Effect of Catalyst Ink and Formation Process on the Multiscale Structure of Catalyst Layers in PEM Fuel Cells
Abstract
1. Introduction
2. Multiscale Structure of CL in PEM Fuel Cell
3. Microstructure and Macroscopic Properties of Catalyst Ink
3.1. Microstructure of Catalyst Ink
3.2. Macroscopic Properties of the Catalyst Ink
- (1)
- Rheology: During the coating process, the ink with the desired viscosity is necessary for high-quality, continuous, and defect-free coating. Some coating techniques, e.g., slot die coating, doctor blade coating, brush coating, or print coating, usually need a relatively viscous ink, often called slurry or paste. A too viscous paste results in poor flowability, leading to a poor distribution of ink on the substrate or weak adhesion of CLs to the membrane [66]. Inkjet printing and spray coating generally need a low viscous ink (e.g., 4.9 mPa s) to avoid blocking the spraying nozzle and decrease the structural defects in the CLs, for example, the ink with a viscosity of 25.3 mPa s [67] may result in the nozzle clogging, making it hard to control the amount of ink sprayed. For spray coating, the viscosity has an influence on the atomization of the catalyst ink. When the viscosity is lower than a certain critical value (e.g., 15 mPa s [68]), increasing the viscosity of ink will decrease the number of large droplets and increase the uniformity of the formed droplets, enhancing spraying quality.
- (i)
- Temperature, the viscosity of solvent, and the volume fraction of solids in the ink. The viscosity of a liquid always decreases with an increase in temperature. A viscous solvent or an increase in the solid volume in the ink lead to an increase in ink viscosity. Here, the solid refers to the catalyst, ionomer, and their formed agglomerates, as well as the solvent trapped in the loose agglomerates, which is equal to a pseudo-solid phase [52]. For a certain solid content, the ink viscosity usually increases with decreasing the size of agglomerates [28,69]. Furthermore, the viscosity will decrease when the strength of the electrostatic repulsion force between agglomerates increases [53].
- (ii)
- Shear rate: When applying a shear force, e.g., during dispersing or coating of the catalyst ink, the fluid properties change, e.g., viscosity, different from the stationary case, which is commonly due to the change of the fluid microstructure caused by shear force [70]. The rheological behavior of fluid could be studied by measuring the change of viscosity or shear stress (τ) with shear rate (γ). Fluid can be categorized into Newtonian fluids with shear-independent viscosity and non-Newtonian fluids. The non-Newtonian flow behavior could be further categorized in shear-thickening fluids and shear-thinning fluids. The Newtonian fluid exhibits a linear function between the applied τ and the rate of strain (or γ). The non-Newtonian fluid could exhibit such a nonlinear function, called the Ostwald–de Waele model or Power Law model:
- (2)
- Surface tension: During the coating process, the surface tension of the ink should be minimized, or it should be at least less than the substrate, to boost the ink’s wettability on the substrate and avoid coating failures. Hence, low surface tension will be beneficial to decrease the formation of defects, enhance the adhesion of CLs to the substrates, and optimize the interface between the CL and substrate. For example, to allow adequate wetting during the coating process onto carbon cloth, the surface tension value of ink should be lower than 32.5 mN/m (the surface tension value of carbon cloth) [80]. During the spraying process, the surface tension of the ink could impact the spray quality to some extent by affecting the small droplets [68].
- (3)
- Stability: Not only the zero-time microstructure and macroscopic properties of a catalyst ink, but also its stability is necessary to be considered to guarantee reproducible coating, which is extremely critical for industrial production. The stability of the catalyst ink includes material stability and colloidal stability. The former includes the oxidation of carbon, ionomer, and organic solvent caused by the catalysis of Pt or dealloying of catalyst during storage, modifying the composition of catalyst ink and as a result, impacting the structure or performance of CL [64,81]. The latter refers to the ability of the agglomerates in the fresh ink to remain dispersion in the solvent. Over time, the well-dispersed agglomerates may collide with each other during random Brownian motion and aggregate together when the collision force is larger than the repulsive force. If the agglomerate size reaches a certain point, sedimentation would take place due to gravity, making the catalyst ink inhomogeneous [34,52].
4. Catalyst Ink: Effect of Its Formulation on Its Microstructure and Macroscopic Properties
4.1. Catalyst and Catalyst Content in the Ink
- (1)
- The surface area and structure of carbon support and the distribution or loading of Pt on carbon [74,84,85,86]: For Pt/Vulcan, the Vulcan carbon black is a solid carbon support; most Pt nanoparticles are on the external surface of Vulcan. However, for Pt/Ketjen, Ketjen carbon black is a porous carbon support and has a high surface area and abundant internal pore; only ∼50% or even less than 50% of Pt nanoparticles reside on the external surface [87,88]. Compared to Pt/Vulcan, Pt/Ketjen has a low Pt surface density. The ionomer exhibits a preferential interaction with Pt relative to carbon through the side chain [50,89]. Therefore, Pt/Vulcan has a more uniform ionomer coverage than Pt/Ketjen due to higher Pt surface density [53,59,60]. In the catalyst ink, the Pt/Vulcan-related agglomerates can be effectively stabilized by ionomer via the electro-steric mechanism. The viscosity of Pt/Ketjen-based ink is generally higher than that of Pt/Vulcan-based ink [52,53]. However, Yoshimune et al. recently observed that increasing Pt loading on carbon would lead to reduced density and increased thickness of the adsorbed ionomer layer in the water/ethanol-based catalyst ink, due to the hydrophobic interaction between the main chain of ionomer and carbon [90]. Furthermore, the support [88,91] (e.g., nanopore volume [88]) or Pt loading on carbon [92,93] will impact the optimal I/C ratio; certainly, the optimal I/C ratio also depends on the type of ionomer (discussed more in Section 4.2), Pt loading in the electrode [94], or operation conditions (e.g., RH [95]). Moreover, pure carbon instead of Pt/C was usually used in some studies to investigate the effect of the structure and surface properties of carbon on the ink microstructure [74], which is improper since the surface properties of carbon may be modified during depositing Pt [53].
- (2)
- The amount and charge type of functional groups on carbon [51,85,96]: For example, hydrophobic carbon tends to form large agglomerates in the polar solvent, e.g., water/alcohol mixed solvent, due to hydrophobic interaction [25]; in contrast, increasing the hydrophilicity of carbon through introducing plenty of functional groups tends to form small agglomerates in polar solvents due to an enhanced repulsion force [51]. To improve the dispersion of Pt on carbon, the pristine carbon materials are usually subjected to the oxidation treatment to increase the surface oxygen-containing groups. This makes the carbon surface negatively charged, increasing the columbic repulsive force with the negatively charged ionomer, which is adverse to the adsorption of ionomer on catalyst [97]. The equilibrium constant of ionomer adsorption on catalyst decreases with increasing the amount of the surface oxygen-containing groups [96]. If introducing -SO3H onto the carbon surface, a similar phenomenon will be incurred [51]. However, if the surface of the carbon in catalyst contains -NHx or N, the interaction between ionomer and catalyst will be enhanced due to the electrostatic attractive interaction, increasing the coverage and uniformity of ionomer on the catalyst.
- (3)
- The modification of Pt nanoparticles: Although it is beneficial for proton transport to Pt, the ionomer film covering Pt increases the kinetic polarization loss due to the poisoning of sulfonate groups [100], and the mass transport polarization loss due to increased local gas transport resistance through ionomer layer and the increased interfacial resistance caused by the interaction of ionomer and Pt, especially in the CL with a low Pt loading [101]. To prevent Pt surface from being covered by ionomer, the Pt surface is covered by alkanethiol, acting as a mask, before mixing catalyst and ionomer (Figure 6). In this case, the ionomer will selectively cover the carbon surface. After the CL is fabricated, the absorbed alkanethiol molecules are removed by voltage cycling, releasing the Pt surface. Therefore, there is barely any ionomer molecules covering the Pt surface, enhancing local gas transport [102,103]. However, the effect of the masking and demasking process on the activity of Pt nanoparticles may need attention.
- (4)
- Catalyst content in the ink: If the catalyst content in the ink is increased, the amount and size of agglomerates increase, and the distance between agglomerates decrease, increasing the viscosity of ink [52,53]. The catalyst content in ink commonly relies on the ink deposition method, e.g., spray coating usually needs a lower catalyst content (the solid concentration is commonly less than 3 wt.%, e.g., ~0.6 wt.% [36], ~1.8 wt.% [104], or 2.5 wt.% [67]) to avoid the blockage of spraying nozzle, while roll-to-roll coating commonly requires a high catalyst content (the solid concentration is commonly larger than 5 wt.%, e.g., 5.76 wt.% [105], or ~4.5–15 wt.% [36], or even 33.76 wt.% for screen printing [80]). However, the ink for spraying cannot be diluted too much, resulting in a low fabrication rate and a waste of solvent to deposit a desired Pt loading. Therefore, the catalyst content should be adjusted for different coating techniques in order to improve the coating quality as well as decreasing the coating time or the fabrication cost of CL.
4.2. Ionomer and I/C Ratio
- (1)
- The type of ionomer: According to the side chain length, the ionomers can be categorized into long-side chain (LSC) ionomer, e.g., Nafion produced by DuPont or perfluoroimide acid (PFIA) ionomer produced by 3M, and short-side chain (SSC) ionomer, e.g., PFSA ionomer produced by 3M and Aquivion ionomer produced by Solvay-Solexis (Figure 7). The commonly used commercial ionomer dispersion solutions and their related parameters are summarized in Table 1. According to the EW, the ionomers can be categorized into low, middle, and high EW ionomer. 3M PFIA ionomer has multiple acid groups per side chain, while the others only have a single acid group at the end of each side chain. Hence, the 3M PFIA ionomer possesses a high ion exchange capacity (IEC). Since it is a newly developed ionomer, there are only a few studies exploring the properties and application of 3M PEIA ionomer [108,109]; therefore, the LSC ionomers mainly refer to Nafion ionomer in this review. The EW of ionomer depends on the number (m) of -CF2- repeat units in the main chain and the molecular weight of the side chain (MWsc), i.e., EW = 50 m + MWsc. For a specific EW, compared to a LSC ionomer, a SSC ionomer has a larger m value, i.e., a larger main chain fraction, resulting in higher crystallinity and thus higher glass transition temperature and enhanced thermal stability [110]. Hence, the SSC ionomer can be prepared with a low EW on the premise of the sufficient residual crystallinity (i.e., adequate stability upon water uptake rather than dissolving or becoming gelatinous), leading to higher proton conductivity. EW and the side chain length are two important parameters influencing the physicochemical properties of ionomer. Recently, Ramaswamy et al. [111] found that EW is the major factor influencing the performance of PEM fuel cell at the high current region rather than side chain length through systematically analyzing the effect of the side chain length and EW of ionomers on the transport resistances of CL. According to the results Young-Chul Park et al. reported, the side chain length may determine the performance of PEM fuel cells at the low current region [107]. The better continuity and uniformity of SSC ionomer on Pt and carbon increase Pt utilization [107].
- (2)
- I/C ratio: If adding a small amount of ionomer in the catalyst ink, i.e., a low I/C ratio, the ionomer molecules adsorb on the catalyst agglomerates, enhancing the columbic repulsion force and steric hindrance of inter-agglomerates [42,53,74], inhibiting aggregation, to some extent similar to a stabilizing agent, decreasing the agglomerate size, and thus improving the colloidal stability [53,54]. When the I/C ratio reaches a certain value, increasing the content of ionomer in the catalyst ink further will not contribute to the size of agglomerates or increase the colloidal stability [34]. It has been reported that the ionomer adsorption on carbon or catalyst follows the Langmuir isotherm for both SSC and LSC ionomer when the ionomer concentration is less than a certain value (at least 1.0 I/C [54]), i.e., there is an adsorption plateau, where the absorbed ionomer content barely increases with increasing I/C ratio [27,54,74]. The amount of the absorbed ionomer on catalyst commonly depends on the surface properties of the catalyst [117], EW of ionomer [27], or the dispersion medium [118]. Moreover, I/C ratio impacts the viscosity of catalyst ink; however, the influencing rule alters with the type of catalyst. For example, in the case of Pt/Vulcan, the ink viscosity slightly decreases (I/C ratio < 0.2) and then increases with the increase in I/C ratio, whereas in the case of Pt/Kejten, the ink viscosity increases over I/C ratio because ionomer flocculates the agglomerates [53].
4.3. Solvent
- (1)
- Dielectric constant and solubility parameter: The dielectric constant and solubility parameters of a solvent primarily influence the dispersion of catalyst and ionomer or the conformation of ionomer (absorbed ionomer and non-absorbed ionomer) [31,131,132]. As the catalyst agglomerates are commonly covered by ionomer in the catalyst ink, solvent affects the dispersion or dispersion stability of catalyst likely through impacting the ionomer coverage on catalyst or the conformation of the adsorbed ionomer [34]. Therefore, it should be mainly through impacting the interaction between solvent and ionomer that the solvent influences the microstructure and macroscopic properties of the ink [127]. Consequently, the studies on the dielectric constant and solubility parameter of solvent primarily focus on the dispersion and conformation of ionomer.
- (2)
- Viscosity: The solvent viscosity has an effect on the ink viscosity and stability, coating process, and drying process [42,126]. The ink containing a viscous solvent is commonly more stable than that containing a thin solvent due to the decreased Brownian motion of agglomerates and thus decreasing their collision chance [42]. The ink for brush coating, blade coating, or print coating usually needs the relatively viscous solvent, while the ink for spray coating needs the solvent with low viscosity. Moreover, the solvent viscosity may impact the sedimentation and stacking of agglomerates during solvent evaporation due to the different fluidity in the solvents with various viscosity and then affect the pore size and pore volume of the CL. In the ink containing a thin solvent, agglomerates have a tendency to be packed densely, forming many small pores in the CLs, whereas in the ink containing a viscous solvent, agglomerates tend to be packed loosely due to the low fluidity, forming many pores but less small pores in CLs [126].
- (3)
- Boiling point and vapor pressure: The higher the boiling point or the lower the vapor pressure, the smaller the solvent evaporation rate at the special temperature or RH, influencing the coating and drying processes. A fast evaporation rate, e.g., methanol, will make the ink unstable during coating process in which the catalyst ink is exposed to air, e.g., brush coating, screen printing, or gravure and flexographic printing. Fast evaporation could also lead to surface cracking in the dried CLs [42]. A low solvent evaporation rate could increase the ink stability during the coating process, increasing the drying time in return [66]. Sometimes it is difficult to completely remove the solvents with a high boiling point, e.g., glycerol [142], or N-methyl-2-pyrrolidone [143]. The residual solvent will block the pores of CLs, decreasing the gas and water transport. Therefore, it is necessary to select the solvents with an appropriate window of boiling point or vapor pressure to guarantee the sufficient stability of ink during the manufacturing process and the complete evaporation of the solvent.
- (4)
- Surface tension: The surface tension of solvent determines the surface tension of the ink to some extent. However, the addition of catalyst and ionomer will change the surface tension [33]. Therefore, the surface tension of the ink is determined by solvent, ionomer, and catalyst.
5. Catalyst Ink: Effect of Its Preparation on Its Microstructure and Macroscopic Properties
5.1. Order of Ingredients Mixing (Adding Order)
5.2. Dispersion Process
- (1)
- The effect on the ink microstructure: Appropriate dispersion process could effectively break up the large agglomerates, reduce the size of agglomerates, and improve the dispersion of catalyst and ionomer in solvent, which will maximize TPB, avoid the formation of cracks, as well as improve the pore volume of CL and thus the mass transport [24,156] (Figure 9b,e). However, if the dispersion process is insufficient, the large agglomerates in the catalyst ink cannot be broken down, leading to an inhomogeneous CL or even causing cracking in the CL (Figure 9a,d) [24,154]. The large agglomerates in the CL would increase the proton, gas, and water transport resistance. Ionomer molecules mainly adsorb on the exterior surface of the catalyst agglomerates and barely penetrate the catalyst agglomerates, especially the large catalyst agglomerates [156]. Therefore, the Pt inside the large agglomerates cannot access ionomer, increasing the proton transport resistance [156]. Due to the low exterior-surface-to-volume ratio, a thick ionomer film may be formed outside the large catalyst agglomerates, increasing the gas transport resistance through the ionomer film [156]. Further, the long gas transport distance in the interior of the large agglomerates also has an adverse effect on the gas and water transport [156].
- (2)
- The effect on the reproducibility, viscosity, or thixotropy of ink: The insufficient dispersion process decreases the reproducibility of the ink and hence the CLs made of the ink [159]. As mentioned in Section 3.2, the ink viscosity relies on its microstructure, e.g., the size of agglomerates. Therefore, the dispersion process influences the ink viscosity. For example, the large agglomerates in the catalyst ink are dispersed as the ball milling time or the sonication amplitude increase, increasing the ink viscosity [28]. When the agglomerates are broken down to a certain size or reach a certain hardness, they are not dispersed further as the ball milling time or the sonication amplitude increase; thus, the ink viscosity no longer changes. The viscosity of the ink prepared by ball milling exceeds the case of ultrasonication due to the better dispersion degree [28]. It was also found that when decreasing the shear rate to zero, the viscosity of the ink prepared by ball milling exhibits a faster reaction, i.e., an excellent thixotropy, than the case of ultrasonication [28].
- (3)
- The effect on the stability of ink: Appropriate and effective dispersion process could form a homogeneous catalyst ink, leading to higher colloidal stability [158,160]. However, a too aggressive dispersion process, e.g., long-time ultrasonic irradiation or ball milling, or high ultrasonic power, will cause the degradation of catalyst and ionomer, e.g., the detachment, aggregation, or Ostwald ripening of Pt nanoparticles (Figure 9c,f), as well as the degradation or decomposition of ionomer [159,161].
6. CL Formation
6.1. Substrate
- (1)
- Catalyst coated membrane (CCM) method: Catalyst ink is deposited onto both sides of the membrane, directly forming CLs on the membrane, known as CCM. The MEA is achieved through hot-pressing the CCM sandwiched between two GDLs (Figure 10a). (Industrially, GDLs may be glued to the edges of a frame and then CCM is sandwiched between two GDLs when assembling the fuel cell stack. Contact of CCM with GDLs is realized by stack compression.) The MEA produced by the CCM method commonly has an excellent interfacial contact between the CL and membrane and a higher Pt utilization due to directly applying the CL onto the membrane, resulting in higher power and durability. Membrane swelling or wrinkling occurs during the coating process, due to direct contact with the solvent in the ink. This can be mitigated to some extent a small or moderate scale fabrication (e.g., in the lab-scale fabrication or in the moderate production where many companies use CCM method at present) by mechanically fixing the membrane (e.g., on a vacuum platform or in a die) or shorten the interaction time between the membrane and solvent via heating and selecting spray coating to deposit the ink. However, although the membrane swelling and the solvent penetration could be decreased through selecting the appropriate solvent [165], it is still a challenge to directly deposit the ink onto the membrane in a continuous, scale production, e.g., roll-to-roll manufacturing process. This is attributed to the difficulty to mechanically stabilize the membrane during the coating process unlike the case of lab-scale fabrication. Moreover, the catalyst ink is applied onto the membrane at once during the roll-to-roll coating, which means a long interaction time between the membrane and solvent before complete evaporation. With the improvement of the membranes, the challenges CCM method faces in the roll-to-roll manufacturing process may be solved in the future.
- (2)
- Gas diffusion electrode (GDE) method: Catalyst ink is deposited onto the MPL, directly forming the CL on a GDL, obtaining a GDE. The MEA is achieved through hot-pressing the membrane sandwiched between two GDEs (Figure 10b). The membrane swelling or wrinkling could be avoided for the GDE method due to indirect contact with the solvent. However, the GDE method typically offers a limited interfacial contact between the CL and membrane compared to the CCM method, likely resulting from the difference in the surface roughness of MPL and membrane. The MPL has a higher origin surface roughness than the membrane (Figure 11(a1,b1)) [75]. When applying the CL onto the membrane, the CL surface facing the membrane is similar to the membrane surface due to the nearly conformal deposition, forming an excellent contact between the CL and membrane. When applying CL onto MPL, the CL surface facing the membrane is uneven and has an increased roughness compared to the MPL (Figure 11(b2)), making it hard for the membrane to contact the entire CL surface and thus causing gaps (similar to the case shown in Figure 11c,d) [166]. This situation will be more serious when the MPL’s surface roughness increases [166]. To improve the interface between the CL and membrane and reduce the interfacial resistance, coating an extra ionomer layer on the top of the GDE was often applied [166,167]. It has been found that the critical ionomer loading increases as the surface roughness of MPLs increases [166]. Further, the hot press is also necessary for fabricating the GDE-based MEAs, which is not necessary for the CCM-based MEAs [167]. Furthermore, catalyst particles may penetrate the pores or cracks of MPL, reducing the catalyst activation and thereby the performance of MEA. The penetration degree depends on the deposition method due to the difference in the viscosity of the used ink or the drying rates for various coating techniques [38].
- (3)
- Decal transfer method (DTM): CL is deposited on an inert decal substrate first and then transferred to the membrane through the hot press, achieving the CCM, known as DTM (Figure 10c). This method was developed to overcome the drawbacks of the CCM method by avoiding the direct contact of the membrane with the catalyst ink. Although the surface of the decal substrate is similar to the membrane, the CL surface facing the membrane is still uneven but has a lower roughness than that produced by the GDE method [75]. From an economy perspective, direct coating (CCM and GDE) is advantageous; however, decal transfer is not desirable due to the increased production cost caused by the transfer process, the usage of decal substrate, and the waste of catalyst and ionomer due to the incomplete transfer caused by the inappropriate process parameters. To increase the transfer yield and decrease the hot-press temperature simultaneously, considerable efforts have been devoted, e.g., using swelling agents to pre-treat the CL before the hot press [168], pre-depositing a breaking layer composed of carbon, or a carbon and Nafion mixture onto the decal substrate [169], selecting appropriate decal substrate [164], or depositing an extra ionomer layer on the top of CL before the hot press [164]. Nevertheless, it is difficult to completely remove the swelling agents since they usually have a high boiling point, e.g., 1,5-pentanediol [168]; the break layer will increase the interfacial resistance between the CL and GDL. Samaneh et al. [164] developed an effective low-temperature decal transfer method with complete transfer yield and the improved interfacial contact between the CL and membrane, through investigating the type of decal substrates, hot-pressing condition, as well as an extra Nafion layer on the top of CL (faces the membrane) and the Nafion ionomer loading. It was found that fluorinated ethylene propylene film is a good decal substrate and superior to the commonly used polytetrafluoroethylene film due to its low friction coefficient and low contact angle. If depositing an outer Nafion layer with a Nafion loading of 0.2 mg/cm2 on the CL before the hot press, the CL can be completely transferred to the membrane at a low hot-pressing temperature of 130 °C, and the extra Nafion layer could improve the interfacial contact between the CL and membrane, which is similar to the case of GDE.
6.2. Coating Process
- (1)
- Spray coating: Spray coating works mainly through applying high energy (e.g., high-pressure gas turbulence or ultrasonic vibration) to break the large catalyst ink droplets into tiny droplets finally deposited onto the substrate. It has several merits, such as relatively high uniformity of the CLs and relatively precise control on the catalyst loading since the CL is deposited layer by layer. However, the spray coating is a slow process, only suitable for depositing CLs on a small or moderate scale [165]. Therefore, spray coating is more suitable for fabricating the CLs in the lab used to evaluate the performance of the advanced materials due to the relatively excellent control on the CL uniformity and the catalyst loading. The most used spray coating method is the gas-assisted spray. To form a fine ink mist with well-defined droplets size, ultrasonic spray and electrospray were developed [104,162,163]. Compared to the CL deposited by the gas-assisted spray, the electrospray-coated CL is much more porous and has an increased ECSA value due to the fine ink droplets formed by the coulomb repulsion [162].
- (2)
6.3. Drying Process
- (i)
- (ii)
- Weak interaction between the ionomer and catalyst [125,175]: Good affinity between ionomer and catalyst enhances the adhesion strength between particles because ionomer acts as a binder, which could avoid cracking. Furthermore, the deformation of ionomer would dissipate the drying stresses in some degree due to the low Young’s modulus of ionomer, decreasing the risk of cracking [74].
- (iii)
- Defects: The defects in the catalyst ink film, e.g., small voids, bubbles, pinholes, uneven film, large catalyst–ionomer agglomerates, or self-organized free ionomer, could induce stress concentration, increasing the risk of cracking [24,81,125]. For example, the large agglomerates in the catalyst ink will induce the non-uniform surface tension during the CL formation, leading to cracking in the dried CL. The large agglomerates may result from the poor dispersion of catalyst ink [24], or the hydrophobic compounds formed from the alcohol oxidation catalyzed by Pt, e.g., 1,1-dipropoxypropane and propyl propionate, which promote the aggregation of catalyst agglomerates in the ink [81]. Therefore, the well-dispersed ink with small and homogeneous agglomerates and depositing an even and uniform ink film on the substrate will be helpful to suppress cracking.
7. Future Direction and Prospects
- (1)
- The catalyst ink microstructure: To uncover how the composition and preparation process of catalyst ink affect the structure of the CL made of the ink, it is necessary to accurately characterize the ink microstructure. The characterization techniques used in the literature have been summarized in Section 3.1. However, because of the opaque, dynamic, and complicated properties, it is difficult to directly characterize the ink microstructure. To date, the characterization techniques used to measure the ink microstructure commonly need to modify its initial concentration, e.g., dilution (e.g., DLS or ELS) or consolidation (e.g., TEM and SEM). Furthermore, some techniques only measure the local microstructure, e.g., TEM or SEM. The results cannot truly assess the microstructure of catalyst ink, a complex system, or are even misleading at times. Hence, it is necessary to introduce or develop some advanced characterization techniques to accurately evaluate the microstructure of catalyst ink without any modification, e.g., XCT, SAS, or CV-SANS. Furthermore, since the macroscopic properties of a catalyst ink are usually determined by its microstructure, the catalyst ink microstructure could be coarsely inferred through testing its macroscopic properties, e.g., the viscosity or rheologic behavior.The microstructure of catalyst ink is primarily governed by the interaction between catalyst, ionomer, and solvent, which could be studied by model and numerical simulations, e.g., molecular dynamic simulation [118] or discrete element method [40]. Therefore, combining the modeling and physical characterization of the ink might allow us understanding the ink microstructure, to optimize the ink formulation and dispersion process.
- (2)
- Inconsistent conclusions reported in the literature: Because the microstructure and macroscopic properties of catalyst ink are determined not only by the types of catalyst, ionomer, and solvent(s) as well as the ratio between them, but also by the preparation process. If only varying one of the variables, such as merely changing catalyst or using different dispersion methods, the prepared catalyst inks may show different microstructure or macroscopic properties. The morphology and structure of the CL made from catalyst ink are co-governed by the microstructure or macroscopic properties of the catalyst ink, the substrate, coating process, and drying process. This makes the influence of the composition and preparation of catalyst ink on the multiscale structure of the CL extremely complicated and increases the level of difficulty uncovering their relationship. Finally, the CL structure, hot-pressing process, and the fuel cell assembly will co-determine the performance and durability of PEM fuel cell [167,170]. For example, if using dipropylene glycol (DPG)/1-propyl alcohol (NPA) and water mixtures as the solvent, the ionomer agglomerates with moderate size close to that of Pt/C agglomerates in the catalyst ink not only form a connected ionomer network, but also maintain the adequate porosity in CL [23,25], offering an improved PEM fuel cells performance. However, in another, the PEM fuel cells with a high performance need large Nafion agglomerates in the IPA/water-based catalyst ink, which leads to a thinner ionomer film on the catalyst surface [26]. In the IPA-, dimethyl sulfoxide (DMSO)-, N-Methyl-2-pyrrolidone (NMP)-, ethylene glycol (EG)-, or propylene glycol (PG)-based catalyst ink, the solvent that could make ionomer uniformly distribute will allow for good PEM fuel cells performance, due to the homogeneous coverage on catalyst agglomerates [126,127]. The reasons why confused conclusions were often reported in the literature are because there are many factors simultaneously influencing the relationship between the formulation and preparation of catalyst ink or the CL formation process and the CL structure and further the performance and durability of the PEM fuel cell. If the other conditions or processes are inconsistent except the main variable in different studies, different or even opposite conclusions may be obtained.
- (3)
- Stability of catalyst ink during storing and reversibility of the aged ink: It is impossible in most situations that the prepared catalyst ink is applied immediately or completely consumed in a short time; in other words, the prepared ink needs to be stored before appliance. To guarantee the consistency of the structure and performance of the CLs fabricated at different periods, the composition and microstructure of catalyst ink should be stable. However, it is generally hard to avoid the material degradation, agglomeration, or sedimentation in the catalyst ink during storage. Consequently, the CLs produced in different periods likely show different structures and hence performances due to the altered microstructure and macroscopic properties of the ink over time, which will have a terrible influence on the performance and durability of PEM fuel cell stack. While the material degradation, especially that caused by Pt catalysis, is hardly inhibited and recovered, the agglomeration and sedimentation are able to be inhibited, retarded, or recovered to some degree. To keep it uniform, Hoffmann et al. permanently stirred the catalyst ink with a magnetic stirrer after dispersion [74]. Stirring may prevent sedimentation to some extent but it is unclear if the agglomeration can be inhibited since stirring cannot effectively break down the large agglomerates in the catalyst ink. Thus, it should be taken into account how to effectively and quickly redisperse the aged ink or recover its uniformity when applying it, and the extent of reversibility.
- (4)
- Studies on the catalyst ink with high solid concentration: At present, most studies on the composition and preparation process of catalyst ink focus on the cases of low solid concentration. Nevertheless, the roll-to-roll coating method, suitable for continuous, scalable production, always requires a slurry with high solid concentration, which may possess different microstructure and macroscopic properties from the ink with low solid concentration. For example, the thin ink nearly shows Newtonian feature; therefore, its rheology may barely impact the ink deposition process and thus the formation of CL. However, the slurry with high solid concentration always exhibits a high shear-thinning degree, which will increase over the solid concentration. In this case, as the rheology of ink will have an important influence on the ink deposition process and the formation of CL, it is necessary to rationally design the ink rheology by modulating the ink recipe and dispersion process. Hence, more attention should be paid to investigate the impact of the ingredient and preparation on the microstructure and macroscopic properties of the concentrated catalyst ink to promote the mass production of MEAs.
- (5)
- Drying process: While the drying process plays a vital role in the formation of the morphology and structure of CL, there are limited studies examining the evolution of ink film into a dried CL during solvent evaporation. Due to the opaque, dynamic, and complicated properties of the ink and the lack of in situ characterization techniques, it is hard to figure out how the particles in ink film move with solvent evaporation and assemble into the final porous structure as well as the nucleation mechanism of cracks. However, clearing the drying process will contribute to fabricating the CLs with a well-desired structure. Therefore, more research is necessary to make sense of the drying process and the reasons causing cracking, especially the drying process of the slurry with high solid concentration.
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velazquez Abad, A.; Dodds, P.E. Green hydrogen characterisation initiatives: Definitions, standards, guarantees of origin, and challenges. Energy Policy 2020, 138, 111300. [Google Scholar] [CrossRef]
- Nicita, A.; Maggio, G.; Andaloro, A.P.F.; Squadrito, G. Green hydrogen as feedstock: Financial analysis of a photovoltaic-powered electrolysis plant. Int. J. Hydrogen Energy 2020, 45, 11395–11408. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Banham, D.; Ye, S. Current Status and Future Development of Catalyst Materials and Catalyst Layers for Proton Exchange Membrane Fuel Cells: An Industrial Perspective. ACS Energy Lett. 2017, 2, 629–638. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Zhao, N.; Fang, B.; Li, H.; Bi, X.T.; Wang, H. Carbon-Supported Pt-Based Alloy Electrocatalysts for the Oxygen Reduction Reaction in Polymer Electrolyte Membrane Fuel Cells: Particle Size, Shape, and Composition Manipulation and Their Impact to Activity. Chem. Rev. 2015, 115, 3433–3467. [Google Scholar] [CrossRef]
- Bu, L.; Zhang, N.; Guo, S.; Zhang, X.; Li, J.; Yao, J.; Wu, T.; Lu, G.; Ma, J.-Y.; Su, D.; et al. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 2016, 354, 1410–1414. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Z.; Cheng, T.; Fortunelli, A.; Chen, C.-Y.; Yu, R.; Zhang, Q.; Gu, L.; Merinov, B.V.; Lin, Z.; et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 2016, 354, 1414–1419. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, X.; Su, Y.-Q.; Wang, L.; Wang, H.; Dang, D.; Chi, B.; Liu, H.; Hensen, E.J.; Lou, X.W.D. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science 2019, 366, 850–856. [Google Scholar] [CrossRef]
- Middelman, E. Improved PEM fuel cell electrodes by controlled self-assembly. Fuel Cells Bull. 2002, 2002, 9–12. [Google Scholar] [CrossRef]
- Murata, S.; Imanishi, M.; Hasegawa, S.; Namba, R. Vertically aligned carbon nanotube electrodes for high current density operating proton exchange membrane fuel cells. J. Power Sources 2014, 253, 104–113. [Google Scholar] [CrossRef]
- Zhang, W.; Minett, A.I.; Gao, M.; Zhao, J.; Razal, J.M.; Wallace, G.G.; Romeo, T.; Chen, J. Integrated High-Efficiency Pt/Carbon Nanotube Arrays for PEM Fuel Cells. Adv. Energy Mater. 2011, 1, 671–677. [Google Scholar] [CrossRef]
- Sun, R.; Xia, Z.; Shang, L.; Fu, X.; Li, H.; Wang, S.; Sun, G. Hierarchically ordered arrays with platinum coated PANI nanowires for highly efficient fuel cell electrodes. J. Mater. Chem. A 2017, 5, 15260–15265. [Google Scholar] [CrossRef]
- Jiang, S.; Yi, B.; Cao, L.; Song, W.; Zhao, Q.; Yu, H.; Shao, Z. Development of advanced catalytic layer based on vertically aligned conductive polymer arrays for thin-film fuel cell electrodes. J. Power Sources 2016, 329, 347–354. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, H.; Wang, Z.; Jia, J.; Miao, S.; Song, W.; Xiao, Y.; Yu, H.; Shao, Z.; Yi, B. Nano-engineering of a 3D-ordered membrane electrode assembly with ultrathin Pt skin on open-walled PdCo nanotube arrays for fuel cells. J. Mater. Chem. A 2018, 6, 6521–6533. [Google Scholar] [CrossRef]
- Kim, O.-H.; Cho, Y.-H.; Kang, S.H.; Park, H.-Y.; Kim, M.; Lim, J.W.; Chung, D.Y.; Lee, M.J.; Choe, H.; Sung, Y.-E. Ordered macroporous platinum electrode and enhanced mass transfer in fuel cells using inverse opal structure. Nat. Commun. 2013, 4, 3473. [Google Scholar] [CrossRef]
- Liu, H.; Qin, J.; Rockward, T.; Wu, J.; Li, J.; Li, G.; Mao, Q.; Lv, Y.; Wang, X.; Zhang, S.; et al. Photo-driven growth of a monolayer of platinum spherical-nanocrowns uniformly coated on a membrane toward fuel cell applications. J. Mater. Chem. A 2020, 8, 23284–23292. [Google Scholar] [CrossRef]
- Li, J.; Tang, H.; Chen, R.; Liu, D.; Xie, Z.; Pan, M.; Jiang, S.P. Highly ordered 3D macroporous scaffold supported Pt/C oxygen electrodes with superior gas-proton transportation properties and activities for fuel cells. J. Mater. Chem. A 2015, 3, 15001–15007. [Google Scholar] [CrossRef]
- Debe, M.K. Tutorial on the Fundamental Characteristics and Practical Properties of Nanostructured Thin Film (NSTF) Catalysts. J. Electrochem. Soc. 2013, 160, F522–F534. [Google Scholar] [CrossRef]
- Tian, Z.Q.; Lim, S.H.; Poh, C.K.; Tang, Z.; Xia, Z.; Luo, Z.; Shen, P.K.; Chua, D.; Feng, Y.P.; Shen, Z.; et al. A Highly Order-Structured Membrane Electrode Assembly with Vertically Aligned Carbon Nanotubes for Ultra-Low Pt Loading PEM Fuel Cells. Adv. Energy Mater. 2011, 1, 1205–1214. [Google Scholar] [CrossRef]
- Shin, H.; Lee, S.; Suk Jung, H.; Kim, J.-B. Effect of ball size and powder loading on the milling efficiency of a laboratory-scale wet ball mill. Ceram. Int. 2013, 39, 8963–8968. [Google Scholar] [CrossRef]
- Holdcroft, S. Fuel Cell Catalyst Layers: A Polymer Science Perspective. Chem. Mater. 2014, 26, 381–393. [Google Scholar] [CrossRef]
- Sharma, R.; Andersen, S.M. Zoom in Catalyst/Ionomer Interface in Polymer Electrolyte Membrane Fuel Cell Electrodes: Impact of Catalyst/Ionomer Dispersion Media/Solvent. ACS Appl. Mater. Interfaces 2018, 10, 38125–38133. [Google Scholar] [CrossRef] [PubMed]
- Doo, G.; Lee, J.H.; Yuk, S.; Choi, S.; Lee, D.-H.; Lee, D.W.; Kim, H.G.; Kwon, S.H.; Lee, S.G.; Kim, H.-T. Tuning the Ionomer Distribution in the Fuel Cell Catalyst Layer with Scaling the Ionomer Aggregate Size in Dispersion. ACS Appl. Mater. Interf. 2018, 10, 17835–17841. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, H.; Onishi, K.; Asami, K.; Yamaguchi, T. Catalyst Slurry Preparation Using a Hydrodynamic Cavitation Dispersion Method for Polymer Electrolyte Fuel Cells. Ind. Eng. Chem. Res. 2019, 58, 19545–19550. [Google Scholar] [CrossRef]
- Takahashi, S.; Mashio, T.; Horibe, N.; Akizuki, K.; Ohma, A. Analysis of the Microstructure Formation Process and Its Influence on the Performance of Polymer Electrolyte Fuel-Cell Catalyst Layers. ChemElectroChem 2015, 2, 1560–1567. [Google Scholar] [CrossRef]
- Ngo, T.T.; Yu, T.L.; Lin, H.-L. Influence of the composition of isopropyl alcohol/water mixture solvents in catalyst ink solutions on proton exchange membrane fuel cell performance. J. Power Sources 2013, 225, 293–303. [Google Scholar] [CrossRef]
- Thoma, M.; Lin, W.; Hoffmann, E.; Sattes, M.-M.; Segets, D.; Damm, C.; Peukert, W. Simple and Reliable Method for Studying the Adsorption Behavior of Aquivion Ionomers on Carbon Black Surfaces. Langmuir 2018, 34, 12324–12334. [Google Scholar] [CrossRef]
- Du, S.; Li, W.; Wu, H.; Abel Chuang, P.-Y.; Pan, M.; Sui, P.-C. Effects of ionomer and dispersion methods on rheological behavior of proton exchange membrane fuel cell catalyst layer ink. Int. J. Hydrogen Energy 2020, 45, 29430–29441. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, F.; Chen, W.; Ding, Z.; Yang, D.; Li, B.; Ming, P.; Zhang, C. The Controllable Design of Catalyst Inks to Enhance PEMFC Performance: A Review. Electrochem. Energy Rev. 2021, 4, 67–100. [Google Scholar] [CrossRef]
- Devivaraprasad, R.; Masuda, T. Solvent-Dependent Adsorption of Perfluorosulfonated Ionomers on a Pt(111) Surface Using Atomic Force Microscopy. Langmuir 2020, 36, 13793–13798. [Google Scholar] [CrossRef]
- Yang, F.; Xin, L.; Uzunoglu, A.; Stanciu, L.; Ilavsky, J.; Son, S.; Xie, J. Investigation of solvent effects on the dispersion of carbon agglomerates and nafion ionomer particles in catalyst inks using ultra small angle X-ray scattering method. ECS Trans. 2016, 75, 361. [Google Scholar] [CrossRef]
- Berlinger, S.A.; McCloskey, B.D.; Weber, A.Z. Inherent Acidity of Perfluorosulfonic Acid Ionomer Dispersions and Implications for Ink Aggregation. J. Phys. Chem. B 2018, 122, 7790–7796. [Google Scholar] [CrossRef] [PubMed]
- Dixit, M.B.; Harkey, B.A.; Shen, F.; Hatzell, K.B. Catalyst layer ink interactions that affect coatability. J. Electrochem. Soc. 2018, 165, F264. [Google Scholar] [CrossRef]
- Shukla, S.; Bhattacharjee, S.; Weber, A.Z.; Secanell, M. Experimental and theoretical analysis of ink dispersion stability for polymer electrolyte fuel cell applications. J. Electrochem. Soc. 2017, 164, F600. [Google Scholar] [CrossRef]
- Hatzell, K.B.; Dixit, M.B.; Berlinger, S.A.; Weber, A.Z. Understanding inks for porous-electrode formation. J. Mater. Chem. A 2017, 5, 20527–20533. [Google Scholar] [CrossRef]
- Mauger, S. Material-Process-Performance Relationships in PEM Catalyst Inks and Coated Layers. 2019. Available online: https://www.hydrogen.energy.gov/pdfs/review19/ta008_ulsh_2019_o.pdf (accessed on 21 March 2022).
- Millington, B.; Whipple, V.; Pollet, B.G. A novel method for preparing proton exchange membrane fuel cell electrodes by the ultrasonic-spray technique. J. Power Sources 2011, 196, 8500–8508. [Google Scholar] [CrossRef]
- Mauger, S.A.; Neyerlin, K.; Yang-Neyerlin, A.C.; More, K.L.; Ulsh, M. Gravure coating for roll-to-roll manufacturing of proton-exchange-membrane fuel cell catalyst layers. J. Electrochem. Soc. 2018, 165, F1012. [Google Scholar] [CrossRef]
- Bodner, M.; García, H.R.; Steenberg, T.; Terkelsen, C.; Alfaro, S.M.; Avcioglu, G.S.; Vassiliev, A.; Primdahl, S.; Hjuler, H.A. Enabling industrial production of electrodes by use of slot-die coating for HT-PEM fuel cells. Int. J. Hydrogen Energy 2019, 44, 12793–12801. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X.; Liu, Z.-s. The effect of ink dilution and evaporation on the microstructures of catalyst layers in polymer electrolyte membrane fuel cells. Int. J. Energy Res. 2019, 43, 6799–6811. [Google Scholar] [CrossRef]
- Mauger, S.A.; Wang, M.; Cetinbas, F.C.; Dzara, M.J.; Park, J.; Myers, D.J.; Ahluwalia, R.K.; Pylypenko, S.; Hu, L.; Litster, S.; et al. Development of high-performance roll-to-roll-coated gas-diffusion-electrode-based fuel cells. J. Power Sources 2021, 506, 230039. [Google Scholar] [CrossRef]
- Huang, D.-C.; Yu, P.-J.; Liu, F.-J.; Huang, S.-L.; Hsueh, K.-L.; Chen, Y.-C.; Wu, C.-H.; Chang, W.-C.; Tsau, F.-H. Effect of dispersion solvent in catalyst ink on proton exchange membrane fuel cell performance. Int. J. Electrochem. Sci. 2011, 6, 2551–2565. [Google Scholar]
- Berlinger, S.A.; Garg, S.; Weber, A.Z. Multicomponent, multiphase interactions in fuel-cell inks. Curr. Opin. Electrochem. 2021, 29, 100744. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, H.; Li, X. Multi-scale Structure of Catalyst Layers in Polymer Electrolyte Membrane Fuel Cells. Electrochem. Energy Rev. 2021; submitted. [Google Scholar]
- Lopez-Haro, M.; Guétaz, L.; Printemps, T.; Morin, A.; Escribano, S.; Jouneau, P.H.; Bayle-Guillemaud, P.; Chandezon, F.; Gebel, G. Three-dimensional analysis of Nafion layers in fuel cell electrodes. Nat. Commun. 2014, 5, 5229. [Google Scholar] [CrossRef] [PubMed]
- Morawietz, T.; Handl, M.; Oldani, C.; Gazdzicki, P.; Hunger, J.; Wilhelm, F.; Blake, J.; Friedrich, K.A.; Hiesgen, R. High-resolution analysis of ionomer loss in catalytic layers after operation. J. Electrochem. Soc. 2018, 165, F3139. [Google Scholar] [CrossRef]
- Ohma, A.; Mashio, T.; Sato, K.; Iden, H.; Ono, Y.; Sakai, K.; Akizuki, K.; Takaichi, S.; Shinohara, K. Analysis of proton exchange membrane fuel cell catalyst layers for reduction of platinum loading at Nissan. Electrochim. Acta 2011, 56, 10832–10841. [Google Scholar] [CrossRef]
- Soboleva, T.; Zhao, X.; Malek, K.; Xie, Z.; Navessin, T.; Holdcroft, S. On the Micro-, Meso-, and Macroporous Structures of Polymer Electrolyte Membrane Fuel Cell Catalyst Layers. ACS Appl. Mater. Interfaces 2010, 2, 375–384. [Google Scholar] [CrossRef]
- Orfanidi, A.; Rheinländer, P.J.; Schulte, N.; Gasteiger, H.A. Ink solvent dependence of the ionomer distribution in the catalyst layer of a PEMFC. J. Electrochem. Soc. 2018, 165, F1254. [Google Scholar] [CrossRef]
- Malek, K.; Mashio, T.; Eikerling, M. Microstructure of Catalyst Layers in PEM Fuel Cells Redefined: A Computational Approach. Electrocatalysis 2011, 2, 141. [Google Scholar] [CrossRef]
- Yang, F.; Xin, L.; Uzunoglu, A.; Qiu, Y.; Stanciu, L.; Ilavsky, J.; Li, W.; Xie, J. Investigation of the Interaction between Nafion Ionomer and Surface Functionalized Carbon Black Using Both Ultrasmall Angle X-ray Scattering and Cryo-TEM. ACS Appl. Mater. Interfaces 2017, 9, 6530–6538. [Google Scholar] [CrossRef]
- Kumano, N.; Kudo, K.; Akimoto, Y.; Ishii, M.; Nakamura, H. Influence of ionomer adsorption on agglomerate structures in high-solid catalyst inks. Carbon 2020, 169, 429–439. [Google Scholar] [CrossRef]
- Khandavalli, S.; Park, J.H.; Kariuki, N.N.; Myers, D.J.; Stickel, J.J.; Hurst, K.; Neyerlin, K.C.; Ulsh, M.; Mauger, S.A. Rheological Investigation on the Microstructure of Fuel Cell Catalyst Inks. ACS Appl. Mater. Interfaces 2018, 10, 43610–43622. [Google Scholar] [CrossRef] [PubMed]
- Yoshimune, W.; Harada, M. Impact of Nonadsorbed Ionomer on Viscosity of Catalyst Inks for Polymer Electrolyte Fuel Cells. Bull. Chem. Soc. Jpn. 2020, 93, 302–307. [Google Scholar] [CrossRef]
- Balu, R.; Choudhury, N.R.; Mata, J.P.; de Campo, L.; Rehm, C.; Hill, A.J.; Dutta, N.K. Evolution of the Interfacial Structure of a Catalyst Ink with the Quality of the Dispersing Solvent: A Contrast Variation Small-Angle and Ultrasmall-Angle Neutron Scattering Investigation. ACS Appl. Mater. Interf. 2019, 11, 9934–9946. [Google Scholar] [CrossRef]
- Suzuki, T.; Tanaka, H.; Hayase, M.; Tsushima, S.; Hirai, S. Investigation of porous structure formation of catalyst layers for proton exchange membrane fuel cells and their effect on cell performance. Int. J. Hydrogen Energy 2016, 41, 20326–20335. [Google Scholar] [CrossRef]
- Lei, C.; Yang, F.; Macauley, N.; Spinetta, M.; Purdy, G.; Jankovic, J.; Cullen, D.A.; More, K.L.; Kim, Y.S.; Xu, H. Impact of Catalyst Ink Dispersing Solvent on PEM Fuel Cell Performance and Durability. J. Electrochem. Soc. 2021, 168, 044517. [Google Scholar] [CrossRef]
- Song, C.-H.; Park, J.-S. Effect of Dispersion Solvents in Catalyst Inks on the Performance and Durability of Catalyst Layers in Proton Exchange Membrane Fuel Cells. Energies 2019, 12, 549. [Google Scholar] [CrossRef]
- Van Cleve, T.; Wang, G.; Mooney, M.; Cetinbas, C.F.; Kariuki, N.; Park, J.; Farghaly, A.; Myers, D.; Neyerlin, K.C. Tailoring electrode microstructure via ink content to enable improved rated power performance for platinum cobalt/high surface area carbon based polymer electrolyte fuel cells. J. Power Sources 2021, 482, 228889. [Google Scholar] [CrossRef]
- Van Cleve, T.; Khandavalli, S.; Chowdhury, A.; Medina, S.; Pylypenko, S.; Wang, M.; More, K.L.; Kariuki, N.; Myers, D.J.; Weber, A.Z.; et al. Dictating Pt-Based Electrocatalyst Performance in Polymer Electrolyte Fuel Cells, from Formulation to Application. ACS Appl. Mater. Interf. 2019, 11, 46953–46964. [Google Scholar] [CrossRef]
- Takahashi, S.; Shimanuki, J.; Mashio, T.; Ohma, A.; Tohma, H.; Ishihara, A.; Ito, Y.; Nishino, Y.; Miyazawa, A. Observation of ionomer in catalyst ink of polymer electrolyte fuel cell using cryogenic transmission electron microscopy. Electrochim. Acta 2017, 224, 178–185. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, H.; Ilavsky, J.; Stanciu, L.; Ho, D.; Justice, M.J.; Petrache, H.I.; Xie, J. Investigation of a Catalyst Ink Dispersion Using Both Ultra-Small-Angle X-ray Scattering and Cryogenic TEM. Langmuir 2010, 26, 19199–19208. [Google Scholar] [CrossRef] [PubMed]
- Kaszuba, M.; Corbett, J.; Watson, F.M.; Jones, A. High-concentration zeta potential measurements using light-scattering techniques. Phil. Trans. R. Soc. A 2010, 368, 4439–4451. [Google Scholar] [CrossRef] [PubMed]
- Uemura, S.; Kameya, Y.; Iriguchi, N.; Yoshida, T.; Shinohara, K.; Hirai, S. Communication—Investigation of catalyst ink degradation by X-ray CT. J. Electrochem. Soc. 2018, 165, F142. [Google Scholar] [CrossRef]
- Yuan, X.-Z.; Nayoze-Coynel, C.; Shaigan, N.; Fisher, D.; Zhao, N.; Zamel, N.; Gazdzicki, P.; Ulsh, M.; Friedrich, K.A.; Girard, F.; et al. A review of functions, attributes, properties and measurements for the quality control of proton exchange membrane fuel cell components. J. Power Sources 2021, 491, 229540. [Google Scholar] [CrossRef]
- Wang, W.; Chen, S.; Li, J.; Wang, W. Fabrication of catalyst coated membrane with screen printing method in a proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2015, 40, 4649–4658. [Google Scholar] [CrossRef]
- Cho, S.; Tamoto, K.; Uchida, M. Effect of an Electrospray-Generated Ionomer Morphology on Polymer Electrolyte Fuel Cell Performance. Energ. Fuel 2020, 34, 14853–14863. [Google Scholar] [CrossRef]
- Li, W.; Bi, X.; Luo, M.; Sui, P.-C. Numerical Investigations on the Ultrasonic Atomization of Catalyst Inks for Proton Exchange Membrane Fuel Cells. J. Electrochem. Soc. 2021, 168, 034502. [Google Scholar] [CrossRef]
- Benretem, A.; Benidir, M.; Chaib, R. Factors influencing slurry rheology. World Pumps 2010, 2010, 30–32. [Google Scholar] [CrossRef]
- Cheng, X.; McCoy, J.H.; Israelachvili, J.N.; Cohen, I. Imaging the Microscopic Structure of Shear Thinning and Thickening Colloidal Suspensions. Science 2011, 333, 1276–1279. [Google Scholar] [CrossRef]
- Schramm, G. A Practical Approach to Rheology and Rheometry; Gebrüder HAAKE GmbH: Karlsruhe, Germany, 1994. [Google Scholar]
- Bird, B.R.; Armstrong, R.C.; Hassager, O. Dynamics of Polymeric Liquids, Volume 1: Fluid Mechanics, 2nd ed.; Wiley-Interscience: New York, NY, USA, 1987. [Google Scholar]
- Herschel, W.H.; Bulkley, R. Konsistenzmessungen von Gummi-Benzollösungen. J. Colloid Polym. Sci. 1926, 39, 291–300. [Google Scholar]
- Hoffmann, E.; Zhang, S.; Thoma, M.; Damm, C.; Peukert, W. Formulation of carbon black-ionomer dispersions for thin film formation in fuel cells. Particuology 2019, 44, 7–21. [Google Scholar] [CrossRef]
- Gomes Bezerra, C.A.; Deiner, L.J.; Tremiliosi-Filho, G. Unexpected Performance of Inkjet-Printed Membrane Electrode Assemblies for Proton Exchange Membrane Fuel Cells. Adv. Eng. Mater. 2019, 21, 1900703. [Google Scholar] [CrossRef]
- Negi, A.S.; Osuji, C.O. New insights on fumed colloidal rheology—Shear thickening and vorticity-aligned structures in flocculating dispersions. Rheol. Acta 2009, 48, 871–881. [Google Scholar] [CrossRef]
- Osuji, C.O.; Kim, C.; Weitz, D.A. Shear thickening and scaling of the elastic modulus in a fractal colloidal system with attractive interactions. Phys. Rev. E 2008, 77, 060402. [Google Scholar] [CrossRef] [PubMed]
- Ney, L.; Singh, R.; Le, H.-P.; Tepner, S.; Keding, R. Modeling the flow behavior of catalyst inks for PEM fuel cells by an evolutionary algorithm. In Proceedings of the Annual European Rheology Conference 2021, Online, 13–15 April 2021. [Google Scholar]
- Guo, Y.; Yang, D.; Li, B.; Yang, D.; Ming, P.; Zhang, C. Effect of Dispersion Solvents and Ionomers on the Rheology of Catalyst Inks and Catalyst Layer Structure for Proton Exchange Membrane Fuel Cells. ACS Appl. Mater. Interf. 2021, 13, 27119–27128. [Google Scholar] [CrossRef]
- Bonifácio, R.N.; Paschoal, J.O.A.; Linardi, M.; Cuenca, R. Catalyst layer optimization by surface tension control during ink formulation of membrane electrode assemblies in proton exchange membrane fuel cell. J. Power Sources 2011, 196, 4680–4685. [Google Scholar] [CrossRef]
- Uemura, S.; Yoshida, T.; Koga, M.; Matsumoto, H.; Yang, X.; Shinohara, K.; Sasabe, T.; Hirai, S. Ink Degradation and Its Effects on the Crack Formation of Fuel Cell Catalyst Layers. J. Electrochem. Soc. 2019, 166, F89–F92. [Google Scholar] [CrossRef]
- Lerche, D.; Sobisch, T. Evaluation of particle interactions by in situ visualization of separation behaviour. Colloids Surf. A Physicochem. Eng. Asp. 2014, 440, 122–130. [Google Scholar] [CrossRef]
- So, M.; Ohnishi, T.; Park, K.; Ono, M.; Tsuge, Y.; Inoue, G. The effect of solvent and ionomer on agglomeration in fuel cell catalyst inks: Simulation by the Discrete Element Method. Int. J. Hydrogen Energy 2019, 44, 28984–28995. [Google Scholar] [CrossRef]
- Park, Y.-C.; Tokiwa, H.; Kakinuma, K.; Watanabe, M.; Uchida, M. Effects of carbon supports on Pt distribution, ionomer coverage and cathode performance for polymer electrolyte fuel cells. J. Power Sources 2016, 315, 179–191. [Google Scholar] [CrossRef]
- Ly, A.; Asset, T.; Atanassov, P. Integrating nanostructured Pt-based electrocatalysts in proton exchange membrane fuel cells. J. Power Sources 2020, 478, 228516. [Google Scholar] [CrossRef]
- Andersen, S.M. Nano carbon supported platinum catalyst interaction behavior with perfluorosulfonic acid ionomer and their interface structures. Appl. Catal. Environ. 2016, 181, 146–155. [Google Scholar] [CrossRef]
- Uchida, M.; Park, Y.-C.; Kakinuma, K.; Yano, H.; Tryk, D.A.; Kamino, T.; Uchida, H.; Watanabe, M. Effect of the state of distribution of supported Pt nanoparticles on effective Pt utilization in polymer electrolyte fuel cells. Phys. Chem. Chem. Phys. 2013, 15, 11236–11247. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Fujii, T.; Harada, C.; Yasumoto, E.; Takeda, K.; Kakinuma, K.; Uchida, M. Effect of Pt and Ionomer Distribution on Polymer Electrolyte Fuel Cell Performance and Durability. ACS Appl. Energy Mater. 2021, 4, 2307–2317. [Google Scholar] [CrossRef]
- Kodama, K.; Motobayashi, K.; Shinohara, A.; Hasegawa, N.; Kudo, K.; Jinnouchi, R.; Osawa, M.; Morimoto, Y. Effect of the Side-Chain Structure of Perfluoro-Sulfonic Acid Ionomers on the Oxygen Reduction Reaction on the Surface of Pt. ACS Catal. 2018, 8, 694–700. [Google Scholar] [CrossRef]
- Yoshimune, W.; Harada, M. Effect of Pt Loading on the Adsorption of Perfluoro-sulfonic Acid Ionomer in Catalyst Ink for Polymer Electrolyte Fuel Cells. Chem. Lett. 2019, 48, 487–490. [Google Scholar] [CrossRef]
- Ahn, C.-Y.; Cheon, J.-Y.; Joo, S.-H.; Kim, J. Effects of ionomer content on Pt catalyst/ordered mesoporous carbon support in polymer electrolyte membrane fuel cells. J. Power Sources 2013, 222, 477–482. [Google Scholar] [CrossRef]
- Choi, S.-H.; Jung, D.-W.; Yoon, S.-O.; Park, S.; Oh, E.-S.; Kim, J. Optimum content of Nafion ionomer for the fabrication of MEAs in PEMFCs with spray-coated Pt/CNT electrodes. Met. Mater. Int. 2011, 17, 811–816. [Google Scholar] [CrossRef]
- Jung, J.-H.; Jung, D.-W.; Kim, J.-B. Optimum ratio between Nafion and 20, 40 wt% Pt/C catalysts for MEAs. J. Korean Electrochem. Soc. 2011, 14, 50–55. [Google Scholar] [CrossRef][Green Version]
- Sasikumar, G.; Ihm, J.W.; Ryu, H. Optimum Nafion content in PEM fuel cell electrodes. Electrochim. Acta 2004, 50, 601–605. [Google Scholar] [CrossRef]
- Jeon, S.; Lee, J.; Rios, G.M.; Kim, H.-J.; Lee, S.-Y.; Cho, E.; Lim, T.-H.; Hyun Jang, J. Effect of ionomer content and relative humidity on polymer electrolyte membrane fuel cell (PEMFC) performance of membrane-electrode assemblies (MEAs) prepared by decal transfer method. Int. J. Hydrogen Energy 2010, 35, 9678–9686. [Google Scholar] [CrossRef]
- Andersen, S.M.; Borghei, M.; Dhiman, R.; Ruiz, V.; Kauppinen, E.; Skou, E. Adsorption Behavior of Perfluorinated Sulfonic Acid Ionomer on Highly Graphitized Carbon Nanofibers and Their Thermal Stabilities. J. Phys. Chem. C 2014, 118, 10814–10823. [Google Scholar] [CrossRef]
- Schmies, H.; Hornberger, E.; Anke, B.; Jurzinsky, T.; Nong, H.N.; Dionigi, F.; Kühl, S.; Drnec, J.; Lerch, M.; Cremers, C.; et al. Impact of Carbon Support Functionalization on the Electrochemical Stability of Pt Fuel Cell Catalysts. Chem. Mater. 2018, 30, 7287–7295. [Google Scholar] [CrossRef]
- Andersen, S.M.; Grahl-Madsen, L. Interface contribution to the electrode performance of proton exchange membrane fuel cells–Impact of the ionomer. Int. J. Hydrogen Energy 2016, 41, 1892–1901. [Google Scholar] [CrossRef]
- Yakovlev, Y.V.; Lobko, Y.V.; Vorokhta, M.; Nováková, J.; Mazur, M.; Matolínová, I.; Matolín, V. Ionomer content effect on charge and gas transport in the cathode catalyst layer of proton-exchange membrane fuel cells. J. Power Sources 2021, 490, 229531. [Google Scholar] [CrossRef]
- Yarlagadda, V.; Carpenter, M.K.; Moylan, T.E.; Kukreja, R.S.; Koestner, R.; Gu, W.; Thompson, L.; Kongkanand, A. Boosting Fuel Cell Performance with Accessible Carbon Mesopores. ACS Energy Lett. 2018, 3, 618–621. [Google Scholar] [CrossRef]
- Orfanidi, A.; Madkikar, P.; El-Sayed, H.A.; Harzer, G.S.; Kratky, T.; Gasteiger, H.A. The Key to High Performance Low Pt Loaded Electrodes. J. Electrochem. Soc. 2017, 164, F418–F426. [Google Scholar] [CrossRef]
- Lee, J.H.; Doo, G.; Kwon, S.H.; Kang, H.; Choi, S.; Yim, S.-D.; Kim, H.-T.; Lee, S.G. Controlling Ionomer Film Morphology through Altering Pt Catalyst Surface Properties for Polymer Electrolyte Membrane Fuel Cells. ACS Appl. Polym. Mater. 2020, 2, 1807–1818. [Google Scholar] [CrossRef]
- Doo, G.; Yuk, S.; Lee, J.H.; Choi, S.; Lee, D.-H.; Lee, D.W.; Hyun, J.; Kwon, S.H.; Lee, S.G.; Kim, H.-T. Nano-scale control of the ionomer distribution by molecular masking of the Pt surface in PEMFCs. J. Mater. Chem. A 2020, 8, 13004–13013. [Google Scholar] [CrossRef]
- Sassin, M.B.; Garsany, Y.; Gould, B.D.; Swider-Lyons, K.E. Fabrication Method for Laboratory-Scale High-Performance Membrane Electrode Assemblies for Fuel Cells. Anal. Chem. 2017, 89, 511–518. [Google Scholar] [CrossRef]
- Mauger, S. Control of Ionomer Distribution in Roll-to-Roll Coated Fuel Cell Catalyst Layers. 2018. Available online: https://www.nrel.gov/docs/fy19osti/72416.pdf (accessed on 21 March 2022).
- Shahgaldi, S.; Alaefour, I.; Zhao, J.; Li, X. Impact of ionomer in the catalyst layers on proton exchange membrane fuel cell performance under different reactant flows and pressures. Fuel 2018, 227, 35–41. [Google Scholar] [CrossRef]
- Park, Y.-C.; Kakinuma, K.; Uchida, H.; Watanabe, M.; Uchida, M. Effects of short-side-chain perfluorosulfonic acid ionomers as binders on the performance of low Pt loading fuel cell cathodes. J. Power Sources 2015, 275, 384–391. [Google Scholar] [CrossRef]
- Shrivastava, U.N.; Fritzsche, H.; Karan, K. Interfacial and Bulk Water in Ultrathin Films of Nafion, 3M PFSA, and 3M PFIA Ionomers on a Polycrystalline Platinum Surface. Macromolecules 2018, 51, 9839–9849. [Google Scholar] [CrossRef]
- Farzin, S.; Sarella, A.; Yandrasits, M.A.; Dishari, S.K. Fluorocarbon-Based Ionomers with Single Acid and Multiacid Side Chains at Nanothin Interfaces. J. Phys. Chem. C 2019, 123, 30871–30884. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid Ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, N.; Kumaraguru, S.; Koestner, R.; Fuller, T.; Gu, W.; Kariuki, N.; Myers, D.; Dudenas, P.J.; Kusoglu, A. Editors’ Choice—Ionomer Side Chain Length and Equivalent Weight Impact on High Current Density Transport Resistances in PEMFC Cathodes. J. Electrochem. Soc. 2021, 168, 024518. [Google Scholar] [CrossRef]
- So, S.; Kang, H.; Choi, D.; Oh, K.-H. Tunable aggregation of short-side-chain perfluorinated sulfonic acid ionomers for the catalyst layer in polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2020, 45, 19891–19899. [Google Scholar] [CrossRef]
- Kim, O.-H.; Oh, S.-H.; Ahn, C.-Y.; Kim, S.; Kim, J.K.; Kim, J.; Yang, S.; Choi, M.; Cho, Y.-H.; Sung, Y.-E. Enhanced Performance of Ionomer Binder with Shorter Side-Chains, Higher Dispersibility, and Lower Equivalent Weight. Fuel Cells 2018, 18, 711–722. [Google Scholar] [CrossRef]
- Peron, J.; Edwards, D.; Haldane, M.; Luo, X.; Zhang, Y.; Holdcroft, S.; Shi, Z. Fuel cell catalyst layers containing short-side-chain perfluorosulfonic acid ionomers. J. Power Sources 2011, 196, 179–181. [Google Scholar] [CrossRef]
- Lei, C.; Bessarabov, D.; Ye, S.; Xie, Z.; Holdcroft, S.; Navessin, T. Low equivalent weight short-side-chain perfluorosulfonic acid ionomers in fuel cell cathode catalyst layers. J. Power Sources 2011, 196, 6168–6176. [Google Scholar] [CrossRef]
- Zhao, N.; Shi, Z.; Girard, F. Superior Proton Exchange Membrane Fuel Cell (PEMFC) Performance Using Short-Side-Chain Perfluorosulfonic Acid (PFSA) Membrane and Ionomer. Materials 2022, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Kim, J.K.; Kim, J.; Yang, S.; Park, J.-E.; Kim, O.-H.; Cho, Y.-H.J.R.A. PtRu/C catalyst slurry preparation for large-scale decal transfer with high performance of proton exchange membrane fuel cells. RSC Adv. 2018, 8, 36313–36322. [Google Scholar] [CrossRef]
- Mashio, T.; Ohma, A.; Tokumasu, T. Molecular Dynamics Study of Ionomer Adsorption at a Carbon Surface in Catalyst Ink. Electrochim. Acta 2016, 202, 14–23. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, X.; Yan, X.; Shen, S.; Ke, C.; Wei, G.; Zhang, J. Respective Influence of Ionomer Content on Local and Bulk Oxygen Transport Resistance in the Catalyst Layer of PEMFCs with Low Pt Loading. J. Electrochem. Soc. 2019, 166, F239–F245. [Google Scholar] [CrossRef]
- Sambandam, S.; Ramani, V. Influence of binder properties on kinetic and transport processes in polymer electrolyte fuel cell electrodes. Phys. Chem. Chem. Phys. 2010, 12, 6140–6149. [Google Scholar] [CrossRef]
- Yandrasits, M. New Fuel Cell Membranes with Improved Durability and Performance. 2017. Available online: https://www.hydrogen.energy.gov/pdfs/review17/fc109_yandrasits_2017_o.pdf (accessed on 21 March 2022).
- 3MTM Ionomers. Available online: https://multimedia.3m.com/mws/media/1895877O/3m-ionomers-725ew-ionomer-800ew-ionomer-tds-experimental.pdf (accessed on 21 March 2022).
- Solvay. Products and Brands. Available online: https://www.solvay.com/en/search?s=Aquivion&facets_query=&f%5B0%5D=fsection%3AProducts (accessed on 21 March 2022).
- Ion Exchange Materials. Available online: https://www.fuelcellearth.com/wp-content/uploads/converted_files/pdf/D520-D521-D1021-D2020-D2021-Product-Bulletin-Chemours.pdf (accessed on 21 March 2022).
- Kumano, N.; Kudo, K.; Suda, A.; Akimoto, Y.; Ishii, M.; Nakamura, H. Controlling cracking formation in fuel cell catalyst layers. J. Power Sources 2019, 419, 219–228. [Google Scholar] [CrossRef]
- Chisaka, M.; Matsuoka, E.; Daiguji, H. Effect of organic solvents on the pore structure of catalyst layers in polymer electrolyte membrane fuel cells. J. Electrochem. Soc. 2010, 157, B1218. [Google Scholar] [CrossRef]
- Kim, T.-H.; Yi, J.-Y.; Jung, C.-Y.; Jeong, E.; Yi, S.-C. Solvent effect on the Nafion agglomerate morphology in the catalyst layer of the proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2017, 42, 478–485. [Google Scholar] [CrossRef]
- Welch, C.; Labouriau, A.; Hjelm, R.; Orler, B.; Johnston, C.; Kim, Y.S. Nafion in dilute solvent systems: Dispersion or solution? ACS Macro Lett. 2012, 1, 1403–1407. [Google Scholar] [CrossRef]
- Burke, J. Solubility Parameters: Theory and Application. AIC Book Pap. Group Annu. 1984, 3, 13–58. [Google Scholar]
- Hoffmann, E.; Fischer, D.; Thoma, M.; Damm, C.; Lobaz, V.; Zhigunov, A.; Peukert, W. Impact of DAA/water composition on PFSA ionomer conformation. J. Colloid Interf. Sci. 2021, 582, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.T.; Yu, T.L.; Lin, H.-L. Nafion-based membrane electrode assemblies prepared from catalyst inks containing alcohol/water solvent mixtures. J. Power Sources 2013, 238, 1–10. [Google Scholar] [CrossRef]
- Lee, J.H.; Doo, G.; Kwon, S.H.; Choi, S.; Kim, H.-T.; Lee, S.G. Dispersion-Solvent Control of Ionomer Aggregation in a Polymer Electrolyte Membrane Fuel Cell. Sci. Rep. 2018, 8, 10739. [Google Scholar] [CrossRef] [PubMed]
- Yeo, R.S. Dual cohesive energy densities of perfluorosulphonic acid (Nafion) membrane. Polymer 1980, 21, 432–435. [Google Scholar] [CrossRef]
- Kim, T.-H.; Yoo, J.H.; Maiyalagan, T.; Yi, S.-C. Influence of the Nafion agglomerate morphology on the water-uptake behavior and fuel cell performance in the proton exchange membrane fuel cells. Appl. Surf. Sci. 2019, 481, 777–784. [Google Scholar] [CrossRef]
- Yang, T.-H.; Yoon, Y.-G.; Park, G.-G.; Lee, W.-Y.; Kim, C.-S. Fabrication of a thin catalyst layer using organic solvents. J. Power Sources 2004, 127, 230–233. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Matsunaga, T.; Amemiya, K.; Ohira, A.; Hasegawa, N.; Shinohara, K.; Ando, M.; Yoshida, T. Dispersion of Rod-like Particles of Nafion in Salt-Free Water/1-Propanol and Water/Ethanol Solutions. J. Phys. Chem. 2014, 118, 14922–14928. [Google Scholar] [CrossRef]
- Mabuchi, T.; Huang, S.-F.; Tokumasu, T. Dispersion of Nafion Ionomer Aggregates in 1-Propanol/Water Solutions: Effects of Ionomer Concentration, Alcohol Content, and Salt Addition. Macromolecules 2020, 53, 3273–3283. [Google Scholar] [CrossRef]
- Shin, S.J.; Lee, J.K.; Ha, H.Y.; Hong, S.A.; Chun, H.S.; Oh, I.H. Effect of the catalytic ink preparation method on the performance of polymer electrolyte membrane fuel cells. J. Power Sources 2002, 106, 146–152. [Google Scholar] [CrossRef]
- Song, W.; Yu, H.; Hao, L.; Yi, B.; Shao, Z. Effect of catalytic ink on sub-freezing endurance of PEMFCs. Int. J. Hydrogen Energy 2010, 35, 11129–11137. [Google Scholar] [CrossRef]
- Berlinger, S.A.; Dudenas, P.J.; Bird, A.; Chen, X.; Freychet, G.; McCloskey, B.D.; Kusoglu, A.; Weber, A.Z. Impact of Dispersion Solvent on Ionomer Thin Films and Membranes. ACS Appl. Polym. Mater. 2020, 2, 5824–5834. [Google Scholar] [CrossRef]
- Kim, Y.; Welch, C.; Mack, N.; Hjelm, R.; Orler, E.; Hawley, M.; Lee, K.; Yim, S.-D.; Johnston, C. Highly durable fuel cell electrodes based on ionomers dispersed in glycerol. Phys. Chem. Chem. Phys. 2014, 16, 5927–5932. [Google Scholar] [CrossRef] [PubMed]
- Chisaka, M.; Daiguji, H. Effect of glycerol on micro/nano structures of catalyst layers in polymer electrolyte membrane fuel cells. Electrochim. Acta 2006, 51, 4828–4833. [Google Scholar] [CrossRef]
- Lee, M.-R.; Lee, H.-Y.; Yim, S.-D.; Kim, C.-S.; Shul, Y.-G.; Kucernak, A.; Shin, D. Effects of Ionomer Carbon Ratio and Ionomer Dispersity on the Performance and Durability of MEAs. Fuel Cells 2018, 18, 129–136. [Google Scholar] [CrossRef]
- Rumble, J.R. CRC Handbook of Chemistry and Physics; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2017. [Google Scholar]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; p. 7. [Google Scholar] [CrossRef]
- Surface Tension. Available online: https://macro.lsu.edu/HowTo/solvents/Surface%20Tension.htm (accessed on 21 March 2022).
- Fuge, E.; Bowden, S.; Jones, W. Some physical properties of diacetone alcohol, mesityl oxide and methyl isobutyl ketone. J. Phys. Chem. 1952, 56, 1013–1016. [Google Scholar] [CrossRef]
- N-Methyl-2-pyrrolidone. Available online: http://www.microkat.gr/msdspd90-99/N-Methyl-2-pyrrolidone.html (accessed on 21 March 2022).
- Viscosity, Surface Tension, Specific Density and Molecular Weight of Selected Liquids. Available online: https://www.accudynetest.com/visc_table.html (accessed on 21 March 2022).
- Lee, J.W.; Park, S.B.; Lee, H. Densities, Surface Tensions, and Refractive Indices of the Water + 1,3-Propanediol System. J. Chem. Eng. Data 2000, 45, 166–168. [Google Scholar] [CrossRef]
- Surface Tension Values of Some Common Test Liquids for Surface Energy Analysis. Available online: http://www.surface-tension.de/ (accessed on 21 March 2022).
- Welch, C.; Labouriau, A.; Hjelm, R.; Mack, N.; Kim, Y. Solvation and gelation process of nafion. In Proceedings of the 224th Electrochemical Society (ECS) Meeting, San Francisco, CA, USA, 27 October–1 November 2013. [Google Scholar] [CrossRef]
- Wang, D.; Cornelius, C.J. Modeling ionomer swelling dynamics of a sulfonated polyphenylene, pentablock copolymers, and nafion. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 435–443. [Google Scholar] [CrossRef]
- Carine, T.; Christine, N.-C. Improvement of active layers homogeneity for the MEA’s (Membrane Electrode Assembly) of PEMFC (Proton Exchange Membrane Fuel Cell): Impact of the ink quality formulation. Nanomater. Sci. Eng. 2020, 2, 135–143. [Google Scholar] [CrossRef]
- Sun, C.-N.; More, K.L.; Veith, G.M.; Zawodzinski, T.A. Composition Dependence of the Pore Structure and Water Transport of Composite Catalyst Layers for Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2013, 160, F1000–F1005. [Google Scholar] [CrossRef]
- Wang, M.; Park, J.H.; Kabir, S.; Neyerlin, K.C.; Kariuki, N.N.; Lv, H.; Stamenkovic, V.R.; Myers, D.J.; Ulsh, M.; Mauger, S.A. Impact of Catalyst Ink Dispersing Methodology on Fuel Cell Performance Using in-Situ X-ray Scattering. ACS Appl. Energy Mater. 2019, 2, 6417–6427. [Google Scholar] [CrossRef]
- Adamski, M.; Peressin, N.; Holdcroft, S.; Pollet, B.G. Does power ultrasound affect Nafion® dispersions? Ultrason. Sonochem. 2020, 60, 104758. [Google Scholar] [CrossRef] [PubMed]
- Bapat, S.; Fricke, S.; Kohsakowski, S.; Goessling, S.; Peinecke, V.; Segets, D. Tailoring of Electrocatalyst Inks for Performance Enhancement in Proton Exchange Membrane Fuel Cells. ECS Trans. 2020, 97, 651–657. [Google Scholar] [CrossRef]
- Pollet, B.G.; Goh, J.T.E. The importance of ultrasonic parameters in the preparation of fuel cell catalyst inks. Electrochim. Acta 2014, 128, 292–303. [Google Scholar] [CrossRef]
- Bapat, S.; Giehl, C.; Kohsakowski, S.; Peinecke, V.; Schäffler, M.; Segets, D. On the State and Stability of Fuel Cell Catalyst Inks. ChemRxiv Camb. Camb. Open Engag. 2021, 32, 3845–3859. [Google Scholar] [CrossRef]
- Pollet, B.G. Let’s Not Ignore the Ultrasonic Effects on the Preparation of Fuel Cell Materials. Electrocatalysis 2014, 5, 330–343. [Google Scholar] [CrossRef]
- Takahashi, K.; Kakinuma, K.; Uchida, M. Improvement of Cell Performance in Low-Pt-Loading PEFC Cathode Catalyst Layers Prepared by the Electrospray Method. J. Electrochem. Soc. 2016, 163, F1182–F1188. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, W.; Wan, L.; Zhang, P.; Li, S.; Gao, Y.; Xu, D.; Zheng, C.; Shang, M. Electrostatic spraying of membrane electrode for proton exchange membrane fuel cell. Curr. Appl. Phys. 2020, 20, 11–17. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Alaefour, I.; Unsworth, G.; Li, X. Development of a low temperature decal transfer method for the fabrication of proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2017, 42, 11813–11822. [Google Scholar] [CrossRef]
- Park, J.; Kang, Z.; Bender, G.; Ulsh, M.; Mauger, S.A. Roll-to-roll production of catalyst coated membranes for low-temperature electrolyzers. J. Power Sources 2020, 479, 228819. [Google Scholar] [CrossRef]
- Wang, M.; Medina, S.; Pfeilsticker, J.R.; Pylypenko, S.; Ulsh, M.; Mauger, S.A. Impact of Microporous Layer Roughness on Gas-Diffusion-Electrode-Based Polymer Electrolyte Membrane Fuel Cell Performance. ACS Appl. Energy Mater. 2019, 2, 7757–7761. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Alaefour, I.; Li, X. Impact of manufacturing processes on proton exchange membrane fuel cell performance. Appl. Energy 2018, 225, 1022–1032. [Google Scholar] [CrossRef]
- Cho, D.H.; Lee, S.Y.; Shin, D.W.; Hwang, D.S.; Lee, Y.M. Swelling agent adopted decal transfer method for membrane electrode assembly fabrication. J. Power Sources 2014, 258, 272–280. [Google Scholar] [CrossRef]
- Krishnan, N.N.; Prabhuram, J.; Hong, Y.T.; Kim, H.J.; Yoon, K.; Ha, H.Y.; Lim, T.H.; Kim, S.K. Fabrication of MEA with hydrocarbon based membranes using low temperature decal method for DMFC. Int. J. Hydrogen Energy 2010, 35, 5647–5655. [Google Scholar] [CrossRef]
- Fouzaï, I.; Gentil, S.; Bassetto, V.C.; Silva, W.O.; Maher, R.; Girault, H.H. Catalytic layer-membrane electrode assembly methods for optimum triple phase boundaries and fuel cell performances. J. Mater. Chem. 2021, 9, 11096–11123. [Google Scholar] [CrossRef]
- Cullen, D.A.; Neyerlin, K.C.; Ahluwalia, R.K.; Mukundan, R.; More, K.L.; Borup, R.L.; Weber, A.Z.; Myers, D.J.; Kusoglu, A. New roads and challenges for fuel cells in heavy-duty transportation. Nat. Energy 2021, 6, 462–474. [Google Scholar] [CrossRef]
- Ferrara, A.; Jakubek, S.; Hametner, C. Energy management of heavy-duty fuel cell vehicles in real-world driving scenarios: Robust design of strategies to maximize the hydrogen economy and system lifetime. Energy Convers. Manag. 2021, 232, 113795. [Google Scholar] [CrossRef]
- Influence of Coating Method on the Performance of Roll-to-Roll Coated PEM Fuel Cell Catalyst Layers. Available online: https://www.nrel.gov/docs/fy20osti/77815.pdf (accessed on 21 March 2022).
- Talukdar, K.; Ripan, M.A.; Jahnke, T.; Gazdzicki, P.; Morawietz, T.; Friedrich, K.A. Experimental and numerical study on catalyst layer of polymer electrolyte membrane fuel cell prepared with diverse drying methods. J. Power Sources 2020, 461, 228169. [Google Scholar] [CrossRef]
- Kusano, T.; Hiroi, T.; Amemiya, K.; Ando, M.; Takahashi, T.; Shibayama, M. Structural evolution of a catalyst ink for fuel cells during the drying process investigated by CV-SANS. Polym. J. 2015, 47, 546–555. [Google Scholar] [CrossRef]
- Bapat, S.; Segets, D. Sedimentation Dynamics of Colloidal Formulations through Direct Visualization: Implications for Fuel Cell Catalyst Inks. ACS Appl. Nano Mater. 2020, 3, 7384–7391. [Google Scholar] [CrossRef]
- Jang, S.; Kim, S.; Kim, S.M.; Choi, J.; Yeon, J.; Bang, K.; Ahn, C.-Y.; Hwang, W.; Her, M.; Cho, Y.-H.; et al. Interface engineering for high-performance direct methanol fuel cells using multiscale patterned membranes and guided metal cracked layers. Nano Energy 2018, 43, 149–158. [Google Scholar] [CrossRef]
- Ahn, C.-Y.; Jang, S.; Cho, Y.-H.; Choi, J.; Kim, S.; Kim, S.M.; Sung, Y.-E.; Choi, M. Guided cracking of electrodes by stretching prism-patterned membrane electrode assemblies for high-performance fuel cells. Sci. Rep. 2018, 8, 1257. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Fowler, M.W.; Simon, L.C.; Grot, S. Morphological features (defects) in fuel cell membrane electrode assemblies. J. Power Sources 2006, 157, 650–656. [Google Scholar] [CrossRef]
- White, R.T.; Wu, A.; Najm, M.; Orfino, F.P.; Dutta, M.; Kjeang, E. 4D in situ visualization of electrode morphology changes during accelerated degradation in fuel cells by X-ray computed tomography. J. Power Sources 2017, 350, 94–102. [Google Scholar] [CrossRef]
- Slowik, V.; Hübner, T.; Schmidt, M.; Villmann, B. Simulation of capillary shrinkage cracking in cement-like materials. Cem. Concr. Compos. 2009, 31, 461–469. [Google Scholar] [CrossRef]
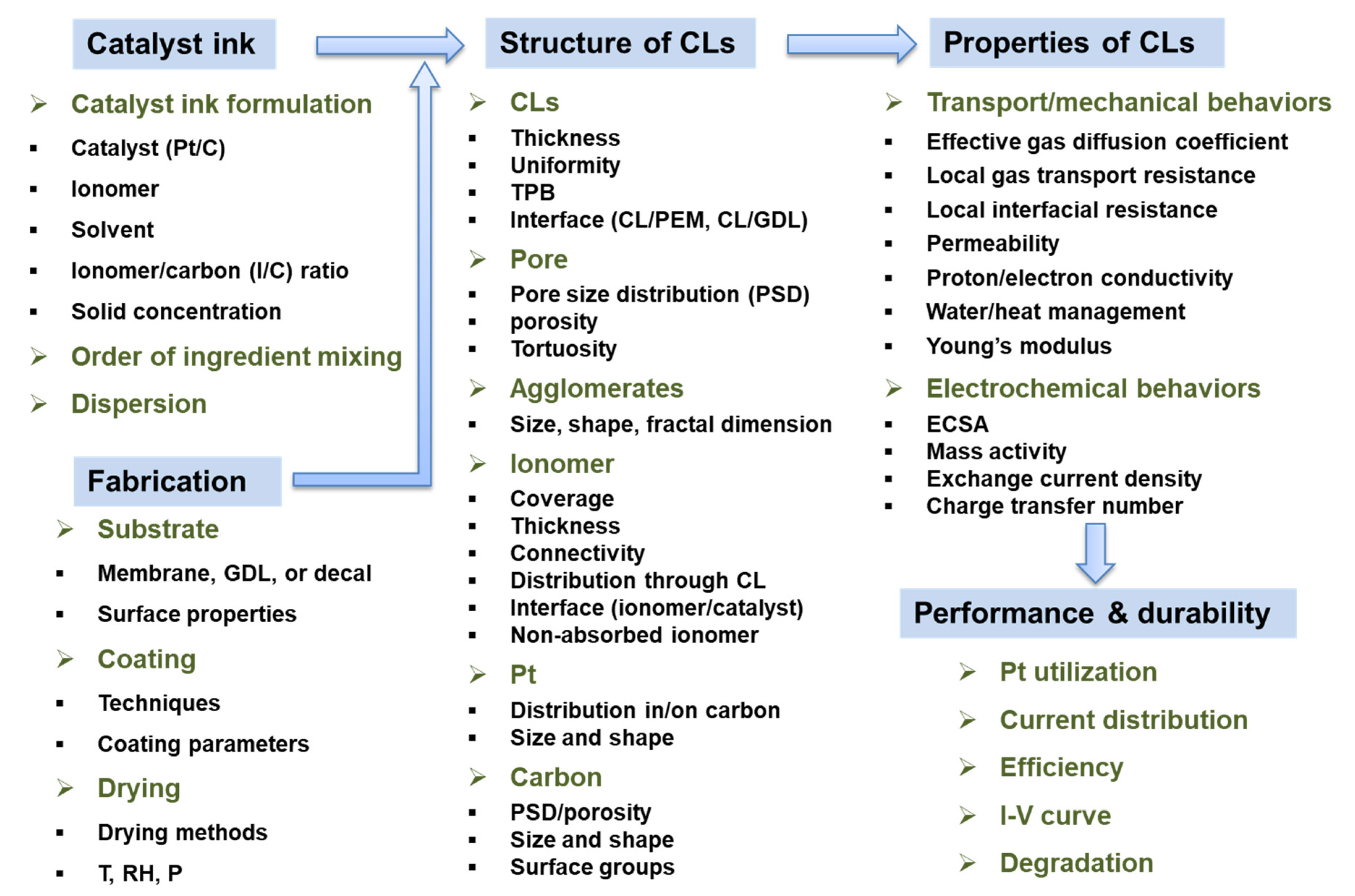







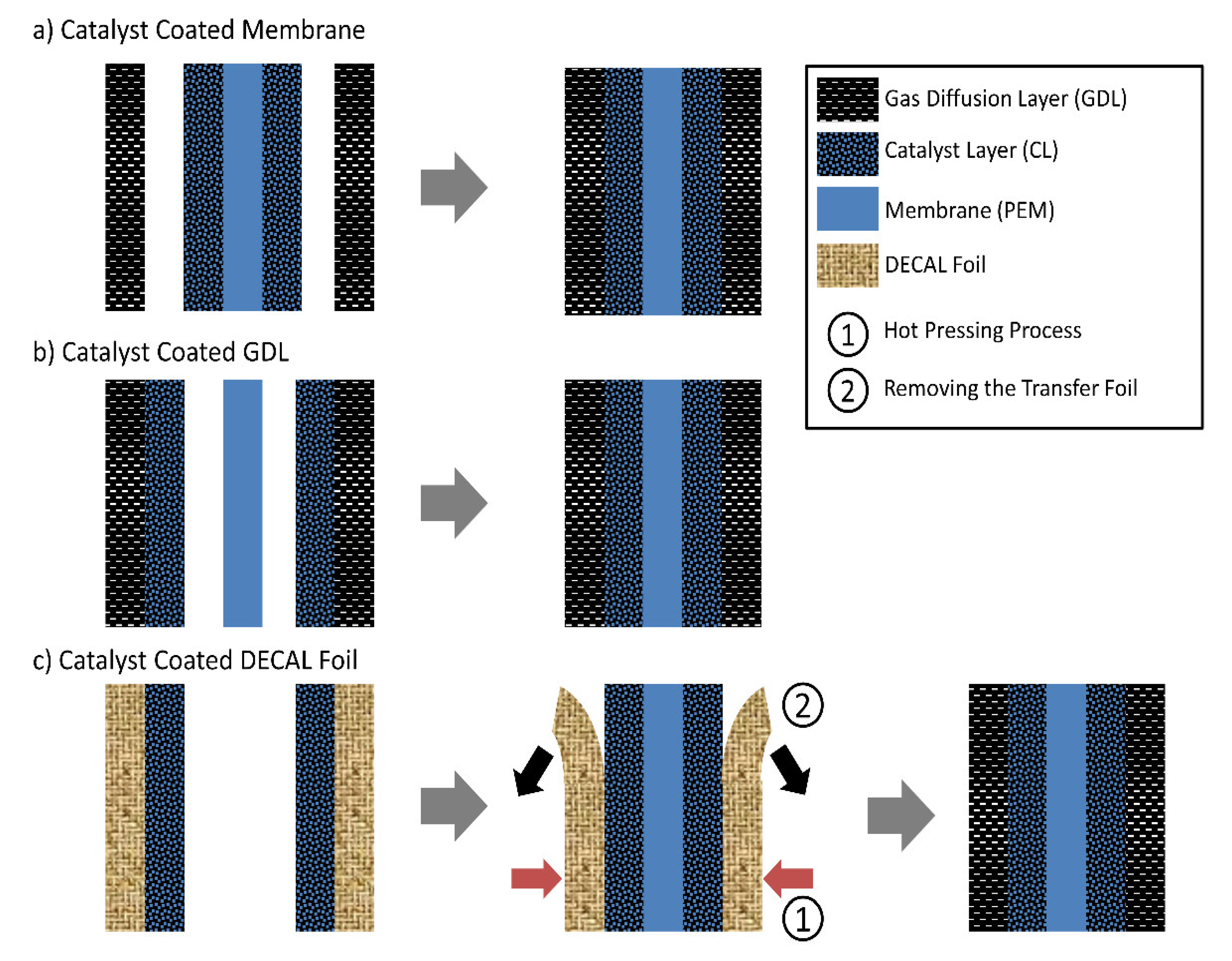



| Ionomer Dispersion | Content (wt.%) | Solvent | EW (g/mol) | IEC (meq/g) | Viscosity (mP s, 25 °C) | |
|---|---|---|---|---|---|---|
| Nafion PFSA | D-520 | 5–5.4 | Water (45 wt.%), 1-propanol (48 wt.%) | 890–970 | 1.03–1.12 | 10–40 (40 s−1) |
| D-1020 | 10–12 | Water (87–90 wt.%) | 2–10 | |||
| D-2020 | 20–22 | Water (34 wt.%), 1-propanol (44 wt.%) | 50–500 | |||
| D-521 | 5–5.4 | Water (45 wt.%), 1-propanol (48 wt.%) | 970–1050 | 0.95–1.03 | 10–40 | |
| D-1021 | 10–12 | Water (87–90 wt.%) | 2–10 | |||
| D-2021 | 20–22 | Water (34 wt.%), 1-propanol (44 wt.%) | 50–500 | |||
| 3M PFSA | 725EW | 15 | 1-propanol/water 60/40 (m/m) | 725 | 1.38 | 50–300(20 °C, 1 s−1) |
| 800EW | 20 | 800 | 1.25 | |||
| 3M PFIA | - | Powder | 620 | 1.61 | — | |
| Aquivion PFSA | D72–25BS | 25 | 99% water, free of ethers % | 700–740 | 1.35–1.43 | <25 |
| D79–25BS | 25 | 790 ± 20 | 1.23–1.30 | <25 | ||
| D83–24B | 24 | >99% water | 810–850 | 1.17–1.23 | 5–15 | |
| D98–25BS | 25 | 99% water, free of ethers % | 940–1020 | 0.98–1.06 | <25 | |
| Solvent | Structure | ε @ 20 °C [144] | Viscosity (cp or mPa s) @ 25 °C [144] | Boiling Point (°C) | Surface Tension (mN/m) @ 25 °C [144] | δ and δtotal * (MPa0.5) @ 25 °C [145] | δD ** [145] | δP ** [145] | δH ** [145] |
|---|---|---|---|---|---|---|---|---|---|
| Diethyl ether | (C2H5)2O | 4.2666 | 0.224 | 34.6 | 16.5 | 15.8 | 14.5 | 2.9 | 5.1 |
| n-Pentane | CH3(CH2)3CH3 | 1.8371 | 0.224 | 36 | 15.45 | 14.5 | 14.5 | 0 | 0 |
| Dichloromethane | CH2Cl2 | 8.93 (25 °C) | 0.413 | 39.6 | 27.20 | 20.2 | 18.2 | 6.3 | 6.1 |
| Acetone | CH3COCH3 | 21.01 | 0.306 | 56 | 22.71 | 19.9 | 15.5 | 10.4 | 7.0 |
| Chloroform | HCCl3 | 4.8069 | 0.537 | 61 | 26.65 | 19.0 | 17.8 | 3.1 | 5.7 |
| Methanol | CH3OH | 33.0 | 0.544 | 65 | 22.17 | 29.6 | 15.1 | 12.3 | 22.3 |
| Tetrahydrofuran (THF) | (CH2)4O | 7.52 (22 °C) | 0.456 | 66 | 26.4 [146] | 19.4 | 16.8 | 5.7 | 8.0 |
| Hexane | CH3(CH2)4CH3 | 1.8865 | 0.3 | 69 | 17.88 | 14.9 | 14.9 | 0 | 0 |
| Ethyl acetate | CH3-COO-CH2-CH3 | 6.0814 | 0.423 | 77 | 23.39 | 18.2 | 15.8 | 5.3 | 7.2 |
| Ethanol | CH3CH3OH | 25.3 | 1.074 | 79 | 21.91 | 26.5 | 15.8 | 8.8 | 19.4 |
| Cyclohexane | C6H12 | 2.0243 | 0.625 | 81 | 24.42 | 16.8 | 16.8 | 0 | 0.2 |
| 2-propanol (IPA) | CH3-CHOH-CH2 | 20.18 | 2.04 | 82 | 20.92 | 23.6 | 15.8 | 6.1 | 16.4 |
| Triethylamine | N(CH2CH3)3 | 2.418 | 0.347 | 90 | 20.22 | 18.8 | 17.8 | 0.4 | 1.0 |
| 1-Propanol (NPA) | CH3CH2CH3OH | 20.8 | 1.945 | 97 | 23.37 | 24.6 | 16.0 | 6.8 | 17.4 |
| n-Heptane | CH3(CH2)5CH3 | 1.9209 | 0.387 | 98 | 19.73 | 15.3 | 15.3 | 0 | 0 |
| 2-Butanol | CH3CH2CHOHCH3 | 17.26 | 3.10 | 99 | 24.13 | 23.2 | 16 | 5.7 | 15.8 |
| Water | H2O | 80.1 | 0.89 | 100 | 72.06 | 47.8 | 15.6 | 16.0 | 42.3 |
| Formic acid | HCOOH | 51.1 (25 °C) | 1.607 | 101 | 37.13 | 24.9 | 14.3 | 11.9 | 16.6 |
| Toluene | C6H5-CH3 | 2.38 (23 °C) | 0.56 | 111 | 27.91 | 18.2 | 18.0 | 1.4 | 2.0 |
| Pyridine | C5H5N | 13.26 | 0.879 | 115 | 36.56 | 21.8 | 19.0 | 8.8 | 5.9 |
| Acetic acid | CH3COOH | 6.2 | 1.056 | 118 | 27.10 | 21.4 | 14.5 | 8.0 | 13.5 |
| 1-Butanol | CH3CH2CH2CH2OH | 17.84 | 2.54 | 118 | 24.13 | 23.2 | 16.0 | 5.7 | 15.8 |
| n-Octane | CH3(CH2)6CH3 | 1.948 | 0.508 | 125 | 21.17 | 15.5 | 15.5 | 0 | 0 |
| n-Butyl acetate | CH3-CO-O-(CH2)3CH3 | 5.07 | 0.685 | 126 | 24.88 | 17.4 | 15.8 | 3.7 | 6.3 |
| Isoamyl alcohol | (H3C)2CHCH2CH2OH | 15.63 | 3.69 | 131 | 23.73 | 21.3 | 15.8 | 5.2 | 13.3 |
| p-Xylene | C6H4(CH3)2 | 2.2735 | 0.603 | 138 | 27.80 | 17.9 | 17.6 | 1.0 | 3.1 |
| N,N-dimethylformamide (DMF) | (CH3)2NCHO | 38.25 | 0.794 | 153 | 35.74 | 24.9 | 17.4 | 13.7 | 11.3 |
| Cyclohexanol | HOCH(CH2)5 | 16.4 | 57.5 | 162 | 33.25 | 22.4 | 17.4 | 4.1 | 13.5 |
| Diacetone alcohol (DAA) | CH3C(O)CH2C(OH)(CH3)2 | 18.2 | 2.8 | 168 | 31 [147] | 20.8 | 15.8 | 8.2 | 10.8 |
| Aniline | C6H5-NH2 | 7.06 | 3.85 | 184 | 42.12 | 22.6 | 19.4 | 5.1 | 10.2 |
| 1,2-Propylene glycol (PG) | HOCH2-CHOH-CH3 | 27.5 (30 °C) | 40.4 | 187 | 35.99 | 30.2 | 16.8 | 9.4 | 23.3 |
| Dimethyl sulfoxide (DMSO) | (CH3)2SO | 47.24 | 1.987 | 189 | 42.92 | 26.7 | 18.4 | 16.4 | 10.2 |
| Octanol | CH3(CH2)7OH | 10.3 | 7.29 | 195 | 26.96 | 20.6 | 16.0 | 5.0 | 11.9 |
| Ethylene glycol (EG) | HOCH2CH2OH | 41.4 | 16.06 | 197 | 48.02 | 32.9 | 17.0 | 11.0 | 26.0 |
| N-Methyl-2-pyrrolidone (NMP) | C5H9NO | 32.55 | 1.65 [148] | 203 | 40.21 | 23.0 | 18.0 | 12.3 | 7.2 |
| Benzyl alcohol | C6H5-CH2OH | 11.92 (30 °C) | 5.47 | 205 | 36.8 [149] | 23.8 | 18.4 | 6.3 | 13.7 |
| Formamide | HCONH2 | 111 | 3.34 | 210 | 57.03 | 16.6 | 17.2 | 26.2 | 19.0 |
| 1,3-propanediol (PDO) | HO(CH2)3OH | 35.1 | 42.3 [126] | 214 | 53.125 [150] | 16.8 | 13.5 | 23.2 | 72.5 |
| Diethylene glycol | (HOCH2CH2)2O | 31.82 | 30.2 | 245 | 44.82 | 29.1 | 16.6 | 12.0 | 20.7 |
| Glycerol | HOCH2-CHOH-CH2OH | 46.53 | 934 | 290 | 64 (20 °C) [151] | 36.1 | 17.4 | 12.1 | 29.3 |
| Nafion N115 | N/A | N/A | N/A | N/A | N/A | 23.5 [152] | 17.4 | 12.5 | 9.6 |
| Nafion N117 | N/A | N/A | N/A | N/A | N/A | 19.9 [153] | 15.1 | 8.9 | 9.4 |
| Aquivion E87-125 | N/A | N/A | N/A | N/A | N/A | 23 [130] | 17.0 | 10.1 | 11.8 |
| Methods | Advantages | Disadvantages |
|---|---|---|
| CCM |
|
|
| GDE |
|
|
| DTM |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Ney, L.; Zamel, N.; Li, X. Effect of Catalyst Ink and Formation Process on the Multiscale Structure of Catalyst Layers in PEM Fuel Cells. Appl. Sci. 2022, 12, 3776. https://doi.org/10.3390/app12083776
Liu H, Ney L, Zamel N, Li X. Effect of Catalyst Ink and Formation Process on the Multiscale Structure of Catalyst Layers in PEM Fuel Cells. Applied Sciences. 2022; 12(8):3776. https://doi.org/10.3390/app12083776
Chicago/Turabian StyleLiu, Huiyuan, Linda Ney, Nada Zamel, and Xianguo Li. 2022. "Effect of Catalyst Ink and Formation Process on the Multiscale Structure of Catalyst Layers in PEM Fuel Cells" Applied Sciences 12, no. 8: 3776. https://doi.org/10.3390/app12083776
APA StyleLiu, H., Ney, L., Zamel, N., & Li, X. (2022). Effect of Catalyst Ink and Formation Process on the Multiscale Structure of Catalyst Layers in PEM Fuel Cells. Applied Sciences, 12(8), 3776. https://doi.org/10.3390/app12083776







