Synthesis and Biological Activity of 1,3,4-Oxadiazoles Used in Medicine and Agriculture
Abstract
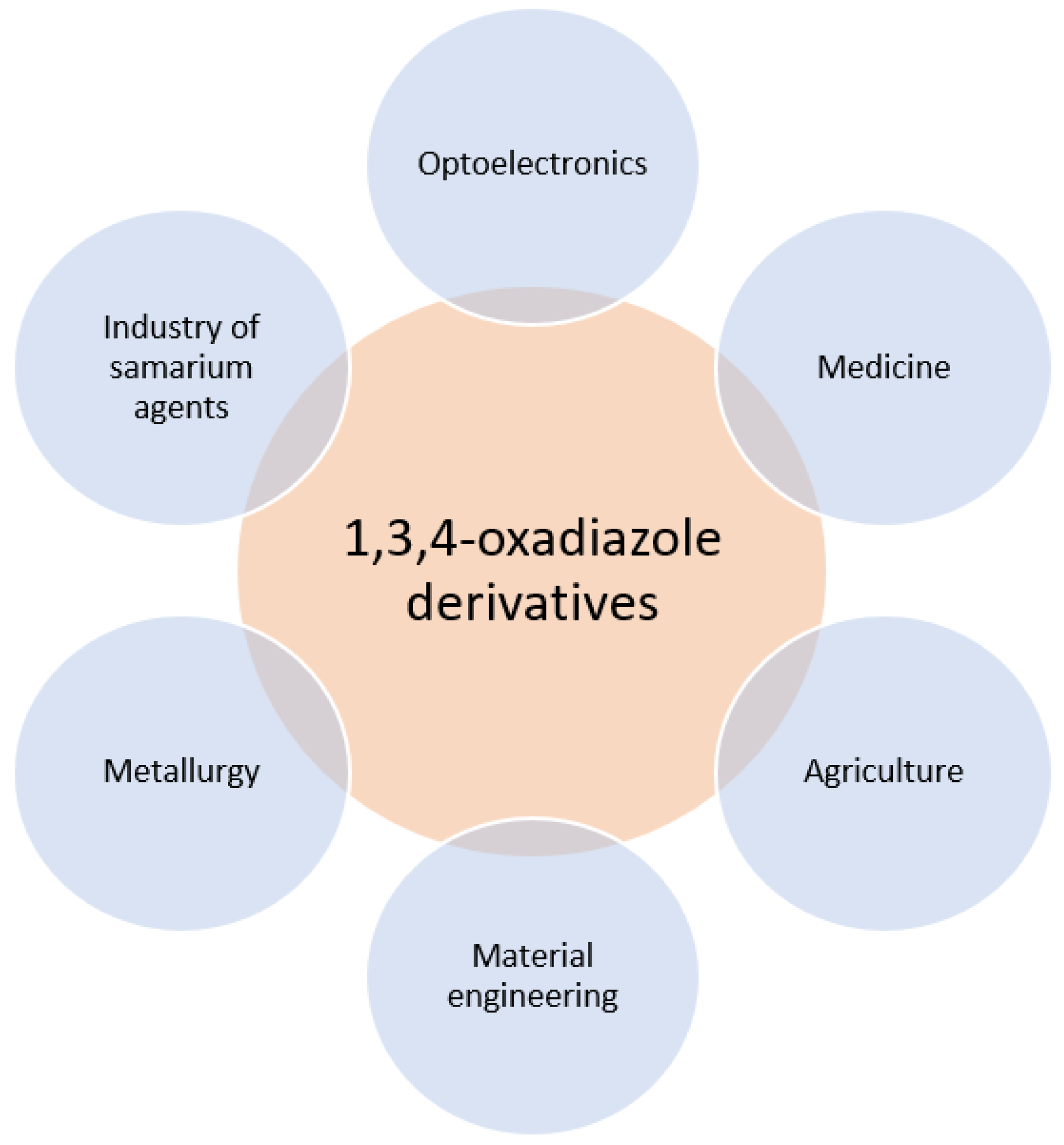
:1. Introduction
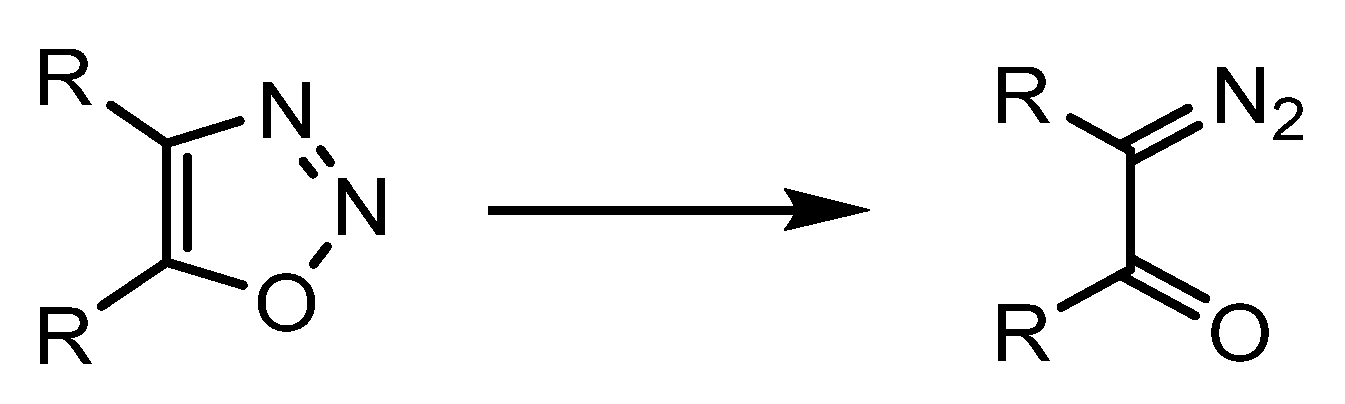
2. Chemistry of Oxadiazoles
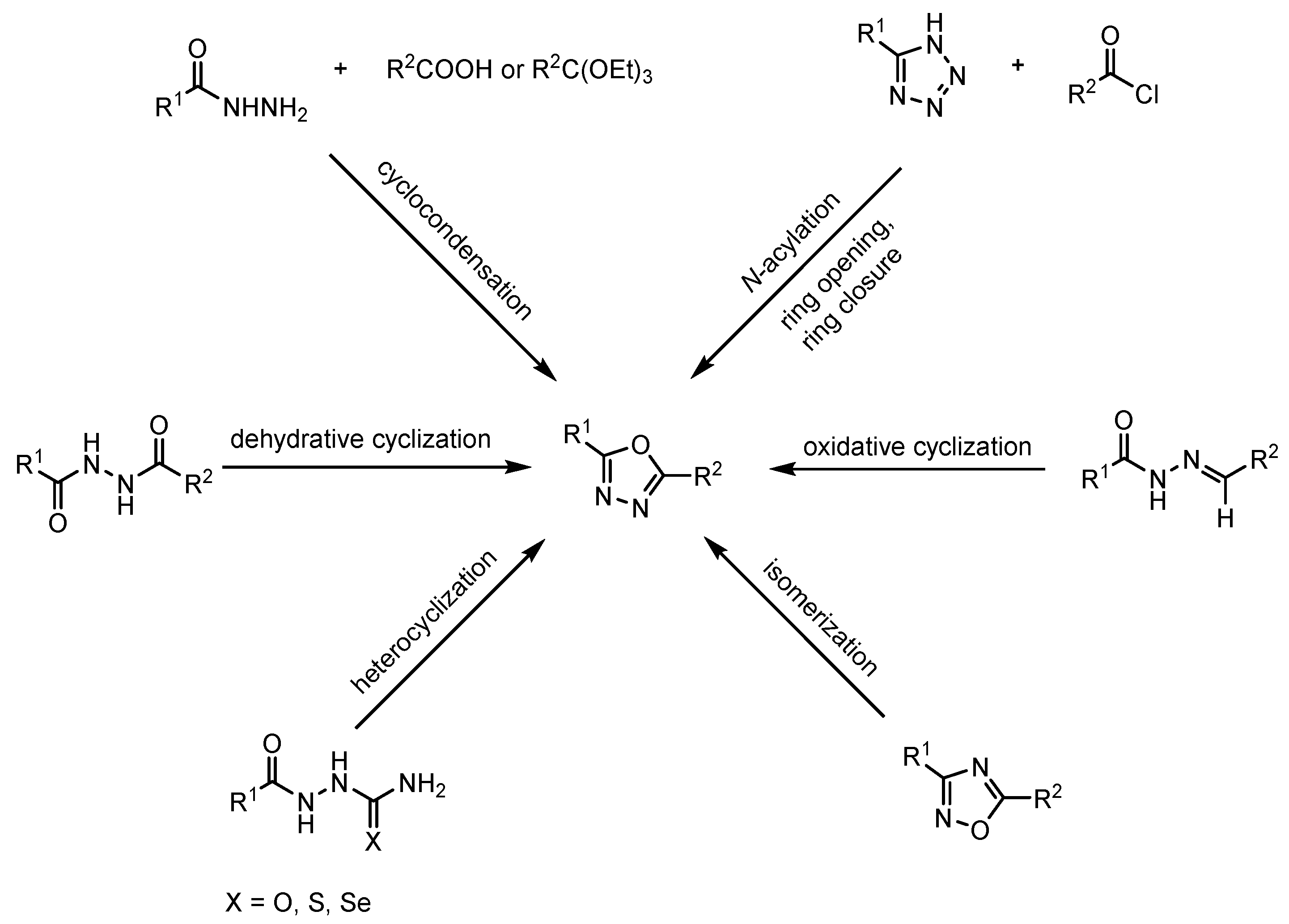
3. General Methods for the Synthesis of 1,3,4-Oxadiazoles
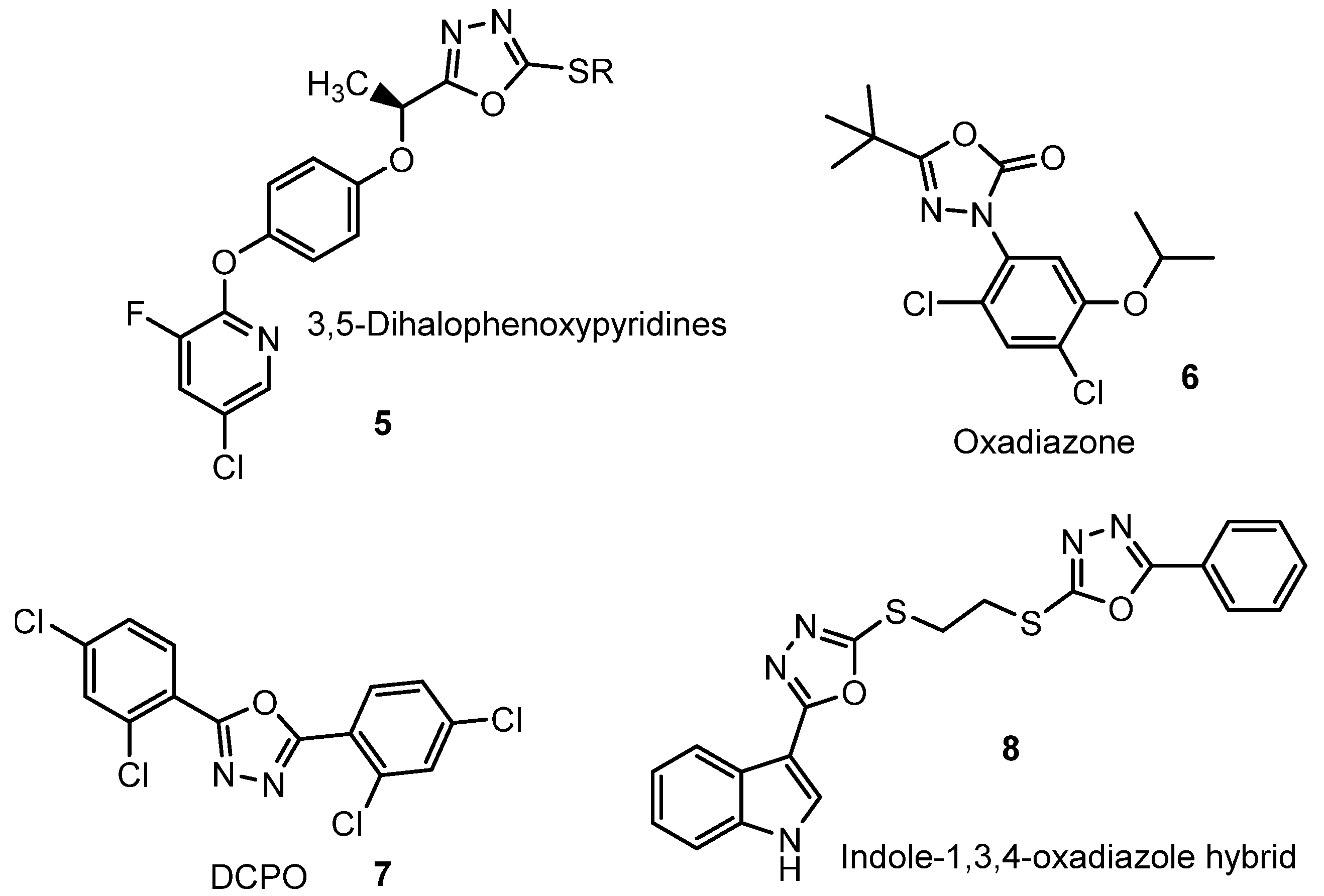
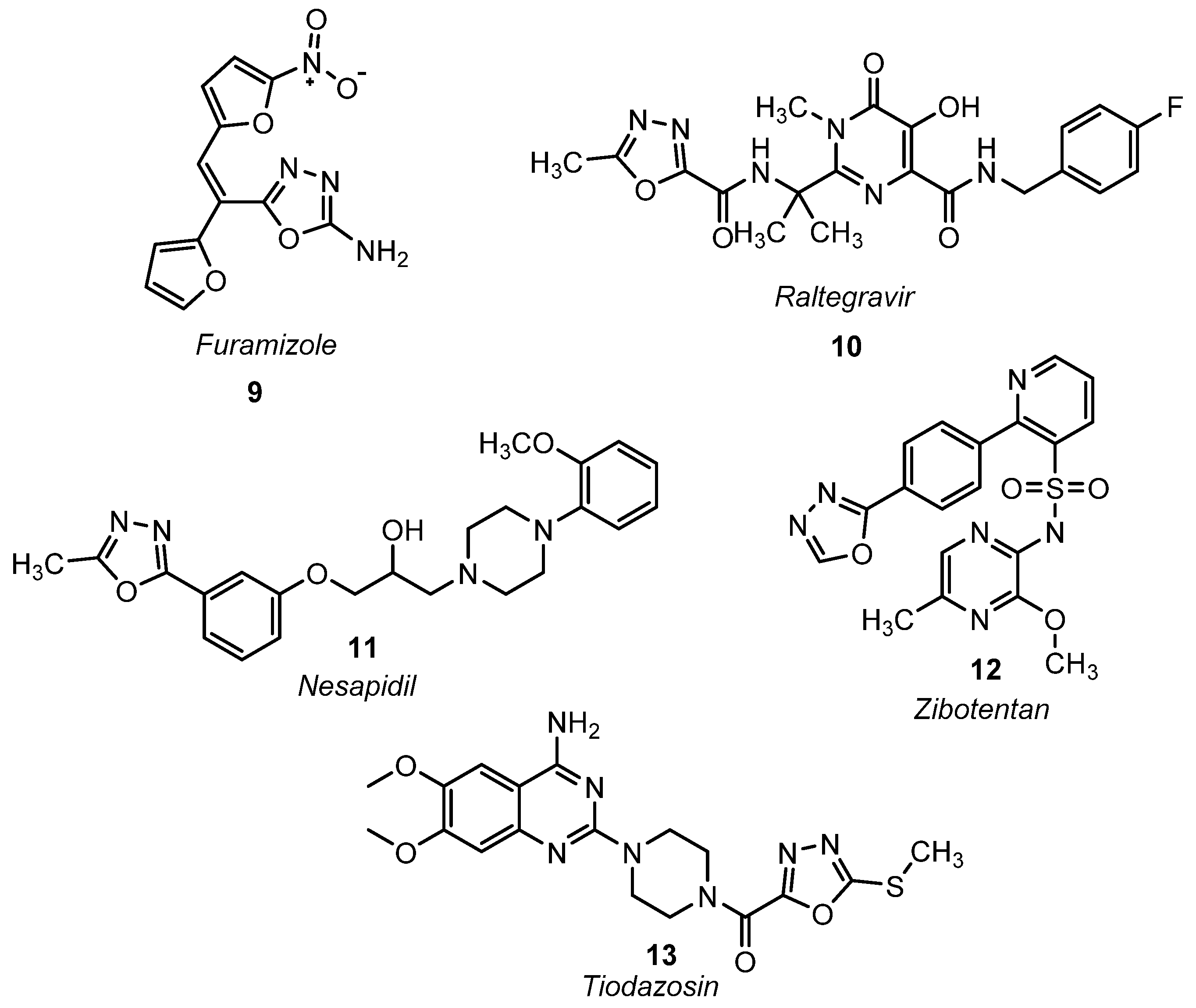
4. 1,3,4-Oxadiazoles Used Commercially in Agriculture and Medicine
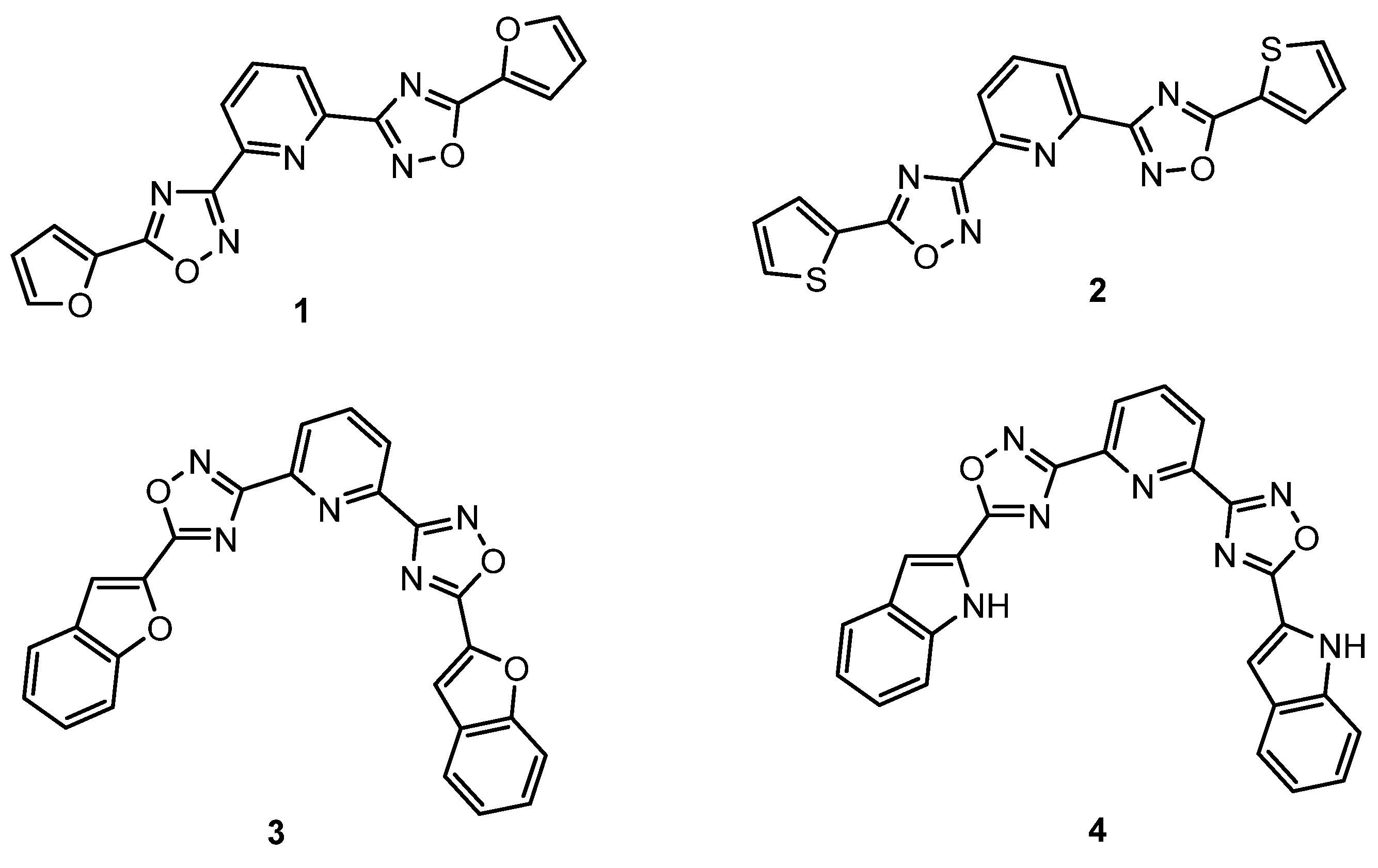
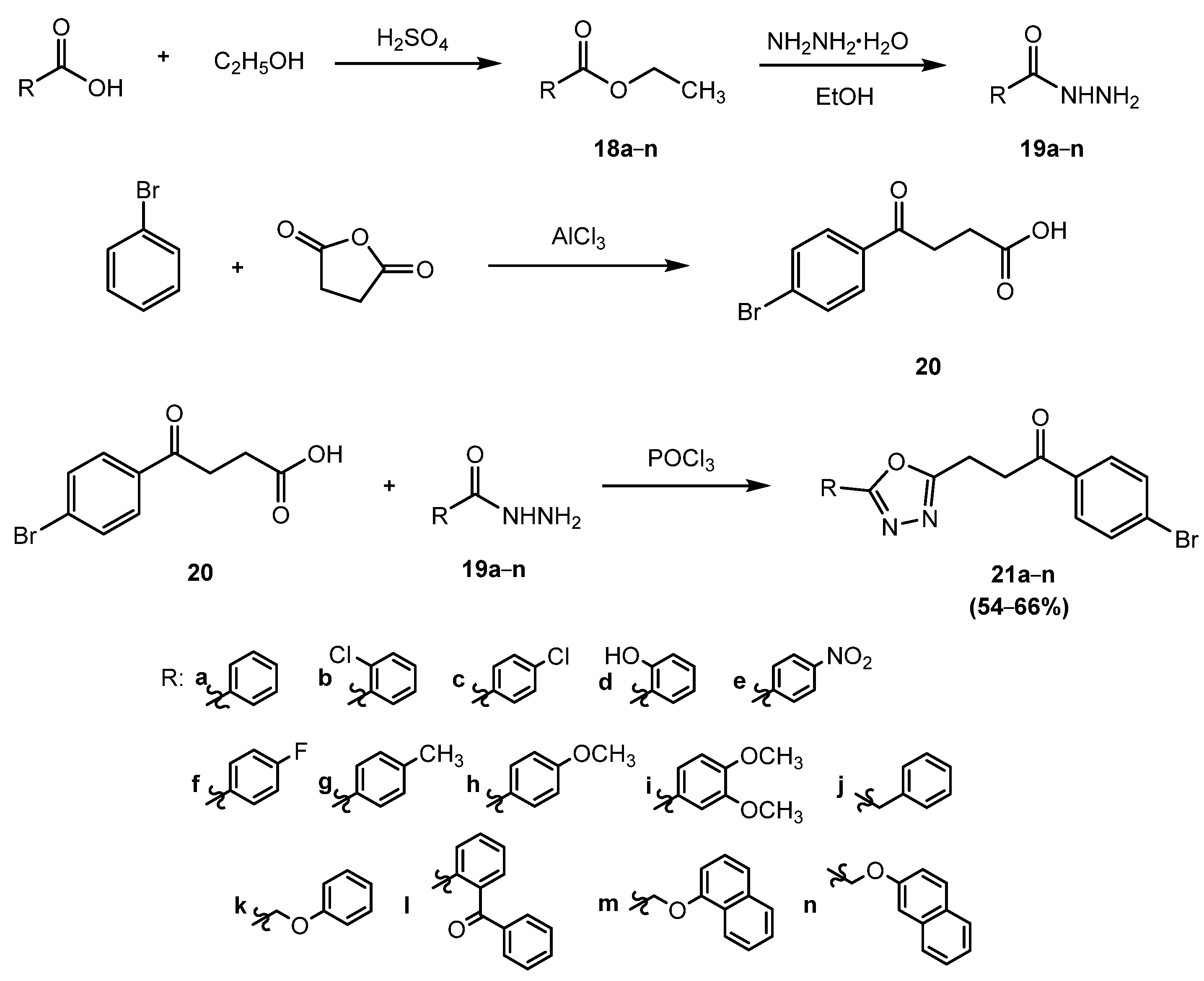
5. Investigation of the Synthesis of New, Biologically Active 1,3,4-Oxadiazoles
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xuan, T.D.; Elzaawely, A.A.; Fukuta, M.; Tawata, S. Herbicidal and fungicidal activities of lactones in Kava (Piper methysticum). J. Agric. Food Chem. 2006, 54, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Yajima, W.; Rahman, M.H.; Das, D.; Suresh, M.R.; Kav, N.N.V. Detection of Sclerotinia sclerotiorum Using a Monomeric and Dimeric Single-Chain Fragment Variable (scFv) Antibody. J. Agric. Food Chem. 2008, 56, 9455–9463. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Henk, D.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef] [PubMed]
- MacLean, D.E.; Lobo, J.M.; Coles, K.; Harding, M.W.; May, W.E.; Peng, G.; Turkington, T.K.; Kutcher, H.R. Fungicide application at anthesis of wheat provides effective control of leaf spotting diseases in western Canada. Crop Prot. 2018, 112, 343–349. [Google Scholar] [CrossRef]
- Obydennov, K.L.; Khamidullina, L.A.; Galushchinskiy, A.N.; Shatunova, S.A.; Kosterina, M.F.; Kalinina, T.A.; Fan, Z.; Glukhareva, T.V.; Morzherin, Y.Y. Discovery of Methyl (5 Z)-[2-(2,4,5-Trioxopyrrolidin-3-ylidene)-4-oxo-1,3-thiazolidin-5-ylidene]acetates as Antifungal Agents against Potato Diseases. J. Agric. Food Chem. 2018, 66, 6239–6245. [Google Scholar] [CrossRef]
- Fungal Disease Frequency. Available online: https://gaffi.org/why/fungal-disease-frequency/ (accessed on 4 April 2022).
- Wu, Y.Y.; Shao, W.B.; Zhu, J.J.; Long, Z.Q.; Liu, L.W.; Wang, P.Y.; Li, Z.; Yang, S. Novel 1,3,4-Oxadiazole-2-carbohydrazides as Prospective Agricultural Antifungal Agents Potentially Targeting Succinate Dehydrogenase. J. Agric. Food Chem. 2019, 67, 13892–13903. [Google Scholar] [CrossRef] [PubMed]
- Akhter, M.; Husain, A.; Azad, B.; Ajmal, M. Aroylpropionic acid based 2,5-disubstituted-1,3,4-oxadiazoles: Synthesis and their anti-inflammatory and analgesic activities. Eur. J. Med. Chem. 2009, 44, 2372–2378. [Google Scholar] [CrossRef]
- Ahsan, M.J.; Samy, J.G.; Khalilullah, H.; Nomani, M.S.; Saraswat, P.; Gaur, R.; Singh, A. Molecular properties prediction and synthesis of novel 1,3,4-oxadiazole analogues as potent antimicrobial and antitubercular agents. Bioorganic Med. Chem. Lett. 2011, 21, 7246–7250. [Google Scholar] [CrossRef]
- Albratty, M.; El-Sharkawy, K.A.; Alhazmi, H.A. Synthesis and evaluation of some new 1,3,4-oxadiazoles bearing thiophene, thiazole, coumarin, pyridine and pyridazine derivatives as antiviral agents. Acta Pharm. 2019, 69, 261–276. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Li, Z.; Han, Y. Synthesis and fungicidal activity against Rhizoctonia solani of 2-alkyl (alkylthio)-5-pyrazolyl-1,3,4-oxadiazoles (thiadiazoles). J. Agric. Food Chem. 2000, 48, 5312–5315. [Google Scholar] [CrossRef]
- Ahsan, M.J.; Choupra, A.; Sharma, R.K.; Jadav, S.S.; Padmaja, P.; Hassan, M.; Al-Tamimi, A.; Geesi, M.H.; Bakht, M.A. Rationale Design, Synthesis, Cytotoxicity Evaluation, and Molecular Docking Studies of 1,3,4-oxadiazole Analogues. Anticancer. Agents Med. Chem. 2017, 18, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Lelyukh, M.; Martynets, M.; Kalytovska, M.; Drapak, I.; Harkov, S.; Chaban, T.; Chaban, I.; Matiychuk, V. Approaches for synthesis and chemical modification of non-condensed heterocyclic systems based on 1,3,4-oxadiazole ring and their biological activity: A review. J. Appl. Pharm. Sci. 2020, 10, 151–165. [Google Scholar]
- Glomb, T.; Świątek, P. Antimicrobial Activity of 1,3,4-Oxadiazole Derivatives. Int. J. Mol. Sci. 2021, 22, 6979. [Google Scholar] [CrossRef] [PubMed]
- Wróblowska, M.; Kudelko, A.; Kuźnik, N.; Łaba, K.; Łapkowski, M. Synthesis of Extended 1,3,4-Oxadiazole and 1,3,4-Thiadiazole Derivatives in the Suzuki Cross-coupling Reactions. J. Heterocycl. Chem. 2017, 54, 1550–1557. [Google Scholar] [CrossRef]
- Bhujabal, Y.B.; Vadagaonkar, K.S.; Kapdi, A.R. Pd/PTABS: Catalyst for Efficient C−H (Hetero)Arylation of 1,3,4-Oxadiazoles Using Bromo(Hetero)Arenes. Asian J. Org. Chem. 2019, 8, 289–295. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, Z.; He, T. Conducting probe atomic force microscopy investigation of anisotropic charge transport in solution cast PBD single crystals induced by an external field. J. Phys. Chem. B 2004, 108, 19198–19204. [Google Scholar] [CrossRef]
- Damaceanu, M.D.; Rusu, R.D.; Bruma, M. Polymers containing 1,3,4-oxadiazoles rings for advanced materials. Mem. Sci. Sect. Rom. Acad. 2011, 34, 1–24. [Google Scholar]
- Ajay Kumar, K.; Jayaroopa, P.; Vasanth Kumar, G. Comprehensive review on the chemistry of 1,3,4-oxadiazoles and their applications. Int. J. ChemTech Res. 2012, 4, 1782–1791. [Google Scholar]
- Siwach, A.; Verma, P.K. Therapeutic potential of oxadiazole or furadiazole containing compounds. BMC Chem. 2020, 14, 70. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Hegarty, A.F.; Elguero, J. Can 1,2,3-Oxadiazole be Stable? Angew. Chem. Int. Ed. Engl. 1985, 24, 713–715. [Google Scholar] [CrossRef]
- Stewart, F.H.C. The chemistry of the sydnones. Chem. Rev. 1964, 64, 129–147. [Google Scholar] [CrossRef]
- Dey, S.; Manogaran, D.; Manogaran, S.; Schaefer, H.F. Quantification of Aromaticity of Heterocyclic Systems Using Interaction Coordinates. J. Phys. Chem. A 2018, 122, 6953–6960. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.K.; Manogaran, D.; Manogaran, S.; Schaefer, H.F. Quantification of Hydrogen Bond Strength Based on Interaction Coordinates: A New Approach. J. Phys. Chem. A 2017, 121, 6090–6103. [Google Scholar] [CrossRef]
- Bird, C.W. Heteroaromaticity, 5, a unified aromaticity index. Tetrahedron 1992, 48, 335–340. [Google Scholar] [CrossRef]
- Baral, N.; Nayak, S.; Pal, S.; Mohapatra, S. 5-(2-Chlorophenyl)-3-(2 H -chromen-3-yl)-1,2,4-oxadiazole. IUCrData 2018, 3, x180129. [Google Scholar] [CrossRef] [Green Version]
- Piccionello, A.P.; Pibiri, I.; Pace, A.; Buscemi, S.; Vivona, N. 1,2,4-Oxadiazoles. Compr. Heterocycl. Chem. IV 2019, 1–43. [Google Scholar] [CrossRef]
- Wang, B.; Xiong, H.; Cheng, G.; Yang, H. Incorporating Energetic Moieties into Four Oxadiazole Ring Systems for the Generation of High-Performance Energetic Materials. Chempluschem 2018, 83, 439–447. [Google Scholar] [CrossRef]
- Terenzi, A.; Barone, G.; Piccionello, A.P.; Giorgi, G.; Guarcello, A.; Portanova, P.; Calvaruso, G.; Buscemi, S.; Vivona, N.; Pace, A. Synthesis, characterization, cellular uptake and interaction with native DNA of a bis(pyridyl)-1,2,4-oxadiazole copper(II) complex. Dalt. Trans. 2010, 39, 9140–9145. [Google Scholar] [CrossRef]
- Bavisotto, C.C.; Nikolic, D.; Gammazza, A.M.; Barone, R.; Cascio, F.L.; Mocciaro, E.; Zummo, G.; de Macario, E.C.; Macario, A.J.; Cappello, F.; et al. The dissociation of the Hsp60/pro-Caspase-3 complex by bis(pyridyl)oxadiazole copper complex (CubipyOXA) leads to cell death in NCI-H292 cancer cells. J. Inorg. Biochem. 2017, 170, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Klingele, J.; Kaase, D.; Schmucker, M.; Meier, L. Unexpected coordination behaviour of 3,5-Di(2-pyridyl)-1,2,4-oxadiazole. Eur. J. Inorg. Chem. 2013, 2013, 4931–4939. [Google Scholar] [CrossRef]
- Piccionello, A.P.; Pace, A.; Buscemi, S. Rearrangements of 1,2,4-Oxadiazole: “One Ring to Rule Them All”. Chem. Heterocycl. Compd. 2017, 53, 936–947. [Google Scholar] [CrossRef]
- Pace, A.; Buscemi, S.; Piccionello, A.P.; Pibiri, I. Recent Advances in the Chemistry of 1,2,4-Oxadiazoles. Adv. Heterocycl. Chem. 2015, 116, 85–136. [Google Scholar]
- Olofson, R.A.; Michelman, J.S. Furazan. J. Org. Chem. 1965, 30, 1854–1859. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Makhova, N.N. 1,2,5-Oxadiazole-Based High-Energy-Density Materials: Synthesis and Performance. Chempluschem 2020, 85, 13–42. [Google Scholar] [CrossRef] [Green Version]
- Dabiri, M.; Salehi, P.; Baghbanzadeh, M.; Bahramnejad, M. A facile procedure for the one-pot synthesis of unsymmetrical 2,5-disubstituted 1,3,4-oxadiazoles. Tetrahedron Lett. 2006, 47, 6983–6986. [Google Scholar] [CrossRef]
- Nesynov, E.P.; Grekov, A.P. The chemistry of 1,3,4-oxadiazole derivatives. Russ. Chem. Rev. 1964, 33, 508–515. [Google Scholar] [CrossRef]
- Redhu, S.; Kharb, R. Recent Updates on Chemistry and Pharmacological aspects of 1,3,4-oxadiazole scaffold. Int. J. Pharm. Innov. 2013, 3, 93–110. [Google Scholar]
- Bettenhausen, J.; Greczmiel, M.; Jandke, M.; Strohriegl, P. Oxadiazoles and phenylquinoxalines as electron transport materials. Synth. Met. 1997, 91, 223–228. [Google Scholar] [CrossRef]
- Adachi, C.; Tsutsui, T.; Saito, S. Organic electroluminescent device having a hole conductor as an emitting layer. Appl. Phys. Lett. 1989, 55, 1489–1491. [Google Scholar] [CrossRef]
- Brocks, G.; Tol, A. Electronic structure of poly-oxadiazoles. J. Chem. Phys. 1997, 106, 6418–6423. [Google Scholar] [CrossRef]
- Naik, L.; Khazi, I.A.M.; Malimath, G.H. Electronic excitation energy transfer studies in binary mixtures of novel optoelectronically active 1,3,4-oxadiazoles and coumarin derivatives. Chem. Phys. Lett. 2020, 749, 137453. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, Q.; Shang, Y.; Yang, C.; Ao, L.; Qin, J.; Ma, D.; Shuai, Z. Multifunctional bipolar triphenylamine/oxadiazole derivatives: Highly efficient blue fluorescence, red phosphorescence host and two-color based white OLEDs. Chem. Commun. 2009, 1, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wang, Q.; Yang, C.; Wang, Q.; Zhang, Z.; Zou, T.; Qin, J.; Ma, D. A simple carbazole/oxadiazole hybrid molecule: An excellent bipolar host for green and red phosphorescent OLEDs. Angew. Chem. Int. Ed. 2008, 47, 8104–8107. [Google Scholar]
- Shang, X.Y.; Wang, S.J.; Ge, X.C.; Xiao, M.; Meng, Y.Z. Synthesis and photoluminescent properties of poly(arylene ether)s containing oxadiazole and fluorene moieties. Mater. Chem. Phys. 2007, 104, 215–219. [Google Scholar] [CrossRef]
- Singh, A.; Kociok-Köhn, G.; Trivedi, M.; Chauhan, R.; Kumar, A.; Gosavi, S.W.; Terashima, C.; Fujishima, A. Ferrocenylethenyl-substituted oxadiazoles with phenolic and nitro anchors as sensitizers in dye sensitized solar cells. New J. Chem. 2019, 43, 4745–4756. [Google Scholar] [CrossRef]
- Venkatakrishnan, P.; Natarajan, P.; Moorthy, J.N.; Lin, Z.; Chow, T.J. Twisted bimesitylene-based oxadiazoles as novel host materials for phosphorescent OLEDs. Tetrahedron 2012, 68, 7502–7508. [Google Scholar] [CrossRef]
- Wang, C.; Jung, G.Y.; Batsanov, A.S.; Bryce, M.R.; Petty, M.C. New electron-transporting materials for light emitting diodes: 1,3,4-oxadiazole-pyridine and 1,3,4-oxadiazole-pyrimidine hybrids. J. Mater. Chem. 2002, 12, 173–180. [Google Scholar] [CrossRef]
- Amir, M.; Kumar, S. Synthesis and evaluation of anti-inflammatory, analgesic, ulcerogenic and lipid peroxidation properties of ibuprofen derivatives. Acta Pharm. 2007, 57, 31–45. [Google Scholar]
- Banik, B.K.; Sahoo, B.M.; Kumar, B.V.V.R.; Panda, K.C.; Jena, J.; Mahapatra, M.K.; Borah, P. Green synthetic approach: An efficient eco-friendly tool for synthesis of biologically active oxadiazole derivatives. Molecules 2021, 26, 1163. [Google Scholar] [CrossRef]
- Ainsworth, C. Oxadiazole. J. Am. Chem. Soc. 1965, 87, 5800–5801. [Google Scholar] [CrossRef]
- Schwarzer, K.; Tullmann, C.P.; Graßl, S.; Górski, B.; Brocklehurst, C.E.; Knochel, P. Functionalization of 1,3,4-Oxadiazoles and 1,2,4-Triazoles via Selective Zincation or Magnesiation Using 2,2,6,6-Tetramethylpiperidyl Bases. Org. Lett. 2020, 22, 1899–1902. [Google Scholar] [CrossRef] [PubMed]
- Tully, W.R.; Gardner, C.R.; Gillespie, R.J.; Westwood, R. 2-(Oxadiazolyl)- and 2-(Thiazolyl)imidazo[1,2-a]pyrimidines as Agonists and Inverse Agonists at Benzodiazepine Receptors. J. Med. Chem. 1991, 34, 2060–2067. [Google Scholar] [CrossRef] [PubMed]
- Short, F.W.; Long, L.M. Synthesis of 5-aryl-2-oxazolepropionic acids and analogs. Antiinflammatory agents. J. Heterocycl. Chem. 1969, 6, 707–712. [Google Scholar] [CrossRef]
- Theocharis, A.B.; Alexandrou, N.E. Synthesis and spectral data of 4,5-bis[5-aryl-1,3,4-oxadiazol-2-yl]-1-benzyl-1,2,3-triazoles. J. Heterocycl. Chem. 1990, 27, 1685–1688. [Google Scholar] [CrossRef]
- Saeed, A. An expeditious, solvent-free synthesis of some 5-aryl-2-(2-hydroxyphenyl)- 1,3,4-oxadiazoles. Chem. Heterocycl. Compd. 2007, 43, 1072–1075. [Google Scholar]
- Liras, S.; Allen, M.P.; Segelstein, B.E. A mild method for the preparation of 1,3,4-oxadiazoles: Triflic anhydride promoted cyclization of diacylhydrazines. Synth. Commun. 2000, 30, 437–443. [Google Scholar] [CrossRef]
- Carlsen, P.H.J.; Jørgensen, K.B. Synthesis of unsymmetrically substituted 4H-1,2,4-triazoles. J. Heterocycl. Chem. 1994, 31, 805–807. [Google Scholar] [CrossRef]
- Tandon, V.K.; Chhor, R.B. An efficient one pot synthesis of 1,3,4-oxadiazoles. Synth. Commun. 2001, 31, 1727–1732. [Google Scholar] [CrossRef]
- Brain, C.T.; Paul, J.M.; Loong, Y.; Oakley, P.J. Novel procedure for the synthesis of 1,3,4-oxadiazoles from 1,2-diacylhydrazines using polymer-supported Burgess reagent under microwave conditions. Tetrahedron Lett. 1999, 40, 3275–3278. [Google Scholar] [CrossRef]
- Majji, G.; Rout, S.K.; Guin, S.; Gogoi, A.; Patel, B.K. Iodine-catalysed oxidative cyclisation of acylhydrazones to 2,5-substituted 1,3,4-oxadiazoles. RSC Adv. 2014, 4, 5357–5362. [Google Scholar] [CrossRef]
- Rostamizadeh, S.; Housaini, S.A.G. Microwave assisted syntheses of 2,5-disubstituted 1,3,4-oxadiazoles. Tetrahedron Lett. 2004, 45, 8753–8756. [Google Scholar] [CrossRef]
- Milcent, R.; Barbier, G. Oxidation of hydrazones with lead dioxide: New synthesis of 1,3,4-oxadiazoles and 4-amino-1,2,4-triazol-5-one derivatives. Chem. Informationsd. 1983, 14, 80–81. [Google Scholar] [CrossRef]
- Jedlovské, E.; Lesko, J. A simple one-pot procedure for the synthesis of 1,3,4-oxadiazoles. Synth. Commun. 1994, 24, 1879–1885. [Google Scholar] [CrossRef]
- Jasiak, K.; Kudelko, A. Oxidative cyclization of N-aroylhydrazones to 2-(2-arylethenyl)-1,3,4-oxadiazoles using DDQ as an efficient oxidant. Tetrahedron Lett. 2015, 56, 5878–5881. [Google Scholar] [CrossRef]
- Prabhu, G.; Sureshbabu, V.V. Hypervalent iodine(V) mediated mild and convenient synthesis of substituted 2-amino-1,3,4-oxadiazoles. Tetrahedron Lett. 2012, 53, 4232–4234. [Google Scholar] [CrossRef] [Green Version]
- Shang, Z.; Reiner, J.; Chang, J.; Zhao, K. Oxidative cyclization of aldazines with bis(trifluoroacetoxy)iodobenzene. Tetrahedron Lett. 2005, 46, 2701–2704. [Google Scholar] [CrossRef]
- Rajapakse, H.A.; Zhu, H.; Young, M.B.; Mott, B.T. A mild and efficient one pot synthesis of 1,3,4-oxadiazoles from carboxylic acids and acyl hydrazides. Tetrahedron Lett. 2006, 47, 4827–4830. [Google Scholar] [CrossRef]
- Kudelko, A.; Zieliński, W. Microwave-assisted synthesis of 2-styryl-1,3,4-oxadiazoles from cinnamic acid hydrazide and triethyl orthoesters. Tetrahedron Lett. 2012, 53, 76–77. [Google Scholar] [CrossRef]
- Kudelko, A.; Zieliński, W.; Ejsmont, K. The reaction of optically active α-aminocarboxylic acid hydrazides with triethyl orthoesters. Tetrahedron 2011, 67, 7838–7845. [Google Scholar] [CrossRef]
- Kudelko, A. Reactions of α-mercaptocarboxylic acid hydrazides with triethyl orthoesters: Synthesis of 1,3,4-thiadiazin-5(6H)-ones and 1,3,4-oxadiazoles. Tetrahedron 2012, 68, 3616–3625. [Google Scholar] [CrossRef]
- Dupau, P.; Epple, R.; Thomas, A.A.; Fokin, V.V.; Sharpless, K.B. Osmium-Catalyzed Dihydroxylation of Olefins in Acidic Media: Old Process, New Tricks. Adv. Synth. Catal. 2002, 344, 421–433. [Google Scholar] [CrossRef]
- Pace, A.; Buscemi, S.; Vivona, N. The synthesis of fluorinated heteroaromatic compounds. Part 1. Five-membered rings with more than two heteroatoms. Org. Prep. Proced. Int. 2005, 37, 447–506. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, J.; Yang, P.; Shi, X.; Li, J. Synthesis of 2-amino-1,3,4-oxadiazoles from isoselenocyanates via cyclodeselenization. Tetrahedron 2011, 67, 5369–5374. [Google Scholar] [CrossRef]
- Shaker, R.M.; Mahmoud, A.F.; Abdel-Latif, F.F. Synthesis and biological activities of novel 1,4-bridged bis-1,2,4-triazoles, bis-1,3,4-thiadiazoles and bis-1,3,4-oxadiazoles. Phosphorus Sulfur Silicon Relat. Elem. 2005, 180, 397–406. [Google Scholar] [CrossRef]
- Tabatabai, S.A.; Lashkari, S.B.; Zarrindast, M.R.; Gholibeikian, M.; Shafiee, A. Design, synthesis and anticonvulsant activity of 2-(2-phenoxy) phenyl-1,3,4-oxadiazole derivatives. Iran. J. Pharm. Res. 2013, 12, 103–109. [Google Scholar]
- Kudelko, A.; Jasiak, K.; Ejsmont, K. Study on the synthesis of novel 5-substituted 2-[2-(pyridyl)ethenyl]-1,3,4-oxadiazoles and their acid-base interactions. Mon. Fur Chem. 2015, 146, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Tajik, H.; Dadras, A. Synthesis and herbicidal activity of novel 5-chloro-3-fluoro-2-phenoxypyridines with a 1,3,4-oxadiazole ring. J. Pestic. Sci. 2011, 36, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Wet-Osot, S.; Phakhodee, W.; Pattarawarapan, M. Application of N-Acylbenzotriazoles in the Synthesis of 5-Substituted 2-Ethoxy-1,3,4-oxadiazoles as Building Blocks toward 3,5-Disubstituted 1,3,4-Oxadiazol-2(3H)-ones. J. Org. Chem. 2017, 82, 9923–9929. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Johnson, D.E. Growth Response of Direct-Seeded Rice to Oxadiazon and Bispyribac-Sodium in Aerobic and Saturated Soils. Weed Sci. 2011, 59, 119–122. [Google Scholar] [CrossRef]
- Zeng, D.; Wang, M.W.; Xiang, M.; Liu, L.W.; Wang, P.Y.; Li, Z.; Yang, S. Design, synthesis, and antimicrobial behavior of novel oxadiazoles containing various N-containing heterocyclic pendants. Pest Manag. Sci. 2020, 76, 2681–2692. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Z.; Wang, Y.; Chen, W.; Huang, Q.; Liu, C.; Song, G. Syntheses and insecticidal activities of novel 2,5-disubstituted 1,3,4-oxadiazoles. J. Fluor. Chem. 2003, 123, 163–169. [Google Scholar] [CrossRef]
- Qian, X.; Cao, S.; Li, Z.; Song, G.; Huang, Q. Oxadiazole Derivatives as Novel Insect-Growth Regulators: Synthesis and Structure-Bioactivity Relationship. In New Discoveries in Agrochemicals; Marshall, C., Hideo, O., Eds.; American Chemical Society: Washington, DC, USA, 2004; Volume 892, pp. 273–282. [Google Scholar]
- Carvalho, F.P. Agriculture, pesticides, food security and food safety. Environ. Sci. Policy 2006, 9, 685–692. [Google Scholar] [CrossRef]
- Tian, K.; Li, X.Q.; Zhang, L.; Gan, Y.Y.; Meng, J.; Wu, S.Q.; Wan, J.L.; Xu, Y.; Cai, C.T.; Ouyang, G.P.; et al. Synthesis of novel indole derivatives containing double 1,3,4-oxadiazole moiety as efficient bactericides against phytopathogenic bacterium Xanthomonas oryzae. Chem. Pap. 2019, 73, 17–25. [Google Scholar] [CrossRef]
- Yang, N.; Yuan, G. One-pot synthesis of 1,3,4-oxadiazol-2(3H)-ones with CO2 as a C1 synthon promoted by hypoiodite. Org. Biomol. Chem. 2019, 17, 6639–6644. [Google Scholar] [CrossRef]
- Shi, W.; Qian, X.; Zhang, R.; Song, G. Synthesis and quantitative structure—Activity relationships of new 2,5-disubstituted-1,3,4-oxadiazoles. J. Agric. Food Chem. 2001, 49, 124–130. [Google Scholar] [CrossRef]
- Rubab, K.; Abbasi, M.A.; Siddiqui, S.Z.; Ashraf, M.; Shaukat, A.; Ahmad, I.; Hassan, S.; Lodhi, M.A.; Ghufran, M.; Shahid, M.; et al. S-Alkylated/aralkylated-2(1H-indol-3-yl-methyl)-1,3,4-oxadiazole-5-thiol derivatives. 2. Anti-bacterial, enzyme-inhibitory and hemolytic activities. Trop. J. Pharm. Res. 2016, 15, 1525–1533. [Google Scholar] [CrossRef] [Green Version]
- Somashekhar, M.; Kotnal, R.B. Recent advances in the application of oxadiazoles in multicomponent reactions from 2015–16: A review. Indo Am. J. Pharm. Sci. 2018, 5, 7632–7644. [Google Scholar]
- Markowitz, M.; Nguyen, B.Y.; Gotuzzo, E.; Mendo, F.; Ratanasuwan, W.; Kovacs, C.; Prada, G.; Morales-Ramirez, J.O.; Crumpacker, C.S.; Isaacs, R.D. Rapid and durable antiretroviral effect of the HIV-1 integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: Results of a 48-week controlled study. J. Acquir. Immune Defic. Syndr. 2007, 46, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Croxtall, J.D.; Keam, S.J. Raltegravir: A Review of its Use in the Management of HIV Infection in Treatment-Experienced Patients. Drugs 2009, 69, 1059–1075. [Google Scholar] [CrossRef]
- Somani, R.; Shirodkar, P. Oxadiazole: A biologically important heterocycle. Der Pharma Chem. 2009, 1, 130–140. [Google Scholar]
- Shepard, D.R.; Dreicer, R. Zibotentan for the treatment of castrate-resistant prostate cancer. Expert Opin. Investig. Drugs 2010, 19, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Trump, D.L.; Payne, H.; Miller, K.; de Bono, J.S.; Stephenson, J., III; Burris, H.A., III; Nathan, F.; Taboada, M.; Morris, T.; Hubner, A. Preliminary study of the specific endothelin a receptor antagonist zibotentan in combination with docetaxel in patients with metastatic castration-resistant prostate cancer. Prostate 2011, 71, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Tomkinson, H.; Kemp, J.; Oliver, S.; Swaisland, H.; Taboada, M.; Morris, T. Pharmacokinetics and tolerability of zibotentan (ZD4054) in subjects with hepatic or renal impairment: Two open-label comparative studies. BMC Clin. Pharmacol. 2011, 11, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forgue, S.T.; Van Harken, D.R.; Smyth, R.D. Determination of tiodazosin concentrations in human plasma with a fluorescence high-performance liquid chromatographic method. J. Chromatogr. B Biomed. Sci. Appl. 1985, 342, 221–227. [Google Scholar] [CrossRef]
- Bala, S.; Kamboj, S.; Kumar, A. Heterocyclic 1, 3, 4-oxadiazole compounds with diverse biological activities: A comprehensive review. J. Pharm. Res 2010, 3, 2993–2997. [Google Scholar]
- Caputo, F.; Corbetta, S.; Piccolo, O.; Vigo, D. Seeking for Selectivity and Efficiency: New Approaches in the Synthesis of Raltegravir. Org. Process Res. Dev. 2020, 24, 1149–1156. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, L.K.; Saraswat, A.; Siddiqui, I.R.; Singh, R.K.P. Electrochemical oxidation of aldehyde-N-arylhydrazones into symmetrical-2,5-disubstituted-1,3,4-oxadiazoles. Res. Chem. Intermed. 2013, 40, 947–960. [Google Scholar] [CrossRef]
- Bhat, K.I.; Sufeera, K.; Chaitanya Sunil Kumar, P. Synthesis, characterization and biological activity studies of 1,3,4-oxadiazole analogs. J. Young Pharm. 2011, 3, 310–314. [Google Scholar] [CrossRef] [Green Version]
- Husain, A.; Ajmal, M. Synthesis of novel 1,3,4-oxadiazole derivatives and their biological properties. Acta Pharm. 2009, 59, 223–233. [Google Scholar] [CrossRef]
- Narella, S.G.; Shaik, M.G.; Mohammed, A.; Alvala, M.; Angeli, A.; Supuran, C.T. Synthesis and biological evaluation of coumarin-1,3,4-oxadiazole hybrids as selective carbonic anhydrase IX and XII inhibitors. Bioorg. Chem. 2019, 87, 765–772. [Google Scholar] [CrossRef]
- Khadri, M.N.; Begum, A.B.; Sunil, M.K.; Khanum, S.A. Synthesis, docking and biological evaluation of thiadiazole and oxadiazole derivatives as antimicrobial and antioxidant agents. Results Chem. 2020, 2, 100045. [Google Scholar]
- Beyzaei, H.; Sargazi, S.; Bagherzade, G.; Moradi, A.; Yarmohammadi, E. Ultrasound-assisted synthesis, antioxidant activity and computational study of 1,3,4-oxadiazol-2-amines. Acta Chim. Slov. 2021, 68, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Jasiak, K.; Kudelko, A.; Biernasiuk, A.; Malm, A. Study on antibacterial and antifungal activity of 2-(2-arylethenyl)-1,3,4-oxadiazoles. Trends Org. Chem. 2008, 18, 21–28. [Google Scholar]
- Khanum, S.A.; Shashikanth, S.; Sudha, B.S.; Deepak, S.A.; Shetty, H.S. Synthesis and anti-mildew activity of 5-(2-aroyl)aryloxymethyl-2-phenyl-1, 3,4-oxadiazoles against downy mildew of pearl millet. Pest Manag. Sci. 2004, 60, 1119–1124. [Google Scholar] [CrossRef]
- Frank, P.V.; Girish, K.S.; Kalluraya, B. Solvent-free microwave-assisted synthesis of oxadiazoles containing imidazole moiety. J. Chem. Sci. 2007, 119, 41–46. [Google Scholar] [CrossRef] [Green Version]
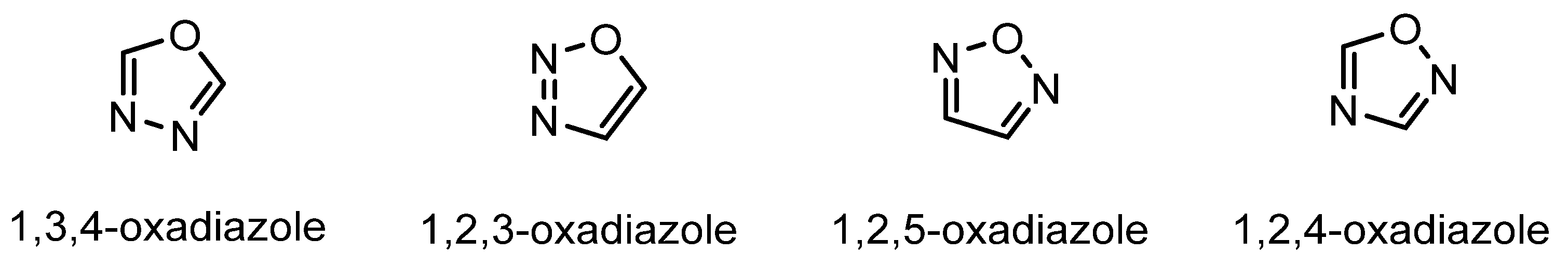





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luczynski, M.; Kudelko, A. Synthesis and Biological Activity of 1,3,4-Oxadiazoles Used in Medicine and Agriculture. Appl. Sci. 2022, 12, 3756. https://doi.org/10.3390/app12083756
Luczynski M, Kudelko A. Synthesis and Biological Activity of 1,3,4-Oxadiazoles Used in Medicine and Agriculture. Applied Sciences. 2022; 12(8):3756. https://doi.org/10.3390/app12083756
Chicago/Turabian StyleLuczynski, Marcin, and Agnieszka Kudelko. 2022. "Synthesis and Biological Activity of 1,3,4-Oxadiazoles Used in Medicine and Agriculture" Applied Sciences 12, no. 8: 3756. https://doi.org/10.3390/app12083756
APA StyleLuczynski, M., & Kudelko, A. (2022). Synthesis and Biological Activity of 1,3,4-Oxadiazoles Used in Medicine and Agriculture. Applied Sciences, 12(8), 3756. https://doi.org/10.3390/app12083756







