Organ-on-a-Chip: Design and Simulation of Various Microfluidic Channel Geometries for the Influence of Fluid Dynamic Parameters
Abstract
:1. Introduction
2. Materials and Methods
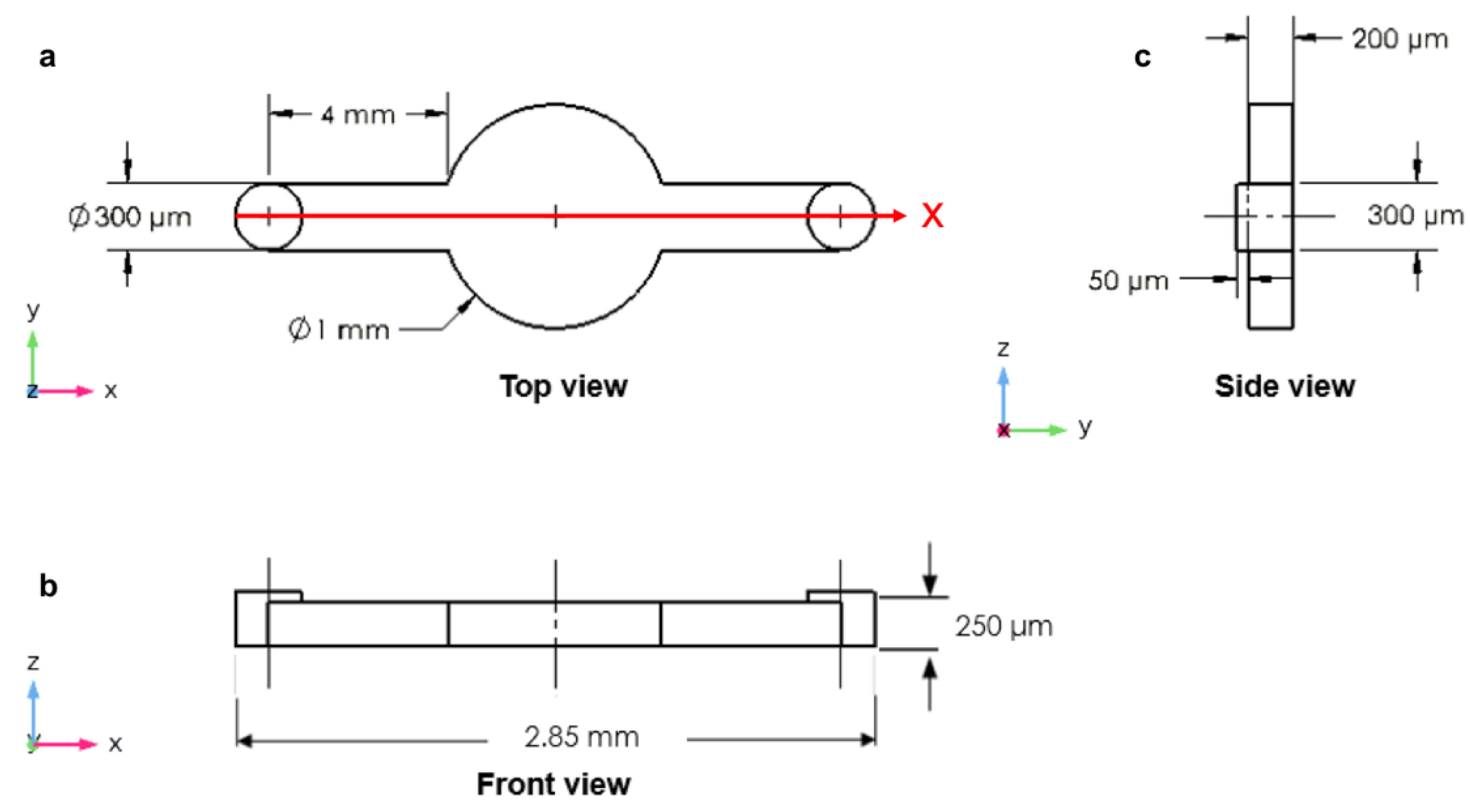
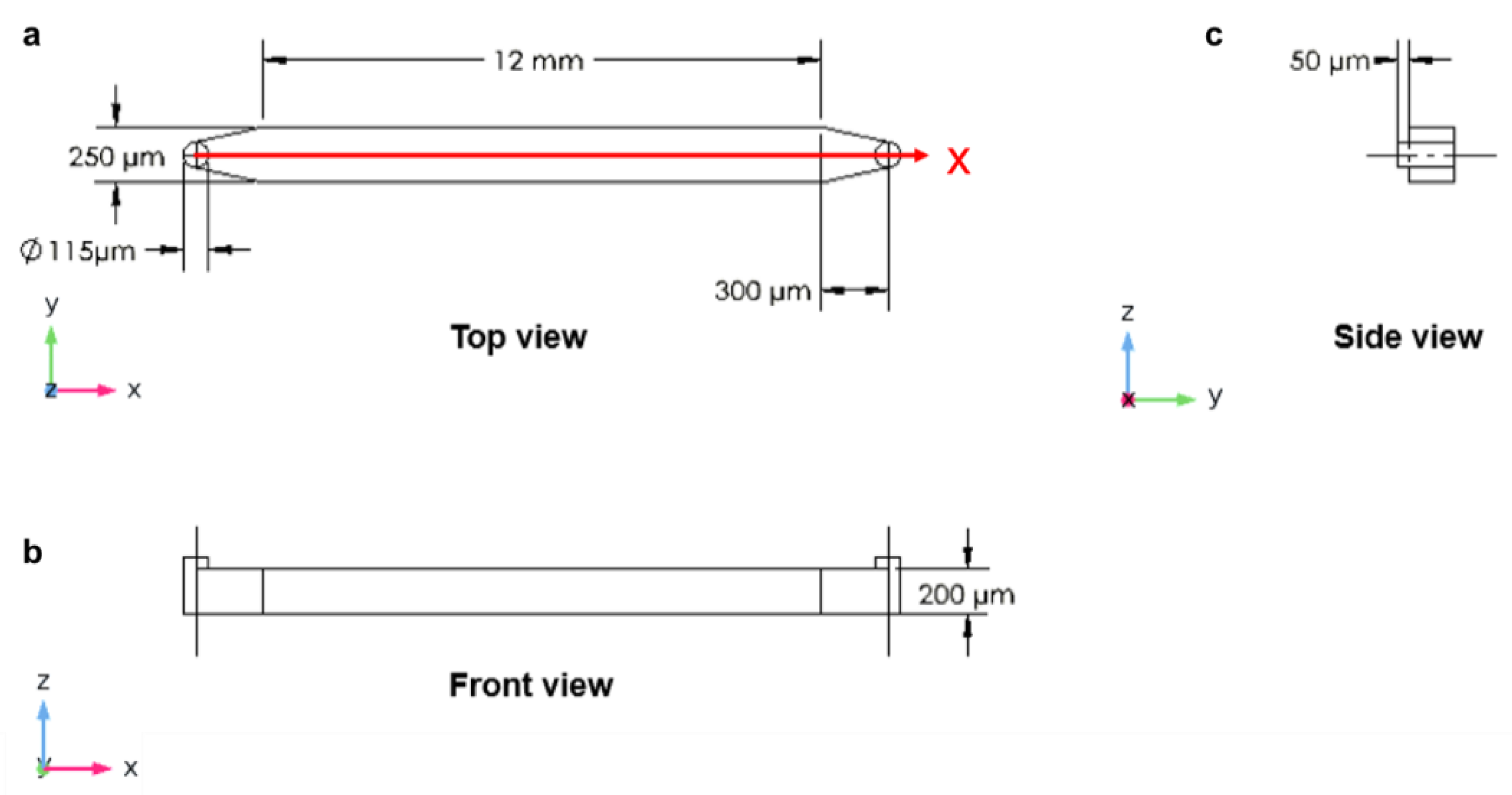
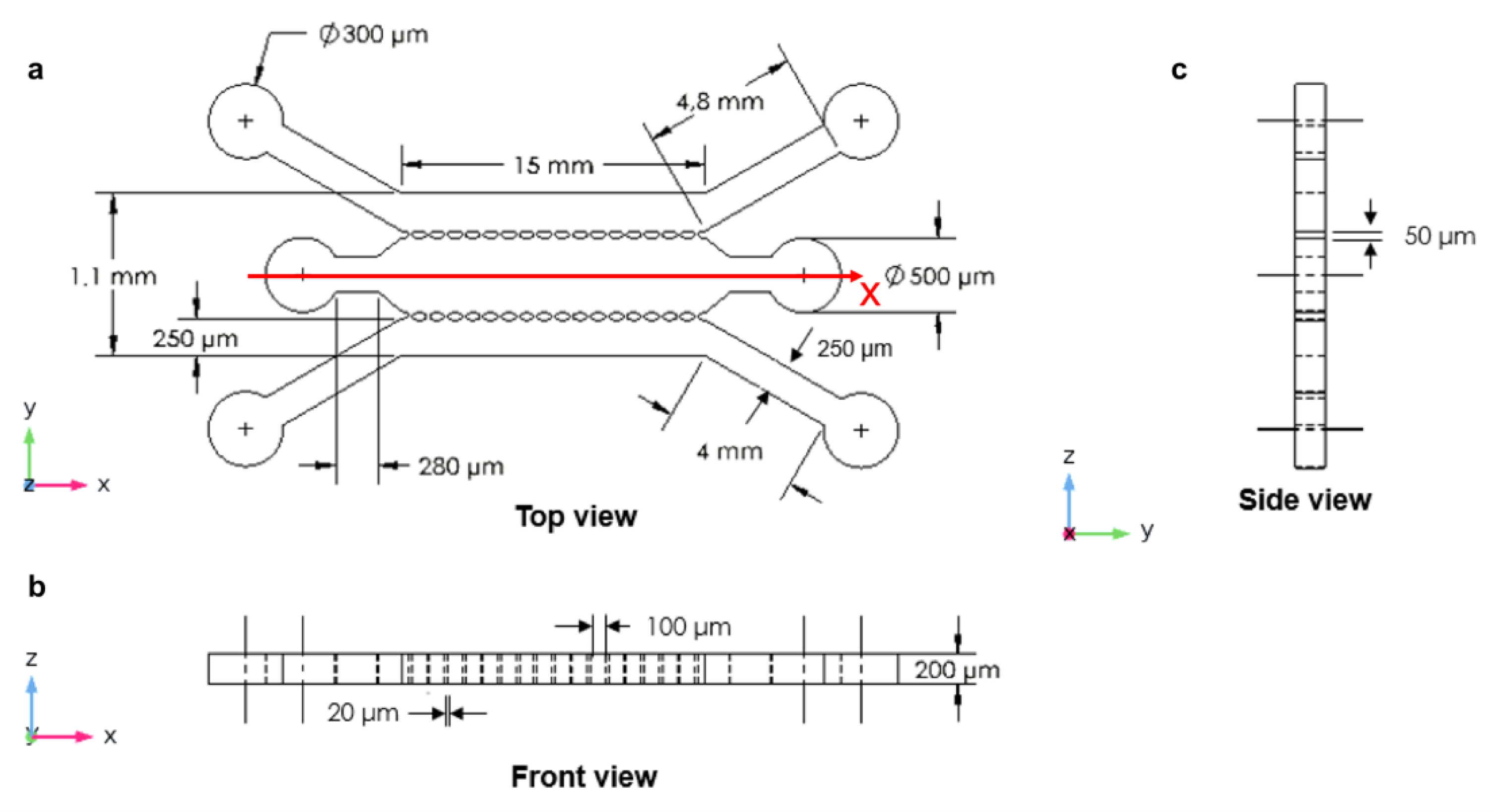
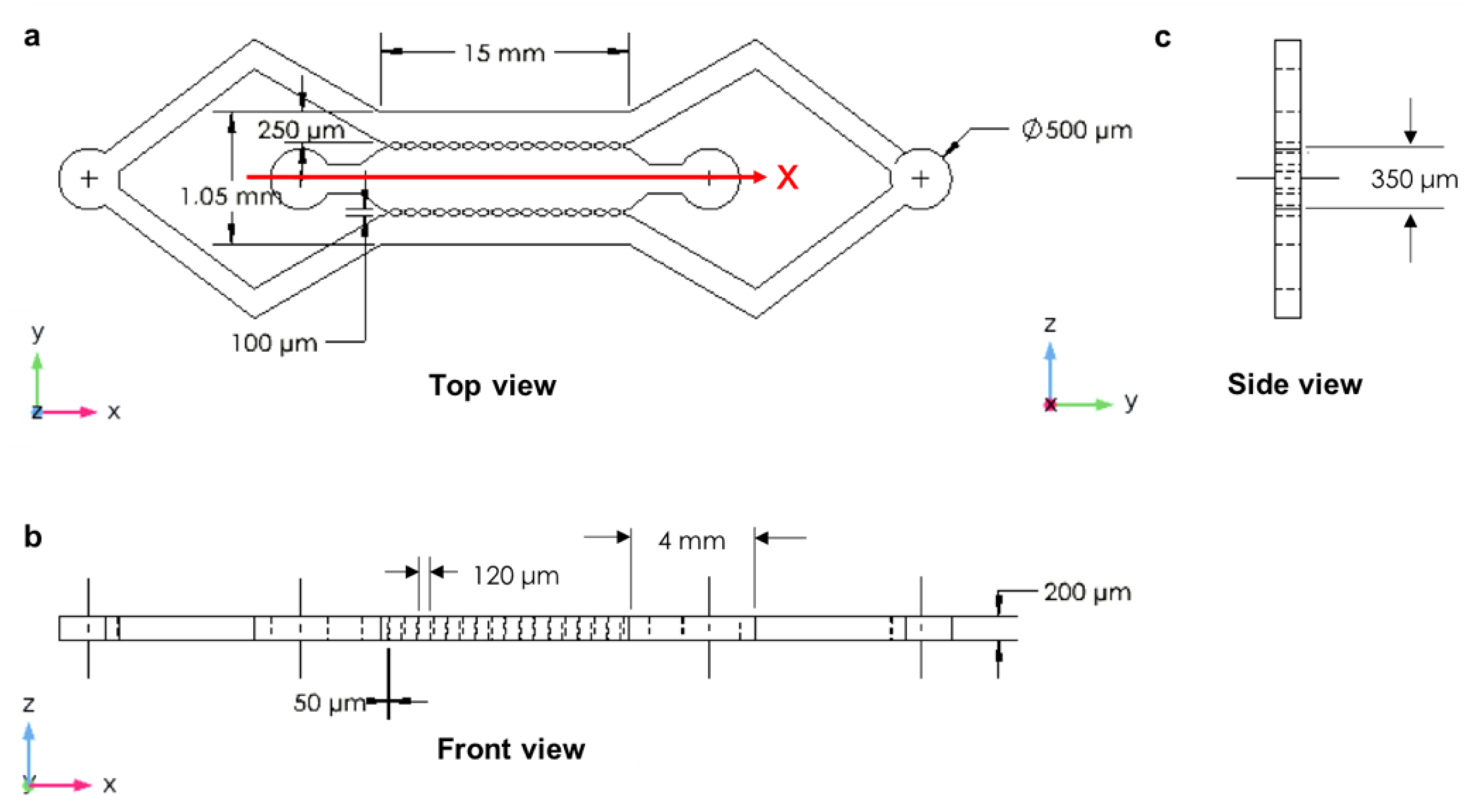
2.1. The Design of Microfluidic Devices
2.2. Computational Fluid Dynamic Simulation
2.3. Velocity Profiles Characteristics
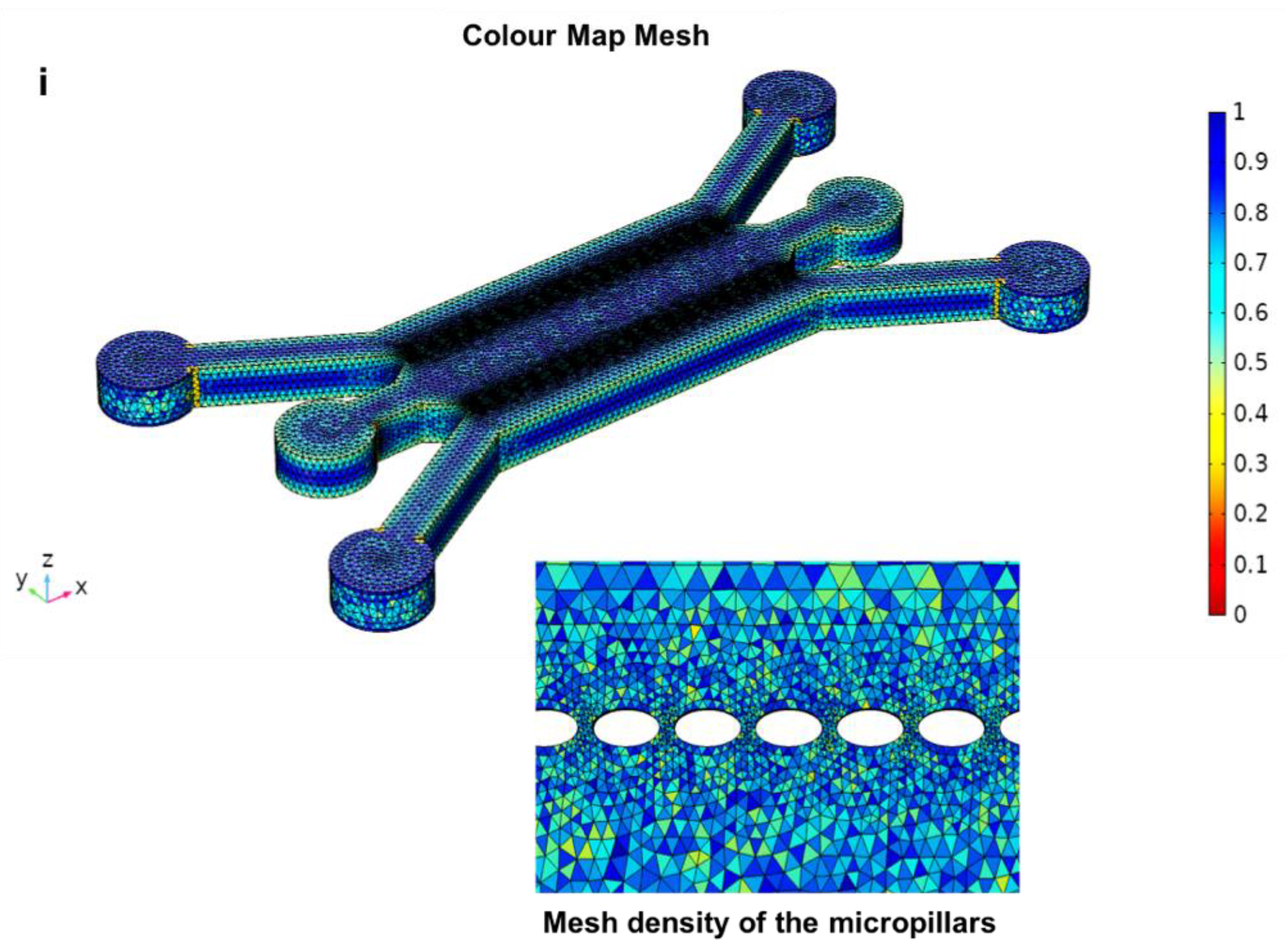
2.4. Computational Meshing for CFD Simulations
2.5. Post-Processing
3. Results and Discussions
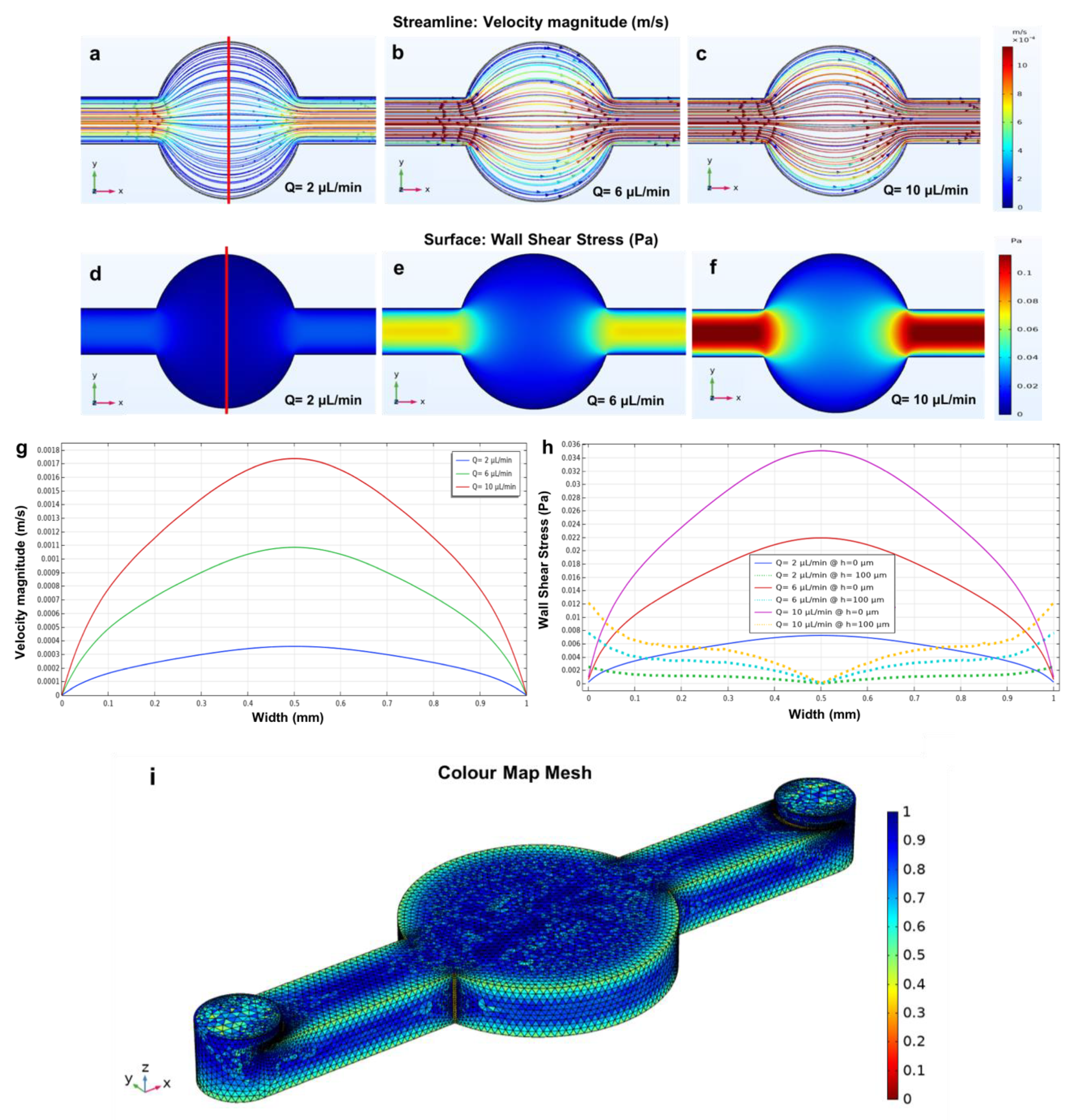
3.1. Circular Microchamber
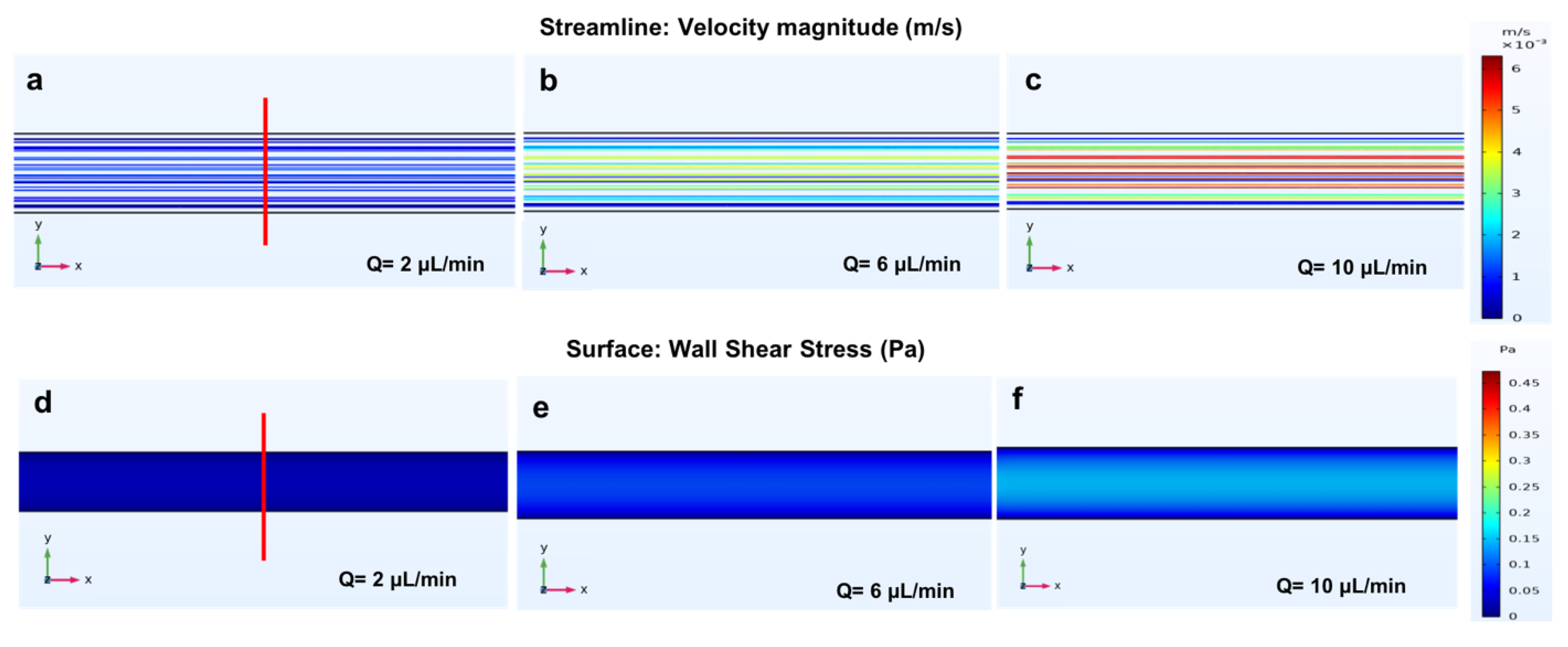
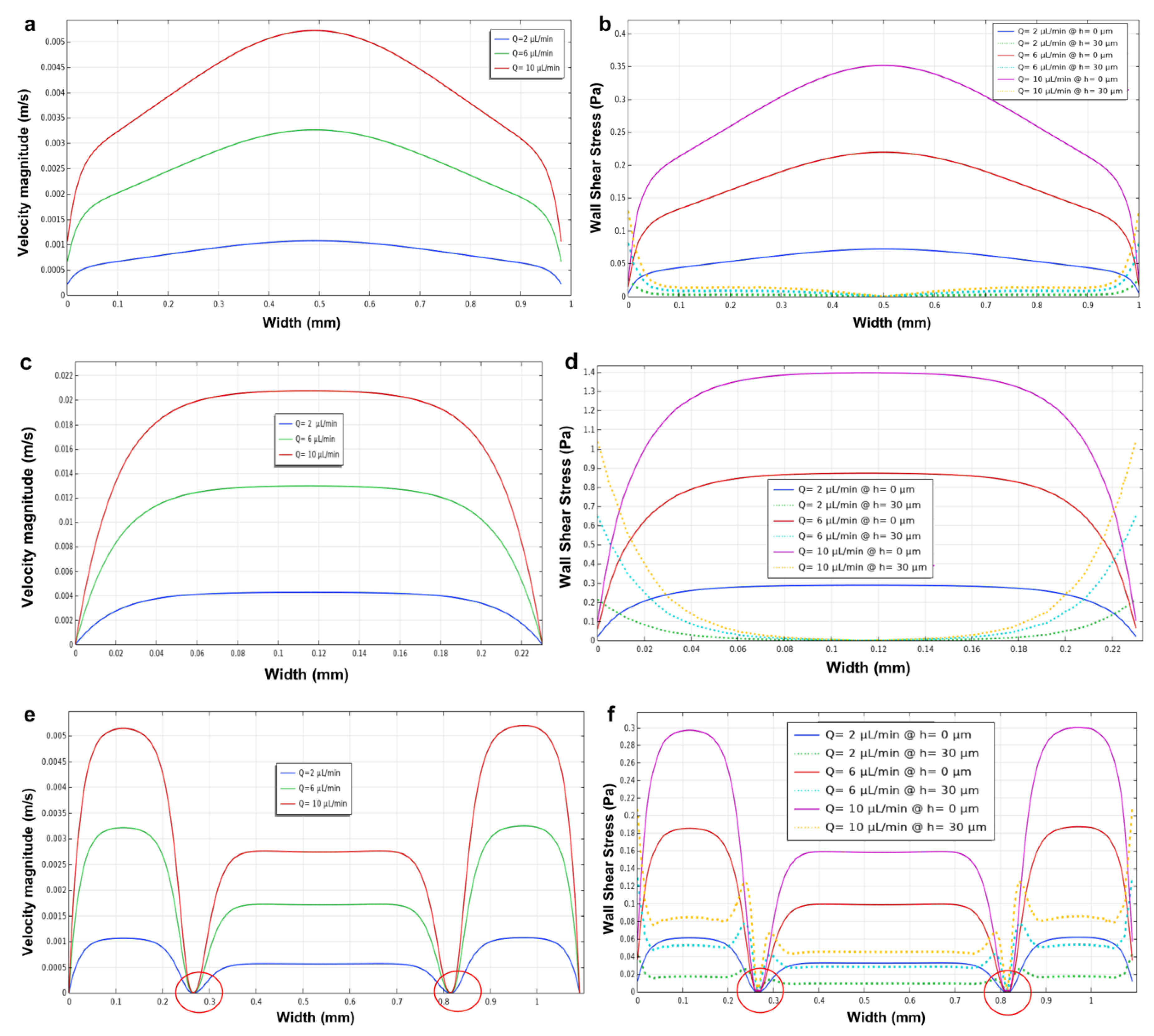
3.2. Longitudinal Microchannel
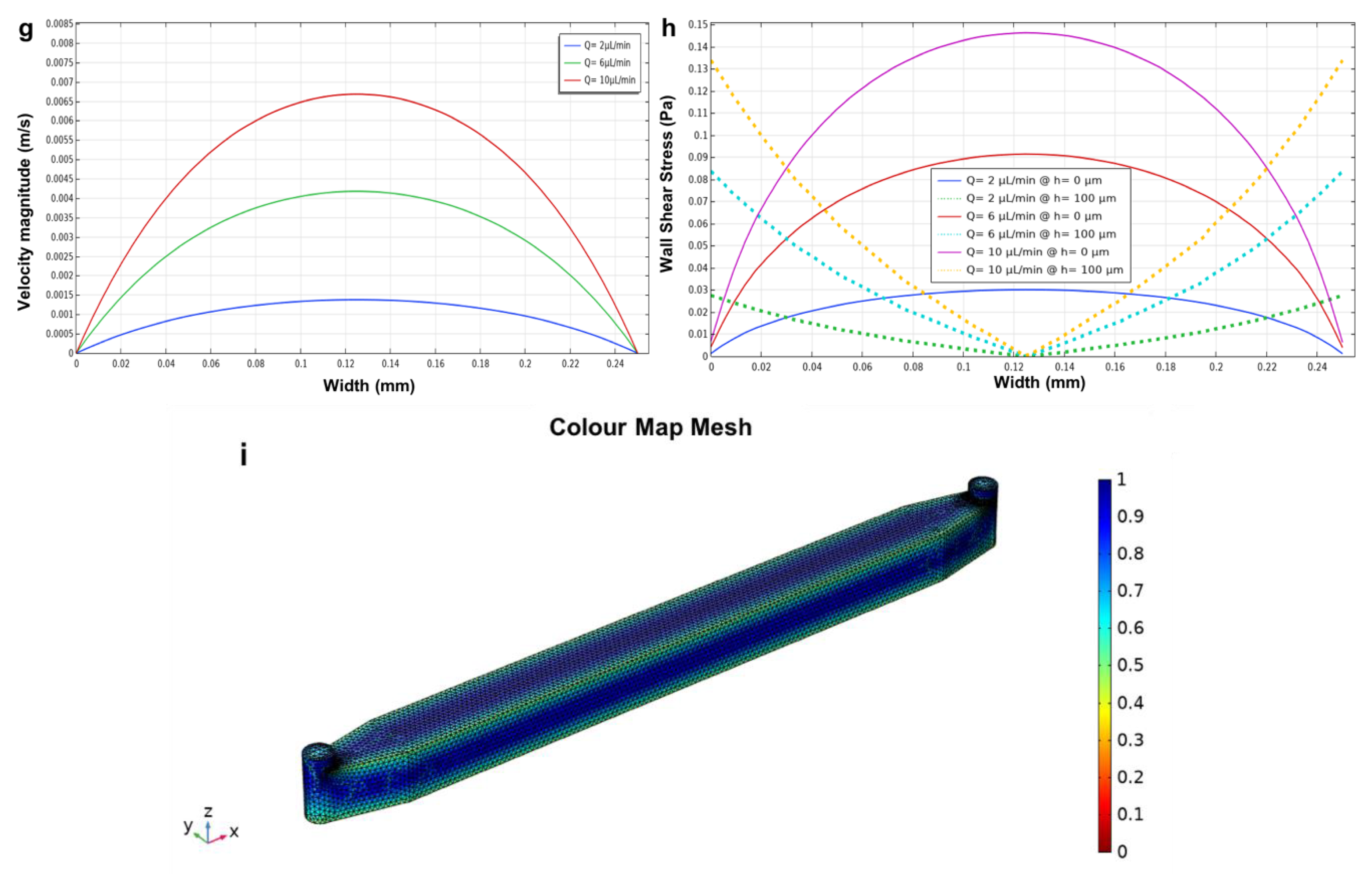
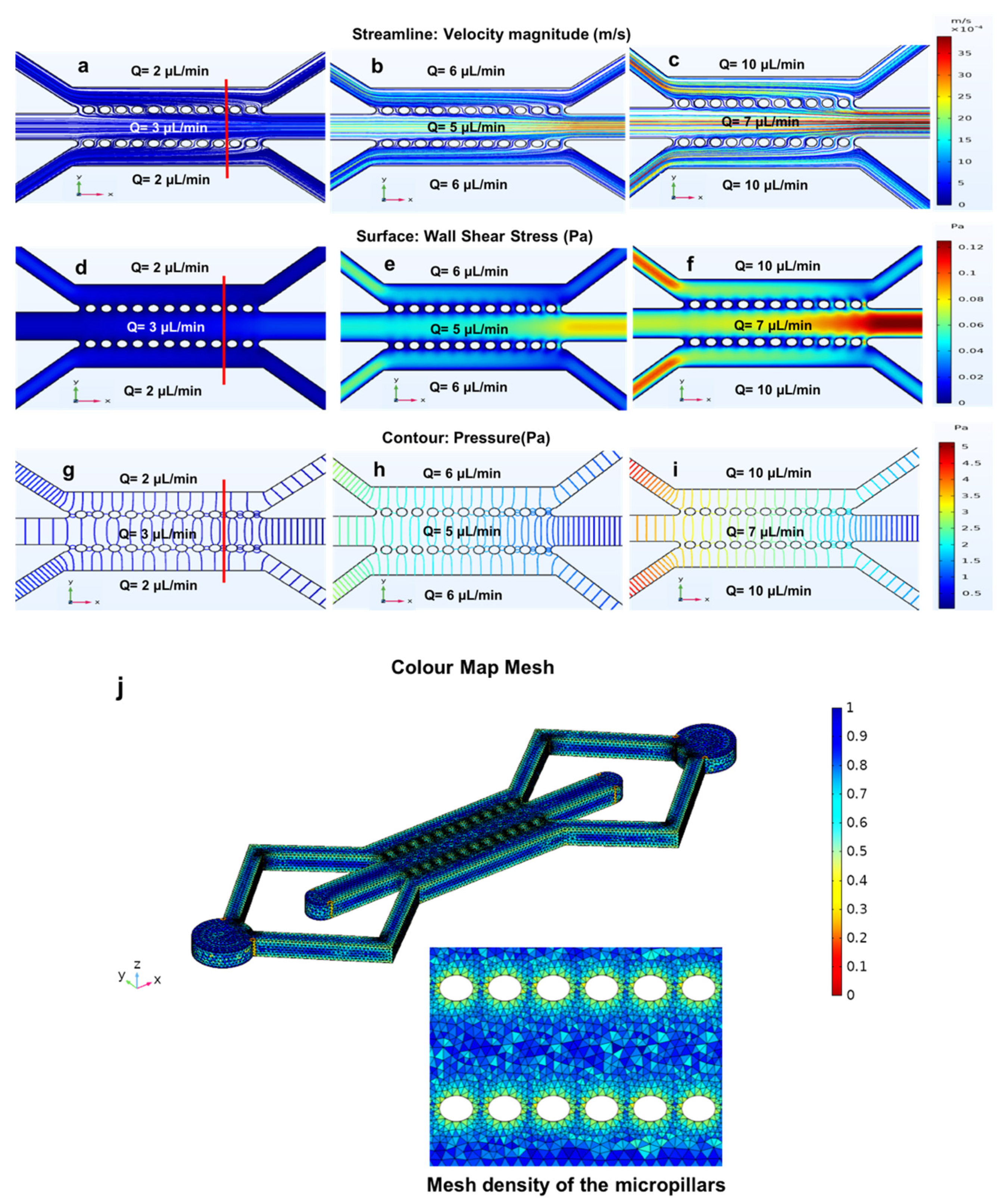
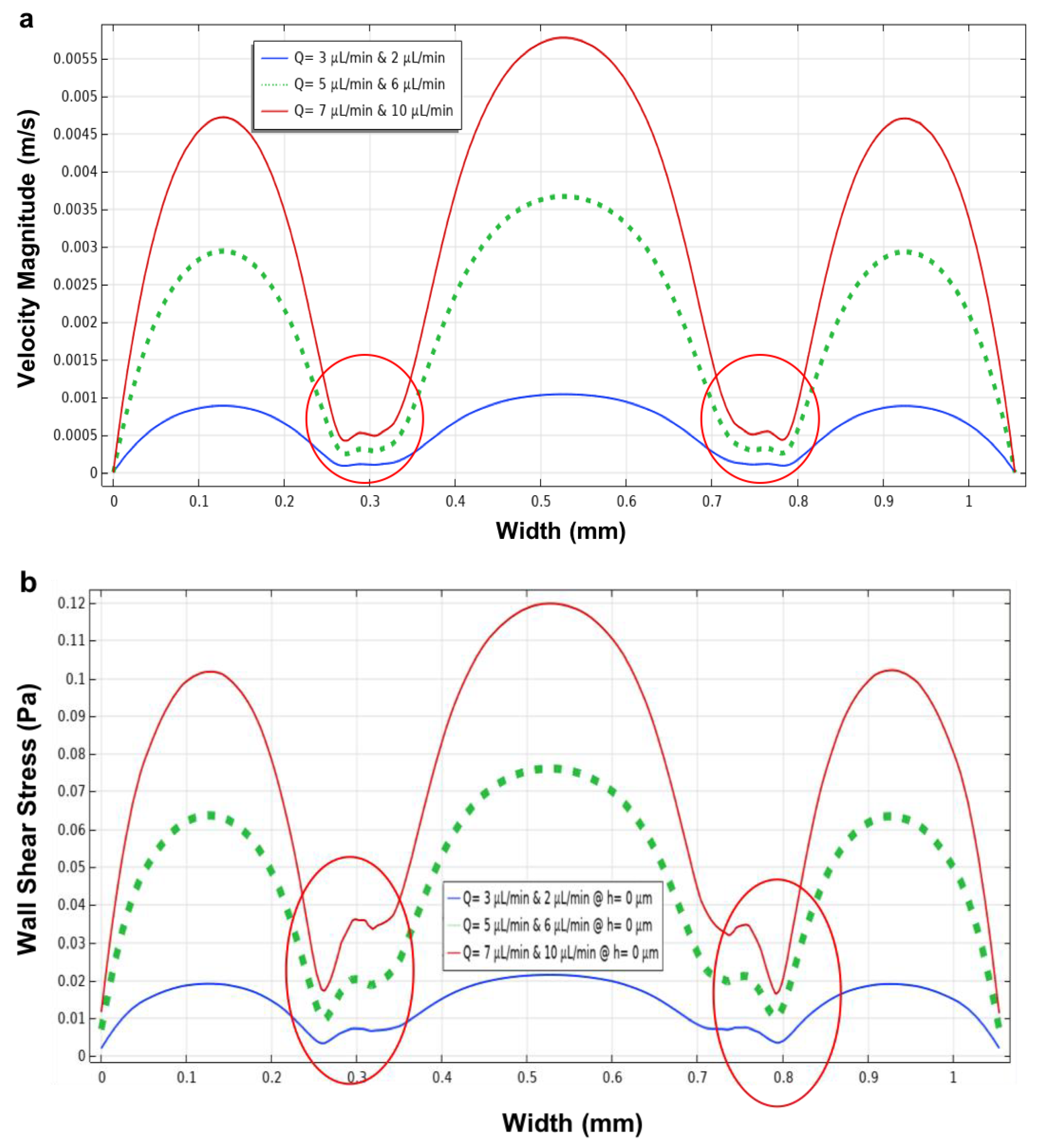
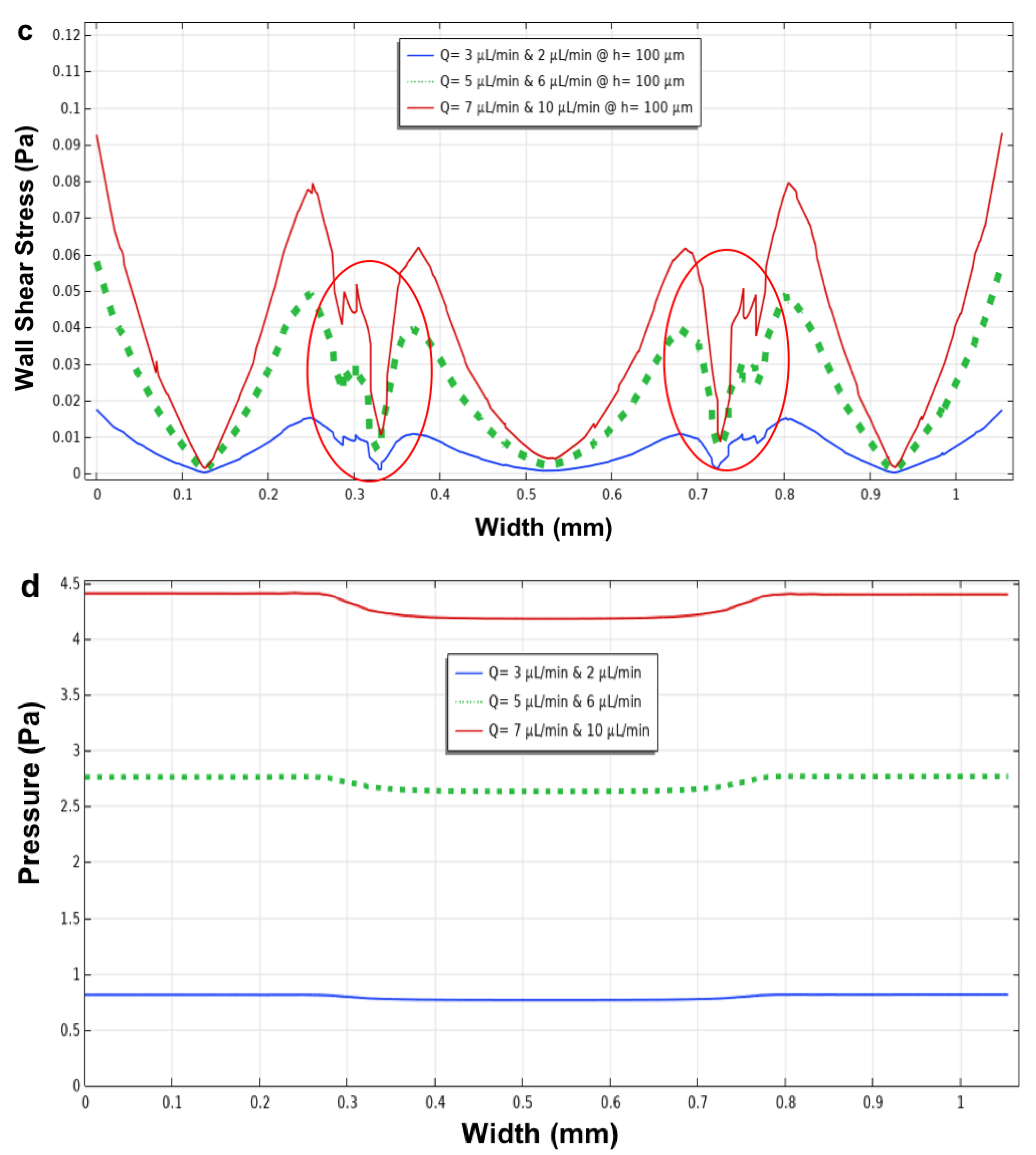
3.3. Three Parallel Microchannels
3.4. The Closed-Microchannel Structure
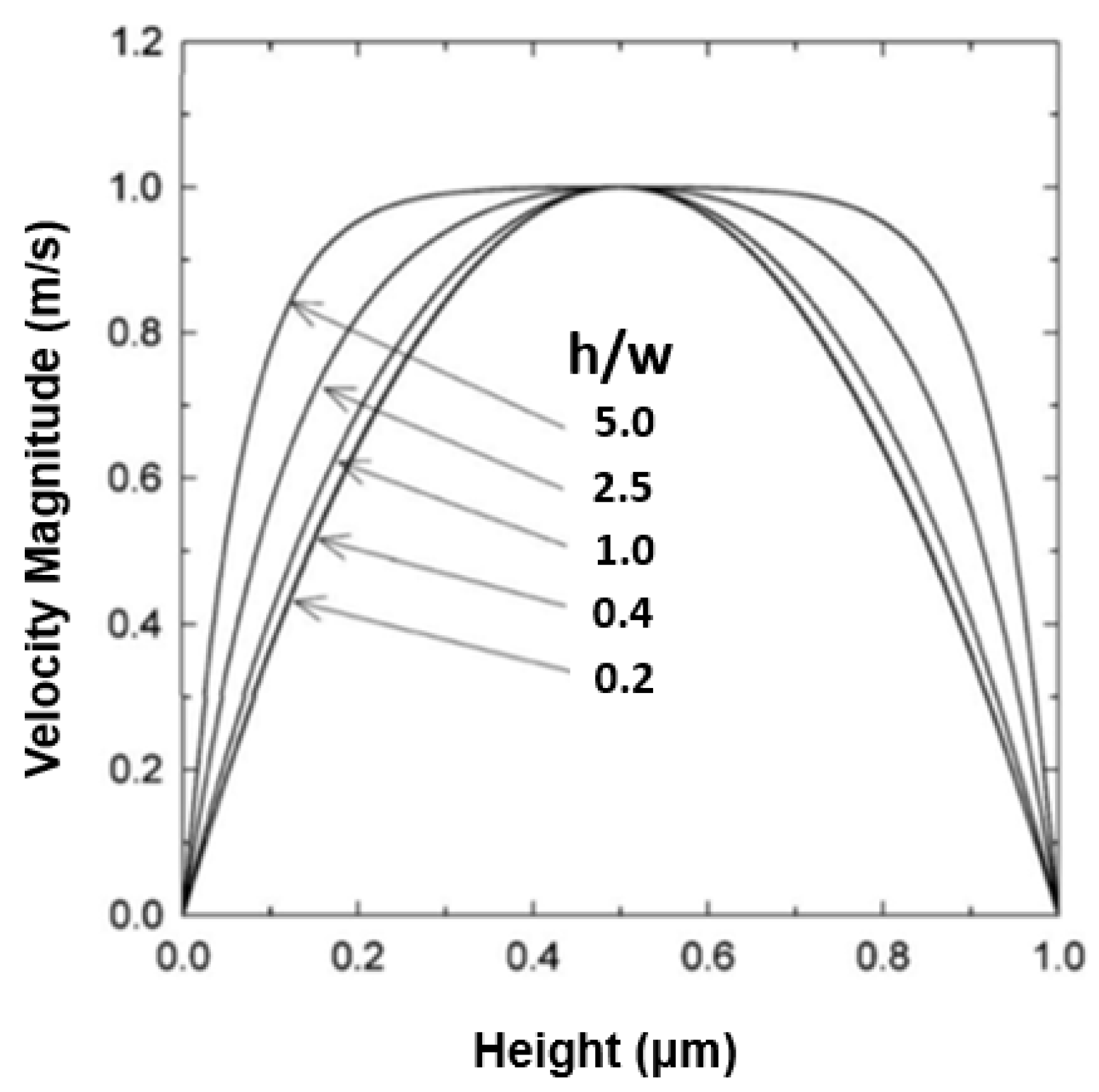
3.5. Effect of Microchannel Height within a Microfluidic Device
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CFD | Computational fluid dynamics |
| ECM | Extracellular matrix |
| MFDs | Microfluidic devices |
| OOC | Organ-on-a-chip |
| PDMS | Polydimethylsiloxane |
| 2D | Two-dimensional |
| 3D | Three-dimensional |
References
- Kaptoge, S.; Pennells, L.; De Bacquer, D.; Cooney, M.T.; Kavousi, M.; Stevens, G.; Riley, L.M.; Savin, S.; Khan, T.; Altay, S.; et al. World Health Organization cardiovascular disease risk charts: Revised models to estimate risk in 21 global regions. Lancet Glob. Health 2019, 7, e1332–e1345. [Google Scholar] [CrossRef] [Green Version]
- Wouters, O.J.; McKee, M.; Luyten, J. Estimated research and development investment needed to bring a new medicine to market. JAMA 2020, 323, 844–853. [Google Scholar] [CrossRef]
- O’Neill, H.S.; Gallagher, L.B.; O’Sullivan, J.; Whyte, W.; Curley, C.; Dolan, E.; Hameed, A.; O’Dwyer, J.; Payne, C.; O’Reilly, D.; et al. Biomaterial-enhanced cell, and drug delivery: Lessons learned in the cardiac field and future perspectives. Adv. Mater. 2016, 28, 5648–5661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, P.; Lemcke, H.; David, R. Stem cell therapy in heart diseases- cell types, mechanisms, and improvement strategies. Cell. Physiol. Biochem. 2018, 48, 2607–2655. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Bonde, M.; Sarinivasan, G. Biodegradable polymer scaffold for tissue engineering. Trends Biomater. Artif. Organs 2011, 25, 20–29. [Google Scholar]
- Curtis, M.W.; Russell, B. Cardiac tissue engineering. J. Cardiovasc. Nurs. 2009, 24, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Marc, N.H.; Hansen, A.; Eschenhagen, T. Cardiac tissue engineering. Circ. Res. 2014, 114, 354–367. [Google Scholar] [CrossRef] [Green Version]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef]
- Yacoub, M.H.; Takkenberg, J.J.M. Will heart valve tissue engineering change the world? Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 60–61. [Google Scholar] [CrossRef]
- Ciofania, G.; Migliore, A.; Raffa, V.; Menciass, A.; Dario, P. Bicompartmental device for dynamic cell coculture: Design, realization and preliminary results. J. Biosci. Bioeng. 2008, 10, 536–544. [Google Scholar] [CrossRef]
- Pasirayi, G.; Auger, V.; Scott, S.M.; Rahman, P.K.S.M.; Islam, M.; O’Hare, L.; Ali, Z. Microfluidic bioreactors for cell culturing: A review. Micro Nanosyst. 2011, 3, 137–160. [Google Scholar] [CrossRef] [Green Version]
- Convery, N.; Gadegaard, N. 30 years of microfluidics. Micro Nano Eng. 2019, 2, 76–91. [Google Scholar] [CrossRef]
- Wasson, E.M.; Dubbin, K.; Moya, M.L. Go with the flow: Modeling unique biological flows in engineered in vitro platforms. Lab Chip 2021, 21, 2095–2120. [Google Scholar] [CrossRef]
- Bhatia, S.; Ingber, D. Microfluidic organs-on-chip. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Kim, H.J.; Ingber, D.E. Gut-on-a-chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr. Biol. 2013, 5, 1130–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, H.; Sakai, Y.; Fujii, T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab. Pharmacokinet. 2018, 33, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Takayama, S.; Ostuni, E.; LeDuc, P.; Naruse, K.; Ingber, D.E.; Whitesides, G.M. Laminar flows: Subcellular positioning of small molecules. Nature 2001, 411, 1016. [Google Scholar] [CrossRef] [Green Version]
- Xi, C.; Yu, S.Z.; Xinping, Z.; Changsheng, L. Organ-on-a-chip platforms for accelerating the evaluation of nanomedicine. Bioact. Mater. 2021, 6, 1012–1027. [Google Scholar] [CrossRef]
- Carvalho, V.; Rodrigues, R.O.; Lima, R.A.; Teixeira, S. Computational Simulations in Advanced Microfluidic Devices: A Review. Micromachines 2021, 12, 1149. [Google Scholar] [CrossRef]
- Sidorov, V.Y.; Samson, P.C.; Sidorova, T.N.; Davidson, J.M.; Lim, C.C.; Wikswo, J.P. I-Wire Heart-on-a-Chip I: Three-dimensional cardiac tissue constructs for physiology and pharmacology. Acta Biomater. 2017, 15, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Ingber, D.E. Developmentally inspired human organs on chips. Development 2018, 145, dev156125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobuszewska, A.; Tomecka, E.; Zukowski, K.; Jastrzebska, E.; Chudy, M.; Dybko, A.; Renaud, P.; Brzozka, Z. Heart-on-a-Chip: An investigation of the influence of static and perfusion conditions on cardiac (H9C2) cell proliferation, morphology, and alignment. SLAS Technol. 2017, 22, 536–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atmaramani, R.; Black, B.J.; Lam, K.H.; Sheth, V.M.; Pancrazio, J.J.; Schmidtke, D.W.; Alsmadi, N.Z. The effect of microfluidic geometry on myoblast migration. Micromachines 2019, 10, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedieh, F.; Jun, Z.; Jordan, N.; Hoang-Phuong, P.; Nam-Trung, N. Stretchable Inertial Microfluidic Device for Tunable Particle Separation. Anal. Chem. 2020, 92, 12473–12480. [Google Scholar] [CrossRef]
- Tomecka, E.; Zukowski, K.; Jastrzebska, E.; Chudy, M.; Brzozka, Z. Microsystem with micropillar array for three-(gel-embedded) and two-dimensional cardiac cell culture. Sens. Actuators B Chem. 2018, 254, 973–983. [Google Scholar] [CrossRef]
- Green, J.V.; Kniazeva, T.; Abedi, M.; Sokhey, D.S.; Taslimb, M.E.; Murthy, S.K. Effect of channel geometry on cell adhesion in microfluidic devices. Lab Chip 2008, 9, 677–685. [Google Scholar] [CrossRef]
- Zohreh, S.; Pooria, A.; Navid, K. Advances in numerical approaches for microfluidic cell analysis platforms. J. Sci. Adv. Mater. Devices 2020, 5, 295–307. [Google Scholar] [CrossRef]
- Letourneau, A.; Kegel, J.; Al-Ramahi, J.; Yachinich, E.; Krause, H.B.; Stewart, C.J.; McClean, M.N. A Microfluidic Device for Imaging Samples from Microbial Suspension Cultures. MethodsX 2020, 7, 100891. [Google Scholar] [CrossRef]
- Wang, X.; Lee, J.; Ali, M.; Kim, J.; Lacerda, C.M.R. Phenotype transformation of aortic valve interstitial cells due to applied shear stresses within a microfluidic chip. Ann. Biomed. Eng. 2017, 45, 2269–2280. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, J.M.; Ahrberg, C.D.; Chung, B.G. Development of the microfluidic device to regulate shear stress gradients. BioChip J. 2018, 12, 294–303. [Google Scholar] [CrossRef]
- Sontheimer-Phelps, A.; Hassell, B.A.; Ingber, D.E. Modelling cancer in microfluidic human organs-on-chips. Nat. Rev. Cancer 2019, 19, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Jigar, P.H.; Kent, N.J.; Knox, A.J.S.; Harris, L.F. Microfluidics in Haemostasis: A Review. Molecules 2020, 25, 833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizgolshani, H.; Coppeta, J.R.; Vedula, E.M.; Marr, E.E.; Cain, B.P.; Luu, R.J.; Lech, M.P.; Kann, S.H.; Mulhern, T.J.; Tandon, V.; et al. High-throughput organ-on-chip platform with integrated programmable fluid flow and real-time sensing for complex tissue models in drug development workflows. Lab Chip 2021, 21, 1454–1474. [Google Scholar] [CrossRef]
- Carvalho, V.; Rodrigues, N.; Ribeiro, R.; Costa, P.F.; Teixeira, J.C.F.; Lima, R.A.; Teixeira, S.F.C.F. Hemodynamic study in 3D printed stenotic coronary artery models: Experimental validation and transient simulation. Comput. Methods Biomech. Biomed. Eng. 2020, 24, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.; Rodrigues, N.; Ribeiro, R.; Costa, P.F.; Lima, R.A.; Teixeira, S.F. 3D printed biomodels for flow visualization in stenotic vessels: An experimental and numerical study. Micromachines 2020, 11, 549. [Google Scholar] [CrossRef]
- Doutel, E.; Viriato, N.; Carneiro, J.; Campos, J.B.; Miranda, J.M. Geometrical effects in the hemodynamics of stenotic and non-stenotic left coronary arteries—Numerical and in vitro approaches. Int. J. Numer. Methods Biomed. Eng. 2019, 35, e3207. [Google Scholar] [CrossRef]
- Mosavati, B.; Oleinikov, A.V.; Du, E. Development of an organ-on-a-chip-device for study of placental pathologies. Int. J. Mol. Sci. 2020, 21, 8755. [Google Scholar] [CrossRef]
- Peng, J.; Fang, C.; Ren, S.; Pan, J.; Jia, Y.; Shu, Z.; Gao, D. Development of a microfluidic device with precise on-chip temperature control by integrated cooling and heating components for single cell-based analysis. Int. J. Heat Mass Transf. 2019, 130, 660–667. [Google Scholar] [CrossRef]
- Hynes, W.F.; Pepona, M.; Robertson, C.; Alvarado, J.; Dubbin, K.; Triplett, M.; Adorno, J.J.; Randles, A.; Moya, M.L. Examining metastatic behavior within 3D bioprinted vasculature for the validation of a 3D computational flow model. Sci. Adv. 2020, 6, eabb3308. [Google Scholar] [CrossRef]
- Bruus, H. Acoustofluidics 1: Governing equations in microfluidics. Lab Chip 2011, 11, 3742–3751. [Google Scholar] [CrossRef] [Green Version]
- Spurk, J.; Aksel, N. Fluid Mechanics, 2nd ed.; Springer: Berlin, Germany, 2008. [Google Scholar]
- Huber, D.; Oskooei, A.; Casadevalli, S.X.; DeMello, A.; Kaigala, G.V. Hydrodynamics in cell studies. Chem. Rev. 2018, 118, 2042–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlichting, H.; Gersten, K.; Krause, E.; Oertel, H. Boundary-Layer Theory, 9th ed.; Springer: Berlin, Germany, 2017; p. 8. [Google Scholar]
- Bukowska, D.M.; Derzsi, L.; Tamborski, S.; Szkulmowski, M.; Garstecki, P.; Wojtkowski, M. Assessment of the flow velocity of blood cells in a microfluidic device using joint spectral and time domain optical coherence tomography. Opt. Express 2013, 21, 24025–24038. [Google Scholar] [CrossRef] [PubMed]
- Tanyeri, M.; Ranka, M.; Sittipolkula, N.; Schroeder, C.M.A. Microfluidic-based hydrodynamic trap: Design and implementation. Lab Chip 2011, 11, 3742–3751. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Chun, M.S.; Jung, H.W. The geometry effect on steady electrokinetic flows in curved rectangular microchannels. Phys. Fluids 2010, 22, 052004. [Google Scholar] [CrossRef] [Green Version]
- Ertl, P.; Sticker, D.; Charwat, V.; Kasper, C.; Lepperdinger, G. Lab-on-a-chip technologies for stem cell analysis. Trends Biotechnol. 2014, 32, 245–253. [Google Scholar] [CrossRef]
- Esch, M.B.; Post, D.J.; Shuler, M.L.; Stokol, T. Characterization of in vitro endothelial linings grown within microfluidic channels. Tissue Eng. Part A 2011, 17, 23–24. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.P.; Herfkens, R.J.; Taylor, C.A. Comparison of abdominal aortic hemodynamic between men and women at rest and during lower limb exercise. Ann. Vasc. Surg. 2003, 37, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.P.; Herfkens, R.J.; Taylor, C.A. Abdominal aortic hemodynamic conditions in healthy subjects aged 50–70 at rest and during lower limb exercise: In vivo quantification using MRI. Atherosclerosis 2003, 168, 323–331. [Google Scholar] [CrossRef]
- Koutsiaris, A.G.; Tachmitzi, S.V.; Batis, N.; Kotoula, M.G.; Karabatsas, C.H.; Tsironi, E.; Chatzoulis, D.Z. Volume flow and wall shear stress quantification in the human conjunctival capillaries and post-capillary venules in-vivo. Biorheology 2007, 44, 375–386. [Google Scholar]
- Tsao, C.; Yeh, L.; Cheng, Y. Mechanical-stress microfluidic device for stem cell stimulation. In Proceedings of the IEEE International Nanoelectronics Conference (INEC), Sapporo, Japan, 28–31 July 2014; pp. 1–2. [Google Scholar] [CrossRef]
- Song, L.; Butler, P.; Wang, Y.; Hu, Y.; Han, D.C.; Usami, S.; Guan, J.L.S.; Chien, S. The role of the dynamics of focal adhesion kinase in the mechanotaxis of endothelial cells. Proc. Natl. Acad. Sci. USA 2002, 99, 3546–3551. [Google Scholar] [CrossRef] [Green Version]
- Nenad, F.; Milica, N.; Tijana, S. Simulation of organ-on-a-chip systems. New Technologies and Future Prospects. Woodhead Publ. Ser. Biomater. 2020, 28, 753–790. [Google Scholar] [CrossRef]
- Sang, S.; Tanga, X.; Feng, Q.; Jian, A.; Zhang, W. A zero-flow microfluidics for long-term cell culture and detection. AIP Adv. 2015, 5, 2158–3226. [Google Scholar] [CrossRef] [Green Version]
- Jessanne, Y.L.; Yue, L.; Seunghyun, K. Numerical Analysis of a Trapezoidal Microchannel for Hydrodynamic Detachment of Cells. Int. J. Eng. Adv. Technol. (IJEAT) 2020, 9, 2249–8958. [Google Scholar]
- Figueroa-Morales, N.; Mino, G.L.; Rivera, A.; Caballero, R.; Clément, E.; Altshuler, E.; Lindner, A. Living on the edge: Transfer and traffic of E. coli in a confined flow. Soft Matter 2015, 11, 6284–6293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, U.; Kim, S.; Park, J.; Jeon, J.S. Lipopolysaccharide-Induced Vascular Inflammation Model on Microfluidic Chip. Micromachines 2020, 11, 747. [Google Scholar] [CrossRef]
- Poon, C. Measuring the density and viscosity of culture media for optimized computational fluid dynamics analysis of in vitro devices. bioRxiv 2020. [Google Scholar] [CrossRef]
- Heldin, C.H.; Rubin, K.; Pietras, K.; Östman, A. High interstitial fluid pressure—An obstacle in cancer therapy. Nat. Rev. Cancer 2004, 4, 806–813. [Google Scholar] [CrossRef]
- Jastrzebska, E.; Flis, S.; Rakowska, A. A Microfluidic System to Study the Cytotoxic Effect of Drugs: The Combined Effect of Celecoxib and 5-Fluorouracil on Normal and Cancer Cells. Microchim. Acta 2013, 180, 895–901. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisapia, F.; Balachandran, W.; Rasekh, M. Organ-on-a-Chip: Design and Simulation of Various Microfluidic Channel Geometries for the Influence of Fluid Dynamic Parameters. Appl. Sci. 2022, 12, 3829. https://doi.org/10.3390/app12083829
Pisapia F, Balachandran W, Rasekh M. Organ-on-a-Chip: Design and Simulation of Various Microfluidic Channel Geometries for the Influence of Fluid Dynamic Parameters. Applied Sciences. 2022; 12(8):3829. https://doi.org/10.3390/app12083829
Chicago/Turabian StylePisapia, Francesca, Wamadeva Balachandran, and Manoochehr Rasekh. 2022. "Organ-on-a-Chip: Design and Simulation of Various Microfluidic Channel Geometries for the Influence of Fluid Dynamic Parameters" Applied Sciences 12, no. 8: 3829. https://doi.org/10.3390/app12083829
APA StylePisapia, F., Balachandran, W., & Rasekh, M. (2022). Organ-on-a-Chip: Design and Simulation of Various Microfluidic Channel Geometries for the Influence of Fluid Dynamic Parameters. Applied Sciences, 12(8), 3829. https://doi.org/10.3390/app12083829




