S.; Stuart, M.E.; Townsend, B.R. Nitrate fluctuations at the water table: Implications for recharge processes and solute transport in the Chalk aquifer. Hydrol. Process. 2015, 29, 3355–3367. [Google Scholar]
- Hefting, M.J.C.; Clément, D.D.; Cosandey, A.C.; Bernal, S.; Cimpian, C.; Tatur, A.; Burt, T.P.; Pinay, G. Water table elevation controls on soil nitrogen cycling in riparian wetlands along a European climatic gradient. Biogeochemistry 2004, 67, 113–134. [Google Scholar]
- Welch, H.L.; Green, C.; Coupe, R.H. The fate and transport of nitrate in shallow groundwater in northwestern Mississippi, USA. Appl. Hydrogeol. 2011, 19, 1239–1252. [Google Scholar] [CrossRef]
- Jurado, A.; Borges, A.V.; Brouyère, S. Dynamics and emissions of N2O in groundwater: A review. Sci. Total Environ. 2017, 584–585, 207–218. [Google Scholar]
- Yang, L.-P.; Li, N.; Zhang, J. Effect of pH on the transport and transformation of “tri-nitrogen” in shallow groundwater. Chin. Agron. Bull. 2017, 33, 56–60. [Google Scholar]
- Zhang, Z.; Furman, A. Redox dynamics at a dynamic capillary fringe for nitrogen cycling in a sandy column. J. Hydrol. 2021, 603, 26899. [Google Scholar] [CrossRef]
- Ashworth, D.J.; Shaw, G. Effects of moisture content and redox potential on in situ Kd values for radioiodine in soil. Sci. Total Environ. 2006, 359, 244–254. [Google Scholar] [CrossRef]
- Li, X.; Yang, T.; Bai, S.; Xi, B.; Zhu, X.; Yuan, Z.; Wei, Y.; Li, W. Research on the Influence of Groundwater Level Fluctuation on the Transport of Nitrogen in Vadose Zone. Agric. Environ. Sci. J. 2013, 32, 2443–2450. [Google Scholar]
- Zhang, D.; Fan, M.; Liu, H.; Wang, R.; Zhao, J.; Yang, Y.; Cui, R.; Chen, A. Effects of shallow groundwater table fluctuations on nitrogen in the groundwater and soil profile in the nearshore vegetable fields of Erhai Lake, southwest China. J. Soils Sediments 2019, 20, 42–51. [Google Scholar] [CrossRef]
- Xin, L.; Rui, Z.; Li, M.; Peng, L.; Zhiping, L.; Zhukun, H.; Qiao, L.; Jinsheng, W. Research on Nitrate Nitrogen (Nitrate) Pollution Changes in the Process of Groundwater Level Rising. China Environ. Sci. 2021, 41, 232–238. [Google Scholar]
- Jia, W.; Yin, L.; Zhang, M.; Zhang, J.; Zhang, X.; Gu, X.; Dong, J. Modified method for the estimation of groundwater evapotranspiration under very shallow water table con-ditions based on diurnal water table fluctuations. J. Hydrol. 2021, 597, 126193. [Google Scholar] [CrossRef]
- Kamon, M.; Endo, K.; Katsumi, T. Measuring the k–S–p relations on DNAPLs migration. Eng. Geol. 2003, 70, 64–65. [Google Scholar] [CrossRef]
- Nedrich, S.M.; Burton, G.A. Indirect effects of climate change on zinc cycling in sediments: The role of changing water levels. Environ. Toxicol. Chem. 2017, 36, 2456–2464. [Google Scholar] [CrossRef]
- Davis, G.B.; Rayner, J.L.; Trefry, M.G.; Fisher, S.J.; Patterson, B.M. Measurement and Modeling of Temporal Variations in Hydrocarbon Vapor Behavior in a Layered Soil Profile. Vadose Zone J. 2005, 4, 265–273. [Google Scholar] [CrossRef]
- Kamon, M.; Li, Y.; Endo, K.; Inui, T.; Katsumi, T. Experimental Study on the Measurement of S-p Relations of LNAPL in a Porous Medium. Soils Found. 2007, 47, 33–45. [Google Scholar] [CrossRef][Green Version]
- Xie, X.; Li, Y.; Xia, B.; Su, Y.; Gu, Q. Study on the hysteresis of saturation-capillary pressure relationship in sand media under fluctuating water level. Acta Pedol. Sin. 2011, 48, 286–294. [Google Scholar]
- Li, X.; Li, J.; Xi, B.; Yuan, Z.; Zhu, X.; Zhang, X. Effects of groundwater level variations on the nitrate content of groundwater: A case study in Luoyang area, China. Environ. Earth Sci. 2015, 74, 3969–3983. [Google Scholar] [CrossRef]
- Chen, A.; Lei, B.; Hu, W.; Wang, H.; Dan, Z. Temporal-spatial variations and influencing factors of nitrogen in the shallow groundwater of the nearshore vegetable field of Erhai Lake, China. Environ. Ence Pollut. Res. 2017, 25, 4858–4870. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, C.E.; Voegelin, A.; Hering, J.G. Manganese oxidation induced by water table fluctuations in a sand column. Environ. Sci. Technol. 2012, 46, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y. Research on Soil Pollutant Transport Model Considering Groundwater Level Fluctuations. Master’s Thesis, Hebei Agricultural University, Baoding, China, 3 June 2015. [Google Scholar]
- Zhang, X.; Wang, P.; Wang, T.; Yu, J.; Liu, X. Hydrochemical characteristics of shallow groundwater in Ejina Oasis and the relationship between water level and depth under water transport conditions. South-to-North Water Divers. Water Conserv. Sci. Technol. 2019, 17, 86–94. [Google Scholar]
- Cao, W.; Yang, H.; Gao, Y.; Nan, T.; Wang, Z.; Xu, S. Prediction of groundwater quality evolution in Baoding Plain in the wa-ter-receiving area of the Middle Route of the South-to-North Water Transfer Project. J. Hydraul. Eng. 2020, 51, 924–935. [Google Scholar]
- Tafteh, A.; Sepaskhah, A.R. Application of HYDRUS-1D model for simulating water and nitrate leaching from continuous and alternate furrow irrigated rapeseed and maize fields. Agric. Water Manag. 2012, 113, 19–29. [Google Scholar] [CrossRef]
- Mo, X.; Peng, H.; Xin, J.; Wang, S. Analysis of urea nitrogen leaching under high-intensity rainfall using HYDRUS-1D. J. Environ. Manag. 2022, 312, 114900. [Google Scholar] [CrossRef]
- Chang, C.; Entz, T. Nitrate Leaching Losses under Repeated Cattle Feedlot Manure Applications in Southern Alberta. J. Environ. Qual. 1996, 25, 145–153. [Google Scholar] [CrossRef]
- Steffy, D.A.; Johnston, C.D.; Barry, D.A. Numerical Simulations and Long-Column Tests of LNAPL Displacement and Trapping by a Fluctuating Water Table. J. Soil Contam. 1998, 7, 325–356. [Google Scholar] [CrossRef]
- Marshall, H.W.H. Ecology of Water-Level Manipulations on a Northern Marsh. Ecology 1963, 44, 331–343. [Google Scholar]
- Wang, Y. Ammonia Nitrogen Adsorption-Desorption Characteristics of Sediments of Different Sizes in the Xiliao River. Master’s Thesis, Liaoning Technical University, Liaoning, China, 15 September 2012. [Google Scholar]
- Sasikala, S.; Tanaka, N.; Wah, H.W.; Jinadasa, K. Effects of water level fluctuation on radial oxygen loss, root porosity, and nitrogen removal in subsurface vertical flow wetland mesocosms. Ecol. Eng. 2009, 35, 410–417. [Google Scholar] [CrossRef]






| Serial Number | Sample | Particle Size (mm) | pH | Organic Matter Content (g·kg−1) |
|---|---|---|---|---|
| 1 | Coarse sand | 0.5–1.0 | 8.5 | 0.241 |
| 2 | Medium sand | 0.25–0.5 | 8.8 | 0.587 |
| 3 | Fine sand | 0.125–0.25 | 9.3 | 1.070 |
| Intake | Coarse Sand | Medium Sand | Fine Sand | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Nitrite Nitrogen | Nitrate | Ammonium Nitrogen | Nitrite Nitrogen | Nitrate | Ammonium Nitrogen | Nitrite Nitrogen | Nitrate | Ammonium Nitrogen | |
| 15 cm | 0.19 | 74.85 | 6.17 | 0.37 | 35.81 | 1.97 | 0.70 | 0.55 | 0.08 |
| 25 cm | 0.15 | 77.14 | 6.97 | 0.27 | 36.47 | 3.07 | 0.84 | 2.20 | 0.09 |
| 35 cm | 0.37 | 47.56 | 7.03 | 0.36 | 78.39 | 11.87 | 0.44 | 12.61 | 5.14 |
| 45 cm | 0.33 | 45.25 | 8.23 | 0.25 | 84.41 | 12.61 | 0.49 | 76.77 | 9.76 |
| Parameter | θr Residual Volume Water Content | θs Saturated Volume of Water Content | α/cm−1 Soil Moisture Characteristic Parameters | n Soil Moisture Characteristic Index | Ks/(cm·d−1) Saturated Hydraulic Conductivity |
|---|---|---|---|---|---|
| Coarse sand | 0.045 | 0.43 | 0.1450 | 2.68 | 712.8 |
| Medium sand | 0.051 | 0.42 | 0.1045 | 2.08 | 550.0 |
| Fine sand | 0.057 | 0.41 | 0.1240 | 2.28 | 350.2 |
| Medium | Solute | ρ/mg·cm−3 Bulk Density | Disp/cm Longitudinal Diffusion | D/cm2·d−1 Solute Diffusion Coefficient | Kd/cm3·mg−1 Adsorption Coefficient |
|---|---|---|---|---|---|
| Coarse sand | Nitrite Nitrogen | 1600 | 1.568 | 1.29085 | 0.000256 |
| Nitrate | 1.69085 | 0.001584 | |||
| Ammonium Nitrogen | 1.69085 | 0.008779 | |||
| Medium sand | Nitrite Nitrogen | 1800 | 1.233 | 1.29085 | 0.000103 |
| Nitrate | 1.69085 | 0.000413 | |||
| Ammonium Nitrogen | 1.69085 | 0.008895 | |||
| Fine sand | Nitrite Nitrogen | 1800 | 1.116 | 1.29085 | 0.000767 |
| Nitrate | 1.69085 | 0.003094 | |||
| Ammonium Nitrogen | 1.69085 | 0.008976 |
| Soil Media | Pollutants | Decisive Factor R2 | RMSE |
|---|---|---|---|
| Coarse sand | Nitrite nitrogen | 0.71284 | 0.0883 |
| Nitrate nitrogen | 0.70572 | 5.4257 | |
| Ammonia nitrogen | 0.82099 | 0.4863 | |
| Medium sand | Nitrite nitrogen | 0.74610 | 0.0486 |
| Nitrate nitrogen | 0.80656 | 9.1317 | |
| Ammonia nitrogen | 0.98810 | 0.5115 | |
| Fine sand | Nitrite nitrogen | 0.87036 | 0.0309 |
| Nitrate nitrogen | 0.98398 | 2.7137 | |
| Ammonia nitrogen | 0.97552 | 0.3860 |
| Observation Hole | Coarse Sand | Medium Sand | Fine Sand | ||||
|---|---|---|---|---|---|---|---|
| Fluctuation Experiment | Situation A | Fluctuation Experiment | Situation A | Fluctuation Experiment | Situation A | ||
| Nitrite nitrogen | 15 cm | 0.0249 | 0.0488 | 0.0348 | 0.0384 | 0.0838 | 0.0896 |
| 25 cm | 0.0197 | 0.0262 | 0.0141 | 0.0168 | 0.1037 | 0.0924 | |
| 35 cm | 0.0159 | 0.0135 | 0.0261 | 0.0263 | 0.0166 | 0.0159 | |
| 45 cm | 0.0276 | 0.0047 | 0.0255 | 0.0328 | 0.0107 | 0.0072 | |
| Nitrate | 15 cm | 5.4152 | 6.2294 | 16.0273 | 22.4563 | 0.0921 | 0.0807 |
| 25 cm | 4.2269 | 6.9961 | 12.8282 | 15.5376 | 0.0932 | 0.3326 | |
| 35 cm | 5.2402 | 14.0076 | 3.8663 | 4.4202 | 24.2231 | 31.5546 | |
| 45 cm | 9.5118 | 11.4918 | 1.9142 | 6.0021 | 0.417 | 0.4754 | |
| Ammonium Nitrogen | 15 cm | 0.7797 | 1.0168 | 0.2074 | 0.2113 | 0.0422 | 0.0441 |
| 25 cm | 0.5452 | 0.7755 | 0.7561 | 0.9278 | 0.027 | 0.0248 | |
| 35 cm | 0.6154 | 0.875 | 1.4036 | 1.8423 | 1.2271 | 1.3702 | |
| 45 cm | 1.0365 | 1.4005 | 1.1279 | 0.8256 | 0.8747 | 0.8622 | |
| Observation Hole | Coarse Sand | Medium Sand | Fine Sand | ||||
|---|---|---|---|---|---|---|---|
| Fluctuation Experiment | Situation B | Fluctuation Experiment | Situation B | Fluctuation Experiment | Situation B | ||
| Nitrite Nitrogen | 15 cm | 0.0249 | 0.0063 | 0.0348 | 0.0316 | 0.0838 | 0.0758 |
| 25 cm | 0.0197 | 0.0143 | 0.0141 | 0.0099 | 0.1037 | 0.1139 | |
| 35 cm | 0.0159 | 0.0183 | 0.0261 | 0.0266 | 0.0166 | 0.0148 | |
| 45 cm | 0.0276 | 0.0206 | 0.0255 | 0.0219 | 0.0107 | 0.0112 | |
| Nitrate | 15 cm | 5.4152 | 2.1756 | 16.0273 | 4.0429 | 0.0921 | 0.1069 |
| 25 cm | 4.2269 | 3.337 | 12.8282 | 8.1699 | 0.0932 | 0.0601 | |
| 35 cm | 5.2402 | 6.0552 | 3.8663 | 2.4465 | 24.2231 | 15.1679 | |
| 45 cm | 9.5118 | 4.4877 | 1.9142 | 2.658 | 0.417 | 0.3406 | |
| Ammonium Nitrogen | 15 cm | 0.7797 | 0.4898 | 0.2074 | 0.2042 | 0.0422 | 0.0391 |
| 25 cm | 0.5452 | 0.4511 | 0.7561 | 0.4871 | 0.027 | 0.029 | |
| 35 cm | 0.6154 | 0.5448 | 1.4036 | 1.4785 | 1.2271 | 1.0044 | |
| 45 cm | 1.0365 | 0.7817 | 1.1279 | 1.3127 | 0.8747 | 0.7587 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, L.; Zou, X.; Qu, J.; Bai, G. Experimental and Simulation Research on the Process of Nitrogen Migration and Transformation in the Fluctuation Zone of Groundwater Level. Appl. Sci. 2022, 12, 3742. https://doi.org/10.3390/app12083742
Li Y, Wang L, Zou X, Qu J, Bai G. Experimental and Simulation Research on the Process of Nitrogen Migration and Transformation in the Fluctuation Zone of Groundwater Level. Applied Sciences. 2022; 12(8):3742. https://doi.org/10.3390/app12083742
Chicago/Turabian StyleLi, Yuepeng, Liuyue Wang, Xun Zou, Jihong Qu, and Gang Bai. 2022. "Experimental and Simulation Research on the Process of Nitrogen Migration and Transformation in the Fluctuation Zone of Groundwater Level" Applied Sciences 12, no. 8: 3742. https://doi.org/10.3390/app12083742
APA StyleLi, Y., Wang, L., Zou, X., Qu, J., & Bai, G. (2022). Experimental and Simulation Research on the Process of Nitrogen Migration and Transformation in the Fluctuation Zone of Groundwater Level. Applied Sciences, 12(8), 3742. https://doi.org/10.3390/app12083742





