Eco Valorization of Eucalyptus globulus Bark and Branches through Liquefaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Chemical Composition
2.3. Liquefaction
3. Results and Discussion
3.1. Chemical Composition
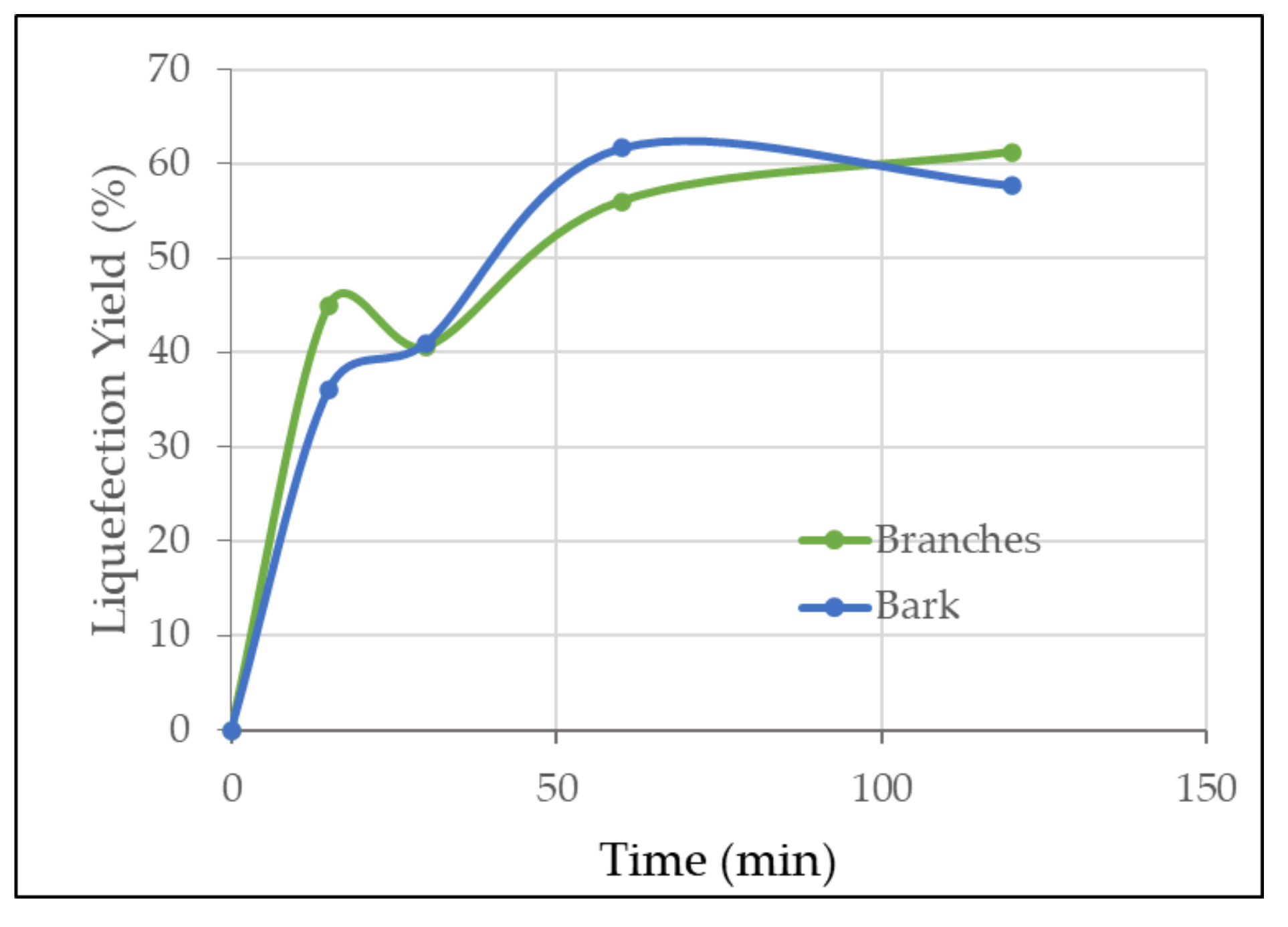
3.2. Liquefaction Optimization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Penín, L.; López, M.; Santos, V.; Alonso, J.L.; Parajó, J.C. Technologies for Eucalyptus Wood Processing in the Scope of Biorefineries: A Comprehensive Review. Bioresour. Technol. 2020, 311, 123528. [Google Scholar] [CrossRef] [PubMed]
- Neiva, D.; Fernandes, L.; Araújo, S.; Lourenço, A.; Gominho, J.; Simões, R.; Pereira, H. Chemical Composition and Kraft Pulping Potential of 12 Eucalypt Species. Ind. Crops Prod. 2015, 66, 89–95. [Google Scholar] [CrossRef]
- CEPI. Key Statistics 2020 European Pulp and Paper Industry; CEPI: Brussels, Belgium, 2020. [Google Scholar]
- Gominho, J.; Lourenço, A.; Miranda, I.; Pereira, H. Chemical and Fuel Properties of Stumps Biomass from Eucalyptus globulus Plantations. Ind. Crops Prod. 2012, 39, 12–16. [Google Scholar] [CrossRef]
- Pereira, H. Variability in The Chemical Composition of Plantation Eucalypts (Eucalyptus globulus Labill.). Wood Fiber Sci. 2007, 20, 82–90. [Google Scholar]
- Coelho, A.L.R. Otimização Da Extração Sólido-Líquido de Antioxidantes de Subprodutos Florestais Pelo Método de Superfície de Resposta. Master’s Thesis, Superior Institute of Technology of Porto, Porto, Portugal, 2013. [Google Scholar]
- Santos, S.A.O.; Villaverde, J.J.; Silva, C.M.; Neto, C.P.; Silvestre, A.J.D. Supercritical Fluid Extraction of Phenolic Compounds from Eucalyptus globulus Labill Bark. J. Supercrit. Fluids 2012, 71, 71–79. [Google Scholar] [CrossRef]
- de Melo, M.M.R.; Oliveira, E.L.G.; Silvestre, A.J.D.; Silva, C.M. Supercritical Fluid Extraction of Triterpenic Acids from Eucalyptus globulus Bark. J. Supercrit. Fluids 2012, 70, 137–145. [Google Scholar] [CrossRef]
- Patinha, D.J.S.; Domingues, R.M.A.; Villaverde, J.J.; Silva, A.M.S.; Silva, C.M.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D. Lipophilic Extractives from the Bark of Eucalyptus grandis × globulus, a Rich Source of Methyl Morolate: Selective Extraction with Supercritical CO2. Ind. Crops Prod. 2013, 43, 340–348. [Google Scholar] [CrossRef]
- Pinto, P.C.; Mota, I.F.; Loureiro, J.M.; Rodrigues, A.E. Membrane Performance and Application of Ultrafiltration and Nanofiltration to Ethanol/Water Extract of Eucalyptus Bark. Sep. Purif. Technol. 2014, 132, 234–243. [Google Scholar] [CrossRef]
- Baptista, M.E.A. Ultrafiltração de Extrato de Casca de Eucalyptus globulus Para Recuperação de Compostos Polifenólicos. Master’s Thesis, University de Porto, Porto, Portugal, 2013. [Google Scholar]
- Domingues, R.M.A.; Sousa, G.D.A.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Eucalyptus globulus Biomass Residues from Pulping Industry as a Source of High Value Triterpenic Compounds. Ind. Crops Prod. 2010, 31, 65–70. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Sousa, G.D.A.; Silva, C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. High Value Triterpenic Compounds from the Outer Barks of Several Eucalyptus Species Cultivated in Brazil and in Portugal. Ind. Crops Prod. 2011, 33, 158–164. [Google Scholar] [CrossRef]
- Neiva, D.M.; Araújo, S.; Gominho, J.; de Carneiro, A.C.; Pereira, H. Potential of Eucalyptus globulus Industrial Bark as a Biorefinery Feedstock: Chemical and Fuel Characterization. Ind. Crops Prod. 2018, 123, 262–270. [Google Scholar] [CrossRef]
- Lima, L.; Miranda, I.; Knapic, S.; Quilhó, T.; Pereira, H. Chemical and Anatomical Characterization, and Antioxidant Properties of Barks from 11 Eucalyptus Species. Eur. J. Wood Prod. 2018, 76, 783–792. [Google Scholar] [CrossRef]
- Gominho, J.; Costa, R.A.; Lourenço, A.; Quilhó, T.; Pereira, H. Eucalyptus globulus Stumps Bark: Chemical and Anatomical Characterization under a Valorisation Perspective. Waste Biomass Valor 2021, 12, 1253–1265. [Google Scholar] [CrossRef]
- Gominho, J.; Costa, R.; Lourenço, A.; Neiva, D.M.; Pereira, H. The Effect of Different Pre-Treatments to Improve Delignification of Eucalypt Stumps in a Biorefinery Context. Bioresour. Technol. Rep. 2019, 6, 89–95. [Google Scholar] [CrossRef]
- Neiva, D.M.; Rencoret, J.; Marques, G.; Gutiérrez, A.; Gominho, J.; Pereira, H.; Río, J.C. del Lignin from Tree Barks: Chemical Structure and Valorization. ChemSusChem 2020, 13, 4537–4547. [Google Scholar] [CrossRef]
- Gominho, J.; Lourenço, A.; Marques, A.V.; Pereira, H. An Extensive Study on the Chemical Diversity of Lipophilic Extractives from Eucalyptus globulus Wood. Phytochemistry 2020, 180, 112520. [Google Scholar] [CrossRef]
- Wang, F.; Ouyang, D.; Zhou, Z.; Page, S.J.; Liu, D.; Zhao, X. Lignocellulosic Biomass as Sustainable Feedstock and Materials for Power Generation and Energy Storage. J. Energy Chem. 2021, 57, 247–280. [Google Scholar] [CrossRef]
- Wang, H.; Chen, H.-Z. A Novel Method of Utilizing the Biomass Resource: Rapid Liquefaction of Wheat Straw and Preparation of Biodegradable Polyurethane Foam (PUF). J. Chin. Inst. Chem. Eng. 2007, 38, 95–102. [Google Scholar] [CrossRef]
- Briones, R.; Torres, L.; Saravia, Y.; Serrano, L.; Labidi, J. Liquefied Agricultural Residues for Film Elaboration. Ind. Crops Prod. 2015, 78, 19–28. [Google Scholar] [CrossRef]
- Xiao, W.; Zhang, X.; Wang, X.; Niu, W.; Han, L. Rapid Liquefaction of Corn Stover with Microwave Heating. BioResources 2015, 10, 4038–4047. [Google Scholar] [CrossRef]
- Zhang, J.; Hori, N.; Takemura, A. Optimization of Agricultural Wastes Liquefaction Process and Preparing Bio-Based Polyurethane Foams by the Obtained Polyols. Ind. Crops Prod. 2019, 138, 111455. [Google Scholar] [CrossRef]
- Jin, Y.; Lai, C.; Kang, J.; Lu, X.; Liu, J.; Lü, Q.-F. Liquefaction of Cornstalk Residue Using 5-Sulfosalicylic Acid as the Catalyst for the Production of Flexible Polyurethane Foams. BioResources 2019, 13, 6970–6982. [Google Scholar] [CrossRef]
- Hu, S.; Wan, C.; Li, Y. Production and Characterization of Biopolyols and Polyurethane Foams from Crude Glycerol Based Liquefaction of Soybean Straw. Bioresour. Technol. 2012, 103, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.M.; Shukry, N. Polyhydric Alcohol Liquefaction of Some Lignocellulosic Agricultural Residues. Ind. Crops Prod. 2008, 27, 33–38. [Google Scholar] [CrossRef]
- Wang, Q.; Tuohedi, N. Polyurethane Foams and Bio-Polyols from Liquefied Cotton Stalk Agricultural Waste. Sustainability 2020, 12, 4214. [Google Scholar] [CrossRef]
- Matos, M.; Barreiro, M.F.; Gandini, A. Olive Stone as a Renewable Source of Biopolyols. Ind. Crops Prod. 2010, 32, 7–12. [Google Scholar] [CrossRef]
- D’Souza, J.; Yan, N. Producing Bark-Based Polyols through Liquefaction: Effect of Liquefaction Temperature. ACS Sustain. Chem. Eng. 2013, 1, 534–540. [Google Scholar] [CrossRef]
- Jiang, W.; Adamopoulos, S.; Hosseinpourpia, R.; Žigon, J.; Petric, M.; Šernek, M.; Medved, S. Utilization Bark for Production of Particleboards. Appl. Sci. 2020, 10, 5253. [Google Scholar] [CrossRef]
- Cruz-Lopes, L.P.; Silva, H.C.; Domingos, I.; Ferreira, J.; Lemos, L.T.; Esteves, B. Optimization of Quercus Cerris Bark Liquefaction. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2016, 10, 1073–1076. [Google Scholar]
- Esteves, B.; Dulyanska, Y.; Costa, C.; Ferreira, J.V.; Domingos, I.; Pereira, H.; de Lemos, L.T.; Cruz-Lopes, L.V. Cork Liquefaction for Polyurethane Foam Production. BioResources 2017, 12, 2339–2353. [Google Scholar] [CrossRef]
- Kim, K.H.; Jo, Y.J.; Lee, C.G.; Lee, E. Solvothermal Liquefaction of Microalgal Tetraselmis Sp. Biomass to Prepare Biopolyols by Using PEG#400-Blended Glycerol. Algal Res. 2015, 12, 539–544. [Google Scholar] [CrossRef]
- Jin, Y.; Ruan, X.; Cheng, X.; Lü, Q. Liquefaction of Lignin by Polyethyleneglycol and Glycerol. Bioresour. Technol. 2011, 102, 3581–3583. [Google Scholar] [CrossRef] [PubMed]
- Kosmela, P.; Hejna, A.; Formela, K.; Haponiuk, J.T.; Piszczyk, Ł. Biopolyols Obtained via Crude Glycerol-Based Liquefaction of Cellulose: Their Structural, Rheological and Thermal Characterization. Cellulose 2016, 23, 2929–2942. [Google Scholar] [CrossRef]
- Braz, A.; Mateus, M.M.; dos Santos, R.G.; Machado, R.; Bordado, J.M.; Correia, M.J.N. Modelling of Pine Wood Sawdust Thermochemical Liquefaction. Biomass Bioenergy 2019, 120, 200–210. [Google Scholar] [CrossRef]
- Mateus, M.M.; Matos, S.; Guerreiro, D.; Debiagi, P.; Gaspar, D.; Ferreira, O.; Bordado, J.C.; Santos, R.G. dos Liquefaction of Almond Husk for Assessment as Feedstock to Obtain Valuable Bio-Oils. Pure Appl. Chem. 2019, 91, 1177–1190. [Google Scholar] [CrossRef]
- Lee, W.-J.; Yu, C.-Y.; Chen, Y.-C. Preparation and Characteristics of Polyurethane Made with Polyhydric Alcohol-Liquefied Rice Husk. J. Appl. Polym. Sci. 2018, 135, 45910. [Google Scholar] [CrossRef]
- Ertas; Fidan Biobased Rigid Polyurethane Foam Prepared from Apricot Stone Shell-Based Polyol for Thermal Insulation Application, Part 1: Synthesis, Chemical, and Physical Properties: BioResources. Available online: https://bioresources.cnr.ncsu.edu/resources/biobased-rigid-polyurethane-foam-prepared-from-apricot-stone-shell-based-polyol-for-thermal-insulation-application-part-1-synthesis-chemical-and-physical-properties/ (accessed on 16 February 2021).
- Zhang, Q.; Zhang, G.; Han, D.; Wu, Y. Renewable Chemical Feedstocks from Peanut Shell Liquefaction: Preparation and Characterization of Liquefied Products and Residue. J. Appl. Polym. Sci. 2016, 133, 43361. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Kairytė, A. Application of Walnut Shells-Derived Biopolyol in the Synthesis of Rigid Polyurethane Foams. Materials 2020, 13, 2687. [Google Scholar] [CrossRef]
- Chang, C.; Liu, L.; Li, P.; Xu, G.; Xu, C. Preparation of Flame Retardant Polyurethane Foam from Crude Glycerol Based Liquefaction of Wheat Straw. Ind. Crops Prod. 2021, 160, 113098. [Google Scholar] [CrossRef]
- Briones, R.; Rodriguez, J.; Labidi, J.; Cunningham, E.; Martin, P. Liquefaction of Corn Husks and Properties of Biodegradable Biopolyol Blends. J. Chem. Technol. Biotechnol. 2020, 95, 2973–2982. [Google Scholar] [CrossRef]
- Jasiūnas, L.; Skvorčinskienė, R.; Miknius, L. Wet and Coarse: The Robustness of Two-Stage Crude Glycerol Mediated Solvothermal Liquefaction of Residual Biomass. Waste Biomass Valor 2020, 11, 2171–2181. [Google Scholar] [CrossRef]
- Yip, J.; Chen, M.; Szeto, Y.S.; Yan, S. Comparative Study of Liquefaction Process and Liquefied Products from Bamboo Using Different Organic Solvents. Bioresour. Technol. 2009, 100, 6674–6678. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.; Lopes, L.C.; Esteves, B. Otimização Da Liquefação Da Madeira de Pinus Pinaster Com Poliálcoois. Silva Lusit. 2013, 21, 177–183. [Google Scholar]
- Niu, M.; Zhao, G.J.; Alma, M.H. Thermogravimetric Studies on Condensed Wood Residues in Polyhydric Alcohols Liquefaction. BioResources 2011, 6, 615–630. [Google Scholar] [CrossRef]
- Pan, H.; Zheng, Z.; Hse, C.Y. Microwave-Assisted Liquefaction of Wood with Polyhydric Alcohols and Its Application in Preparation of Polyurethane (PU) Foams. Eur. J. Wood Wood Prod. 2012, 70, 461–470. [Google Scholar] [CrossRef]
- Esteves, B.; Cruz-Lopes, L.; Ferreira, J.; Domingos, I.; Nunes, L.; Pereira, H. Optimizing Douglas-Fir Bark Liquefaction in Mixtures of Glycerol and Polyethylene Glycol and KOH. Holzforschung 2018, 72, 25–30. [Google Scholar] [CrossRef]
- Alma, M.; Shiraishi, N. Preparation of Polyurethane-like Foams from NaOH-Catalyzed Liquefied Wood. Eur. J. Wood Wood Prod. 1998, 56, 245–246. [Google Scholar] [CrossRef]
- Yao, Y.; Yoshioka, M.; Shiraishi, N. Water-absorbing Polyurethane Foams from Liquefied Starch. J. Appl. Polym. Sci. 1996, 60, 1939–1949. [Google Scholar] [CrossRef]
- Kobayashi, M.; Tukamoto, K.; Tomita, B. Application of Liquefied Wood to a New Resin System-Synthesis and Properties of Liquefied Wood/Epoxy Resins. Holzforschung 2000, 54, 93–100. [Google Scholar] [CrossRef]
- Juhaida, M.F.; Paridah, M.T.; Mohd Hilmi, M.; Sarani, Z.; Jalaluddin, H.; Mohamad Zaki, A.R. Liquefaction of Kenaf (Hibiscus cannabinus L.) Core for Wood Laminating Adhesive. Bioresour. Technol. 2010, 101, 1355–1360. [Google Scholar] [CrossRef]
- Fernandes, F.; Matos, S.; Gaspar, D.; Silva, L.; Paulo, I.; Vieira, S.; Pinto, P.C.R.; Bordado, J.; Galhano dos Santos, R. Boosting the Higher Heating Value of Eucalyptus globulus via Thermochemical Liquefaction. Sustainability 2021, 13, 3717. [Google Scholar] [CrossRef]
- Mateus, M.M.; Guerreiro, D.; Ferreira, O.; Bordado, J.C.; Galhano dos Santos, R. Heuristic Analysis of Eucalyptus globulus Bark Depolymerization via Acid-Liquefaction. Cellulose 2017, 24, 659–668. [Google Scholar] [CrossRef]
- Vale, M.; Mateus, M.M.; Galhano dos Santos, R.; Nieto de Castro, C.; de Schrijver, A.; Bordado, J.C.; Marques, A.C. Replacement of Petroleum-Derived Diols by Sustainable Biopolyols in One Component Polyurethane Foams. J. Clean. Prod. 2019, 212, 1036–1043. [Google Scholar] [CrossRef]
- TAPPI. Solvent Extractives of Wood and Pulp. TAPPI T204 Cm-07; TAPPI Press: Atlanta, GA, USA, 2007. [Google Scholar]
- TAPPI. T 222 Om-02. Acid-Insoluble Lignin in Wood and Pulp; TAPPI: Atlanta, GA, USA, 2002. [Google Scholar]
- Roman, K.; Barwicki, J.; Rzodkiewicz, W.; Dawidowski, M. Evaluation of Mechanical and Energetic Properties of the Forest Residues Shredded Chips during Briquetting Process. Energies 2021, 14, 3270. [Google Scholar] [CrossRef]
- Kilulya, K.F.; Msagati, T.A.M.; Mamba, B.B.; Catherine Ngila, J.; Bush, T. Effect of Site, Species and Tree Size on the Quantitative Variation of Lipophilic Extractives in Eucalyptus Woods Used for Pulping in South Africa. Ind. Crops Prod. 2014, 56, 166–174. [Google Scholar] [CrossRef]
- Vázquez, G.; Fontenla, E.; Santos, J.; Freire, M.S.; González-Álvarez, J.; Antorrena, G. Antioxidant Activity and Phenolic Content of Chestnut (Castanea Sativa) Shell and Eucalyptus (Eucalyptus globulus) Bark Extracts. Ind. Crops Prod. 2008, 28, 279–285. [Google Scholar] [CrossRef]
- Miranda, I.; Gominho, J.; Mirra, I.; Pereira, H. Fractioning and Chemical Characterization of Barks of Betula Pendula and Eucalyptus globulus. Ind. Crops Prod. 2013, 41, 299–305. [Google Scholar] [CrossRef]
- Yadav, K.R.; Sharma, R.K.; Kothari, R.M. Bioconversion of Eucalyptus Bark Waste into Soil Conditioner. Bioresour. Technol. 2002, 81, 163–165. [Google Scholar] [CrossRef]
- Roman, K.; Roman, M.; Szadkowska, D.; Szadkowski, J.; Grzegorzewska, E. Evaluation of Physical and Chemical Parameters According to Energetic Willow (Salix viminalis L.) Cultivation. Energies 2021, 14, 2968. [Google Scholar] [CrossRef]
- Akyuz, M.; Sahin, A.; Alma, A.; Bektap, I.; Usta, A. Conversion of Tree Bark into Bakelite-like Thermosetting Materials by Phenolation. In Proceedings of the XII World Forest Congress, Québec City, QC, Canada, 21–28 September 2003; p. 0425-A1. [Google Scholar]
- Yona, A.M.C.; Budija, F.; Kričej, B.; Kutnar, A.; Pavlič, M.; Pori, P.; Tavzes, Č.; Petrič, M. Production of Biomaterials from Cork: Liquefaction in Polyhydric Alcohols at Moderate Temperatures. Ind. Crops Prod. 2014, 54, 296–301. [Google Scholar] [CrossRef]
- Maldas, D.; Shiraishi, N. Liquefaction of Wood in the Presence of Polyol Using NaOH as a Catalyst and Its Application to Polyurethane Foams. Int. J. Polym. Mater. 1996, 33, 61–71. [Google Scholar] [CrossRef]
- Soares, B.; Gama, N.; Freire, C.; Barros-Timmons, A.; Brandão, I.; Silva, R.; Pascoal Neto, C.; Ferreira, A. Ecopolyol Production from Industrial Cork Powder via Acid Liquefaction Using Polyhydric Alcohols. ACS Sustain. Chem. Eng. 2014, 2, 846–854. [Google Scholar] [CrossRef]
- Kurimoto, Y.; Tamura, Y. Species Effects on Wood-Liquefaction in Polyhydric Alcohols. Holzforschung 1999, 53, 617–622. [Google Scholar] [CrossRef]
- Hu, S.; Luo, X.; Li, Y. Polyols and Polyurethanes from the Liquefaction of Lignocellulosic Biomass. ChemSusChem 2014, 7, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Kahlerras, Z.; Irinislimane, R.; Bruzaud, S.; Belhaneche-Bensemra, N. Elaboration and Characterization of Polyurethane Foams Based on Renewably Sourced Polyols. J. Polym. Environ. 2020, 28, 3003–3018. [Google Scholar] [CrossRef]




| Parameter | Bark Composition (%) | Branches Composition (%) | |
|---|---|---|---|
| Ashes | 14.2 ± 1.2 | 10.6 ± 0.8 | |
| Extract. | Dichloromethane | 1.2 ± 0.2 | 3.0 ± 0.2 |
| Ethanol | 2.9 + 0.3 | 3.1 ± 0.2 | |
| Hot water | 4.4 ± 1.0 | 4.8 ± 0.5 | |
| Proteins a | -- | 0.3 ± 0.1 | |
| Tannins b | 11.4 ± 6.8 | 16.8 ± 0.6 | |
| Klason Lignin c | 15.6 ± 0.3 | 17.9 ± 1.6 | |
| Cellulose | 40.5 ± 1.0 | 41.3 ± 3.7 | |
| Hemicelluloses | 23.1 | 22.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, A.; Cruz-Lopes, L.; Dulyanska, Y.; Domingos, I.; Ferreira, J.; Evtuguin, D.; Esteves, B. Eco Valorization of Eucalyptus globulus Bark and Branches through Liquefaction. Appl. Sci. 2022, 12, 3775. https://doi.org/10.3390/app12083775
Fernandes A, Cruz-Lopes L, Dulyanska Y, Domingos I, Ferreira J, Evtuguin D, Esteves B. Eco Valorization of Eucalyptus globulus Bark and Branches through Liquefaction. Applied Sciences. 2022; 12(8):3775. https://doi.org/10.3390/app12083775
Chicago/Turabian StyleFernandes, Ana, Luísa Cruz-Lopes, Yuliya Dulyanska, Idalina Domingos, José Ferreira, Dmitry Evtuguin, and Bruno Esteves. 2022. "Eco Valorization of Eucalyptus globulus Bark and Branches through Liquefaction" Applied Sciences 12, no. 8: 3775. https://doi.org/10.3390/app12083775
APA StyleFernandes, A., Cruz-Lopes, L., Dulyanska, Y., Domingos, I., Ferreira, J., Evtuguin, D., & Esteves, B. (2022). Eco Valorization of Eucalyptus globulus Bark and Branches through Liquefaction. Applied Sciences, 12(8), 3775. https://doi.org/10.3390/app12083775








